Abstract
Various horticultural plants synthesize polyols as major products of photosynthesis in addition to sucrose and starch and use polyols and sucrose as translocated sugars. The parallel presence of two translocated sugars and their metabolic pathway is specific and complicates the comprehension of their roles in physiology and response to stress. This review first describes the metabolism of sorbitol, focusing on sorbitol-specific metabolizing proteins and their physiological roles in Rosaceae fruit trees. In addition, research on sorbitol as a signal molecule and sorbitol-metabolizing proteins regulated by sugar is discussed. A series of studies regarding various Rosaceae fruit trees has revealed the relationship of sorbitol accumulation with abiotic stresses, including drought, salt, cold, and micronutrient deficiency stresses. On the basis of acknowledging the metabolism of sorbitol, the biochemical mechanism of sorbitol accumulation in response to abiotic stress has been investigated. Furthermore, recent molecular analyses are providing direct evidence of the correlation of the proteins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Typically, most plant species synthesize sucrose and starch as major products of photosynthesis and use sucrose as a translocated sugar. However, various horticultural plants synthesize polyols as the major products in addition to sucrose and starch and use polyol and sucrose as translocated sugars. For examples of such polyols, sorbitol is synthesized and transported in the Rosaceae, mannitol in the Apiaceae, Combretaceae, Oleaceae, and Rubiaceae, and galactitol in the Celastraceae (Loescher and Everard 1996; Zimmermann and Ziegler 1975). The parallel presence of two translocated sugars and their metabolic pathway complicates the comprehension of their roles in physiology and response to stress and regulation of the metabolism. Research on clarification must advance because the plants are economically important. This review focuses on a series of studies on the metabolism of sorbitol in Rosaceae fruit trees and its role in abiotic stress tolerance.
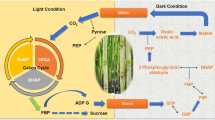
2 Metabolism of Sorbitol
Sorbitol is a major product of photosynthesis and a primary translocated sugar in Rosaceae fruit trees. Sorbitol accounts for more than 80 % of newly fixed carbohydrates during the light period in apples (Wang et al. 1997). It also accounts for 60 % to 90 % of the carbon exported from source leaves (Loescher 1987), and its concentration in phloem sap reaches approximately 560 mM, whereas that of sucrose is about 140 mM (Moing et al. 1997). A sorbitol-specific metabolism is mainly the result of four proteins, including sorbitol-6-phosphate dehydrogenase (S6PDH), sorbitol-6-phosphate phosphatase (S6PP), sorbitol transporter (SOT), and sorbitol dehydrogenase (SDH). Briefly, sorbitol is synthesized by S6PDH and S6PP in source leaves, translocated through phloem, and catabolized by SDH in fruit. SOT functions in the translocation of sorbitol across the plasma membrane in these processes. Recently, apple genomic information of these proteins has been shown; it has been reported that there are 16 S6PDH or S6PDH-like genes, 17 SDH or SDH-like genes, and 38 SOT or SOT-like genes (Velasco et al. 2010). Also, genomic information is available for the peach; manual annotation has identified two S6PDH, seven SDH, and ten SOT (International Peach Genome Initiative 2013). Genomic information shows that those of apple and peach comprise large gene families, suggesting the importance of the specific sorbitol metabolism in Rosaceae fruit trees.
2.1 Sorbitol-Metabolizing Proteins
2.1.1 NADP-Sorbitol-6-Phosphate Dehydrogenase (S6PDH) (EC 1.1.1.200)
S6PDH is a key enzyme in the process of synthesizing sorbitol in source organs (Hirai 1981; Loescher et al. 1982; Yamaki and Ishikawa 1986). It synthesizes sorbitol-6-phosphate (S6P) via reduction of glucose-6-phosphate (G6P), which is also a precursor of sucrose as another photosynthetic product. S6PDH has been purified and characterized from mature leaves of loquat and apple seedlings (Hirai 1981; Kanayama and Yamaki 1993). S6PDH activity is in both directions, reduction of G6P and oxidation of S6P (Kanayama and Yamaki 1993). The maximum velocity of the former is much higher than that of the latter, and the oxidation of S6P proceeds very slowly at a neutral pH. These biochemical results support the belief that S6PDH has a major function in the biosynthesis of sorbitol.
