Abstract
Plants assimilate carbon during photosynthesis using light energy to reduce atmospheric CO2 and to produce sugars and chemical energy (ATP). Sugars are partly incorporated directly into starch granules in leaf chloroplasts for short-term storage or are exported to non-photosynthetic organs for long-term storage. Indeed, starch accumulation in photosynthetic tissues is transient since it undergoes recurrent cycles of synthesis and degradation following day/night oscillation. Transient starch is synthesized during the day while photosynthesis is active and is degraded at night to provide carbon and energy to the plant when photosynthesis is inactive. Conversely, storage starch accumulates over long periods in storage organs such as seeds or tubers where it is degraded to sustain germination before photosynthesis becomes effective. Transient and storage starch syntheses occur in plastid stroma and involve dedicated enzymatic activities typically supported by several genetically independent isoforms. Although highly similar, both processes hold specific features regarding synthesis and regulation. In this chapter, we describe the mechanism of starch synthesis in photosynthetic tissues (mostly leaves) and its regulation. Several aspects are specifically highlighted here such as: (1) the function of starch synthases for the initiation of starch synthesis and the elongation of the amylopectin- and amylose-forming glucans, (2) the implication of branching enzymes and debranching enzymes for the formation of branch points and the control of their distribution within the polysaccharides, and (3) the regulation of the pathway in leaves especially by the circadian clock , the redox state of the cell and the influence of physiological factors, and the formation of protein-protein complexes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Initiation of Starch Synthesis in Leaves: The Central Role of Starch Synthases
Starch biosynthesis occurs in the stroma of the chloroplast in plant photosynthetic tissues. It involves several enzymes all encoded by the nuclear genome of the plant and subsequently transferred to the plastid after translation. The first committed step of starch synthesis in plants is the synthesis of the unique precursor: ADP-glucose. This glucosyl-nucleotide is synthesized by the condensation of ATP and glucose-1-phosphate and the release of inorganic pyrophosphate (PPi). This reaction is catalyzed by the ADP-glucose pyrophosphorylase (AGPase ), a heterotetrameric enzyme of the stroma. The synthesis of ADP-glucose is the rate-limiting step of starch synthesis, AGPase activity being tightly regulated at different levels (see Sect. 6.4 in this chapter for more details). Once synthesized, this nucleotide sugar will provide the building blocks (i.e., the glucose residues) for the synthesis of amylose and amylopectin molecules. The biosynthetic enzymes responsible for the elaboration of these polymers have been extensively studied, and their proposed functions in leaves will be described in Sects. 6.2 and 6.3 of this chapter. In contrast, mechanisms underlying the initial steps of the formation of a new starch granule remain unclear. However, this aspect of the metabolism is of major importance because it will determine the leaf ability to store photosynthetic products. Two distinct processes may operate at these early steps of the granule biogenesis: (1) de novo synthesis of one or several so-called primers, consisting of α(1 → 4) glucans long enough to be utilized by starch synthases (the elongating enzymes responsible for the synthesis of α(1 → 4) bounds of starch polymers), and (2) assembly of the amylopectin and/or amylose molecules and/or their primers (in potential association with other components such as proteins) to initiate the formation of the quaternary structure of starch (D’Hulst and Mérida 2010) (see Chap. 9 for in-depth description of these aspects).
Contrary to starch, glycogen (the storage glucan in animal, fungi, and bacteria) displays a homogenous structure, which does not require specific organization of the polymers. Nevertheless, priming is also necessary to initiate the synthesis of a new particle. This process is either performed by glycogenins in animals and fungi (Blumenfeld et al. 1983; Lomako et al. 2004) or by glycogen synthases in bacteria (Ugalde et al. 2003). In both cases, the enzyme catalyzes glucose transfer on a tyrosine residue within its own sequence (Lomako et al. 1988; Ugalde et al. 2003). This self-glucosylation step produces a primer which is subsequently elongated by glycogen synthases regardless of the nature of the organism. Although eight sequences were identified in Arabidopsis by homology with the mammalian enzyme (Chatterjee et al. 2005), only one of these loci encodes a protein containing a predicted chloroplast transit peptide. However, the product of this gene was later shown to localize to the Golgi apparatus, where it is involved in xylan metabolism (Mortimer et al. 2010; Rennie et al. 2012).
On the other hand, soluble starch synthases (SSs) have been proposed to be responsible for glucan priming in plant leaves (Roldán et al. 2007; Szydlowski et al. 2009). Four SS isoforms, SS1 , SS2 , SS3, and SS4, are found in plant genomes. Chloroplasts of ss4 Arabidopsis mutants accumulate one (or two in some cases) starch granule when 5–7 granules per chloroplast are observed in wild-type plants (Roldán et al. 2007). This decrease in the number of initiation events induces the overaccumulation of ADP-glucose, which indirectly leads to photooxidative stress and altered plant growth (Ragel et al. 2013). This phenotype is unique and specifically related to the inactivation of the SS4 locus among SS genes, defining the corresponding enzyme as a major contributor for starch initiation (Szydlowski et al. 2009; D’Hulst and Mérida 2010). Moreover, most leaf chloroplasts of ss3 ss4 double mutant plants do not accumulate starch (Szydlowski et al. 2009). This observation suggests a dominant role for SS3 in the initiation of the residual granule observed in ss4 plastids. However, the presence of residual starch in a few chloroplasts of ss3 ss4 mutant plants (Crumpton-Taylor et al. 2013) indicates that SS1 and SS2 isoforms may also participate in the priming reaction although at less efficiency. Indeed, when the latter isoforms are inactivated in an ss4 mutant background, some chloroplasts are also empty of starch (Szydlowski et al. 2009). Self-glucosylation experiments were performed in vitro with SS3 or SS4 recombinant proteins expressed in E. coli (Szydlowski et al. 2009). For both enzymes, no activity could be detected in the experimental conditions used. However, the self-glucosylation ability of starch synthases in planta or with the use of native proteins remains to be investigated.
Starch initiation begins during the early stages of leaf development (Crumpton-Taylor et al. 2012). Furthermore, chloroplasts of immature wild-type Arabidopsis leaves contain more starch granules compared to mature leaves (Crumpton-Taylor et al. 2012). However, this is not the case in ss4 mutant plants in which the synthesis of the residual granule occurs only in mature leaves (Crumpton-Taylor et al. 2013). This suggests that two temporally distinct mechanisms may operate for starch initiation. The first, involving SS4 , takes place at the early developmental stages when leaves start to expand. The second only occurs in mature leaves and involves predominantly the SS3 isoform. The question arises on whether the latter mechanism takes place in a wild-type context (i.e., when SS4 is also present) or only occurs in the ss4 mutant background, and remains to be investigated. Indeed, although most initiation events happen during early leaf development in wild-type plants, de novo synthesis of starch at leaf maturity was also reported (Crumpton-Taylor et al. 2012). Similarly, it is difficult to predict whether SS1 and SS2 are partially redundant with SS3 for this function or if their absence would destabilize complexes comprising SS3 and consequently alter its activity (D’Hulst and Mérida 2010). Indeed, these three isoforms possess functional redundancies regarding their glucan-elongating activities in Arabidopsis leaves (Zhang et al. 2008; Szydlowski et al. 2011). On the other hand, although evidence is missing for their existence in leaf chloroplasts, multiprotein complexes involving the three isoforms were identified in amyloplasts of the wheat and maize endosperms (Hennen-Bierwagen et al. 2008; Tetlow et al. 2008). Systematic analysis of starch accumulation, as well as of activities and expression levels of the starch biosynthetic enzymes during leaf development, in the wild-type, and the mutant combinations including mutations at the four SS loci, will help to address these questions.
Many assumptions can be made regarding the initial process that leads to the three-dimensional architecture of starch. Structural information on the nature and organization of the molecules composing the hilum (the nucleus of a granule) is missing. This is due to the physicochemical properties of starch polysaccharides and limitation of the available methods. However, it is tempting to speculate that mature granule morphology is determined by the growth direction of starch polymers during granule expansion and thus derives from their orientation at the hilum. Consequently, modifications of mature granule shape would reflect alteration of the hilum composition and/or structure. ss4 mutant granules do not display the characteristic flattened lenticular shape of Arabidopsis wild-type starch (Roldán et al. 2007). They appear as swollen spherical particles, resembling to most of studied storage starches from different botanical origins (Jane et al. 1994). In this mutant, the lack of SS4 may provoke hilum destabilization, leading to radial expansion of the polymers composing residual starch. Indeed, electron micrographs of ss4 mutant starch reveal a less electron-dense zone of several tens of nm of diameter in the hilum region that is not observed in wild-type starch (Roldán et al. 2007). However, no evidence yet allows discriminating between direct or indirect involvement of SS4 in shaping this area of the granule. The variable N-terminal region of SS4 sequence contains several predicted long coiled-coil domains known to favor physical interaction between proteins (Rose and Meier 2004). Indeed, interaction of SS4 with fibrillin 1a and 1b (FBN1s) was recently reported (Gámez-Arjona et al. 2014). These proteins are located to plastoglobules (plastoglobules are proteolipidic structures associated to thylakoid membranes), suggesting that starch initiation would occur at these specific regions of the chloroplast. One could speculate that SS4 , in interaction with FBN1s and other proteins and/or other classes of molecules (such as lipids of the plastoglobules), forms a nucleation center, leading to de novo initiation of a starch granule. Nevertheless, native SS4 has never yet been identified in association with starch. Moreover, fluorescence signal of AtSS4-GFP fusion proteins is only detectable in specific areas at the periphery of starch granules when expressed in chloroplasts of Arabidopsis leaf cells (Szydlowski et al. 2009). As mentioned above, residual starch of ss4 mutant plants is predominantly initiated by the SS3 isoform. It is not known if this population of granules is present in wild-type Arabidopsis plants or if SS4 directly participates to its elaboration in a wild-type context. Further work is thus required to assess the potential implication of SS4 in the nucleation of a starch granule.
