Abstract
Prior to recent advances in therapeutics, various aspects of rare diseases, such as their etiology, natural history, and evaluation items, were not well understood. Yet, in reality, only a few studies have been conducted on rare diseases, given the difficulty of obtaining a sufficient patient sample size for data collection. In this study, we performed questionnaire and retrospective medical record surveys, as well as a prospective natural history study of patients with UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase gene (GNE) myopathy, which is also referred to as distal myopathy with rimmed vacuoles (DMRV). This study aimed to identify evaluation tools for use in upcoming clinical trials and to clarify the natural history of GNE myopathy and life-threatening risk factors related to disease progression in a historical control. Moreover, in order to inform patients with GNE myopathy, physicians, researchers, and companies of progress in clinical research, we established a patient registry system. Our efforts are aimed not only at preparing for upcoming clinical trials, but also to provide information regarding the nature of the disease and advice on preferable patient care. International cooperation will improve the understanding of GNE myopathy and promote clinical progress by providing access to a larger cohort.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Clinical progress can present challenges to physicians, who need to adapt and accurately diagnose and evaluate diseases in view of new developments. To date, many clinical trials have been conducted to evaluate the natural history of muscle disorders.
GNE myopathy, also known as distal myopathy with rimmed vacuoles (DMRV), is an early adult-onset myopathy with slow progression that preferentially affects the tibialis anterior muscle and commonly spares the quadriceps femoris muscles [1, 2]. The disease is caused by a mutation in the GNE gene, which encodes a bifunctional enzyme [uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc) 2-epimerase (GNE) and N-acetylmannosamine kinase (NMK)] that catalyzes two rate-limiting reactions in cytosolic sialic acid synthesis [3–7]. Oral sialic acid metabolite treatment prevents muscle atrophy and weakness in a mouse GNE myopathy model [8]. A recent phase I clinical trial with oral sialic acid was conducted in Japan (ClinicalTrials.gov; identifier, NCT01236898), and a phase II study is currently underway in the United States and Israel (ClinicalTrials.gov; identifier, NCT01517880).
To prepare for global phase III clinical trials, we believe it is necessary to gain a better understanding of the natural history of GNE, as well as its progression. To this end, we first conducted questionnaire and medical record surveys among patients with GNE to obtain a rough understanding of the natural history of the disease. Second, we performed a prospective study on the natural history of patients with confirmed GNE myopathy in order to identify evaluation items that can be used to monitor disease progression. Finally, we generated a patient registry of Japanese patients with GNE myopathy with the aim of recruiting patients to clinical trials and as an information tool on healthcare and research progress for patients, physicians, researchers, and companies. Through these efforts, we provide a view of the clinical progress in GNE myopathy and discuss the significance of rare-disease translational research for physicians.
10.1.1 Retrospective Questionnaire and Medical Record Surveys
10.1.1.1 Aims
To date, no natural history study has been conducted on the genetics of the GNE gene. Indeed, some studies to date have denied significant genotype–phenotype correlations in GNE myopathy [7]. However, we suspected that disease severity might be related to the genotype of the GNE gene, given the existence of severely affected, early onset patients, as well as mildly affected, late onset patients. Some patients also exhibit respiratory dysfunction, although there have been no reports on the involvement of respiratory dysfunction in GNE myopathy. Against this backdrop, we assessed (1) disease onset, ambulatory status, and respiratory dysfunction and (2) genotype–phenotype correlations. This study was approved by the Medical Ethics Committee of the National Center of Neurology and Psychiatry (NCNP).
10.1.1.2 Study Design
We first performed a questionnaire survey of patients with confirmed GNE myopathy. The questionnaire was provided to patients by physicians at eight hospitals specializing in neuromuscular diseases, including the NCNP. This study was a retrospective and cross-sectional analysis and involved 71 patients with genetically confirmed GNE myopathy. Clinical information was collected from patients using the questionnaire, and genetic information was acquired from available medical records. We then conducted a medical record survey of NCNP patients in order to obtain their clinical characteristics. The presence or absence of respiratory failure was of particular interest in this aspect of the study.
