Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive fibrosing disease. Acute exacerbation of IPF (AE-IPF) is an abrupt and rapid deterioration by unidentifiable causes which occurs during chronic clinical course of IPF. The estimated incidence is 5–15 % per year in IPF patients. Moreover, AE-IPF is not only associated with a very high mortality rate (over 70 %), but it is also an important determinant of overall prognosis in patients with IPF. Pathologically, AE-IPF is characterized by acute diffuse alveolar damage (DAD) lesion that develops close to the chronic progressive usual interstitial pneumonia (UIP) lesion. Because DAD is also a common pathological feature observed in acute respiratory distress syndrome (ARDS), acute interstitial pneumonia (AIP), and AE-IPF, it is possible to regard AE-IPF as ARDS developed due to IPF. Although the trigger factors for AE-IPF are often unclear, sometimes the triggers such as surgery, corticosteroid dose reduction, or viral infection are evident. Evidence-based and effective therapies are lacking for AE-IPF, with the current management guidelines recommending only supportive care and corticosteroid use. Novel treatments for AE-IPF that have been investigated in small-scale studies, including the use of polymyxin B-immobilized fiber column (PMX) hemoperfusion, may be promising. When AE with DAD pathology develops once, treatment is extremely difficult; therefore, development of effective prevention methods is required. To this date, there is emerging evidence from clinical trials of investigational treatments for chronic phase of IPF which may reduce the incidence of AE-IPF.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
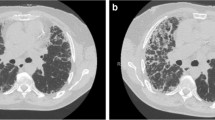
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive fibrosing disease. It has a poor prognosis, with a median survival period of approximately 3 years from the time of diagnosis [1, 2]. The typical natural history of IPF is the slow progression of the disease that eventually leads to respiratory failure and death. In some cases of IPF, abrupt and rapid deteriorations that have no identifiable cause occur during the chronic clinical course (Fig. 3.1). In Japan, clinical case reports of an acute exacerbation of IPF (AE-IPF) appeared from the 1970s, and the concept of AEX-IPF has since become widely recognized by pulmonologists. In1984, Yoshimura et al. [3] reported a clinical study of 35 cases, while Kondo et al. [4] published the first literature on AE of IPF in English in 1989, followed by Kondoh et al. [5] in 1993. In contrast, this concept was not accepted in Europe and America for a long time. This may have been for the following reasons: (1) The acute worsening was interpreted as a part of the natural progress of IPF, and (2) it was not easy to distinguish it from other conditions with similar presentations such as pneumonia, pulmonary emboli, or cardiac failure. Nevertheless, the concept of AE-IPF was first described in the American Thoracic Society/European Respiratory Society (ATS/ERS) International Multidisciplinary Consensus Classification in 2001 [6], and it has since been recognized worldwide [7–10]. When AE-IPF develops, not only it is associated with a very high mortality rate, but it is also an important determinant of overall prognosis in patients with IPF [11].
Clinical disease courses in patients with IPF. The typical natural history of IPF is the slow progression of the disease with a median survival period of approximately 3 years from the time of diagnosis (a). In some cases of IPF, abrupt and rapid deteriorations, triggered by unidentifiable causes or obvious causes, occur during the chronic clinical course (b). The deterioration is termed an acute exacerbation of IPF (AE-IPF). AE-IPF can occur even before diagnosis of IPF (c)
2 Definition
Diagnostic criteria were first established in 1995 and later revised in 2004 by The Study Group on Diffuse Pulmonary Disorders, Scientific Research/Refractory Disease-Overcoming Research Business, Japan Ministry of Health, Labor and Welfare. According to these criteria, diagnosis requires the following: (1) progressive dyspnea over 1 month or less, (2) new pulmonary infiltrates seen on a high-resolution computed tomography (HRCT) scan with evidence of underlying usual interstitial pneumonia (UIP), (3) worsening hypoxemia (i.e., a fall in PaO2 of >10 mmHg), and (4) the absence of an underlying cause such as pulmonary infection, pneumothorax, malignancy, pulmonary embolism, and cardiac failure. A study group in the United States reported a similar definition in 2007 [10] (Table 3.1), which includes an item to exclude pulmonary infection by endotracheal aspirate or bronchoalveolar lavage (BAL) to this criterion. Although the presence of overt infection is considered to be an exclusion criterion, occult infection could be a trigger factor for AE-IPF. In fact, bacterial and viral pneumonias are often seen in patients with AE-IPF and may be the trigger factor. Therefore, although it is important to exclude simple pulmonary infection from AE-IPF, the existence of infection should not preclude the diagnosis of an AE [12].