It is intriguing that SS4 is absolutely required for determining the correct number of starch granules per chloroplast. As mentioned above, plastids of mature ss4 mutant leaves generally contain one (sometimes two) granule at the end of the light period (Roldán et al. 2007), whereas wild-type plants accumulate 5.5 ± 0.3 granules per chloroplast on average (Crumpton-Taylor et al. 2012). Individual chloroplast volume in wild-type Arabidopsis leaves is widely heterogeneous (Crumpton-Taylor et al. 2012). However, the number of starch granules per plastid is strongly correlated with the stromal volume of the latter (Crumpton-Taylor et al. 2012). These data indicate a tight relationship between the unit volume of stroma and the number of initiation events. Such relationship might thus be disturbed in Arabidopsis ss4 mutant leaves since no modification of average plastid volume has been reported for the corresponding transgenic plant lines. With regard to the lack of knowledge about granule nucleation and hilum composition, it is difficult to predict the molecular basis of SS4 involvement in modulating this process. A recent study assessed the effect of an increased plastid volume (i.e., by genetically manipulating plastid division) on starch accumulation in an ss4 mutant background (Crumpton-Taylor et al. 2013). Mutations at ARC loci (ARC proteins are components of the plastid division machinery) lead to decreased plastid number per mesophyll cell and strongly increased chloroplast volume (Crumpton-Taylor et al. 2013). Starch initiation was only restored in few chloroplasts of immature leaves of arc ss4 double mutant plants (Crumpton-Taylor et al. 2013), confirming that the link between stromal volume and occurrence of granule initiation is disturbed in the absence of SS4. On the other hand, no evidence relating SS4 -driven control of starch granule number to plastid division has been obtained. Addressing the latter question, as well as deciphering the underlying mechanisms, represents the next challenge in understanding starch initiation in plant leaves.
2 The Synthesis of Amylopectin Is Complex and Requires at Least Elongating, Branching, and Debranching Enzymes
Amylopectin is composed of glucose residues linked together by α(1 → 4) and α(1 → 6) O-glycosidic linkages (see Chap. 1 for exhaustive description of the fine structure of amylopectin). Although amylopectins from different botanical and/or organ origins share common features (notably the universal 9–10 nm periodicity of crystalline/amorphous lamellae alternation) (Jenkins et al. 1993), the crystallinity of leaf starches studied so far differs from that of other sources such as cereal or leguminous storage organs. Amylopectin double helices interact with water molecules within starch crystals, and their organization is more relaxed in the former (displaying the B allomorph) compared to the latter (displaying the A allomorph or a mix between A and B allomorphs, respectively) (Imberty et al. 1988; Buléon et al. 1998). These differences most likely account for glucan chain length distribution (Hizukuri 1985) and repartition of α-1,6 linkages within amylopectin (Jane et al. 1997; Gérard et al. 2000). It is widely accepted that determination of these features requires a core set of enzymes comprising at least α(1 → 4)-glucan α-4-glucosyltransferase (starch synthases, SSs), α(1 → 4)-glucan:α(1 → 4)-glucan-6-glucanotransferase (starch branching enzymes, BEs), α-dextrin endo-α(1 → 6)-glucosidase, and α(1 → 6)-glucanohydrolase (starch debranching enzymes, DBEs) activities. On the other hand, α(1 → 4)-glucan glucanohydrolase (α-amylase) and α(1 → 4)-glucan maltohydrolase (β-amylase) were proposed to also participate in amylopectin synthesis in plant leaves (Streb et al. 2008; Wu et al. 2014) as well as disproportionating enzymes (4-α-glucanotransferase) (Colleoni et al. 1999a, b) and plastidial starch phosphorylase (Dauvillée et al. 2006). Understanding why amylopectin is differently shaped with regard to its organ/botanical origin relies on the determination of functions, specificities, redundancies, interactions, and regulation of these enzymes in the different systems. In addition to studies of leaf-starch metabolism in different crops of interest, the use of the model plant Arabidopsis thaliana, as well as of the associated biomolecular tools, allowed elucidating several of these aspects in leaves. In particular, recent research had benefited from reverse genetics and T-DNA insertion mutant collections, which have permitted rapid investigation of a gene function. Importantly, this approach allowed combining mutations at numerous loci of interest to assess redundancies and/or interactions between isoforms. In the next paragraphs we will focus on the function of SS, BE, and DBE in the synthesis of amylopectin although other enzymes may be also involved in that process.
2.1 Starch Synthases Involved in Amylopectin Synthesis
Similar to what is observed for glycogen metabolism, elongation of starch glucans requires α(1 → 4)-glucan α-4-glucosyltransferase activities. In plants, the corresponding enzymes are called starch synthases and transfer the glucose residue of ADP-glucose to the nonreducing end of an elongating glucan to build the α(1 → 4) bonds. Plant genomes contain six sequences phylogenetically related to glycogen synthases, namely, SS1 , SS2 , SS3 , SS4 , SS5, and GBSS1 (Deschamps et al. 2008). The granule-bound starch synthase, GBSS1, is only active when physically associated with starch (Rongine De Fekete et al. 1960). Moreover, this enzyme is responsible for amylose biosynthesis (Nelson and Rines 1962) and will be described in a dedicated section of this chapter. As stated above, SS4 is involved in starch initiation and the control of granule number per plastid (Roldán et al. 2007). However, no impact of the ss4 mutation on amylopectin structure in plant leaves has been reported. Furthermore, there is no evidence for the involvement of SS5 in any aspect of starch metabolism. Thus, to our knowledge, only SS1, SS2, and SS3 isoforms contribute to the determination of amylopectin glucan chain length and will be highlighted in this section.
The three isoforms share a conserved C-terminal catalytic domain of about 450 amino acid residues in length that is similar to glycogen synthase sequences. Glycogen and starch synthases form part of the GT-5 (retaining glucosyl transferase) family of the CAZy (carbohydrate-active enzyme) classification (Cantarel et al. 2009) and contain two strictly conserved motifs of five amino acid residues in length. These motifs, K-X-G-G-L and X-X-G-G-L, are located to the N-terminal and C-terminal extremities of the catalytic domain, respectively (Cao et al. 1999). The former is responsible for sugar-nucleotide binding as well as being involved in the catalytic mechanism (Furukawa et al. 1990, 1993; Buschiazzo et al. 2004). On the other hand, SS3 protein sequence differs from those of SS1 and SS2 , as illustrated by a longer N-terminal variable region upstream from the catalytic domain. SS3 contains three starch-binding domains (SBDs) which favors physical interaction with starch polymers and thus contributes to its activity (Palopoli et al. 2006; Valdez et al. 2008).
SS1 isoform seems to have only little or no impact on amylopectin synthesis in storage organs of potato and barley (Kossmann et al. 1999). Indeed, no major modification of amylopectin structure was reported in the corresponding RNAi lines or mutant, respectively (Tyynelä and Schulman 1993; Kossmann et al. 1999). In potato, this may be related to very low expression levels of the corresponding gene in wild-type tubers (Kossmann et al. 1999). However, SS1 is highly expressed in leaves of this species (Kossmann et al. 1999) but its function in this organ has not been studied so far. Similarly, SS1 expression levels are much higher in rice leaves when compared to seeds (Hirose and Terao 2004). Nevertheless, contrary to what is observed in potato tuber, amylopectin chain length distribution is altered in the rice endosperm of the corresponding mutant (Fujita et al. 2006). ss1 Arabidopsis mutant plants display a comparable phenotype (Delvalle et al. 2005). Leaves of the latter accumulate starch with major structural modifications, comprising a decrease of the amylopectin/amylose ratio and alteration of the polymodal chain length distribution of amylopectin. Relative proportion of the shortest chains (DP 8 to 12) is substantially reduced in mutant amylopectin compared to the wild type (Delvalle et al. 2005). This phenotype is accompanied by an increase in the proportion of chains with a DP comprised between 12 and 23 glucose residues. Thus, this enzyme was proposed to be the determinant activity for the synthesis of the smallest chains of amylopectin molecules (Delvalle et al. 2005). Such a function in planta corroborates data from in vitro study of the recombinant enzyme from maize expressed in E. coli (Commuri and Keeling 2001). Moreover, chain length distribution analysis after β-amylolysis showed that these small glucans are the outer chains of the molecule (Delvalle et al. 2005). On the other hand, these structural modifications alter neither the starch crystallinity nor allomorph configurations (Delvalle et al. 2005).
Genomes of some plant species contain several isoforms of SS2 while others contain only a single gene encoding this enzyme. In particular, maize contains two isoforms, namely, SS2a and SS2b (Harn et al. 1998), and three isoforms are found in the rice genome, SS2-1, SS2-2, and SS2-3 (Hirose and Terao 2004; Jiang et al. 2004). Like the SS1 from potato and rice, the latter isoforms are differentially expressed in leaves and storage organs (Harn et al. 1998; Jiang et al. 2004). Indeed, maize SS2b or rice SS2-2 are expressed in leaves, while maize SS2a or rice SS2-3 are expressed in the endosperm (Harn et al. 1998; Jiang et al. 2004). On the other hand, rice SS2-1 is expressed in both types of organs during the whole life cycle, whereas other isoforms are expressed at different stages of development (Jiang et al. 2004). These expression profiles may account for different functions of each isoform in starch synthesis in these species. However, all ss2 mutants characterized so far display the same phenotype regardless of the organ considered. Modification of starch composition and structure was assessed in storage organs of pea (Craig et al. 1998), barley (Morell et al. 2003), and maize (Zhang et al. 2004), as well as in Arabidopsis leaves (Zhang et al. 2008). In each case the phenotype was similar in decreasing amylopectin/amylose ratio and, in turn, starch quantity, decreasing glucans of DP between 12 and 30 while increasing the proportion of short glucans, and alteration of starch crystallinity (Craig et al. 1998; Morell et al. 2003; Zhang et al. 2004; Szydlowski et al. 2011). Thus, SS2 seems to be responsible for the synthesis of glucans of 12 < DP < 30 regardless of the species/organ considered so far.
