Abstract
Indocyanine green (ICG)-based fluorescence imaging has been recently applied to liver surgery to detect hepatocellular carcinoma (HCC). This technique is quite safe and simple. Briefly, ICG is intravenously injected at a dose of 0.5 mg/kg body weight as a routine preoperative liver function test before surgery. On the day of surgery, after laparotomy, the liver surface is examined by a commercially available near-infrared (NIR) camera system with the surgical lights turned off. HCC nodules are usually detected as intense fluorescent signals by this method. In this chapter, we introduce the clinical applications of ICG fluorescence imaging in HCC surgery and review the clinical study literature to date. This technique is highly sensitive and complementary to conventional modalities for the detection of HCC. Although there are some limitations, such as the low penetration depth and the detection of false-positive lesions, this technique is expected to be indispensable for the diagnosis and treatment of HCC in the near future, with further developments in basic research and improvements of the devices.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. Following improvements in surgical techniques and preoperative imaging modalities, long-term survival has been achieved in some patients treated with hepatic resection [1]. However, even when curative resection is performed, 70–80 % of patients experience tumor recurrence within 5 years postoperatively [2–4]. A possible explanation for this high early recurrence rate is the presence of small metastatic lesions that are missed using current preoperative and intraoperative detection methods [5, 6]. Therefore, conducting a more sensitive and accurate intrahepatic imaging evaluation of these types of lesions is essential for determining the extent of hepatic resection.
Recently, intraoperative imaging using near-infrared (NIR) fluorescence light has been applied to various surgeries. Indocyanine green (ICG) is a NIR fluorescent dye approved by the Food and Drug Administration for use in cardiocirculatory and liver function diagnostic procedures. Several studies have reported the usefulness of ICG intraoperative fluorescence imaging for detecting sentinel nodes in cases of breast, gastric, and colon cancer [7–9]. In 2007, we incidentally found that HCC tumors show very intense fluorescent signals using this method in patients given with ICG several days prior to surgery as a routine preoperative liver function test. [10] This technique provides not only real-time visualization of HCC nodules but also highly sensitive identification of small and grossly unidentifiable HCC nodules, enhancing the accuracy of liver resection.
This review describes the techniques, current applications, and future perspectives regarding ICG fluorescence imaging for HCC.
2 Technique of ICG Fluorescence Imaging for HCC Detection
The technique for ICG fluorescence imaging of HCC is quite safe and simple. The patient is administered with ICG (Diagnogreen Inj., Daiichi Pharmaceutical, Tokyo, Japan) intravenously at a dose of 0.5 mg/kg body weight as a routine preoperative liver function test prior to undergoing surgery [11]. The interval between ICG injection and surgery is usually within 14 days in HCC patients. On the day of surgery, after laparotomy, the liver surface is observed using a commercially available near-infrared (NIR) camera system with the surgical lights turned off. This system activates ICG with emitted light at a wavelength of 760 nm and filters out light with a wavelength below 820 nm. The light source is a light-emitting diode (LED), and the detector is a charge-coupled device (CCD) camera. The camera unit of the device is handled directly, and real-time fluorescence images are obtained on the monitor in the operating room.
3 Detection of HCC Nodules Using ICG Fluorescence Imaging
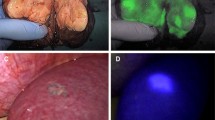
HCC nodules are usually detected as intense fluorescent signals using intraoperative ICG fluorescence imaging (Fig. 29.1). In a preliminary study, we reported that ten primary HCC nodules showed intense fluorescent signals and were easily detected using this method [10]. At the same time, Ishizawa et al. reported detecting 63 primary HCC nodules using the same technique [12]. Previously published studies of HCC detection are summarized in Table 29.1 [10, 12–14]. The rate of HCC detection on intraoperative fluorescence imaging is reported to be 52–100 % and is strongly dependent on the distance from the liver surface to the tumor. There is a technical limitation in that the tissue penetration depth of the fluorescence emitted by ICG is only 5–10 mm. Morita et al. demonstrated that the average distance from the liver surface to tumors unidentified on intraoperative fluorescence imaging is 10.4 (0–27) mm [13]. In that study, 26 tumors unidentified on intraoperative fluorescence imaging were identified in the sectioned specimens using ex vivo fluorescence imaging. Van der Vorst et al. reported that, in their study, 71 liver metastases, all of which were ≤6.2 mm from the liver surface, were detected on intraoperative ICG fluorescence imaging, whereas 26 liver metastases identified on conventional imaging could not be detected using intraoperative ICG fluorescence imaging, all of which were deeper than 8 mm from the liver surface [15]. Therefore, this technique is expected to improve the performance of imaging devices in terms of achieving deeper visualization.