In AE-IPF without a prior diagnosis of IPF, differentiation from acute interstitial pneumonia (AIP) represents an important diagnostic challenge. However, if the typical honeycomb findings of IPF are observed in the lungs by HRCT, an AE-IPF is diagnosable.
3 Pathophysiology
AE-IPF is considered to be a sudden acceleration of the disease or acute lung injury by an unknown cause that is superimposed on the diseased lung. Pathologically, AE-IPF is characterized by an acute-onset diffuse alveolar damage (DAD) that develops close to chronic UIP lesion. Given that DAD is also a common pathological feature of acute respiratory distress syndrome (ARDS) and AIP, it is possible to regard AE-IPF as ARDS due to IPF. In IPF, the lung tissue without fibrosis is primed by inflammatory cytokines produced by chronic inflammation, and it seems to be susceptible to develop DAD through some unknown trigger. Therefore, it is easy to understand AE-IPF as complication of ARDS-like DAD in IPF rather than as deterioration of IPF itself. This is supported by the fact that AE occurs not only in IPF (UIP) but also in other chronic interstitial pneumonias such as nonspecific interstitial pneumonia [13, 14].
4 Trigger Factors
Although the triggers for AE-IPF are often unclear, surgical operation, corticosteroid dose reduction, and viral infection can present as obvious triggers [11]. Diagnostic procedures such as BAL and video-assisted thoracoscopic surgery (VATS) are also potential triggers [15]. Gastroesophageal reflux disease (GERD) is another potential cause of AE-IPF [10].
According to the diagnostic criteria, pulmonary infection should be excluded; however, in practice, viral and bacterial infections are often obvious triggers [16, 17]. Simon-Blancal et al. [18] demonstrated that AE-IPF was more frequent in winter and spring than during summer and fall, suggesting that unidentified infections might be an important trigger. Despite this, a study using standard PCR analysis of BAL fluid demonstrated that common respiratory viruses were detected in only four of the 43 patients with AE-IPF, with no evidence of viral infection in most cases [19]. However, limitations with this finding mean that viruses cannot be definitively excluded as the cause of AE-IPF [20].
Some medications have been reported to be triggers for AE-IPF. Implicated drugs include biologic (anakinra, etanercept, and infliximab), nonbiologic (ambrisentan), immunomodulatory (interferon alpha/beta, everolimus, and leflunomide), and anticancer agents [21–23]. In fact, patients with lung fibrosis are prone to drug-induced lung injury. However, because drug-induced DAD-type lung injury can develop in patients without lung fibrosis, it is debatable whether such cases should be regarded as drug-induced AE-IPF or simply as comorbid drug-induced lung injury.
Pulmonary resection in patients with lung cancer and interstitial lung disease can provoke AE-IPF at higher rates and with higher mortality. A systematic review showed that the incidence of postoperative AE-IPF ranged from 0 to 20.8 %, with mortality ranging from 37.5 to 100 % [24]. A large-scale multi-institutional cohort study reported that AE-IPF occurred in 164 of 1763 (9.3 %) patients with non-small cell lung cancer who underwent pulmonary resection and that it was the leading cause of 30-day mortality (71.7 %), with an overall mortality rate of 43.9 % when it developed [25]. Surgical procedures show the strongest association with AE-IPF; for example, using wedge resection as a reference, lobectomy or segmentectomy has an odds ratio of 3.83, and bi-lobectomy or pneumonectomy has an odds ratio of 5.70 (P < 0.001). In high-risk patients, surgical procedures associated with a higher risk of AE-IPF should be chosen cautiously. No benefit was found for perioperative steroids and sivelestat prophylaxis in that study.
Suzuki H et al. [26] analyzed the HRCT findings of patients with IPF to identify radiological characteristics of IPF susceptible to acute exacerbation after surgery for lung cancer. They demonstrated that the degree of fibrosis on preoperative HRCT was significantly higher in the exacerbation group (P < 0.003).
5 Epidemiology
The incidence of AE-IPF varies greatly between studies (8.5–60 %), and the precise determination is made difficult by the use of retrospective analyses of selected cases. The incidence also changes depending on whether pulmonary infection is completely excluded. AE-IPF is also increasingly common because of the spreading recognition that it is a common clinical feature of IPF. Recent data from randomized controlled trials have provided incidences that are more conservative, with rates of 14 % over 9 months and 4.8, 5.4, and 9.6 % over 1 year in the control groups [27–29]. Using retrospective data from a large observational cohort of 461 patients, 1- and 3-year incidences are suggested to be 14 % and 21 %, respectively [11]. A population-based analysis demonstrated the rate of AE was 0.13 cases per person year [30].