10.1.1.3 Results
Mean age at symptom onset was 25.2 ± 9.2 years (range, 12–58 years; median, 24.5 years), 52.0 % (n = 37/71) were ambulant (41.3 ± 12.8 years), 15.5 % (n = 11/71, 40.0 ± 13.6 years) could walk without assistance, and 35.2 % required assistance (n = 25/71, 41.8 ± 12.7 years). The median age at which assistance was required for walking was 30.0 ± 1.4 years, the median age of wheelchair users was 36.0 ± 2.7 years, and the median age at loss of ambulation was 45.0 ± 4.2 years. Durations from disease onset to walking with assistance, wheelchair use, and loss of ambulation were 7.0 ± 0.4 years, 11.5 ± 1.2 years, and 17.0 ± 2.1 years, respectively. We also identified potential genotype–phenotype correlations. V572L homozygotes (i.e., mutation in the NMK domain), the most frequent mutation in Japan, had more severe phenotypes than V572L/D176V (i.e., mutations in both GNK/MNK domains) compound heterozygotes, the most frequent type of compound heterozygote in Japan. It was unclear whether each individual mutation contributed to these differences or whether the combination of mutations was important; GNK/MNK mutations tended to be more severely affected. The medical record survey revealed that some patients had respiratory dysfunction which correlated with disease severity. Some reports have suggested that patients with advanced disease may require respiratory support [8, 9]. Differences in disease severity between V572L homozygotes and D176V/V572L compound heterozygotes were also found in a study using the NCNP muscle bank [10].
Unresolved questions include genotype–phenotype correlations in patients with mutations other than V572L and D176V/V572L, as well as genotype–phenotype relationships with respect to cardiac impairment observed in the mouse GNE model [11].
10.1.2 Prospective Natural History Study
10.1.2.1 Aims
This study aimed to identify evaluation tools for use in upcoming clinical trials. As mentioned above, a phase II study is currently underway in the United States and Israel. We believe that further insight into the natural history of the disease will be beneficial for preparing for phase III clinical trials. Thus, we aimed to identify evaluation items that can be used to detect disease progression within a year, with respect to observation duration of clinical trials.
10.1.2.2 Patients and Methods
A total of 24 Japanese patients (9 men and 15 women) participated in this study. Two women were siblings and the rest were unrelated. Mean age at the time of data collection was 43.0 ± 12.9 years (mean ± SD), and mean age at disease onset was 25.9 ± 10.3 years (range, 15–58 years; median, 24 years). Of the 24 patients, 9 (36.0 %) were ambulant, 8 completed the 6-min walk test (6MWT) without assistance, 1 required assistance (e.g., cane and/or ankle brace) and could not complete the 6MWT, and 15 (64.0 %) had lost ambulation.
All patients rested for more than 2 h before each muscle strength test. Measurements using a handheld dynamometer for knee extension in the sitting position, grip power, and pinch power were repeated three times on both the right and left sides, and all six measurements were averaged for data analysis. Muscle strength tests, including manual muscle testing (MMT) and gross motor function measure (GMFM, Japanese version; range, 0–100 [%]), were performed to examine 17 muscle groups (neck flexion, truncal flexion, shoulder abduction, shoulder adduction, shoulder flexion, shoulder extension, elbow flexion, elbow extension, wrist flexion, wrist extension, hip flexion, thigh adduction, thigh abduction, knee extension, knee flexion, ankle dorsiflexion, and plantar flexion). Results of right and left MMTs were averaged, except for those corresponding to neck and truncal flexion. The summed MMT value (range, 0–85) was obtained from the sum of the 17 muscle groups. The 6MWT was performed among patients who were able to walk without assistance.
10.1.2.3 Results
At baseline, eight, eight, and six patients were too weak to complete measurements for HHD, grip power, and pinch power, respectively. In two patients, noninvasive positive pressure ventilation (NPPV) for respiratory failure was started at night due to newly diagnosed respiratory dysfunction and hypoxemia during hospitalization for baseline evaluation. None of the patients presented with disease-specific cardiac dysfunction.
A significant reduction in summed MMT (p < 0.01), grip power (p = 0.034), and the percentage of forced vital capacity (%FVC) (p = 0.030) and a nonsignificant reduction in 6MWT (p = 0.061) scores, GMFM (p = 0.089), and CK (p = 0.087) were observed (Fig. 10.1). Among all muscles examined, shoulder extension (p = 0.017) and abduction (p = 0.029) and knee flexion (p = 0.010) showed significant annual decreases. Seven of eight ambulant patients showed deteriorations in 6 MW distance within 1 year (Fig. 10.1a). Changes in %FVC (p = 0.034) were greater in nonambulant patients than in ambulant patients [12].
