Abstract
Autophagy is a eukaryotic, nonspecific degradation mechanism and serves as part of the innate immune system of host cells. In Helicobacter pylori-infected host cells, autophagy is activated by the bacterial vacuolating cytotoxin A (VacA) via the following pathway: VacA binds to low-density lipoprotein receptor-related protein-1, induces intracellular glutathione deficiency, and enhances activation of protein kinase B (Akt), which in turn induces Mdm2-mediated p53 degradation and then activates autophagy. Translocated bacterial proteins, VacA and CagA, are degraded by autophagy. However, autophagy is not activated in the CD44v9-expressing gastric cancer stem-like cells, leading to the specific accumulation of intracellular CagA. Therefore, the presence of CD44v9-expressing cells in H. pylori-infected patients is associated with the risk of developing gastric cancer. Autophagy in the host gastric epithelial cells plays an important role in H. pylori infectious disease via degradation of VacA and CagA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Induction of Autophagy by VacA in Host Epithelial Cells Infected with Helicobacter pylori
Autophagy is a nonspecific degradation mechanism seen in eukaryotic cells. Targets for degradation by autophagy include intracellular components such as mitochondria, where targets are surrounded by the cellular double membrane to form transient autophagosomes and are degraded by fusion with lysosomes. Autophagy is an important cellular recycling mechanism to overcome temporary starvation, in addition to removing intracellular foreign materials. In particular, intracellular parasitic bacteria such as group A streptococci, Shigella spp., Salmonella spp., and Listeria monocytogenes are degraded by autophagy. In fact, autophagy serves as part of the innate immune system of host cells. In recent years, it has been revealed that autophagy is activated in Helicobacter pylori-infected cells, interestingly, by an exotoxin known as vacuolating cytotoxin A (VacA), which is produced by the bacterium and is involved in ulceration. VacA is biologically versatile and shows apoptosis-inducing activity via mitochondrial pathway [1]. In a recent study, Terebiznik et al. indicated that VacA induces autophagy [2, 3]. VacA is composed of p55 fragments involved in receptor recognition and an N-terminal (p33) fragment involved in vacuolization. Further, the p55 fragment can be divided into m1 type (m1VacA) and m2 type (m2VacA) due to the difference in primary structure. Receptor protein-tyrosine phosphatase (RPTP) α and β have been identified as receptors for VacA [4, 5]. Additionally, Yahiro et al. recently indicated that low-density lipoprotein receptor-related protein-1(LRP1) is a receptor for VacA and this binding is important for induction of autophagy [6]. Our study demonstrated that although m1VacA bound to LRP1, m2VacA did not [7]. Thus, m2VacA probably does not induce autophagy.
Interestingly, m1VacA causes a reduction in intracellular glutathione (GSH) levels in the host epithelial cells, resulting in the accumulation of intracellular reactive oxygen species (ROS) [7]. It is well known that accumulation of intracellular ROS activates autophagy. In fact, autophagy induced by m1VacA is repressed by treatment with antioxidants such as N-acetylcysteine [7]. In addition, m1VacA also induces p53 downregulation during autophagy. Tasdemir et al. reported that p53 inactivation by chemical inhibition or knockdown induces autophagy via inhibition of mTOR [8]. Our study showed that p53 downregulation by m1VacA is necessary for the induction of autophagy [7]. In fact, the autophagic pathway via m1VacA is as follows: m1VacA induces GSH deficiency by binding to LRP1 and enhances activation of protein kinase B (Akt), which in turn induces Mdm2-mediated p53 degradation, activating autophagy [7] (Fig. 5.1).
Recent studies have revealed that p62 binds to LC3 on the autophagosomal membrane to target ubiquitinated aggregates for selective degradation [9]. Yahiro et al. demonstrated that LC3-II is colocalized with p62 on the autophagosomal puncta induced by VacA [6]. Therefore, autophagy activated by VacA is considered selective and involves targeting by ubiquitination. Further studies are needed to clarify the selective mechanisms of VacA-activated autophagy.
2 Significance of Autophagy in Host Epithelial Cells Infected with H. pylori
H. pylori is a significant risk factor for the development of peptic ulcers and gastric cancer; therefore, in this chapter, we focus on the role of autophagy in H. pylori infection.
VacA binds to host cell-surface receptors and is internalized via endocytosis. An in vitro study demonstrated that VacA induces vacuolization and apoptosis in host epithelial cells [1]. These activities have been implicated to cause induced gastric mucosal injury in gastric ulcers during H. pylori infection [10]. Recently, it has been reported that intracellular VacA is degraded by autophagy activated by VacA itself [2, 3]. Therefore, decreased activation of autophagy by genetic polymorphisms of autophagy-related genes ATG16L1 contributes to increase in VacA-mediated toxicity [2, 3].