There are no significant differences in disease duration, pulmonary function, age, gender, or smoking history between patients with and without AE [9, 31]. By multivariate analysis, Song et al. [11] demonstrated that low forced vital capacity (FVC) levels and the absence of a history of smoking were risk factors for AE-IPF; however, the role of smoking in AE-IPF is controversial [32, 33]. Pulmonary hypertension may also be a risk factor for AE-IPF [34].
The mortality rates reported in small case series have been very poor, and they are as high as 85 % [7, 35–39]. In a summary of 16 studies, Collard et al. [10] reported an overall mortality rate of 70 %, while a systematic review reported 1- and 3-month mortality rates of 60 % and 67 %, respectively [40]. Kishaba et al. [41] reported that extensive disease on chest HRCT, including traction bronchiectasis, honeycombing, ground-glass opacity, and consolidation, was associated with particularly poor mortality in AE-IPF. Indeed, the 3-month mortality was 80.6 % among patients with extensive HRCT-related disease findings, which compared negatively with the mortality of 54.5 % in patients with limited disease (P = 0.007).
6 Histopathology
The histopathology of AE is characterized by an underlying fibrotic interstitial pneumonia with superimposed DAD [9, 10]. The latter usually appears in a relatively normal area without prior honeycombing, and it is histologically the same as DAD that occurs without a background of UIP such as that observed in AIP and ARDS. Kang et al. [42] investigated the pathological differences depending on the underlying risk, determining the degree of α-smooth muscle actin-positive or collagen type I-positive alveolar interstitial myofibroblasts in the proliferative phase of DAD by immunohistochemical staining. Only two of seven patients with septic ARDS showed interstitial myofibroblast proliferation as opposed to 15 of 16 patients with drug-induced ARDS. Only three patients had AIP, but all patients showed myofibroblast proliferation. These findings indicated that DAD can be divided into two subphenotypes: less fibrogenic and more fibrogenic. The former is seen in patients with septic ARDS and a high incidence of multi-organ dysfunction syndrome (MODS), while the latter is seen in those with drug-induced ARDS, AIP, and AE-IPF with a low incidence of MODS.
7 Radiological Assessment
The most common radiological finding in patients with AE-IPF is the presence of new bilateral ground-glass opacities or of consolidation superimposed on the underlying UIP (i.e., subpleural reticular and honeycombing densities) [43]. Acute exacerbations of either IPF or other chronic interstitial lung diseases can closely resemble ARDS in both clinical presentation and chest radiographic abnormalities. Similar to ARDS, pathological findings are dominated by DAD, although the prognosis is substantially worse. The diagnosis of AE-IPF is suggested by a careful review of previous chest radiographic images, by the discovery of subpleural reticular changes intermixed with alveolar opacities on a chest CT scan obtained shortly after the onset of ARDS, or by surgical lung biopsy.
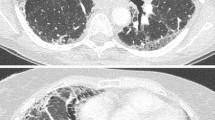
Akira et al. [44] classified the new onset parenchymal abnormalities into three patterns: peripheral, multifocal, and diffuse (Fig. 3.2). By multivariate analysis, the strongest correlations were observed between CT patterns (combined diffuse and multifocal versus peripheral) and survival (odds ratio, 4.629; P = 0.001). Diffuse and multifocal ground-glass patterns appear to predict a worse survival in patients with AE-IPF compared with those with peripheral patterns. In contrast to these findings, Silva et al. [45] were unable to show any relationship between radiographic pattern and survival. Data that are more recent suggest that the extent of lesion on HRCT is a more important determinant of the outcome than the distribution of lesion [46]. Using the HRCT score, which was calculated based on normal attenuation areas and extent of abnormalities (i.e., areas of ground-glass attenuation and/or consolidation with or without traction bronchiectasis or bronchiolectasis and areas of honeycombing), survival among patients was worse with an HRCT score of ≥245 than those with a lower score (log-rank test, P < 0.0001).