Two isoforms of SS3 are found in the rice genome, namely, SS3-1 and SS3-2 (Dian et al. 2005). SS3-1 is expressed in both leaves and the endosperm at early stages of development, while the other isoform is only expressed in the endosperm (Dian et al. 2005). The function of SS3 in storage starch biosynthesis was investigated in maize (Gao et al. 1998) and potato (Abel et al. 1996) by mutagenesis and RNA interference, respectively. In addition, the phenotype of an ss3 mutant of Chlamydomonas was also characterized (Fontaine et al. 1993). In each case, although phenotypes of RNAi potato lines were moderated, inactivation of SS3 leads to similar phenotypes. The latter include a decrease in starch content and enrichment of glucans of DP < 10 and DP > 40 as well as more or less pronounced alteration of starch granule morphology (Fontaine et al. 1993; Abel et al. 1996; Gao et al. 1998). According to structural analysis of mutant starch, the function of SS3 in amylopectin synthesis in Arabidopsis leaves seems to be similar to its respective counterpart in storage organs, although with less impact on the chain length distribution (Zhang et al. 2005). However, contrary to the other species, starch content in ss3 mutant Arabidopsis leaves is significantly higher than that of the wild-type at the end of the light period (Zhang et al. 2005). These data suggest that, in addition to its role in the synthesis of the longest glucan chains of amylopectin (i.e., with a DP comprised approximately between 10 and 40 depending on the species considered), SS3 plays some regulatory function in starch biosynthesis in leaves (Zhang et al. 2005). Indeed, the inactivation of SS3 in Arabidopsis is also associated with an increase of total SS activity in leaves which may explain this phenotype (Zhang et al. 2005).
As described in the previous paragraphs, forward and reverse genetic approaches showed that SS1 , SS2, and SS3 possess some specificity in leaves and preferentially elongate the shortest, medium, and longest glucans of the amylopectin molecule, respectively. However, depending on the species considered, the number of isoforms in a given species, and expression profiles of the corresponding genes in leaves or storage organs, their contribution to the synthesis of the molecule can be modulated. Other levels of complexity account for redundancies between isoforms especially between SS2 and SS3 (Zhang et al. 2008) or between SS1 and SS3 (Szydlowski et al. 2011) for the synthesis of glucans of 12 < DP < 28 or 6 < DP < 10, respectively, as shown by phenotypic analysis of the corresponding double mutant plants of Arabidopsis . In leaves of these mutants, modifications of amylopectin structure not only reflect the addition of single mutant phenotypes but are more exacerbated for the 12 < DP < 28 and 6 < DP < 10 populations of glucans (Zhang et al. 2008; Szydlowski et al. 2011). Moreover, β-amylolysis of amylopectins from ss1 ss2 and ss1 ss3 double mutant leaves suggests that SS activities not only dictate the chain length distribution but also contribute to the placement of α(1 → 6) bonds within amylopectin (Szydlowski et al. 2011). This was recently confirmed by studying mutant combinations in Arabidopsis including mutations at SS and DBE loci (Pfister et al. 2014) (this will be described in more details in the section dedicated to DBEs). It is finally worth noting that complexes including SSs and BEs have been identified in the wheat and maize endosperms (Grimaud et al. 2008; Tetlow et al. 2008). Although such physical interactions have yet to be reported for the leaf enzyme counterparts, functional interactions between SSs and BEs were recently studied in vitro (Brust et al. 2014). Importantly, combination of SS1 and BE activities leads to the production of branched glucans with a polymodal distribution similar to that seen for Arabidopsis leaf starch (Brust et al. 2014).
2.2 Formation of the α(1 → 6) Linkages in Starch: The Specific Function of Branching Enzymes
The enzymes responsible for the formation of α(1 → 6) bonds of amylopectin are α(1 → 4)-α-D-glucan:(1 → 4)-α-D-glucan-6-glycosyl transferases (EC 2.4.1.18), also known as starch branching enzymes (BEs or Q factor). BE activity was initially identified in potato (Haworth et al. 1944). The latter cleave an α(1 → 4) bond of a preexisting glucan chain and transfer the resulting fragment in α(1 → 6) position by an intra- or intermolecular mechanism. Plants generally contain two or three genetically independent isoforms of BE that were subdivided into two classes based on their amino acid sequences (Burton et al. 1995). Biochemical characterization of their activities indicates that BEI (or BE B) isoforms are more active on amylose and can transfer longer glucans compared to BEII (or BE A) enzymes. On the other hand, BEII isoforms are more active on amylopectin than BEIs (Guan and Preiss 1993; Takeda et al. 1993).
Both BEI and BEII are expressed in potato and pea leaves with most of the branching activity supported by BEII (Smith et al. 1990; Jobling et al. 1999). Mutation at the rugosus locus of pea leads to the disappearance of an A-class branching enzyme (BEII) associated with a tenfold decrease of total leaf branching enzyme activity (Smith et al. 1990; Tomlinson et al. 1997). When this mutant is cultivated under high irradiance, the rate of leaf-starch synthesis decreased by 40 % compared to the wild-type due to a reduction of the rate of photosynthesis (Smith et al. 1990). Moreover, the iodine-λmax of amylopectin from rugosus mutant leaves and the average chain length of the molecule increased (Tomlinson et al. 1997).
The role of the other isoform, BEI , in starch metabolism has been poorly studied in dicots, and downregulation of this enzyme has only a small impact on starch metabolism (Flipse et al. 1996). Indeed, no differences in the amylopectin content or chain length distribution could be detected in tuber starch from amylose-free potato lines with reduced BEI expression. However, physicochemical properties of starch suspension showed differences compared to the wild-type suggesting that minor (or uncharacterized) structural modifications may occur when the expression of BEI is reduced (Flipse et al. 1996).
Monocots differ from dicots since they contain two types of BEII enzymes, namely, BEIIa and BEIIb (Dang and Boyer 1988). Both genes are tissue specific and their expression differs during seed development. After purification of BEs from maize leaves and kernels, it was suggested that BEI and BEIIa are expressed in both organs, whereas BEIIb expression is restricted to the endosperm (Dang and Boyer 1988). These results were later confirmed indicating that BEIIa and BEIIb are genetically independent and differentially expressed in leaves and endosperm (Fisher et al. 1996a, b; Gao et al. 1996, 1997). These expression patterns were corroborated in other cereals such as barley and rice where BEIIa is expressed in most plant organs (endosperm, leaf, and root) and the embryo, while BEIIb expression was limited to the endosperm (Yamanouchi and Nakamura 1992; Sun et al. 1998; Nishi et al. 2001). Since BEIIb expression is restricted to the endosperm, mutants lacking this enzyme accumulate high-amylose starch with structurally modified amylopectin in this organ (Garwood et al. 1976; Hedman and Boyer 1982), whereas they cannot be differentiated from the wild-type regarding leaf starch (Dang and Boyer 1989). In maize leaves, BEIIa is the major branching enzyme activity. Indeed in be2a mutant lines, the branching activity is decreased by sevenfold at the middle of the light phase. Since BEIIb is not expressed in leaves, the residual activity is most probably due to BEI, and, although BEI proteins were not detected by immunoblot, the corresponding mRNA was identified by RT-PCR (Yandeau-Nelson et al. 2011).
The influence of BEIIa activity on transitory starch metabolism was evident by the phenotype of mutator insertion lines lacking the corresponding enzyme. Leaves of these mutants produce amylopectin with reduced branching compared to the wild-type while kernel starch was unaffected by the mutation. Moreover, an accelerated senescence phenotype was associated with the lack of BEIIa in leaves (Blauth et al. 2001). Yandeau-Nelson et al. (2011) reported that be2a mutant lines accumulate wild-type amount of starch in leaves during the day but have reduced degradation rate compared to the wild-type. Indeed, only 40 % of starch is degraded at night in the mutant, while more than 80 % is degraded in the wild-type. The amount of starch remaining at the end of the day is even larger in the senescent regions of be2a leaves. This reduction of the degradation process is likely caused by alteration of starch granule structure and composition. Indeed, starch produced by be2a lines differs from wild-type starch in several aspects. Scanning electron microscopy analyses of purified starch granules revealed “lobular and fused” structures instead of the uniform discs observed in the wild-type (Yandeau-Nelson et al. 2011). This alteration of the granule shape is probably caused by a decrease in the amylopectin to amylose ratio. Moreover, structural analysis of mutant starch revealed that amylopectin is enriched in intermediate to long glucans (DP > 25) and contains fewer short glucan chains (Dinges et al. 2003).
The function of BEI in monocot leaves is unclear since be1 null mutants of maize produce starch that are undistinguishable from that of the wild-type (Blauth et al. 2002). Among the dicots, Arabidopsis represents a particular case since two genes, BE2.1 (or BE3) and BE2.2 (or BE2), both encoding A-class branching enzymes are expressed in leaves and are involved in transient starch synthesis (Fisher et al. 1996a, b). Expression of both genes differs throughout the diurnal cycle. Indeed, BE2.1 expression is regulated by light while that for BE2.2 remains unaffected (Khoshnoodi et al. 1998; Smith et al. 2004). However, accumulation of transcripts of both genes is favored when glucose, fructose, and sucrose are supplied during the day (Khoshnoodi et al. 1998). Interestingly, no enzyme of the BEI family can be found in the genome of this species.
Phenotypic analysis of Arabidopsis mutant lines lacking either BE2 or BE3 indicates that these enzymes possess redundant functions. Indeed, each single mutant contains starch and amylose contents similar to those of the wild-type. Only modifications in the chain length distribution of amylopectin were reported: relative proportions of short chains (DP 5–8) were slightly reduced and those of intermediate chains (DP 9–15) were slightly increased. However, when mutations affecting both enzymes are combined, plants fail to accumulate starch, indicating that no other enzyme can compensate for their functions. In this double mutant, starch is substituted by high levels of α-maltose, and plants display a severe plant growth retardation as well as pale leaf phenotype (Dumez et al. 2006). All data collected so far regarding transitory starch metabolism suggest that BEIIa and BEII are the major starch-branching enzymes involved in amylopectin synthesis in monocots and dicots, respectively, whereas the potential function of BEI in leaf remains to be elucidated.
2.3 Maturation of the Polysaccharide Structure: Debranching as a Mandatory Controlling Step
The semicrystalline nature of amylopectin is a consequence of the specific distribution of α(1 → 6) linkages within this polymer. Indeed, branch points are concentrated in amorphous lamellae but are extremely rare in other regions of the molecule, where linear glucans can intertwine to form crystalline double helices.