Annual changes in motor function. Right column, ambulant patients; left column, nonambulant patients. a 6MWT; b, c summed MMT; d, e grip power; and f, g %FVC. All patients, with the exception of one (*), showed deterioration in 6MWT. (a) Only one patient with improved 6MWT succeeded in weight control and had more opportunities to walk relative to baseline. Both ambulant (b) and nonambulant (c) patients showed deterioration in summed MMT. The decrease in grip power was greater in ambulant patients (d, e), whereas the decrease in %FVC was greater in nonambulant patients
10.1.2.4 Limitations and Conclusions
Summed MMT, grip power, and %FVC significantly changed over the course of a year. Although results of the 6MWT were not significant, which likely reflects the small number of ambulant patients, a larger cohort may clearly detect the deterioration. The 6MWT and summed MMT are important end-point item candidates for clinical trials because they can be used to determine annual changes in disease progression. Our study showed reductions in respiratory function, especially among nonambulant patients, suggesting that %FVC is a useful outcome measure for nonambulant patients. Our data suggest that GNE myopathy does not involve cardiomyopathy, although cardiac involvement was previously implicated in a mouse model [11].
Limitations include the small number of patients and short study period. In rare diseases such as GNE myopathy, large-scale studies tend to be difficult.
In conclusion, 6MWT, summed MMT, GMFM, grip power tests, and %FVC may be good clinical evaluation tools for clinical trials and to correlate with disease progression, although %FVC and grip power should be used according to ambulation status.
10.1.3 Patient Registry Development
10.1.3.1 Aims
Our previous studies made clear the limitations of natural history studies, which involved small sample populations. In order to gain further insight into GNE myopathy for the purpose of improving therapy and care, more patient data are required. To this end, we aimed to develop a nationwide patient registry for GNE myopathy in order to facilitate the planning of clinical trials and recruitment of candidates and to disseminate standard care and current information to patients, physicians, and researchers.
10.1.3.2 Study Design
Medical records of genetically confirmed patients with GNE myopathy at the NCNP were retrospectively reviewed in order to obtain data reflecting the severity and progression of the disease. Items selected for the registration sheet included age, sex, age at onset, past history and complications, family history, body weight and height, pathological findings from muscle biopsy, grip power, walking ability, respiratory function, cardiac function, willingness to join upcoming clinical trials, and participation in patient associations. A copy of the original genetic analysis report was required of each patient.
10.1.3.3 Institution, Organization, Registration Method, Data Collection, and Ethical Approval
In 2009, we developed a national registry for neuromuscular diseases (Registry of Muscular Dystrophy, Remudy; http://www.Remudy.jp/, see also Chap. 11) in Japan in collaboration with the TREAT-NMD Alliance in order to aid in the recruitment of eligible patients for clinical trials, provide information regarding the natural history and epidemiology of diseases, and serve as a source of information on current clinical care [13]. Remudy is supported by Intramural Research Grants (23-4/26-7) for Neurological and Psychiatric Disorders from the NCNP. Registry information was provided to interested individuals and their informed consent was obtained. Individuals whose data were included were informed that inclusion in the database confers no obligation to the patient and that they will be removed from the registry immediately upon request. They were also told that refusal to participate would not affect subsequent medical care. Study objectives, design, risks, and benefits of participation were explained to all patients, and their written informed consent was obtained prior to enrollment.
10.1.3.4 Results
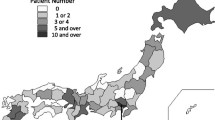
As of the end of October 2013, a total of 121 Japanese patients with GNE myopathy (55 men and 66 women) had registered. Mean ages at data collection and disease onset were 44.9 ± 13.2 years (median, 43 years; range, 21–85 years) and 27.9 ± 9.6 years (median, 26 years; range, 12–61 years), respectively. The registry included patients from throughout Japan (38/47 prefectures) who were recruited through a collaboration with 92 attending physicians from 73 institutes (Fig. 10.2). Three patients had a past history of idiopathic thrombocytopenia (ITP).