High-resolution computed tomography (HRCT) patterns of acute exacerbation of IPF: (a) peripheral pattern, (b) multifocal pattern, (c) diffuse pattern (Adapted from Ref. [44])
8 Laboratory Tests and Biomarkers
When the acute exacerbation occurs, cough and dyspnea become acutely worse within one month. Blood test typically shows increases in the white cell count and both C-reactive protein and lactate dehydrogenase levels. In addition, markers of fibrosis are increased, including surfactant protein-A (SP-A), surfactant protein-D (SP-D), and sialylated carbohydrate antigen (KL-6). Measurement of serum KL-6 level is very useful when assessing disease activity and prognosis in IPF, and it is a useful predictive marker for AE-IPF. Ohshimo et al. [47] reported that baseline serum KL-6 levels were significantly higher in patients who later developed AE-IPF than in those with stable IPF (P < 0.0001). At a KL-6 cutoff level of 1300 U/mL, the sensitivity, specificity, accuracy, and likelihood ratio for predicting AE-IPF were 92 %, 61 %, 66 %, and 2.36, respectively. In a Kaplan-Meier analysis, they reported that patients with baseline serum KL-6 levels of ≥1300 U/mL experienced earlier onset of AE-IPF (P = 0.002). Thus, it is suggested that baseline serum KL-6 (both continuous and at a cutoff level of ≥1300 U/mL) is a sensitive independent predictive factor for the onset of AE-IPF. However, it is essential that care be taken while prescribing steroids or immunosuppressive therapy in IPF because of the risk of opportunistic infections by pneumocystis pneumonia or cytomegalovirus. In patients with pneumocystis pneumonia, although serum KL-6 rises in a similar manner to AE-IPF, blood β-d-glucan levels rise concurrently.
Pneumonia represents the most problematic differential in the diagnosis of AE-IPF. According to the current diagnostic criteria, pulmonary infection must be ruled out by endotracheal aspiration or BAL. However, performing bronchoscopy in patients with AE-IPF is associated with a high risk of worsening the respiratory condition, with BAL itself recognized to be a trigger factor for AE-IPF. In practice, it is often difficult to differentiate between AE-IPF and bacterial pneumonia, and a surrogate marker has, therefore, been sought to exclude infectious pathology. To this end, serum procalcitonin (PCT) is useful while attempting to differentiate between typical bacterial and nonbacterial causes of inflammation. Nagata et al. [48] reported that serum PCT levels in AE-IPF were significantly lower than those in bacterial pneumonia with IP (0.62 ± 1.30 vs. 8.31 ± 14.83 ng/mL; P < 0.05). Thus, serum PCT is a useful surrogate marker for discriminating between AE-IPF and concurrent bacterial pneumonia with IPF.
In addition, circulating fibrocytes have been reported to be a good biomarker of fibrosis. Fibrocytes are circulating bone marrow-derived spindle-shaped cells, produce extracellular matrix components, and may play an important role in wound repair and tissue fibrosis. Fibrocytes were defined as cells positive for CD45 and collagen-1 by flow cytometry. Circulating fibrocytes defined as CD45-positive and collagen-1-positive cells by flow cytometry are increased threefold in patients with stable IPF compared with healthy controls [49]. During AE-IPF, fibrocyte counts have been shown to further increase to an average of 15 % of peripheral blood leukocytes. The increased fibrocyte counts then tend to decrease to pre-exacerbation levels in patients who recover. Circulating fibrocyte counts can also indicate prognosis; patients with IPF and fibrocytes of >5 % had a poor prognosis when compared with those with fibrocytes of <5 % (mean survival time, 7.5 months vs. 27 months; P = 0.0001).
9 Pharmacological Treatments
To date, no evidence-based effective therapy has been established for AE-IPF. Indeed, international consensus guidelines produced by the ATS, ERS, Japanese Respiratory Society, and Latin American Thoracic Association only recommend management supportive care and corticosteroid use [50].
Empirical treatment with high-dose corticosteroid therapy is generally used in AE-IPF without any clear evidence that they are effective. No randomized controlled trials have been conducted using corticosteroids in AE-IPF. Methylprednisolone pulse therapy [1 g intravenously (IV)] is usually given on days 1–3 with a maintenance prednisolone dose equivalent to approximately 40 mg daily [5]. The 3-day pulse therapy is repeated weekly on 1–4 occasions until the patient’s condition stabilizes.
Steroid pulse therapy came to be used for respiratory diseases based on its biological plausibility and experience, with a similar method used for renal and collagen diseases [51]. In Japan, this therapy has been used for IPF and AE-IPF since 1978. It is also usually used in the treatment of other lung diseases presenting with DAD, such as ARDS and AIP. Although the rapidly progressive clinical condition is often temporarily stabilized by the pulse therapy, thereby improving oxygenation, there is no evidence that it improves mortality. Indeed, the mortality rate remains high, despite therapy. In recent years, a low-dose steroid therapy has been employed (methylprednisolone, 1 mg/kg/day) in reference to trials in the treatment of ARDS [52], but the benefits remain unclear. Given that the pathological evidence of DAD is an extremely poor prognostic factor, it seems that the effect of steroids may be insufficient. Nevertheless, surgical lung biopsies reveal that some cases of AE-IPF have organizing pneumonia rather than DAD [53], in which case glucocorticoid therapy might be effective.