As described in Chap. 5, this organization results from the concerted action of starch synthases, branching, and debranching enzymes. In plants, debranching enzymes (DBE) are members of the GH13 (glycoside hydrolase) family of the CAZy classification. DBEs specifically cleave α(1 → 6) linkages and are subdivided into two classes depending on their substrate specificity. Isoamylases (ISA) act on amylopectin and glycogen, while pullulanases (PU) (also called limit dextrinases, LDA) are active on amylopectin and pullulan (a highly branched polyglucan composed of maltotriosyl residues linked together by α(1 → 6) bonds) but not on glycogen. Genes encoding debranching enzymes are well conserved throughout the Chloroplastida. All organisms studied so far contain one pullulanase- and three isoamylase-encoding genes (ISA1 , ISA2, and ISA3 ) (Deschamps et al. 2008). PU and ISA3 are mainly involved in starch degradation and have minor role in polysaccharide synthesis. Indeed, as observed in maize or Arabidopsis , pullulanase null mutants accumulate normal amount of starch in photosynthetic tissues, and the structure of amylopectin is close to that of the wild-type reference. However, pullulanase is able to participate in transient starch synthesis under certain circumstances. This function has been highlighted when mutations affecting PU and ISA1 were combined, leading to less starch and to the accumulation of more soluble polysaccharide (Dinges et al. 2003; Wattebled et al. 2005, 2008; Streb et al. 2008).
There is no doubt that ISA3 is essential for starch degradation in Arabidopsis leaves. Nevertheless, its involvement in starch synthesis is similar to that of pullulanase and was inferred when ISA1 and/or ISA2 proteins were also missing. Indeed, in a wild-type context, ISA3 has no obvious function in starch synthesis (Wattebled et al. 2005; Delatte et al. 2006). Since ISA3 and pullulanase do not influence starch synthesis in a wild-type context, the debranching activity involved in transient starch anabolism is mainly dependent on the presence of ISA1 and ISA2 proteins. These proteins behave very differently. In all organisms, ISA2 is not active and lacks essential catalytic amino acids (Table 6.1) conserved within the GH13 family (Hussain et al. 2003; Hennen-Bierwagen et al. 2012). In all plants, ISA2 proteins can form active hetero-oligomer complexes with ISA1. However, depending on its tissular or botanical origin, ISA1 can also be active by itself in the form of a homo-oligomer (see below).
In Arabidopsis leaves, the isoamylase activity involved in starch synthesis requires the presence of both ISA1 and ISA2 proteins and was called ISO1 (Wattebled et al. 2005). Mutant plants lacking ISA1 and/or ISA2 display the same phenotype including a minimal decrease of 80 % of the starch content at the end of the day (Zeeman et al. 1998; Delatte et al. 2005; Wattebled et al. 2005). In these plants, the reduction of starch content is associated with the accumulation of a highly branched soluble polysaccharide in mesophyll cells. This material resembles glycogen and was therefore called phytoglycogen. Moreover, the residual starch, mainly accumulated by epidermal and bundle sheath cells, is profoundly modified at the level of granule size and amylopectin structure (Delatte et al. 2005). Indeed, starch granules are much smaller than in the wild-type and amylopectin is enriched in very short chains (DP 3–5).
In monocots such as rice or maize, ISA1 is active in the endosperm either in complex with ISA2 or with itself as a homo-oligomer. Inactivation of ISA1 in maize, barley, or rice endosperms generates a phenotype similar to that observed in the corresponding mutant of Arabidopsis (James et al. 1995; Nakamura et al. 1996; Dinges et al. 2001; Burton et al. 2002). However, since ISA1 can form active homo-oligomers (i.e., without the requirement of ISA2), isa2 mutants of maize and rice display a wild-type starch phenotype (Utsumi and Nakamura 2006; Kubo et al. 2010; Utsumi et al. 2011).
Studies were conducted in rice and maize to assess whether the homo-oligomer active form of ISA1 is a specific feature of cereal endosperms or also exists in leaves. Interestingly, in rice, only hetero-oligomers were purified from leaves, whereas both homo- and hetero-oligomers were catalytically active in the endosperm (Utsumi et al. 2011). The lack of homo-oligomers in leaves could result either from a predominant expression of ISA2 or from the rapid degradation of the heteromeric form. Indeed, ISA1 homo-complexes are less stable than ISA1/ISA2 hetero-complexes. The former are quickly inactivated at 40 °C, while the latter remain active more than 30 min in these conditions (Utsumi et al. 2011). ISA1 homo-oligomer may also behave differently in leaf. Indeed, in maize leaves, ISA1 homo-oligomers appear as a weak and diffuse band on specific native-gel activity assays, whereas it is clearly visible as a sharp blue band when extracted from the endosperm (Lin et al. 2013). The presence of the ISA1 homo-oligomer in leaf was further confirmed by western blot. Phenotypes induced by the absence of ISA1 or ISA2 on polysaccharides were determined. Unlike Arabidopsis , isa1 or isa2 maize mutants do not accumulate phytoglycogen. However, they display severe reduction in starch content, reduced average granule size, and modification of amylopectin, similar to what is observed in Arabidopsis. Interestingly, these phenotypes are more pronounced in the isa1 mutant than in the isa2 mutant. The presence of tiny amounts of ISA1 homo-oligomers can partially counterbalance the lack of ISA2 but cannot restore a wild-type phenotype conversely to what occurs in the endosperm (Lin et al. 2013).
To determine if the ability of ISA1 from cereals to function as a homo-oligomer is species dependent, ISA1 from maize or rice were expressed in isa1 isa2 Arabidopsis double mutant plants. The isa1 isa2 double mutant accumulates large amounts of phytoglycogen while the starch content is strongly reduced. Expression of ISA1 from maize or rice in the isa1 isa2 mutant restores wild-type starch synthesis (Facon et al. 2013; Streb and Zeeman 2014). This result indicates that ISA1 from cereals can function without ISA2 even in a heterologous physiological context. This property is likely the consequence of evolutionary divergence between monocots and dicots (Facon et al. 2013).
Finally, three isoamylase activities were detected by zymogram in crude extracts of Chlamydomonas reinhardtii . Genetic studies revealed that two out of these three activities are composed by ISA1 and ISA2 proteins. The latter are assembled into hetero-oligomer, while the third activity corresponds to ISA1 homo-oligomers (Dauvillée et al. 2001a, b; Sim et al. 2014). The ISA1 homo-oligomer can sustain starch synthesis to a near wild-type level when growth conditions mimic the leaf cell physiological context (Dauvillée et al. 2001a, b). Recently the crystal structure of the ISA1 homo-oligomer was elucidated and revealed that it is composed of two ISA1 subunits associated by their C-terminal domains (Sim et al. 2014), as previously suggested for the maize leaf enzyme (Facon et al. 2013). Sequence alignment comparison indicates that the dimeric organization of the ISA1 homo-oligomer may be conserved in all plants (Sim et al. 2014).
2.4 Physical and Functional Interactions
Amylopectin crystallization is a complex mechanism that is not yet completely elucidated. The involvement of isoamylases in this process is indisputable as shown by mutant analysis. Data accumulated since 20 years fully support the trimming model of amylopectin synthesis (Ball et al. 1996; Myers et al. 2000). However, other factors can also affect amylopectin crystallization. Indeed, enzymes of the starch pathway do not work independently but physically and/or functionally interact with each other. These interactions have been mostly studied in storage tissues (see Chap. 8) although the intricate interaction between enzymes of the starch pathways is also evident in leaves. For instance, in maize leaves, the absence of ISA1 or PU was shown to cause the loss of BE2a activity although the protein content is unmodified, suggesting that the activity of BE2a is altered because of posttranslational modifications (Dinges et al. 2003). In Arabidopsis the implication of starch synthases for controlling the distribution of α(1 → 6) linkages within amylopectin has been suggested after the study of mutant lines defective for several starch synthases. Indeed, defects in both SS1 and SS2 or both SS1 and SS3 , but not in the corresponding single mutants, modify amylopectin branch points distribution (Szydlowski et al. 2011). The precise reason of such modification is not yet known. However, the destabilization of protein complexes involving BEs (and consequently BEs activity) may be one explanation. More recently functional interactions between starch synthases and debranching enzymes during starch synthesis have been exemplified by the combination of mutations affecting SS1, SS2, SS3, and ISA1 in Arabidopsis (Pfister et al. 2014). It is proposed that outer chain length is controlled by starch synthases activity and is a key factor to ensure further amylopectin crystallization. It is then postulated that isoamylases are involved in the degradation of incorrectly synthesized glucans that remain water soluble when starch synthase activity is altered. However, the higher water-soluble polysaccharides content observed in ss2- ss3- isa-triple mutant could simply arise because of ISA activity deficiency. Moreover in maize endosperm, combination of mutations for SS3 (du1-ref) and ISA2 (isa2-339) induces higher water-soluble polysaccharides accumulation without reduction of debranching enzyme activity (Lin et al. 2012) ruling out the involvement of debranching enzymes for the removing of water-soluble polysaccharides.
3 Amylose Synthesis Is Controlled by a Dedicated Elongating Enzyme Located Within the Starch Granule
In addition to amylopectin, starch also contains amylose, which represents between 20 and 30 % of the average granule dry weight. Similar to amylopectin, amylose is composed of glucose residues linked together by α(1 → 4) and β(1 → 6) O-glycosidic bonds. However, the proportion of α-1,6 linkages is smaller (less than 1 %) in the molecule of amylose compared to the moderately branched amylopectin molecule (5–6 %). The contribution of amylose to the crystallinity of starch is not yet fully understood. On one hand, amylose can form single helices in vitro (Godet et al. 1995) and some correlations between amylose contents and starch crystallinity were observed in Chlamydomonas (Wattebled et al. 2002) or maize (Cheetham and Tao 1998). On the other hand, one could argue that a direct effect of amylose on starch crystallinity cannot be discriminated from an indirect effect of amylose on amylopectin crystallinity in planta.
Studies in some species like tobacco, for example, suggested that amylose content is lower in leaf starch compared to storage starches (Matheson 1996). Furthermore, amylose polymers appeared to be on average smaller in transitory starch from leaves of this species. Nevertheless, it is difficult to generalize since leaf starch from other species can contain as much or more amylose than storage starches (BeMiller and Whistler 2009). Moreover, there is no report on variation in structure and/or content of this polymer within a broad population of leaf starches from different botanical origins.
