Abstract
A combination of pharmacologically induced hypertension and degeneration of elastic lamina by lysyl oxidase inhibition can cause aneurysm formations at two aneurysm-prone regions of the aorta. This model with or without modification has been successfully used to study pathophysiology of aortic aneurysms. Phenotypic differences between thoracic and abdominal aortic aneurysms in this model may indicate that different pharmacological strategies may be needed to prevent growth and rupture of aneurysms at these two different locations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Rupture of aortic aneurysm results in severe mortality and morbidity. Surgical or endovascular intervention for unruptured aortic aneurysms is to prevent future rupture. However, these procedures still carry significant risks of adverse events. Therefore, pharmacological stabilization of aneurysms that prevents growth and rupture of aortic aneurysms has been proposed [1]. In order to develop such strategy, an animal model that recapitulates key features of human aortic aneurysms is extremely useful.
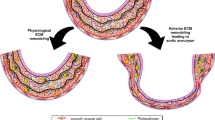
There is a close association between systemic hypertension and aortic aneurysm formations in humans [2, 3]. In addition, degeneration and disorganization of elastic lamina are characteristic histological changes observed in both thoracic and abdominal aortic aneurysms [4, 5]. Incidence of aortic aneurysms increases with age [6, 7]. Aging-related degeneration of elastic lamina mat represents a precursory change that precedes aneurysm formation [8].
In animals, degeneration of elastic lamina can be induced by administration of beta-aminopropionitrile (BAPN), an inhibitor of lysyl oxidase [9]. Lysyl oxidase cross-links elastin fibers and collagen fibers. While aging decreases lysyl oxidase activity in humans [10], BAPN is considered as a lathyrogen because its effects closely mimic human aging [11].
2 Animal Model
We combined hypertension and degeneration of elastic lamina by lysyl oxidase inhibitor, BAPN, to induce thoracic and abdominal aortic aneurysms in mice [12]. This model has been successfully used by multiple independent groups with or without modifications [13–19].
In this model, hypertension can be induced by two well-established methods of pharmacologically induced hypertension—angiotensin-II-induced hypertension and deoxycorticosterone acetate (DOCA)-salt hypertension. BAPN (150 mg/kg/day), a lysyl oxidase inhibitor, can be administered for the first 2 weeks through a subcutaneously implanted osmotic pump (Alzet, Durect Corp).
Figure 11.1 shows representative aortic aneurysms from this model. Aortic aneurysms in this model develop at the ascending thoracic aorta and abdominal aorta, the two locations where most human aortic aneurysms can be found [7, 12]. In addition, thoracic and abdominal aortic aneurysms in this model show site-specific morphological and histological characteristics [12]. Another key advantage of this new model is the use of wild-type mice, which makes it easier to examine roles of different signaling pathways compared to using knockout and transgenic mice.
Representative aortic aneurysms. Macroscopically, thoracic and abdominal aortic aneurysms in this model resembled human aortic aneurysms with their site-specific morphology. Thoracic aortic aneurysms were saccular-shaped with localized dilation at the great curvature, while abdominal aortic aneurysms were fusiform-shaped aneurysms with a thick vascular wall. (a) normal thoracic aorta; (b, c) unruptured thoracic aortic aneurysm; (d) ruptured thoracic aortic aneurysm; (e) normal abdominal aorta; (f, g) unruptured abdominal aortic aneurysm; (h) ruptured abdominal aortic aneurysm; (i) dissecting aortic aneurysm. Scale bar: 1 mm (This figure is reproduced with permission from the publisher (Kanematsu and Kanematsu [12]))
Using this study, we showed that the combination of hypertension and degeneration of elastic lamina by lysyl oxidase inhibition in mice resulted in formation of aortic aneurysms that recapitulate key features of human aortic aneurysms with site-specific phenotypes. In addition, we showed critical roles of high blood pressure in the formation of aortic aneurysms, establishing a causal link between hemodynamic conditions and aortic aneurysm formation in animals.