Immunosuppressants, such as cyclosporine A (CsA), cyclophosphamide, or tacrolimus, are used together when the reaction of steroid alone is poor. These drugs can also be used in combination with a steroid from the beginning. Several investigators reported better survival in patients treated with this type of combination therapy [54–56]. Although these studies have mostly been retrospective, have included small samples, and have used various definitions of AE-IPF, they suggest that the use of immunosuppressants in combination with corticosteroids is more effective than corticosteroid monotherapy. Sakamoto et al. [54] reported that the mean survival period after the first onset of AE-IPF was 285 days in a CsA-treated group and 60 days in a non-CsA-treated group. Thus, prognosis was significantly better in the CsA-treated group. In this study, a low dosage of CsA (100–150 mg/day) was started at the same time as the pulse therapy. Morawiec et al. [55] reported their experience with cyclophosphamide pulse therapy for AE-IPF in a small case series. Patients with AE-IPF were treated with a methylprednisolone pulse (1,000 mg) on days 1–3, before the escalating regimen of cyclophosphamide was started on day 4 with an initial intravenous dose of 500 mg. The dose of cyclophosphamide was then increased by 200 mg every 2 weeks up to the maximum dose of 1,500 mg.
The majority of patients with AE-IPF receive empiric broad-spectrum antibiotics against respiratory pathogens, even if there is no obvious infection. This is based on the following clinical rationale: (1) an underlying infection can be easily missed on microbiological testing, (2) the mortality rate is very high, (3) many patients present with fever and flu-like symptoms and have elevated blood neutrophil counts and CRP levels, and (4) antimicrobial therapy has a low risk of complications.
Because a coagulation disorder subsequent to vascular injury could be important for the pathogenesis of AE-IPF, anticoagulant therapy may be effective. A small prospective clinical trial of anticoagulation with warfarin and low molecular weight heparin in patients with IPF reportedly improved survival in the group receiving anticoagulation mostly by reducing the mortality associated with AE-IPF [57]. In that study, the mortality associated with AE-IPF was significantly reduced in the anticoagulant group when compared with the non-anticoagulant group (18 % vs. 71 %, respectively; P = 0.008). Conversely, a randomized, double-blind, placebo-controlled study of warfarin as a treatment for IPF had to be terminated early because of higher mortality in the warfarin arm and a low likelihood of benefit [58]. Therefore, at present, anticoagulant therapy in patients with IPF is discouraged.
Combination therapy with sivelestat, a neutrophil elastase inhibitor, and corticosteroids was examined in a multicenter, double-blind prospective phase II study in Japan [59]. In total, 78 cases were divided into placebo, low-dose, and high-dose groups. High doses of sivelestat treatment for 14 days improved oxygenation and global clinical status but not mortality. A subsequent phase III trial has since confirmed the clinical utility for AE-IPF [60]. Currently sivelestat is clinically available for lung injury with systemic inflammatory response syndrome (SIRS) and it is often used for AE-IPF [61].
Pharmacological treatments such as thrombomodulin [62] have also been studied. However, their efficacy in the treatment of AE-IPF is based on a few small, retrospective studies that do not provide conclusive evidence of benefit.
10 Therapy with Polymyxin B-Immobilized Fiber Column
One possible treatment for AE-IPF currently under study is a direct hemoperfusion with a polymyxin B (PMX)-immobilized fiber column (PMX-DHP). In this technique, PMX-DHP columns not only absorb endotoxins and reactive oxygen species, and other substances, but also selectively remove activated neutrophils that cause endothelial injury [63–65]. Several case studies indicated that PMX-DHP treatment may improve oxygenation and survival in patients with AE-IPF. Abe et al. [66] reported a multicenter retrospective analysis (18 institutions in Japan) of 160 patients with interstitial pneumonia (including 73 with IPF) who had acute disease exacerbations treated by PMX. In patients with AE-IPF, the ratio of PaO2/FiO2 was significantly improved after treatment with PMX compared with that before treatment (173.9 ± 105.4 to 195.2 ± 106.8 Torr; P = 0.003). Recently, Enomoto et al. [67] reported 31 patients with AE-IPF; they found a significantly greater improvement in PaO2/FiO2 ratio in those treated with PMX-DHP (n = 14) after 2 days of treatment than in those who did not receive PMX-DHP treatment (mean ± SEM, 58.2 ± 22.5 vs. 0.7 ± 13.3; P = 0.034). The 12-month survival rate was also significantly higher in patients treated with PMX-DHP (48.2 % vs. 5.9 %; P = 0.041). These studies suggest that PMX-DHP therapy is promising and that large randomized controlled trials are needed.