Contrary to amylopectin glucans, amylose is biosynthesized by only one starch synthase, the GBSS. The latter is only active when it is physically associated with starch (Rongine De Fekete et al. 1960). This isoform is thus located within the starch granule in planta and can represent up to 95 % of the total starch-bound protein content. GBSS is a processive enzyme using ADP-glucose as a substrate and contains the catalytic amino acid residues conserved in all starch synthases (Denyer et al. 1999a, b; Edwards et al. 1999) (see above section for more details). However, studies performed with Chlamydomonas, potato, and pea starches showed that GBSS uses the long external glucans of amylopectin as acceptor substrates (van de Wal et al. 1998) and that malto-oligosaccharides enhance amylose synthesis (Denyer et al. 1996).
In waxy mutants of wheat, amylose synthesis sometimes still occurs in some organs while storage starch is free of amylose (Nakamura et al. 1998), suggesting that two isoforms of GBSS, encoded by two different loci, may be differentially expressed according to the organ considered. Indeed, wheat contains two GBBS isoforms, namely, GBSS1 and GBSS2 (Vrinten and Nakamura 2000). In this species, GBSS2 is encoded by a locus different from that encoding GBSS1. The former is expressed in leaves, while the latter is highly expressed in the endosperm (Vrinten and Nakamura 2000). Thus, GBSS2 seems to be the main enzyme responsible for amylose synthesis in wheat leaves. Conversely to wheat, Arabidopsis genome contains only one isoform of GBSS which is regulated both at the transcriptional and activity levels during day/night cycles (Tenorio et al. 2003).
4 The Regulation of Starch Synthesis and Degradation in Leaves
As described in the previous paragraphs and chapters, starch metabolism is relatively complex. It is controlled by a quite broad set of enzymes responsible for the formation and the cleavage of only two O-glycosidic linkages between glucose residues. Although the functions of many of these enzymes have been unraveled during the last two decades, the regulation of their activities has remained elusive. However, the regulation of enzyme activities is a key aspect because it strictly controls starch accumulation and degradation. Consequently it also controls carbon flux within the cell and particularly in leaves where starch metabolism is submitted to diurnal fluctuations. Indeed starch synthesis occurs during the illuminated period when photosynthesis is active. The polymer is subsequently degraded in the dark providing carbon and energy to the whole plant, sustaining its development and maintaining the carbon balance even when photosynthesis is off. Consequently, starch metabolism is highly regulated in leaves, and the storage polysaccharide appears as a regulator for plant growth and yield although it is still debating how this regulation occurs (Sulpice et al. 2009; Gibson et al. 2011). Regulation occurs at different levels including gene transcription and enzyme activities. This requires different mechanisms such as control by the circadian clock , transcriptional regulation of gene expression, redox control of protein activities, as well as posttranslational modifications, protein-protein complexes formation, and other physiological regulations.
It is now well established that both starch accumulation and degradation occur almost linearly (albeit with opposite signs) in Arabidopsis leaves throughout the diurnal cycle (Caspar et al. 1985; Gibon et al. 2004) and that about 95 % of starch is degraded at the end of the night (Graf and Smith 2011). However, starch accumulates to a lesser extent in plants cultivated under short-day condition. Moreover, the rate of starch degradation is negatively correlated to the length of the dark period (Lu et al. 2005). Thus, plants adjust the rate of starch degradation according to the ratio between night and day lengths and consequently to the level of starch accumulated at the end of the light period (Lu et al. 2005). Interestingly, plants immediately accommodate the rate of starch degradation after an unexpected arrival of night (Graf et al. 2010). Such adjustment avoids sugar starvation during the dark period and is under the control of the circadian clock as shown by the impairment of the rate of starch degradation in the clock-altered cca1/lhy mutant of Arabidopsis (Graf et al. 2010). Indeed, assuming that the length of the whole diurnal cycle is not modified, leaf cells can anticipate the arrival of day and night but can also rapidly adjust to changes in night length preventing carbon/sugar starvation (Graf et al. 2010). It is not clear at which level the circadian clock impacts starch degradation. The expression of starch enzymes is likely to be tuned by the clock as suggested by others (Zabawinski et al. 2001; Smith et al. 2004; Lu et al. 2005; Ral et al. 2006). However, the protein abundance of some of the starch-degrading enzymes (e.g., DPE2 , PHS2, and SEX1 ) is not modified throughout the cycle (Lu et al. 2005) suggesting that posttranslational regulation of enzyme activity is responsible for the control of starch degradation rather than enzyme amounts.
Although the circadian clock is probably an important regulator of starch breakdown, it is likely not the only controlling step of starch degradation in leaves. Indeed, immediate adjustment of the rate of starch degradation still occurs in the cca1/lhy clock mutant when unexpected early night is applied (Scialdone et al. 2013). It has been suggested that two molecules provide information on (1) the concentration of starch at end of day and (2) on the expected time of dawn. Such molecules have not yet been identified, although phosphate groups at C6 and C3 positions on some of the glucose residues of starch were proposed as candidates to regulate the rate of starch degradation (Scialdone et al. 2013). It is not clear whether or not the same process applies for the control of starch synthesis.
The rate of starch degradation is also compromised in the ss4 mutant of Arabidopsis . As described above, SS4 is one of the major factors controlling the initiation of starch synthesis. The lack of SS4 leads to the accumulation of fewer (one, rarely two, starch granule per chloroplast) but larger starch granules in chloroplasts (Roldán et al. 2007). The rates of both starch synthesis and degradation as well as plant growth are reduced in the ss4 mutant compared to the wild-type, although the circadian clock and the starch-degrading machinery are unaffected in this mutant (except for the activities of both starch phosphorylases PHS1 and PHS2 which are enhanced) (Roldán et al. 2007). It was suggested that the surface of the starch granules represents a key factor in controlling the rate of starch synthesis and degradation (Roldán et al. 2007; D’Hulst and Mérida 2010). Indeed, if one compares starch granules to perfect spheres (this assumption has no impact on the following rationale, although granules resemble oblate spheroids) and considering homogenous distribution of starch within these spheres, for a given volume of starch at the end of the day, the cumulated surface of six granules (the average number of granules per wild-type chloroplasts) is about 2× larger than that of a single granule. Thus, the surface accessibility for synthesis and degradation is different between the wild-type and the ss4 mutant. Taking into account that starch content is decreased in the latter, this surface is about 1.6 times larger in the wild-type at the end of the day. This may explain the reduction in both the rates of starch synthesis and degradation as well as why starch is not fully depleted at the end of the night in the ss4 mutant.
It is not yet clear whether the number of granules in plant leaves is unaffected throughout a day/night cycle. However, if this parameter is constant, the total starch volume and the surface available for enzymes of the degradation pathway will be lower at anticipated night arrival in a wild-type background. Moreover, based on the same postulates and data published by Lu et al. (2005), the ratio between the total granule surface after 12 h of light and 8 h of light would be 1.6. Thus, such a reduction of the granule surface may also account, even partly, for the reduced rate of starch degradation and to the immediate control of this process when the day length is unexpectedly shortened. Interestingly, SS4 gene expression seems to be controlled by the transcription factors AtCOL (constans like) and AtIDD5 (indeterminate domain 5) (Ingkasuwan et al. 2012). In the corresponding mutants of Arabidopsis (i.e., col and idd5), starch granule size and number per chloroplast were altered as well as plastid morphology (as observed by TEM on ultrathin sections of leaves). The inference of such regulation is still not understood and requires further investigation but may provide new insights in the regulation of starch granule initiation and thus starch synthesis as well as degradation control.
Regulation of starch metabolism in leaves also occurs through other mechanisms. For instance, it is suspected that enzymes of the starch pathway physically interact to form heteromultimeric complexes in plant storage organs (Hennen-Bierwagen et al. 2008; Tetlow et al. 2008; Liu et al. 2012). In some instances, their formation is conditioned by the posttranslational modification (phosphorylation) of the enzymes involved (Makhmoudova et al. 2014). However, conversely to sink organs, their formation in leaves has been poorly studied. Apart from the formation of ADP-glucose pyrophosphorylase (AGPase ) (see Chap. 11 for details) and debranching enzymes (isoamylases) complexes (see Sect. 6.2.3 for details), no other protein-protein interaction has been formerly described so far in leaves. It is assumed that the control of the formation of these complexes is crucial for the tuned regulation of starch synthesis and degradation and thus plant homeostasis.
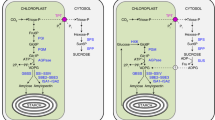
Redox control of enzymes is also one of the regulatory steps of starch metabolism in leaves. The first example described was the control of AGPase (a more complete description of AGPase activity and regulation is presented in Chap. 11), the enzyme catalyzing the synthesis of ADP-glucose. This reaction is considered as the rate-limiting step for starch synthesis. AGPase activity was shown to be regulated in photosynthetic tissues such as leaves of A. thaliana (Crevillen et al. 2003), barley (Kleczkowski 2000), rice (Lee et al. 2007), and maize (Huang et al. 2014) or Chlamydomonas reinhardtii (Ball et al. 1991). The regulation results from the ratio between concentrations of allosteric effectors such as 3-phosphoglyceric acid (3-PGA) and inorganic phosphate (Pi). AGPase activity is also modulated by the redox state of the protein as shown in potato, pea, and Arabidopsis leaves (Hendriks et al. 2003) and previously demonstrated in potato tubers (Ballicora et al. 2000; Tiessen et al. 2002). Indeed, AGPase is a heterotetramer composed of two small (catalytic) and two large (regulatory) subunits. In the oxidized state, the catalytic subunits of the leaf and potato tuber enzyme can form a dimer maintained by a disulfide bond between two Cys12 cysteine residues (Fu et al. 1998; Hädrich et al. 2012). Dimerization occurs at night and leads to a strong reduction of enzyme activity (Hendriks et al. 2003). The disulfide bond is reduced during the light period, but also in the dark when leaves are supplied with sucrose. AGPase activity is consequently increased as well as starch synthesis. Interestingly, AGPase activity is under the control of the circadian clock as demonstrated in Chlamydomonas reinhardtii (Zabawinski et al. 2001). Regulation by the clock applies at both gene expression and enzyme activity suggesting that the circadian clock modulates the whole starch metabolism.