Daugherty et al. developed an abdominal aortic aneurysm model in genetically atherosclerosis-prone mice by continuously infusing angiotensin-II [20, 21]. In their angiotensin-II-induced aortic aneurysm model, apolipoprotein E (ApoE)-knockout mice or fat-fed low-density lipoprotein (LDL) receptor knockout mice was used [20, 21]. Morphological and histological characteristics of angiotensin-II-induced abdominal aortic aneurysms in these knockout mice were similar to the abdominal aortic aneurysms in our model, indicating that common molecular mechanisms potentially exist between these two models in respect to abdominal aortic aneurysms. Interestingly, angiotensin-II infusion in ApoE-knockout or LDL receptor knockout mice did not cause thoracic aortic aneurysm [20, 21]. In contrast, aneurysm formation in our model occurred not only in the abdominal aorta but also in the thoracic aorta involving the ascending aorta.
Direct application of calcium chloride to the descending thoracic aorta through thoracotomy can cause aneurysmal formation in the aortic segment that was exposed to calcium chloride [22]. The advantage of the calcium application model is that aneurysmal dilatation occurred in almost all animals [22]. However, the aneurysmal dilatation in their model was mild, i.e., 25 % dilatation comparing to 50 % in our model. More importantly, in our model, both abdominal and thoracic aneurysms were induced by the same pharmacological treatments. Our model may be more suitable for studying differential underlying mechanisms and treatment strategies between thoracic and abdominal aortic aneurysms.
In our model, the aneurysms at the two aneurysm-prone regions were induced by the same systemic pharmacological treatment. However, they exhibited different morphological and histological features that closely resembled human aortic aneurysms at the respective locations. Morphological and histological differences observed between thoracic and abdominal aortic aneurysms in this model may suggest that differential responses to the combination of hypertension and lysyl oxidase inhibition at these two regions of the aorta lead to different phenotypes of aneurysms.
Morphological and histological differences between thoracic and abdominal aortas in this model and in humans may be due to the differences in developmental origins of smooth muscle cells [23, 24]. Embryologically programmed differences of vascular smooth muscle cells may determine the site-specific phenotypes of aneurysms at the two regions [23–25]. More importantly, different pharmacological strategies may be needed to prevent growth and rupture of aneurysms at these two different locations.
Interestingly, normalization of blood pressure by an antihypertensive agent dramatically reduced the incidence of aneurysms and almost completely abolished histological changes associated with angiotensin-II and BAPN treatment in this model. We were able to reproduce thoracic and abdominal aortic aneurysms when DOCA-salt hypertension was used. Captopril did not reduce the incidence of aortic aneurysm in DOCA-salt-hypertensive mice, further suggesting critical roles of hypertension in this model.
It should be noted that although our mouse model replicated key features of thoracic and abdominal aortic aneurysms in humans, aneurysms in this model did not form spontaneously but were induced by two pharmacological interventions, which potentially bypassed some of the early critical events that lead to aortic aneurysm in humans.
3 Conclusions
A combination of pharmacologically induced hypertension and degeneration of elastic lamina by lysyl oxidase inhibition can cause aneurysm formations at two aneurysm-prone regions of aorta. Using this model, we established critical roles of hypertension in the formation of aortic aneurysms. Phenotypic differences between thoracic and abdominal aortic aneurysms in this model may indicate that different pharmacological strategies may be needed to prevent growth and rupture of aneurysms at these two different locations.
References
Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, Thompson MM. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33(12):2858–64. PubMed.
Dapunt OE, Galla JD, Sadeghi AM, Lansman SL, Mezrow CK, de Asla RA, Quintana C, Wallenstein S, Ergin AM, Griepp RB. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 1994;107(5):1323–32; discussion 32–3. PubMed.
Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126(6):441–9. PubMed.
Tang PC, Coady MA, Lovoulos C, Dardik A, Aslan M, Elefteriades JA, Tellides G. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation. 2005;112(8):1098–105. PubMed.
Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150(3):993–1007. Epub 1997/03/01. PubMed PMID: 9060837; PMCID: 1857880.
Albornoz G, Coady MA, Roberts M, Davies RR, Tranquilli M, Rizzo JA, Elefteriades JA. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82(4):1400–5. Epub 2006/09/26. doi: S0003-4975(06)01020-4 [pii] 10.1016/j.athoracsur.2006.04.098. PubMed.
Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111(6):816–28. Epub 2005/02/16. doi: 111/6/816 [pii] 10.1161/01.CIR.0000154569.08857.7A. PubMed.
Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39(1):13–20. PubMed.
McCallum HM. Experimental lathyrism in mice. J Pathol Bacteriol. 1965;89:625–36. PubMed.
Behmoaras J, Slove S, Seve S, Vranckx R, Sommer P, Jacob MP. Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation Res. 2008;11(5):883–9. PubMed.
Davies I, Schofield JD. Connective tissue ageing: the influence of a lathyrogen (beta-aminopropionitrile) on the life span of female C57BL/Icrfat mice. Exp Gerontol. 1980;15(5):487–94. PubMed.
Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. hypertension. 2010;55(5):1267–74. Epub 2010/03/10. doi: HYPERTENSIONAHA.109.140558 [pii] 10.1161/HYPERTENSIONAHA.109.140558. PubMed PMID: 20212272; PMCID: PMC2859958.
Remus EW, O’Donnell RE, Jr., Rafferty K, Weiss D, Joseph G, Csiszar K, Fong SF, Taylor WR. The role of lysyl oxidase family members in the stabilization of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2012;303(8):H1067–75. doi: 10.1152/ajpheart.00217.2012. PubMed PMID: 22904155; PMCID: 3469640.
Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, Weiss SJ, Itoh H, Hori S, Aikawa N, Okada Y. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126(25):3070–80. doi:10.1161/CIRCULATIONAHA.112.097097. PubMed.
Kurobe H, Hirata Y, Matsuoka Y, Sugasawa N, Higashida M, Nakayama T, Maxfield MW, Yoshida Y, Shimabukuro M, Kitagawa T, Sata M. Protective effects of selective mineralocorticoid receptor antagonist against aortic aneurysm progression in a novel murine model. J Surg Res. 2013;185(1):455–62. doi:10.1016/j.jss.2013.05.002. PubMed.
Kurobe H, Matsuoka Y, Hirata Y, Sugasawa N, Maxfield MW, Sata M, Kitagawa T. Azelnidipine suppresses the progression of aortic aneurysm in wild mice model through anti-inflammatory effects. J Thorac Cardiovasc Surg. 2013;146(6):1501–8. doi:10.1016/j.jtcvs.2013.02.073. PubMed.
Takayanagi T, Crawford KJ, Kobayashi T, Obama T, Tsuji T, Elliott KJ, Hashimoto T, Rizzo V, Eguchi S. Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clinical science. 2014;126(11):785–94. doi: 10.1042/CS20130660. PubMed PMID: 24329494; PMCID: 4019733.
Obama T, Tsuji T, Kobayashi T, Fukuda Y, Takayanagi T, Taro Y, Kawai T, Forrester SJ, Elliott KJ, Choi E, Daugherty A, Rizzo V, Eguchi S. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin Sci. 2015;128(9):559–65. doi:10.1042/CS20140696. PubMed.
Anzai A, Shimoda M, Endo J, Kohno T, Katsumata Y, Matsuhashi T, Yamamoto T, Ito K, Yan X, Shirakawa K, Shimizu-Hirota R, Yamada Y, Ueha S, Shinmura K, Okada Y, Fukuda K, Sano M. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res. 2015;116(4):612–23. doi:10.1161/CIRCRESAHA.116.304918. PubMed.
Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105(11):1605–12. PubMed.
Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann N Y Acad Sci. 1999;892:108–18. Epub 2000/06/08. PubMed.
Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, Spinale FG. A murine model of thoracic aortic aneurysms. J Surg Res. 2003;115(1):157–63. Epub 2003/10/24. doi: S0022480403001938 [pii]. PubMed.
Guo DC, Papke CL, He R, Milewicz DM. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:339–52. PubMed.
Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(9):1621–6. PubMed.
Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–58. PubMed.
Sources of Funding
The project described was supported by R01NS055876 (TH) and R01NS082280 (TH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS).
Disclosures
No conflicts.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Kanematsu, M., Kanematsu, Y., Hashimoto, T. (2016). Mouse Models of Aortic Aneurysm. In: Sata, M. (eds) Mouse Models of Vascular Diseases. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55813-2_11
Download citation
DOI: https://doi.org/10.1007/978-4-431-55813-2_11
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55811-8
Online ISBN: 978-4-431-55813-2
eBook Packages: MedicineMedicine (R0)