The CagA effector protein is delivered into H. pylori-attached host epithelial cells via type IV secretion system (TFSS). Translocated CagA binds to and dysregulates SHP-2 tyrosine phosphatase and specifically interacts with the polarity-regulating kinase partitioning-defective 1 (Par1b)/microtubule affinity-regulating kinase 2 (MARK2) to disrupt tight junctions and cause a loss of epithelial apical-basolateral cell polarity. Additionally, it has been reported that CagA-transgenic mice developed gastrointestinal carcinomas. These observations indicate that CagA effector protein is a bacterial oncoprotein, involved in gastric carcinogenesis. However, translocated CagA is degraded by autophagy induced by VacA, and, as a result, CagA does not persist in host gastric epithelial cells [7].
Thus, it is thought that autophagy induced by VacA contributes to a reduction in gastric mucosal injury and gastric cancer risk associated with H. pylori infection, via degradation of VacA and CagA. On the other hand, characteristic alterations in the host cell, involved in the repression of autophagy, induce the accumulation of intracellular VacA and CagA. Thus, it is presumed that inhibition of autophagy may lead to increased gastric cancer risk via specific accumulation of CagA. Therefore, autophagy response in host gastric epithelial cells is considered to play an important role in the development of gastric carcinogenesis.
3 Suppression of Autophagy and Accumulation of CagA in CD44v9–Expressing Cancer Stem-Like Cells
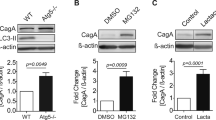
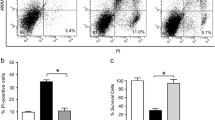
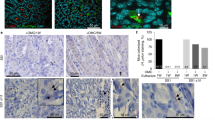
CD44 is a cell-surface marker associated with cancer stem cells in various tumors. Gastric cancer stem-like cells express a variant isoform of CD44 (CD44v9). A recent study reported that the recurrence rate of early gastric cancer (EGC) is significantly higher in CD44v9-positive individuals than in CD44v9-negative individuals [11]. CD44v9-expressing gastric cancer cells suppress ROS accumulation via control of intracellular GSH levels by stabilizing xCT, a cystine transporter [12]. Since induction of autophagy by VacA requires the reduction of GSH, we hypothesize that autophagy is not activated by VacA in CD44v9-expressing cells. In fact, intracellular GSH levels in CD44v9-expressing cells are not decreased by VacA [7]. Further, increase of Akt, Mdm2 phosphorylation, and p53 degradation are also not observed in CD44v9-expressing cells; hence, autophagy is not induced by VacA [7]. In addition, translocated CagA accumulates in CD44v9-expressing cells (Fig. 5.2), which is reversed with sulfasalazine, a potent xCT inhibitor and a well-known anti-inflammatory drug for rheumatic arthritis and inflammatory bowel disease. Moreover, autophagy is activated in CD44v9-expressing cells by treatment with sulfasalazine [7]. These observations reveal that specific accumulation of intracellular CagA in CD44v9-expressing cancer stem-like cells is caused by the repression of autophagy. Therefore, the presence of CD44v9-expressing cells in H. pylori-infected patients is associated with the risk of developing gastric cancer.
References
Yamasaki E, Wada A, Kumatori A, et al. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J Biol Chem. 2006;281:11250–9.
Terebiznik MR, Raju D, Vazquez CL, et al. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5:370–9.
Raju D, Hussey S, Ang M, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142:1160–71.
Yahiro K, Niidome T, Kimura M, et al. Activation of Helicobacter pylori VacA toxin by alkaline or acid conditions increases its binding to a 250-kDa receptor protein-tyrosine phosphatase beta. J Biol Chem. 1999;274:36693–9.
Yahiro K, Wada A, Nakayama M, et al. Protein-tyrosine phosphatase alpha, RPTP alpha, is a Helicobacter pylori VacA receptor. J Biol Chem. 2003;278:19183–9.
Yahiro K, Satoh M, Nakano M, et al. Low-density Lipoprotein Receptor-related Protein-1 (LRP1) mediates autophagy and apoptosis caused by Helicobacter pylori VacA. J Biol Chem. 2012;287:31104–15.
Tsugawa H, Suzuki H, Saya H, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–77.
Tasdemir E, Maiuri MC, Galluzzi L, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–87.
Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–69.
Fujikawa A, Shirasaka D, Yamamoto S, et al. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375–81.
Hirata K, Suzuki H, Imaeda H, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379–86.
Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Tsugawa, H., Suzuki, H. (2016). Autophagy. In: Suzuki, H., Warren, R., Marshall, B. (eds) Helicobacter pylori. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55705-0_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55705-0_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55704-3
Online ISBN: 978-4-431-55705-0
eBook Packages: MedicineMedicine (R0)