ICG fluorescence imaging for primary HCC. Gross appearance (left) and intraoperative ICG fluorescence imaging (right) of liver surfaces. Fluorescence imaging by using a commercially available near-infrared (NIR) camera system (a) Photodynamic Eye (PDE; Hamamatsu Photonics K.K., Hamamatsu, Japan) and (b) HyperEye Medical System (HEMS, Mizuho Co., Tokyo, Japan)
Following resection and sectioning, all liver tumors are examined using ICG fluorescence imaging ex vivo. The detection rate is reported to be almost 100 % [10, 12–14]. Ishizawa et al. reported that, in false-negative cases of ICG fluorescence imaging, the interval between the intravenous injection of ICG and surgery is exceptionally long compared with that seen in the remaining identified patients [14]. On the other hand, they found that the signal intensity of the noncancerous liver parenchyma was higher especially in patients with advanced cirrhosis who had received the ICG injection within 24 h before surgery [12]. A previously performed study in rats showed an optimal cancer-to-liver contrast in the group where ICG was administered at 72 h prior to surgery [16]. Further studies are needed to determine the optimal interval between ICG injection and surgery for obtaining good cancer-to-liver contrast.
4 Correlation Between the ICG Fluorescence Pattern and HCC Differentiation
The fluorescence patterns observed under NIR are classified into three groups: total fluorescent type, partial fluorescent type, and rim fluorescent type (Fig. 29.2). Several studies have reported a significant correlation between HCC differentiation and the fluorescence pattern [13, 14]. Combining these studies, a total of 346 patients were included (total type (n = 150), partial type (n = 145), and rim type (n = 51)). Among the rim fluorescent-type patients, three cases (6 %) were classified as well-differentiated carcinoma, and 22 cases (43 %) were classified as poorly differentiated carcinoma. On the other hand, in the other fluorescent-type groups (total and partial), 85 cases (29 %) involved well-differentiated carcinoma, and five cases (2 %) involved poorly differentiated carcinoma. These results suggest that the rate of the rim fluorescent type is significantly lower among cases of well-differentiated carcinoma and higher among cases of poorly differentiated carcinoma compared to that of the other fluorescent types. It is hypothesized that the mechanisms of ICG accumulation in HCC nodules are associated with ICG transporter expression levels. Ishizawa et al. reported the expression of Na+/taurocholate cotransporting polypeptide (NTCP) and organic anion-transporting polypeptide 8 (OATP8), the major uptake transporters for ICG [17], in the cancerous tissues of HCC lesions showing total and partial fluorescence patterns, but not lesions showing rim-type fluorescence, according to a gene expression and immunostaining analysis [14]. These results suggest that, in differentiated HCC tissues, the portal uptake function is preserved, whereas the biliary excretion of ICG is likely impaired. It has also been suggested that most poorly differentiated HCCs exhibit rim-type fluorescence on ICG fluorescence images as a result of an impaired portal uptake function in cancerous tissues. However, further research is needed to confirm the mechanism and pattern of ICG accumulation in HCC nodules.