The redox control of starch synthesis in leaves is not limited to the regulation of AGPase activity. Such type of regulation was also reported for several Arabidopsis enzymes such as: ISA1 /ISA2 , LDA, SS1 , SS3 , BE2, BAM3 (β-amylase), AMY3 (α-amylase), GWD (glucan water dikinase), and DSP4 (SEX4 ) (phosphoglucan phosphatase) (Mikkelsen et al. 2005; Sokolov et al. 2006; Sparla et al. 2006; Valerio et al. 2011; Glaring et al. 2012; Seung et al. 2013; Silver et al. 2013). This suggests that the whole pathway is under redox control allowing a tight regulation of enzyme activities during the day/night cycle. For instance, AMY3 is inactive in its oxidized form (when a disulfide bond is formed between the Cys499 and Cys587 residues) and is reactivated by thioredoxins (Seung et al. 2013). Recently a plastid -localized NADPH-dependent thioredoxin reductase C (NTRC ) was found to interact with AGPase in Arabidopsis (Michalska et al. 2009; Lepistö et al. 2013). Moreover, starch accumulation is impaired in ntrc mutant plants particularly when the latter are cultivated under short-day conditions. Conversely, overexpression of NTRC enhanced plant growth and biomass indicating a direct control of starch synthesis by this thioredoxin reductase (Toivola et al. 2013). Interestingly, the over expression of the plastidial thioredoxin f in tobacco increases leaf-starch accumulation by up to 700 % (Sanz-Barrio et al. 2013). However, high starch accumulation is not a consequence of AGPase redox activation as proposed earlier for Arabidopsis (Li et al. 2012). Indeed, it was suggested that the redox control of SS3/SS4 (starch synthases 3 and 4) is responsible for this increase of the starch content in Arabidopsis leaves (Li et al. 2011).
Finally, starch metabolism in Arabidopsis leaf is physiologically controlled by the concentration of trehalose-6-phosphate (Tre6P). Tre6P is a nonreducing disaccharide composed by two glucose residues and found at very low level in Arabidopsis leaves. It is synthesized from Glc-6-P and UPG-glucose by the trehalose-6-phosphate synthase (AtTPS1) (Avonce et al. 2004). AtTPS1 was proposed to act as a regulator for glucose signaling in Arabidopsis. An osmoprotectant function of trehalose was excluded because of its very low levels in plants. Indeed, Tre6P concentration in leaves increases during the day but also when the dark period is unexpectedly extended for 6 h as well as in a pgm starchless mutant (Lunn et al. 2006). Increased concentration of Tre6P is accompanied by a modulation of AGPase redox state in favor of the reduced active form of the enzyme. Consequently starch content is increased after 4 h of illumination (except in the pgm mutant) (Lunn et al. 2006). This observation confirmed previous results indicating that the redox modulation of AGPase activity is controlled by Tre6P, the concentration of which being in turn regulated by sugars (Kolbe et al. 2005). Interestingly, oscillation of sugar levels during day/night cycles acts as a metabolic feedback to the circadian clock (Haydon et al. 2013). Thus, over its function in carbon and energy storage, starch, which metabolism is controlled by the circadian clock, may also contribute to the entrainment and the robustness of the clock in photosynthetic tissues. However, the molecular mechanisms underlying this regulation are still not unraveled.
References
Abel GJW, Springer F, Willmitzer L et al (1996) Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.). Plant J 10:981–991
Avonce N, Leyman B, Mascorro-Gallardo JO et al (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136:3649–3659
Ball S, Marianne T, Dirick L et al (1991) A Chlamydomonas reinhardtii low-starch mutant is defective for 3-phosphoglycerate activation and orthophosphate inhibition of ADP-glucose pyrophosphorylase. Planta 185:17–26
Ball S, Guan H-P, James M et al (1996) From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell 86:349–352
Ballicora MA, Frueauf JB, Fu Y et al (2000) Activation of the potato tuber ADP-glucose pyrophosphorylase by thioredoxin. J Biol Chem 275:1315–1320
BeMiller JN, Whistler RL (2009) Starch: chemistry and technology. Elsevier Science, New York
Blauth SL, Yao Y, Klucinec JD et al (2001) Identification of Mutator insertional mutants of starch-branching enzyme 2a in corn. Plant Physiol 125:1396–1405
Blauth SL, Kim K-N, Klucinec J et al (2002) Identification of Mutator insertional mutants of starch-branching enzyme 1 (sbe1) in Zea mays L. Plant Mol Biol 48:287–297
Blumenfeld ML, Whelan WJ, Krisman CR (1983) The initiation of glycogen biosynthesis in rat heart. Eur J Biochem 135:175–179
Brust H, Lehmann T, D'Hulst C et al (2014) Analysis of the functional interaction of Arabidopsis starch synthase and branching enzyme isoforms reveals that the cooperative action of SSI and BEs results in glucans with polymodal chain length distribution similar to amylopectin. PLoS ONE 9:e102364
Buléon A, Gérard C, Riekel C et al (1998) Details of the crystalline ultrastructure of C-starch granules revealed by synchrotron microfocus mapping. Macromolecules 31:6605–6610
Burton RA, Bewley JD, Smith AM et al (1995) Starch branching enzymes belonging to distinct enzyme families are differentially expressed during pea embryo development. Plant J 7:3–15
Burton RA, Jenner H, Carrangis L et al (2002) Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J 31:97–112
Buschiazzo A, Ugalde JE, Guerin ME et al (2004) Crystal structure of glycogen synthase: homologous enzymes catalyze glycogen synthesis and degradation. EMBO J 23:3196–3205
Cantarel BL, Coutinho PM, Rancurel C et al (2009) The carbohydrate-active enzymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238
Cao H, Imparl-Radosevich J, Guan H et al (1999) Identification of the soluble starch synthase activities of maize endosperm. Plant Physiol 120:205–216
Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79:11–17
Chatterjee M, Berbezy P, Vyas D et al (2005) Reduced expression of a protein homologous to glycogenin leads to reduction of starch content in Arabidopsis leaves. Plant Sci 168:501–509
Cheetham NWH, Tao L (1998) Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohydr Polym 36:277–284
Colleoni C, Dauvillee D, Mouille G et al (1999a) Genetic and biochemical evidence for the involvement of alpha −1,4 glucanotransferases in amylopectin synthesis. Plant Physiol 120:993–1004
Colleoni C, Dauvillee D, Mouille G et al (1999b) Biochemical characterization of the chlamydomonas reinhardtii alpha −1,4 glucanotransferase supports a direct function in amylopectin biosynthesis. Plant Physiol 120:1005–1014
Commuri PD, Keeling PL (2001) Chain-length specificities of maize starch synthase I enzyme: studies of glucan affinity and catalytic properties. Plant J 25:475–486
Craig J, Lloyd JR, Tomlinson K et al (1998) Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos. Plant Cell Online 10:413–426
Crevillen P, Ballicora MA, Merida A et al (2003) The different large subunit isoforms of Arabidopsis thaliana ADP-glucose pyrophosphorylase confer distinct kinetic and regulatory properties to the heterotetrameric enzyme. J Biol Chem 278:28508–28515
Crumpton-Taylor M, Grandison S, Png KMY et al (2012) Control of starch granule numbers in Arabidopsis chloroplasts. Plant Physiol 158:905–916
Crumpton-Taylor M, Pike M, Lu K-J et al (2013) Starch synthase 4 is essential for coordination of starch granule formation with chloroplast division during Arabidopsis leaf expansion. New Phytol 200:1064–1075
D’Hulst C, Mérida Á (2010) The priming of storage glucan synthesis from bacteria to plants: current knowledge and new developments. New Phytol 188:13–21
Dang PL, Boyer CD (1988) Maize leaf and kernel starch synthases and starch branching enzymes. Phytochemistry 27:1255–1259
Dang P, Boyer C (1989) Comparison of soluble starch synthases and branching enzymes from leaves and kernels of normal and amylose-extender maize. Biochem Genet 27:521–532
Dauvillée D, Colleoni C, Mouille G et al (2001a) Two loci control phytoglycogen production in the monocellular green alga Chlamydomonas reinhardtii. Plant Physiol 125:1710–1722
Dauvillée D, Colleoni C, Mouille G et al (2001b) Biochemical characterization of wild-type and mutant isoamylases of Chlamydomonas reinhardtii supports a function of the multimeric enzyme organization in amylopectin maturation. Plant Physiol 125:1723–1731
Dauvillée D, Chochois V, Steup M et al (2006) Plastidial phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J 48:274–285
Delatte T, Trevisan M, Parker ML et al (2005) Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J 41:815–830
Delatte T, Umhang M, Trevisan M et al (2006) Evidence for distinct mechanisms of starch granule breakdown in plants. J Biol Chem 281:12050–12059
Delvalle D, Dumez S, Wattebled F et al (2005) Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J 43:398–412
Denyer K, Clarke B, Hylton C et al (1996) The elongation of amylose and amylopectin chains in isolated starch granules. Plant J 10:1135–1143
Denyer K, Waite D, Edwards A et al (1999a) Interaction with amylopectin influences the ability of granule-bound starch synthase I to elongate malto-oligosaccharides. Biochem J 342:647–653
Denyer K, Waite D, Motawia S et al (1999b) Granule-bound starch synthase I in isolated starch granules elongates malto-oligosaccharides processively. Biochem J 340:183–191
Deschamps P, Moreau H, Worden AZ et al (2008) Early gene duplication within chloroplastida and its correspondence with relocation of starch metabolism to chloroplasts. Genetics 178:2373–2387
Dian W, Jiang H, Wu P (2005) Evolution and expression analysis of starch synthase III and IV in rice. J Exp Bot 56:623–632
Dinges JR, Colleoni C, Myers AM et al (2001) Molecular structure of three mutations at the maize sugary1 locus and their allele-specific phenotypic effects. Plant Physiol 125:1406–1418