Thirty-nine of 121 patients (32.3 %) harbored a homozygous mutation in GNE, and 78 of 121 (64.5 %) had a compound heterozygous mutation. Only one heterozygous mutation was found in four (3.3 %) patients. Among those with a homozygous mutation, 82 % (32/39), 8 % (3/39), and 5 % (2/39) harbored p. V572L, p. C13S, and p. M172T mutations, respectively. A homozygous mutation of p. D176V was identified in only one patient. Of those carrying two heterozygous mutations, 31 % (24/78) had p. D176V/p. V572L mutations, while the remaining patients had other combinations of mutations.
Mean age at disease onset was 27.7 ± 9.6 years (median, 27.5 years; interquartile range, 15–61), 20 % (24/121) were ambulant without assistance, 37 % (45/121) required assistance (e.g., canes and/or braces), and 43 % (52/121) had lost ambulation. Mean age at loss of ambulation was 35.4 ± 11.3 years. Kaplan–Meier analysis revealed median durations from disease onset to walking with assistance, wheelchair use, and loss of ambulation of 8.9 years (95 % CI, 6.3–9.7), 14.0 years (95 % CI, 11.8–16.2), and 21.0 years (95 % CI, 15.4–26.6), respectively.
Information on pulmonary and cardiac function was available for 65 % (79/121) and 34 % (41/121) of patients, respectively. Of those examined, 33 % (26/79) had respiratory dysfunction (%FVC < 80), and two were using nocturnal NPPV. %FVC was significantly correlated with disease duration (ρ = 0.479, p < 0.01) and serum CK levels (ρ = 0.573, p < 0.01). None of those who underwent ultrasound cardiographic examination had cardiac dysfunction (ejection fraction, 50–82 %; fraction shortening (FS), 25–50 %). Mean serum CK level was 459.1 ± 355.0 IU/L (median, 202; range, 11–3133).
The patient registry is useful in that it allows for recruiting patients and resolving data deviation in comparison with analyses by isolated institutions. For example, the age at disease onset in the Remudy cohort was later than that determined from an analysis of medical records at the NCNP Hospital (26.8 ± 9.0 years). In a previous questionnaire-based study of core muscle disease center patients, we reported median durations from disease onset to walking with assistance, wheelchair use, and loss of ambulation of 7.0 ± 0.4 years, 11.5 ± 1.2 years, and 17.0 ± 2.1 years, respectively [8], which were all shorter than the durations determined in the present study. We speculate that this discrepancy reflects the more advanced disease status of patients at neuromuscular disease-specialized center hospitals. Patients with GNE myopathy were widely distributed throughout Japan, with 1.7 patients per hospital and 1.3 patients per physician. There were fewer patients per physician or per hospital than for dystrophinopathy (5.8 patients per hospital and 3.6 per physician). Thus, while patients with GNE myopathy appeared to be dispersed throughout Japan, those with dystrophinopathy were concentrated in specialized hospitals, given the need for cardiopulmonary care.
We have been publishing bulletins every 3 months and sending them to patients and physicians who join Remudy. The bulletin includes useful information regarding clinical care, translational medicine, and clinical trials, as well as articles introducing specialists and specialized hospitals for muscle diseases. These contents are available on the Remudy home page. Patient recruitment has also started for additional phase I clinical trials via the Remudy GNE myopathy registry home page [14].
10.2 Discussion
Through the three studies presented herein, we revealed various new aspects of GNE myopathy. First, unlike previous reports, we found that GNE myopathy is associated with respiratory dysfunction. Indeed, some patients required a respirator, indicating that respiratory dysfunction can be life threatening in this population. Physicians should take careful note of any abnormalities in respiratory function, which may serve as an evaluation tool for disease progression and therapeutic effects in the late stages of the disease. Notably, respiratory failure was not considered to be associated with GNE myopathy, despite GNE myopathy being one of the most common muscle diseases in Japan. One reason for this is that physicians may be of the mindset that respiratory failure does not derive from myopathy itself, given the small number of affected patients they see. Second, given that GNE myopathy is progressive, walk tests such as the 6MWT and other quantitative methods, and respiratory function for nonambulant patients, may be useful evaluation items for annual disease progression and clinical trials. Finally, patient registries are useful for rare diseases such as GNE myopathy, as it allows for the building of a larger cohort, which can be used to assess more accurately etiologic aspects of the disease compared to medical record surveys from specialized hospitals. Such registries are also useful in that they allow for recruiting patients and resolving data deviation in comparison with analyses by isolated institutions.