5 Newly Detected Lesions on ICG Fluorescence Imaging
ICG fluorescence imaging technique can be used to achieve the enhanced detection of small HCC nodules undetectable with conventional detection methods, including preoperative CT, intraoperative ultrasound (IOUS), palpation, and visual inspection (Fig. 29.3). Previously published studies of newly detected HCC nodules are also summarized in Table 29.1 [10, 12–14]. The detection rate of new HCC lesions on intraoperative ICG fluorescence imaging is reported to be 10–40 %. Despite recent advances in preoperative imaging modalities, this technique detected new HCC nodules in 40 % of the patients in our preliminary study. Because most small HCC nodules (<10 mm in diameter) are pathologically well differentiated [18], lack typical imaging patterns for HCC, and are not always hypervascular [19], it is sometimes very difficult to diagnose these lesions as HCC preoperatively. In fact, all of the new HCC nodules detected in our study were very small (3–6 mm), and most of them were diagnosed as well-differentiated HCC. Sahani et al. reported that one of the main limitations of IOUS is the hampered detection of superficial and small tumors [20]. ICG fluorescence imaging performs well in cases of superficial and small tumors, although it is unable to visualize deeper tumors. Therefore, intraoperative ICG fluorescence imaging should be viewed as a complementary adjuvant to conventional imaging techniques for the preoperative and intraoperative detection of HCC.
On the other hand, previous studies have demonstrated that nonmalignant lesions (regenerative nodules, dysplastic nodules, bile duct proliferation, cysts, fibrosis, and normal liver parenchyma) display ICG uptake [10, 12–14]. The false-positive rate on intraoperative ICG fluorescence imaging is reported to be 38–83 % (Table 29.1). Since ICG is not a cancer-specific dye, it is difficult to distinguish HCC from benign lesions using ICG fluorescence imaging. Therefore, further basic studies, including molecular, genetic, and immunohistochemical research, are needed to clarify the mechanisms and patterns of ICG accumulation in HCC nodules.
6 Detection of Extrahepatic HCC Tumors Using ICG Fluorescence Imaging
HCC tumors are sometimes found outside the liver, in cases of extrahepatic metastasis or ectopic HCC, a rare clinical entity defined as HCC arising from extrahepatic liver tissue. Extrahepatic HCC tumors also demonstrate ICG uptake and emit fluorescence when illuminated by NIR light. We experienced two rare cases of HCC metastasis. The first case involved solitary and metachronous metastasis to the appendix arising from HCC [21], while the second case comprised abdominal wall metastasis of HCC. The tumors showed intense fluorescent signals on intraoperative ICG fluorescence imaging (Fig. 29.4a, b). In particular, in the second case, small metastasis lesions were observed around the main tumor on ICG fluorescence imaging and were completely resected with negative margins under guidance with this system. We also previously reported the usefulness of ICG fluorescence imaging for detecting ectopic HCC arising in the left triangular ligament of the liver [22]. In that report, the tumor was examined using a prototype laparoscopic near-infrared camera system (Hamamatsu Photonics K.K., Hamamatsu, Japan) and was found to show bright fluorescent signals (Fig. 29.4c). The tumor was subsequently resected with an adequate margin under guidance with the above system. Satou et al. reported that, of 28 lesions for which ICG fluorescence was performed intraoperatively, 24 lesions exhibited fluorescence and were proven to be HCC metastases pathologically [23]. Five of these lesions were newly identified on ICG fluorescent imaging. Therefore, ICG fluorescence imaging is also a useful tool for intraoperatively detecting HCC tumors outside the liver.
7 Future Perspectives
As described above, there are two major challenges when identifying HCC using ICG fluorescent imaging: the low tissue penetration depth and the detection of false-positive lesions. The available literature currently reports a penetration depth ranging from approximately 5–10 mm. The ability to visualize tumors located in deeper regions would provide further improvements in the accuracy of resection of HCC lesions. In particular, ICG fluorescence imaging may be of great value in laparoscopic and robotic liver surgery, as it is not possible to palpate the liver. Moreover, modifying the ICG with a cancer-specific antibody would not only enhance the accuracy of this technique for tumor diagnosis and localization but also provide a novel photosensitive substance for use in photodynamic therapy. Since ICG is also a photosensitive substance, the development of novel photodynamic therapy that takes advantage of this property and the accumulation of ICG in HCC tumor may be possible [24]. Therefore, further improvements in camera systems and fluorophores are needed for ICG fluorescence imaging to become an indispensable technique for the diagnosis and treatment of HCC.
8 Conclusion
ICG fluorescence imaging for HCC detection is already useful in clinical settings for hepatic surgeons. With further developments in basic research and improvements of the devices, this technique is expected to be indispensable for the diagnosis and treatment in HCC surgery.