Dinges JR, Colleoni C, James MG et al (2003) Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 15:666–680
Dumez S, Wattebled F, Dauvillee D et al (2006) Mutants of Arabidopsis lacking starch branching enzyme II substitute plastidial starch synthesis by cytoplasmic maltose accumulation. Plant Cell 18:2694–2709
Edwards A, Borthakur A, Bornemann S et al (1999) Specificity of starch synthase isoforms from potato. Eur J Biochem 266:724–736
Facon M, Lin Q, Azzaz AM et al (2013) Distinct functional properties of isoamylase-type starch debranching enzymes in monocot and dicot leaves. Plant Physiol 163:1363–1375
Fisher D, Gao M, Kim K-N et al (1996a) Two closely related cDNAs encoding starch branching enzyme from Arabidopsis thaliana. Plant Mol Biol 30:97–108
Fisher DK, Gao M, Kim KN et al (1996b) Allelic analysis of the maize amylose-extender locus suggests that independent genes encode starch-branching enzymes IIa and IIb. Plant Physiol 110:611–619
Flipse E, Suurs L, Keetels CJAM et al (1996) Introduction of sense and antisense cDNA for branching enzyme in the amylose-free potato mutant leads to physico-chemical changes in the starch. Planta 198:340–347
Fontaine T, D'Hulst C, Maddelein ML et al (1993) Toward an understanding of the biogenesis of the starch granule. Evidence that Chlamydomonas soluble starch synthase II controls the synthesis of intermediate size glucans of amylopectin. J Biol Chem 268:16223–16230
Fu Y, Ballicora MA, Leykam JF et al (1998) Mechanism of reductive activation of potato tuber ADP-glucose pyrophosphorylase. J Biol Chem 273:25045–25052
Fujita N, Yoshida M, Asakura N et al (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140:1070–1084
Furukawa K, Tagaya M, Inouye M et al (1990) Identification of lysine 15 at the active site in Escherichia coli glycogen synthase. Conservation of Lys-X-Gly-Gly sequence in the bacterial and mammalian enzymes. J Biol Chem 265:2086–2090
Furukawa K, Tagaya M, Tanizawa K et al (1993) Role of the conserved Lys-X-Gly-Gly sequence at the ADP-glucose-binding site in Escherichia coli glycogen synthase. J Biol Chem 268:23837–23842
Gámez-Arjona FM, Raynaud S, Ragel P et al (2014) Starch synthase 4 is located in the thylakoid membrane and interacts with plastoglobule-associated proteins in Arabidopsis. Plant J 80(2):305–316
Gao M, Fisher D, Kim K-N et al (1996) Evolutionary conservation and expression patterns of maize starch branching enzyme I and IIb genes suggests isoform specialization. Plant Mol Biol 30:1223–1232
Gao M, Fisher DK, Kim KN et al (1997) Independent genetic control of maize starch-branching enzymes IIa and IIb (Isolation and characterization of a Sbe2a cDNA). Plant Physiol 114:69–78
Gao M, Wanat J, Stinard PS et al (1998) Characterization of dull1, a maize gene coding for a novel starch synthase. Plant Cell Online 10:399–412
Garwood DL, Shannon JC, Creech RG (1976) Starches of endosperm possessing different alleles at the amylose-extender locus in Zea mays L. Cereal Chem 53:355–364
Gérard C, Planchot V, Colonna P et al (2000) Relationship between branching density and crystalline structure of A- and B-type maize mutant starches. Carbohydr Res 326:130–144
Gibon Y, Bläsing OE, Palacios-Rojas N et al (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39:847–862
Gibson K, Park J-S, Nagai Y et al (2011) Exploiting leaf starch synthesis as a transient sink to elevate photosynthesis, plant productivity and yields. Plant Sci 181:275–281
Glaring MA, Skryhan K, Kötting O et al (2012) Comprehensive survey of redox sensitive starch metabolising enzymes in Arabidopsis thaliana. Plant Physiol Biochem 58:89–97
Godet MC, Bizot H, Buléon A (1995) Crystallization of amylose—fatty acid complexes prepared with different amylose chain lengths. Carbohydr Polym 27:47–52
Graf A, Smith AM (2011) Starch and the clock: the dark side of plant productivity. Trends Plant Sci 16:169–175
Graf A, Schlereth A, Stitt M et al (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci 107:9458–9463
Grimaud F, Rogniaux H, James MG et al (2008) Proteome and phosphoproteome analysis of starch granule-associated proteins from normal maize and mutants affected in starch biosynthesis. J Exp Bot 59:3395–3406
Guan HP, Preiss J (1993) Differentiation of the properties of the branching isozymes from maize (Zea mays). Plant Physiol 102:1269–1273
Hädrich N, Hendriks JHM, Kötting O et al (2012) Mutagenesis of cysteine 81 prevents dimerization of the APS1 subunit of ADP-glucose pyrophosphorylase and alters diurnal starch turnover in Arabidopsis thaliana leaves. Plant J 70:231–242
Harn C, Knight M, Ramakrishnan A et al (1998) Isolation and characterization of the zSSIIa and zSSIIb starch synthase cDNA clones from maize endosperm. Plant Mol Biol 37:639–649
Haworth WN, Peat S, Bourne EJ (1944) Synthesis of amylopectin. Nature 154:236
Haydon MJ, Mielczarek O, Robertson FC et al (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502:689–692
Hedman K, Boyer C (1982) Gene dosage at the amylose-extender locus of maize: effects on the levels of starch branching enzymes. Biochem Genet 20:483–492
Hendriks JHM, Kolbe A, Gibon Y et al (2003) ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol 133:838–849
Hennen-Bierwagen TA, Liu F, Marsh RS et al (2008) Starch biosynthetic enzymes from developing maize endosperm associate in multisubunit complexes. Plant Physiol 146:1892–1908
Hennen-Bierwagen TA, James MG, Myers AM (2012) Involvement of debranching enzymes in starch biosynthesis. In: Tetlow IJ (ed) Starch: origins, structure and metabolism, vol 5. Society for Experimental Biology, London
Hirose T, Terao T (2004) A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.). Planta 220:9–16
Hizukuri S (1985) Relationship between the distribution of the chain length of amylopectin and the crystalline structure of starch granules. Carbohydr Res 141:295–306
Huang B, Hennen-Bierwagen TA, Myers AM (2014) Functions of multiple genes encoding ADP-glucose pyrophosphorylase subunits in maize endosperm, embryo, and leaf. Plant Physiol 164:596–611
Hussain H, Mant A, Seale R et al (2003) Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans. Plant Cell 15:133–149
Imberty A, Chanzy H, Pérez S et al (1988) The double-helical nature of the crystalline part of A-starch. J Mol Biol 201:365–378
Ingkasuwan P, Netrphan S, Prasitwattanaseree S et al (2012) Inferring transcriptional gene regulation network of starch metabolism in Arabidopsis thaliana leaves using graphical Gaussian model. BMC Syst Biol 6:100
James MG, Robertson DS, Myers AM (1995) Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell Online 7:417–429
Jane J-L, Kasemsuwan T, Leas S et al (1994) Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke 46:121–129
Jane J-L, Wong K-S, McPherson AE (1997) Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr Res 300:219–227
Jenkins PJ, Cameron RE, Donald AM (1993) A universal feature in the structure of starch granules from different botanical sources. Starch-Stärke 45:417–420
Jiang H, Dian W, Liu F et al (2004) Molecular cloning and expression analysis of three genes encoding starch synthase II in rice. Planta 218:1062–1070
Jobling SA, Schwall GP, Westcott RJ et al (1999) A minor form of starch branching enzyme in potato (Solanum tuberosum L.) tubers has a major effect on starch structure: cloning and characterisation of multiple forms of SBE A. Plant J 18:163–171
Khoshnoodi J, Larsson CT, Larsson H et al (1998) Differential accumulation of Arabidopsis thaliana Sbe2.1 and Sbe2.2 transcripts in response to light. Plant Sci 135:183–193
Kleczkowski LA (2000) Is leaf ADP-glucose pyrophosphorylase an allosteric enzyme? Biochim Biophys Acta Gen Subj 1476:103–108
Kolbe A, Tiessen A, Schluepmann H et al (2005) Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc Natl Acad Sci U S A 102:11118–11123
Kossmann J, Abel GJW, Springer F et al (1999) Cloning and functional analysis of a cDNA encoding a starch synthase from potato (Solanum tuberosum L.) that is predominantly expressed in leaf tissue. Planta 208:503–511
Kubo A, Colleoni C, Dinges JR et al (2010) Functions of heteromeric and homomeric isoamylase-type starch-debranching enzymes in developing maize endosperm. Plant Physiol 153:956–969
Lee S-K, Hwang S-K, Han M et al (2007) Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol Biol 65:531–546
Lepistö A, Pakula E, Toivola J et al (2013) Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. J Exp Bot 64:3843–3854
Li J, Ezquer I, Bahaji A et al (2011) Microbial volatile-induced accumulation of exceptionally high levels of starch in Arabidopsis leaves is a process involving NTRC and starch synthase classes III and IV. Mol Plant-Microbe Interact 24:1165–1178
Li J, Almagro G, Muñoz FJ et al (2012) Post-translational redox modification of ADP-glucose pyrophosphorylase in response to light is not a major determinant of fine regulation of transitory starch accumulation in Arabidopsis leaves. Plant Cell Physiol 53:433–444
Lin Q, Huang B, Zhang M et al (2012) Functional interactions between starch synthase III and isoamylase-type starch-debranching enzyme in maize endosperm. Plant Physiol 158:679–692
Lin Q, Facon M, Putaux J-L et al (2013) Function of isoamylase-type starch debranching enzymes ISA1 and ISA2 in the Zea mays leaf. New Phytol 200:1009–1021
Liu F, Ahmed Z, Lee EA et al (2012) Allelic variants of the amylose extender mutation of maize demonstrate phenotypic variation in starch structure resulting from modified protein-protein interactions. J Exp Bot 63:1167–1183
Lomako J, Lomako WM, Whelan WJ (1988) A self-glucosylating protein is the primer for rabbit muscle glycogen biosynthesis. FASEB J 2:3097–3103
Lomako J, Lomako WM, Whelan WJ (2004) Glycogenin: the primer for mammalian and yeast glycogen synthesis. Biochim Biophys Acta Gen Subj 1673:45–55
Lu Y, Gehan JP, Sharkey TD (2005) Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol 138:2280–2291