11 Prevention
Prevention of AE-IPF may be the most effective approach to management. Clinical trials of several investigational treatments for IPF have evaluated whether daily treatment of chronic phase of IPF reduces the incidence of AE-IPF.
Pirfenidone, an antifibrotic molecule, has shown inconsistent effects on AE-IPF. A phase II study in Japanese patients with IPF was terminated after 9 months of a planned 1-year follow-up because of a higher frequency of AE-IPF in the placebo group than in the pirfenidone group [27]. However, in a phase III trial in Japanese patients, no significant differences were observed in the incidence of AE-IPF over 52 weeks between patients treated with pirfenidone and those treated with placebo [28]. Subsequent larger clinical trials of pirfenidone in patients with IPF (CAPACITY-1 and CAPACITY-2) also failed to confirm any reduction in the incidence of AE-IPF [68]. Therefore, at present, pirfenidone cannot be considered to be effective in preventing the onset of AE-IPF.
Nintedanib is a tyrosine kinase inhibitor of platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), and fibroblast growth factor receptor (FGFR) that has been developed for the treatment of IPF. In a phase II trial (the TOMORROW trial, lasting for 12 months), a lower incidence of AE-IPF was observed in patients treated with nintedanib than those treated with placebo [69]. Two subsequent replicate 52-week, randomized, double-blind, phase III trials (INPULSIS-1 and INPULSIS-2) were performed [29]. However, in INPULSIS-1, there was no significant difference between the nintedanib and placebo groups in the time to the first AE (hazard ratio in the nintedanib group, 1.15; P = 0.67), with similar proportions of patients with AE-IPF in the treatment and placebo groups (6.1 % and 5.4 %, respectively). In contrast, INPULSIS-2 reported a significant increase in the time to the first AE in the nintedanib group when compared with the placebo group (hazard ratio, 0.38; P = 0.005), and the proportion of patients with AE was also lower in the nintedanib group than in the placebo group (3.6 % vs. 9.6 %). In the prespecified pooled analysis, there was no significant difference between the nintedanib and placebo groups in the time to first AE (hazard ratio, 0.64; P = 0.08) and also in the proportion of patients with AE (4.9 % in the nintedanib group and 7.6 % in the placebo group). Because nintedanib did not show a consistent effect on the incidence of AE in these trials, its effectiveness remains unclear.
Antacid drugs have been considered to be useful for preventing AE-IPF because gastroesophageal reflux disease (GERD) is a risk factor for both IPF and AE-IPF. A retrospective analysis using data from controls in three randomized controlled trials showed that patients taking antacid treatments (proton pump inhibitors or histamine receptor-2 blockers) at baseline had fewer AE-IPF than those who were not (zero vs. nine events after a mean follow-up of 30 weeks; P < 0.01) [70]. The decrease in FVC at 30 weeks was also smaller in the patients who were treated with antacid drugs than those who were not treated (−0.06 vs. −0.12L; P = 0.05). These findings suggest that antacid treatment could be beneficial in patients with IPF. Controlled clinical trials are needed to confirm those observational findings.
Postoperative AE-IPF is also common and associated with a high mortality rate. Therefore, appropriate measures should be employed to prevent the onset of AE. Various preventive measures have been considered to date, but none has been established. In general, high-pressure ventilation, high oxygen concentrations, long operation times, and overhydration during operation should be avoided.
12 Conclusions
AE-IPF and AE of other fibrotic lung diseases are life-threatening events that decrease overall survival and are associated with high mortality rates. They have been recognized in Japan since the 1970s but have only recently been recognized in Europe and America. One reason for this is that there was the difference of recognition whether AE was regarded as a part of the natural progression of the underlying disease or as a newly developed distinct condition. The other possibilities for the reason are a racial factor and an environmental factor. However, this is not likely because recent reports demonstrated that there are no great differences in the incidence of AE-IPF between Japan and other countries.