The cDNA encoding S6PDH is first cloned from apple seedlings (Kanayama et al. 1992). It has been shown that in peach and pear the level of S6PDH mRNA is higher in source mature leaves than in sink young leaves, concomitant with the alteration of enzyme activity and protein level (Sakanishi et al. 1998; Suzue et al. 2006). Tobacco, which does not have the potential to produce sorbitol, when transformed with apple cDNA encoding S6PDH synthesizes sorbitol (Tao et al. 1995), and, in transgenic apple plants silenced for S6PDH, the sorbitol content in leaves drastically decreases (Kanamaru et al. 2004; Teo et al. 2006). These results confirm that S6PDH is a key enzyme in the sorbitol biosynthetic pathway in Rosaceae fruit trees.
Subcellular localization of S6PDH was revealed in apple cotyledons using differential centrifugation and linear sucrose density gradient centrifugation; it is localized in both cytosol and chloroplast (Yamaki 1981). Immunogold electron microscopy analysis confirms the localization of S6PDH in apple mature leaves (Liang et al. 2012). These results are consistent with S6PDH localized in source organs, including mature leaves, primarily being synthesizers of sorbitol as a major product of photosynthesis.
The roles of S6PDH in apple trees and fruit have definitely been revealed using apple transformed with S6PDH (Teo et al. 2006). S6PDH-suppressed apples show a decrease in the vegetative growth and acid content of fruit and an increase in the total soluble solid content of fruit, whereas S6PDH overexpression shows the opposite result. Thus, S6PDH is a critical enzyme for determining the vegetative growth and fruit quality through the degree of sorbitol synthesis.
2.1.2 Sorbitol-6-Phosphate Phosphatase (S6PP) (EC 3.1.3.50)
S6PP synthesizes sorbitol via dephosphorylation of S6P, which is the final step in synthesizing sorbitol. Although Grant and ap Rees (1981) first suggested the presence of S6PP in leaves of apple seedlings, it had remained undetermined. The necessity of the enzyme for synthesizing sorbitol was questionable because there are nonspecific phosphatases in cells and it could dephosphorylate S6P to sorbitol. Actually, transgenic plants, which do not have the potential to produce sorbitol, introduced for the S6PDH gene, including tobacco and persimmon, can synthesize sorbitol without the S6PP gene (Gao et al. 2001; Sheveleva et al. 1998; Tao et al. 1995). Zhou et al. (2003) purified and characterized S6PP from mature apple leaves, which confirms the presence and the necessity of S6PP. S6PP is highly specific for S6P and is regulated by sorbitol through negative feedback inhibition.
2.1.3 Sorbitol Transporter (SOT)
A sugar transporter is necessary for the functional transport of sugars across membranes. SOT has important roles in unloading sorbitol in sink organs, including fruit, young leaves, and flowers (Gao et al. 2003, 2005). cDNAs of SOT were first isolated from sour cherry fruit: PcSOT1 and PcSOT2. Using heterologous expression of the genes in yeast, it has been proved that sorbitol transporters actually act as plasma membrane sorbitol/H+ symporters. The expression of PcSOT1 during the development of fruit is high when the growth and sugar accumulation rates of fruit are high, suggesting the sorbitol transporter is important in sugar accumulation and sink strength. On the other hand, PcSOT2 is mainly expressed only early in fruit development, suggesting that isogenes seem to all have their own role in the development of fruit such as apples (Li et al. 2012c; Teo et al. 2006). In addition, the genes are also expressed in young leaves and are low in mature leaves. These facts show that SOT works in sink organs (Gao et al. 2003). SOT is also suggested to be related to the occurrence of a watercore in apples. Expressions of MdSOT1 and MdSOT2 are typically high in sink tissues but low in watercore-affected fruit tissues. Decreased ability to transport sorbitol into fruit parenchyma tissues because of the decreased expression would result in sorbitol accumulation in the intercellular space and occurrence of watercore (Gao et al. 2005). In apple source leaves, the expression of MdSOT3, MdSOT4, and MdSOT5 has been identified, suggesting that these MdSOTs have different functions (Watari et al. 2004).
2.1.4 NAD-Sorbitol Dehydrogenase (SDH) (EC 1.1.1.14)
SDH is a key enzyme of sorbitol catabolism in sink organs, including the fruit and immature leaves, which converts sorbitol to fructose (Loescher et al. 1982; Yamaki and Ishikawa 1986; Yamaki and Moriguchi 1989). It has been shown that the activity of SDH is positively correlated with sink strength of fruit throughout the development of peaches (Lo Bianco and Rieger 2002). SDH has been purified to homogeneity and characterized from Japanese pear (Oura et al. 2000). The K m values for sorbitol are much lower than for fructose. This biochemical result supports the belief that SDH favors the conversion of sorbitol to fructose.