Lunn JE, Feil R, Hendriks JHM et al (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397:139–148
Makhmoudova A, Williams D, Brewer D et al (2014) Identification of multiple phosphorylation sites on maize endosperm starch branching enzyme IIb, a key enzyme in amylopectin biosynthesis. J Biol Chem 289:9233–9246
Matheson NK (1996) The chemical structure of amylose and amylopectin fractions of starch from tobacco leaves during development and diurnally-nocturnally. Carbohydr Res 282:247–262
Michalska J, Zauber H, Buchanan BB et al (2009) NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci 106:9908–9913
Mikkelsen R, Mutenda KE, Mant A et al (2005) a-Glucan, water dikinase (GWD): a plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc Natl Acad Sci U S A 102:1785–1790
Morell MK, Kosar-Hashemi B, Cmiel M et al (2003) Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J 34:173–185
Mortimer JC, Miles GP, Brown DM et al (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci 107:17409–17414
Myers AM, Morell MK, James MG et al (2000) Recent progress toward understanding biosynthesis of the amylopectin crystal. Plant Physiol 122:989–998
Nakamura Y, Umemoto T, Takahata Y et al (1996) Changes in structure of starch and enzyme activities affected by sugary mutations in developing rice endosperm. Possible role of starch debranching enzyme (R-enzyme) in amylopectin biosynthesis. Physiol Plant 97:491–498
Nakamura T, Vrinten P, Hayakawa K et al (1998) Characterization of a granule-bound starch synthase isoform found in the pericarp of wheat. Plant Physiol 118:451–459
Nelson OE, Rines HW (1962) The enzymatic deficiency in the waxy mutant of maize. Biochem Biophys Res Commun 9:297–300
Nishi A, Nakamura Y, Tanaka N et al (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127:459–472
Palopoli N, Busi MV, Fornasari MS et al (2006) Starch-synthase III family encodes a tandem of three starch-binding domains. Protein Struct Funct Bioinf 65:27–31
Pfister B, Lu K-J, Eicke S et al (2014) Genetic evidence that chain length and branch point distributions are linked determinants of starch granule formation in Arabidopsis. Plant Physiol 165:1457–1474
Ragel P, Streb S, Feil R et al (2013) Loss of starch granule initiation has a deleterious effect on the growth of Arabidopsis plants due to an accumulation of ADP-glucose. Plant Physiol 163:75–85
Ral J-P, Colleoni C, Wattebled F et al (2006) Circadian clock regulation of starch metabolism establishes GBSSI as a major contributor to amylopectin synthesis in Chlamydomonas reinhardtii. Plant Physiol 142:305–317
Rennie EA, Hansen SF, Baidoo EEK et al (2012) Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases. Plant Physiol 159:1408–1417
Roldán I, Wattebled F, Lucas MM et al (2007) The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J 49:492–504
Rongine De Fekete MA, Leloir LF, Cardini CE (1960) Mechanism of starch biosynthesis. Nature 187:918–919
Rose A, Meier I (2004) Scaffolds, levers, rods and springs: diverse cellular functions of long coiled-coil proteins. Cell Mol Life Sci CMLS 61:1996–2009
Sanz-Barrio R, Corral-Martinez P, Ancin M et al (2013) Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnol J 11:618–627
Scialdone A, Mugford ST, Feike D et al (2013) Arabidopsis plants perform arithmetic division to prevent starvation at night. eLife 2:e00669
Seung D, Thalmann M, Sparla F et al (2013) Arabidopsis thaliana AMY3 is a unique redox-regulated chloroplastic α-amylase. J Biol Chem 288:33620–33633
Silver DM, Silva LP, Issakidis-Bourguet E et al (2013) Insight into the redox regulation of the phosphoglucan phosphatase SEX4 involved in starch degradation. FEBS J 280:538–548
Sim L, Beeren SR, Findinier J et al (2014) Crystal structure of the Chlamydomonas starch debranching enzyme isoamylase ISA1 reveals insights into the mechanism of branch trimming and complex assembly. J Biol Chem 289(33):22991–23003
Smith A, Neuhaus HE, Stitt M (1990) The impact of decreased activity of starch-branching enzyme on photosynthetic starch synthesis in leaves of wrinkled-seeded peas. Planta 181:310–315
Smith SM, Fulton DC, Chia T et al (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136:2687–2699
Sokolov LN, Dominguez-Solis JR, Allary A-L et al (2006) A redox-regulated chloroplast protein phosphatase binds to starch diurnally and functions in its accumulation. Proc Natl Acad Sci 103:9732–9737
Sparla F, Costa A, Lo Schiavo F et al (2006) Redox regulation of a novel plastid-targeted beta-amylase of Arabidopsis. Plant Physiol 141:840–850
Streb S, Zeeman SC (2014) Replacement of the endogenous starch debranching enzymes ISA1 and ISA2 of Arabidopsis with the rice orthologs reveals a degree of functional conservation during starch synthesis. PLoS ONE 9:9
Streb S, Delatte T, Umhang M et al (2008) Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase. Plant Cell 20:3448–3466
Sulpice R, Pyl E-T, Ishihara H et al (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci 106:10348–10353
Sun C, Sathish P, Ahlandsberg S et al (1998) The two genes encoding starch-branching enzymes IIa and IIb are differentially expressed in barley. Plant Physiol 118:37–49
Szydlowski N, Ragel P, Raynaud S et al (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21:2443–2457
Szydlowski N, Ragel P, Hennen-Bierwagen TA et al (2011) Integrated functions among multiple starch synthases determine both amylopectin chain length and branch linkage location in Arabidopsis leaf starch. J Exp Bot 62:4547–4559
Takeda Y, Guan H-P, Preiss J (1993) Branching of amylose by the branching isoenzymes of maize endosperm. Carbohydr Res 240:253–263
Tenorio G, Orea A, Romero J et al (2003) Oscillation of mRNA level and activity of granule-bound starch synthase I in Arabidopsis leaves during the day/night cycle. Plant Mol Biol 51:949–958
Tetlow IJ, Beisel KG, Cameron S et al (2008) Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol 146:1878–1891
Tiessen A, Hendriks JHM, Stitt M et al (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14:2191–2213
Toivola J, Nikkanen L, Dahlström KM et al (2013) Overexpression of chloroplast NADPH-dependent thioredoxin reductase in Arabidopsis enhances leaf growth and elucidates in-vivo function of reductase and thioredoxin domains. Front Plant Sci 4:389
Tomlinson KL, Lloyd JR, Smith AM (1997) Importance of isoforms of starch-branching enzyme in determining the structure of starch in pea leaves. Plant J 11:31–43
Tyynelä J, Schulman AH (1993) An analysis of soluble starch synthase isozymes from the developing grains of normal and shx cv. Bomi barley (Hordeum vulgare). Physiol Plant 89:835–841
Ugalde JE, Parodi AJ, Ugalde RA (2003) De novo synthesis of bacterial glycogen: agrobacterium tumefaciens glycogen synthase is involved in glucan initiation and elongation. Proc Natl Acad Sci U S A 100:10659–10663
Utsumi Y, Nakamura Y (2006) Structural and enzymatic characterization of the isoamylase1 homo-oligomer and the isoamylase1–isoamylase2 hetero-oligomer from rice endosperm. Planta 225:75–87
Utsumi Y, Utsumi C, Sawada T et al (2011) Functional diversity of isoamylase oligomers: the ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm. Plant Physiol 156:61–77
Valdez HA, Busi MV, Wayllace NZ et al (2008) Role of the N-terminal starch-binding domains in the kinetic properties of starch synthase III from Arabidopsis thaliana. Biochemistry 47:3026–3032
Valerio C, Costa A, Marri L et al (2011) Thioredoxin-regulated β-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J Exp Bot 62:545–555
van de Wal M, D’Hulst C, Vincken J-P et al (1998) Amylose is synthesized in vitro by extension of and cleavage from amylopectin. J Biol Chem 273:22232–22240
Vrinten PL, Nakamura T (2000) Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol 122:255–264
Wattebled F, Buléon A, Bouchet B et al (2002) Granule-bound starch synthase I. A major enzyme involved in the biogenesis of B-crystallites in starch granules. Eur J Biochem 269:3810–3820
Wattebled F, Dong Y, Dumez S et al (2005) Mutants of Arabidopsis lacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin. Plant Physiol 138:184–195
Wattebled F, Planchot V, Dong Y et al (2008) Further evidence for the mandatory nature of polysaccharide debranching for the aggregation of semicrystalline starch and for overlapping functions of debranching enzymes in Arabidopsis leaves. Plant Physiol 148:1309–1323
Wu AC, Ral J-P, Morell MK et al (2014) New perspectives on the role of α- and β-amylases in transient starch synthesis. PLoS ONE 9:e100498
Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol 33:985–991
Yandeau-Nelson MD, Laurens L, Shi Z et al (2011) Starch-branching enzyme IIa is required for proper diurnal cycling of starch in leaves of maize. Plant Physiol 156:479–490
Zabawinski C, Van Den Koornhuyse N, D'Hulst C et al (2001) Starchless mutants of Chlamydomonas reinhardtii lack the small subunit of a heterotetrameric ADP-glucose pyrophosphorylase. J Bacteriol 183:1069–1077
Zeeman SC, Umemoto T, Lue W-L et al (1998) A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell 10:1699–1712
Zhang X, Colleoni C, Ratushna V et al (2004) Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa. Plant Mol Biol 54:865–879
Zhang X, Myers AM, James MG (2005) Mutations affecting starch synthase III in Arabidopsis alter leaf starch structure and increase the rate of starch synthesis. Plant Physiol 138:663–674
Zhang X, Szydlowski N, Delvalle D et al (2008) Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol 8:96
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
D’Hulst, C., Wattebled, F., Szydlowski, N. (2015). Starch Biosynthesis in Leaves and Its Regulation. In: Nakamura, Y. (eds) Starch. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55495-0_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-55495-0_6
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55494-3
Online ISBN: 978-4-431-55495-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)