A critical problem in the management of AE-IPF is the differentiation from comorbidities, such as pneumonia, pulmonary thromboembolism, pneumothorax, or cardiac failure, which may present similarly with abrupt, acute deterioration of respiratory failure in the context of chronic disease progression. In that setting, the emergence of new bilateral lung shadows not caused by apparent infection should be considered to be the decisive factor in favor of AE. Indeed, because they are pathologically characterized by DAD, AE-IPF is easy to understand when one considers them as ARDS due to IPF. Although the diagnosis of AE-IPF requires that infection be excluded, concomitant infection may play a role such as that when ARDS is caused by infection. Triggers for AE-IPF also appear to be variable and unclear.
Despite trials of various therapies, no concrete therapy has been established to date. Given the high incidence of AE-IPF following lung resection surgery, a high-priming state probably makes the patient susceptible to a second attack. Unfortunately, when an AE with DAD has occurred once, subsequent treatment becomes extremely difficult. Therefore, efforts need to focus on the development of effective prevention methods. Although emerging evidence from clinical trials suggests that some treatments may reduce the incidence of AE-IPF in the context of chronic IPF, robust evidence is needed to confirm their efficacy before they can be put into routine practice.
References
Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203.
Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med. 2000;161:1172–8.
Yoshimura K, Nakatani T, Nakamori Y, et al. Acute exacerbation in idiopathic interstitial pneumonia. Nihon Kyobu Shikkan Gakkai Zasshi. 1984;22:1012–20. Japanese.
Kondo A, Saiki S. Acute exacerbation in idiopathic interstitial pneumonia. In: Interstitial pneumonia of unknown etiology. Tokyo: University of Tokyo Press; 1989. p. 34–42.
Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103:1808–12.
American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304.
Ambrosini V, Cancellieri A, Chilosi M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J. 2003;22:821–6.
Martinez FJ, Safrin S, Weycker D, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–7.
Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27:143–50.
Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–43.
Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:56–63.
Huie TJ, Olson AL, Cosgrove GP, et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: aetiology and outcomes. Respirology. 2010;15:909–17.
Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009;103:846–53.
Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–20.
Hiwatari N, Shimura S, Takishima T, et al. Bronchoalveolar lavage as a possible cause of acute exacerbation in idiopathic pulmonary fibrosis patients. Tohoku J Exp Med. 1994;174:379–86.
Molyneaux PL, Cox MJ, Willis-Owen SA, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–13.
Umeda Y, Morikawa M, Anzai M, et al. Acute exacerbation of idiopathic pulmonary fibrosis after pandemic influenza A (H1N1) vaccination. Intern Med. 2010;49:2333–6.
Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration. 2012;83:28–35.
Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1698–702.
Kolb MR, Richeldi L. Viruses and acute exacerbations of idiopathic pulmonary fibrosis: rest in peace? Am J Respir Crit Care Med. 2011;183:1583–4.
Ling VY, Mortimore M, Serisier DJ. Suspected acute exacerbation of idiopathic pulmonary fibrosis associated with interferon alpha therapy for hepatitis C: case report. Springerplus. 2013;2:101.
Malouf MA, Hopkins P, Snell G, et al. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology. 2011;16:776–83.
Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, et al. Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum. 2011;41:256–64.
Watanabe A, Kawaharada N, Higami T. Postoperative acute exacerbation of IPF after lung resection for primary lung cancer. Pulm Med. 2011;2011:960316.
Sato T, Teramukai S, Kondo H, et al. Japanese Association for Chest Surgery. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147:1604–11.
Suzuki H, Sekine Y, Yoshida S, et al. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today. 2011;41:914–21.
Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7.
Taniguchi H, Ebina M, Kondoh Y, Pirfenidone Clinical Study Group in Japan, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9.
Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82.
Fernandez Perez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137:129–37.
Stern JB, Mal H, Groussard O, et al. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120:213–19.
Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases related versus idiopathic. Respiration. 2012;83:20–7.
Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35:105–11.
Judge EP, Fabre A, Adamali HI, et al. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. 2012;40:93–100.
Panos RJ, Mortenson RL, Niccoli SA, et al. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med. 1990;88:396–404.
Akira M, Hamada H, Sakatani M, et al. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 1997;168:79–83.
Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128:3310–15.
Nava S, Rubini F. Lung and chest wall mechanics in ventilated patients with end stage idiopathic pulmonary fibrosis. Thorax. 1999;54:390–5.
Fumeaux T, Rothmeier C, Jolliet P. Outcome of mechanical ventilation for acute respiratory failure in patients with pulmonary fibrosis. Intensive Care Med. 2001;27:1868–74.