The direct motivation to conduct these studies stemmed from the need to prepare for upcoming clinical trials and follow the development of new therapies and improved care methods. Comparisons with animal disease models will also provide insight into new symptoms, which in turn may provide insight into patient care. We note that our patients were very cooperative, as they were aware of the importance of these studies in driving the development of new therapies.
We recognize that a large cohort study is essential to gain a better understanding of rare diseases, including progression and potential risk factors. Yet our data were not sufficient to make conclusive genotype–phenotype correlations, so this will be an important aspect for further elucidation of the disease pathogenesis. Thus, studies similar to those described herein with a larger cohort are warranted.
Similar to our collaborations with the dystrophinopathy registry, we are currently in discussions to join the international registry of GNE myopathy of the TREAT-NMD Alliance [ClinicalTrials.gov; identifier, NCT01784679, http://www.treat-nmd.eu/gne/patient-registries/patient-registries/]. Our Japanese registry and the TREAT-NMD Alliance registry work in close collaboration and will serve as irreplaceable infrastructures that accelerate research, therapy development, and trial readiness, in addition to increasing opportunities for collaboration and improving global patient care.
References
Nonaka I, Sunohara N, Satoyoshi E et al (1985) Autosomal recessive distal muscular dystrophy: a comparative study with distal myopathy with rimmed vacuole formation. Ann Neurol 17:51–59
Argov Z, Yarom R (1984) “Rimmed vacuole myopathy” sparing the quadriceps. a unique disorder in Iranian Jews. J Neurol Sci 64:33–43
Nishino I, Noguchi S, Murayama K et al (2002) Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology 59:1689–1693
Eisenberg I, Avidan N, Potikha T et al (2001) The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet 29:83–87
Kayashima T, Matsuo H, Satoh A et al (2002) Nonaka myopathy is caused by mutations in the UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase gene (GNE). J Hum Genet 47:77–79
Keppler OT, Hinderlich S, Langner J et al (1999) UDP–GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science 284:1372–1376
Malicdan MC, Noguchi S, Hayashi YK et al (2009) Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med 15:690–695
Mori-Yoshimura M, Monma K, Suzuki N et al (2012) GNE myopathy (distal myopathy with rimmed vacuoles) patients with mutations in the UDP-GlcNAc 2-epimerase and in the N-acetylmannosamine kinase domains of the GNE gene exhibit less severe phenotypes than patients with mutations only in MNK domain. J Neurol Sci 318(1–2):100–105
Mori-Yoshimura M, Oya Y, Hayashi YK, Noguchi S, Nishino I, Murata M (2013) Respiratory dysfunction in patients severely affected by GNE myopathy (distal myopathy with rimmed vacuoles). Neuromuscul Disord 23:84–88
Cho A, Hayashi YK, Monma K et al (2014) Mutation profile of the GNE gene in Japanese patients with distal myopathy with rimmed vacuoles (GNE myopathy). J Neurol Neurosurg Psychiatry 85:914–917
Malicdan MC, Noguchi S, Nonaka I et al (2007) A Gene knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 16:2669–2682
Mori-Yoshimura M, Oya Y, Yajima H et al (2014) GNE myopathy: a prospective natural history study of disease progression. Neuromuscul Disord 24:380–386
Nakamura H, Kimura E, Mori-Yoshimura M, Komaki H, Matsuda Y, Goto K, Hayashi YK, Nishino I, Takeda SI, Kawai M (2013) Characteristics of Japanese Duchenne and Becker muscular dystrophy patients in a novel Japanese national registry of muscular dystrophy (Remudy). Orphanet J Rare Dis 19:60
UMIN CTR website. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000013489&language=J
Acknowledgments
We thank members of the Patients Association for Distal Myopathies (PADM) in Japan. This work was partly supported by Health and Labor Sciences Research Grants for research on rare and intractable diseases from the Ministry of Health, Labor, and Welfare and Intramural Research Grants (26-7) for Neurological and Psychiatric Disorders from the NCNP.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Mori-Yoshimura, M. (2016). Clinical Aspects of GNE Myopathy and Translational Medicine. In: Takeda, S., Miyagoe-Suzuki, Y., Mori-Yoshimura, M. (eds) Translational Research in Muscular Dystrophy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55678-7_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-55678-7_10
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55677-0
Online ISBN: 978-4-431-55678-7
eBook Packages: MedicineMedicine (R0)