References
Poon RT, Fan ST, Lo CM et al (2001) Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 234:63–70
Llovet JM, Fuster J, Bruix J (1999) Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 30:1434–1440
Sasaki Y, Yamada T, Tanaka H et al (2006) Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg 244:771–780
Katz SC, Shia J, Liau KH et al (2009) Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 249:617–623
Sakon M, Umeshita K, Nagano H et al (2000) Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg 135:1456–1459
Shimada M, Takenaka K, Taguchi K et al (1998) Prognostic factors after repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg 227:80–85
Kitai T, Inomoto T, Miwa M, Shikayama T (2005) Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 12:211–215
Miyashiro I, Miyoshi N, Hiratsuka M et al (2008) Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol 15:1640–1643
Noura S, Ohue M, Seki Y et al (2008) Evaluation of the lateral sentinel node by indocyanine green for rectal cancer based on micrometastasis determined by reverse transcriptase-polymerase chain reaction. Oncol Rep 20:745–750
Gotoh K, Yamada T, Ishikawa O et al (2009) A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol 100:75–79
Makuuchi M, Kosuge T, Takayama T et al (1993) Surgery for small liver cancers. Semin Surg Oncol 9:298–304
Ishizawa T, Fukushima N, Shibahara J et al (2009) Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 115:2491–2504
Morita Y, Sakaguchi T, Unno N et al (2013) Detection of hepatocellular carcinomas with near-infrared fluorescence imaging using indocyanine green: its usefulness and limitation. Int J Clin Oncol 18:232–241
Ishizawa T, Masuda K, Urano Y et al (2014) Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann Surg Oncol 21:440–448
van der Vorst JR, Schaafsma BE, Hutteman M et al (2013) Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 119:3411–3418
van der Vorst JR, Hutteman M, Mieog JS et al (2012) Near-infrared fluorescence imaging of liver metastases in rats using indocyanine green. J Surg Res 174:266–271
de Graaf W, Hausler S, Heger M et al (2011) Transporters involved in the hepatic uptake of (99m) Tc-mebrofenin and indocyanine green. J Hepatol 54:738–745
Kenmochi K, Sugihara S, Kojiro M (1987) Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver 7:18–26
Takayasu K, Muramatsu Y, Mizuguchi Y et al (2004) Imaging of early hepatocellular carcinoma and adenomatous hyperplasia (dysplastic nodules) with dynamic ct and a combination of CT and angiography: experience with resected liver specimens. Intervirology 47:199–208
Sahani DV, Kalva SP, Tanabe KK et al (2004) Intraoperative US in patients undergoing surgery for liver neoplasms: comparison with MR imaging. Radiology 232:810–814
Imada S, Noura S, Ohue M et al (2013) Recurrence of hepatocellular carcinoma presenting as an asymptomatic appendiceal tumor: report of a case. Surg Today 43:685–689
Kanzaki R, Yamada T, Gotoh K et al (2010) Ectopic Hepatocellular Carcinoma Arising in the Left Triangular Ligament of the Liver. Case Rep Gastroenterol 4:138–143
Satou S, Ishizawa T, Masuda K et al (2013) Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol 48:1136–1143
Kaneko J, Inagaki Y, Ishizawa T et al (2014) Photodynamic therapy for human hepatoma-cell-line tumors utilizing biliary excretion properties of indocyanine green. J Gastroenterol 49:110–116
Acknowledgment
This work was supported in part by a grant from Osaka foundation for the prevention of cancer and cardiovascular diseases and by a grant from the Otsuka Research Fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Gotoh, K. et al. (2016). Intraoperative Detection of Hepatocellular Carcinoma Using Indocyanine Green Fluorescence Imaging. In: Kusano, M., Kokudo, N., Toi, M., Kaibori, M. (eds) ICG Fluorescence Imaging and Navigation Surgery. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55528-5_29
Download citation
DOI: https://doi.org/10.1007/978-4-431-55528-5_29
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55527-8
Online ISBN: 978-4-431-55528-5
eBook Packages: MedicineMedicine (R0)