Agarwal R, Jindal SK. Acute exacerbation of idiopathic pulmonary fibrosis: a systematic review. Eur J Int Med. 2008;19:227–35.
Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192:141–9.
Kang D, Nakayama T, Togashi M, et al. Two forms of diffuse alveolar damage in the lungs of patients with acute respiratory distress syndrome. Hum Pathol. 2009;40:1618–27.
Hyzy R, Huang S, Myers J, et al. Acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2007;132:1652–8.
Akira M, Kozuka T, Yamamoto S, et al. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;15(178):372–8.
Silva CI, Muller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging. 2007;22:221–9.
Fujimoto K, Taniguchi H, Johkoh T, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol. 2012;22:83–92.
Ohshimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med. 2014;108:1031–9.
Nagata K, Tomii K, Otsuka K, et al. Serum procalcitonin is a valuable diagnostic marker in acute exacerbation of interstitial pneumonia. Respirology. 2013;18:439–46.
Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94.
Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824.
Keogh BA, Bernardo J, Hunninghake GW, et al. Effect of intermittent high dose parenteral corticosteroids on the alveolitis of idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1983;127(1):18–22.
Meduri GU, Golden E, Freire AX, et al. Effect of intermittent high dose parenteral corticosteroids on the alveolitis of idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1983;127:18–22.
Churg A, Muller NL, Silva CI, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol. 2007;31:277–84.
Sakamoto S, Homma S, Miyamoto A, et al. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49:109–15.
Morawiec E, Tillie-Leblond I, Pansini V, et al. Exacerbations of idiopathic pulmonary fibrosis treated with corticosteroids and cyclophosphamide pulses. Eur Respir J. 2011;38:1487–9.
Horita N, Akahane M, Okada Y, et al. Tacrolimus and steroid treatment for acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2011;50:189–95.
Kubo H, Nakayama K, Yanai M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–82.
Noth I, Anstrom KJ, Calvert SB, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95.
Ishii Y, Kitamura S, Kira S, et al. A phase II clinical study of a neutrophil elastase inhibitor; ONO-5046・Na on acute exacerbation in IIP patients. J Clin Therap Med. 1998;14:397–420 (in Japanese).
Ishii Y, Kitamura S, Ando M. A phase III clinical study of a neutrophil elastase inhibitor; ONO-5046・Na on acute exacerbation in IIP patients. J Clin Therap Med. 1998;14:421–46 (in Japanese).
Nakamura M, Ogura T, Miyazawa N, et al. Outcome of patients with acute exacerbation of idiopathic interstitial fibrosis (IPF) treated with sivelestat and the prognostic value of serum KL-6 and surfactant protein D. Nihon Kokyuki Gakkai Zasshi. 2007;45:455–9 (in Japanese).
Tsushima K, Yamaguchi K, Kono Y, et al. Thrombomodulin for acute exacerbations of idiopathic pulmonary fibrosis: a proof of concept study. Pulm Pharmacol Ther. 2014;29:233–40.
Kase Y, Obata T, Okamoto Y, et al. Removal of 2-arachidonylglycerol by direct hemoperfusion therapy with polymyxin B immobilized fibers benefits patients with septic shock. Ther Apher Dial. 2008;12:374–80.
Kohro S, Imaizumi H, Yamakage M, et al. Anandamide absorption by direct hemoperfusion with polymixin B-immobilized fiber improves the prognosis and organ failure assessment score in patients with sepsis. J Anesth. 2006;20:11–6.
Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–52.
Abe S, Azuma A, Mukae H, et al. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med. 2012;51:1487–91.
Enomoto N, Mikamo M, Oyama Y, et al. Treatment of acute exacerbation of idiopathic pulmonary fibrosis with direct hemoperfusion using a polymyxin B-immobilized fiber column improves survival. BMC Pulm Med. 2015;15:15.
Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–9.
Woodcock HV, Molyneaux PL, Maher TM. Reducing lung function decline in patients with idiopathic pulmonary fibrosis: potential of nintedanib. Drug Des Devel Ther. 2013;7:503–10.
Lee JS, Collard HR, Anstrom KJ, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomized controlled trials. Lancet Respir Med. 2013;1:369–76.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Ishii, Y. (2016). Acute Exacerbation of IPF. In: Nakamura, H., Aoshiba, K. (eds) Idiopathic Pulmonary Fibrosis. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55582-7_3
Download citation
DOI: https://doi.org/10.1007/978-4-431-55582-7_3
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55581-0
Online ISBN: 978-4-431-55582-7
eBook Packages: MedicineMedicine (R0)