cDNA-encoding SDH has been cloned from fruit of Rosaceae fruit trees, including loquats, peaches, pears, plums, and apples (Bantog et al. 2000; Guo et al. 2012; Yamada et al. 1998, 2001, 2006). Expression analyses of SDH during fruit development show that the activity is regulated at the transcriptional level because the gene expression pattern corresponds to the SDH activity and confirms that SDH is important in fruit maturation and sugar accumulation (Bantog et al. 2000; Yamada et al. 2001, 2006). SDH is important in fruit set and early fruit development as well as maturation in apples (Nosarzewski et al. 2004). In those cases, SDH is expressed not only in the cortex but also in the seeds and is derived from SDH genes, which are differentially expressed in seeds and the cortex (Nosarzewski and Archbold 2007). In other sink organs, young leaves of pear, the expression of the SDH mRNA level is not coincident with the activity, although the activity is high (Suzue et al. 2006).
In source leaves of apples, expression of the SDH isogene, MdSDH1, has also been detected (Nosarzewski et al. 2004; Park et al. 2002). Immunohistochemical analysis shows that SDH is distributed both in the flesh and in the vascular tissue of the fruit and in the vascular tissue and mesophyll tissue of the young and old leaves (Wang et al. 2009), suggesting that SDH is localized not only in sink organs but also in source organs. Immunogold electron microscopy analysis revealed the subcellular localization of SDH. It is localized mainly in the cytoplasm and chloroplast of the fruit and leaves, although, interestingly, SDH is also localized in vacuoles in young and mature leaves (Wang et al. 2009). The fact that sorbitol is a primary soluble carbohydrate that can be widely metabolized strongly supports the importance of sorbitol in Rosaceae fruit trees.
The importance of SDH in sink organs is directly confirmed using transgenic apple trees with reduced SDH activity, which show vegetative disorders, such as shorter growth, precocious spring leaf loss, loss of apical dominance, and excessive growth of axillary shoots close to the apex because of an altered fructose:sorbitol ratio in immature leaves. These results suggest that reduced SDH activity in immature leaves, a sink organ, could affect sugar partitioning and, as a result, vegetative growth (Martinelli et al. 2011).
2.2 Phloem Loading and Unloading
Loading strategies into the minor vein in plants are categorized in three pathways and mechanisms: passive loading, polymer trapping, and active transport (Rennie and Turgeon 2009). In apples, which use sorbitol and sucrose as translocated sugars, there are abundant plasmodesmata at all interfaces in the minor vein phloem (Rennie and Turgeon 2009) and much higher concentrations of sorbitol and sucrose in leaves (Cheng et al. 2005). Radiolabeled sorbitol, sucrose, or CO2 is not detected in the minor veins when apple leaf tissues are exposed to them because of ready diffusion. These facts show that the movement of sugar alcohol from the mesophyll into the phloem in apples is symplastic and passive (Reidel et al. 2009). Additionally, Reidel et al. (2009) point out that the presence of an active uptake mechanism for a solute in the phloem does not, in itself, prove that the phloem-loading route is apoplastic and that sorbitol transporters in apple leaves are involved in retrieving sorbitol that leaks from phloem cells into the apoplast.
In the fruit of apples, the presence of plasmodesmata between the sieve element and the companion cell and between parenchyma cells but not between the companion and parenchyma cells suggests that phloem unloading of sorbitol and sucrose is related with an apoplastic step between the sieve element–companion cell complex and parenchyma cells (Zhang et al. 2004). The presence of a sorbitol transporter on the plasma membrane, which transports sorbitol into the cytosol of parenchyma cells using the proton motive force, also supports apoplastic unloading (Gao et al. 2003, 2005).
3 Regulation of Sorbitol Metabolism
3.1 Regulation of Sorbitol Metabolism by Environmental Factors
Partitioning of photoassimilates into sorbitol is dependent on environmental conditions that affect photosynthesis, including the concentration of CO2 and light intensity. In mature apple leaves, when photosynthesis increases with an increase in CO2, sorbitol and starch concentrations increase, but sucrose concentrations are stable; this means that the photoassimilate is partitioned into sorbitol rather than sucrose (Pan et al. 1998; Wang et al. 1999). In contrast, in mature peach leaves, when photosynthesis increases with an increase in light intensity, sucrose and starch concentrations increase more drastically than does that of sorbitol. In this case, the photoassimilate seems to be partitioned into sucrose rather than sorbitol (Escobar-Gutiérrez and Gaudillèr 1997). The photoperiod affects carbon partitioning in Rosaceae fruit trees. In mature apple leaves, as the photoperiod increases, sorbitol concentrations increase concomitant with glucose, fructose, and starch concentrations, and the relative partitioning of 14C into only sorbitol increases. However, sucrose concentrations and that into sucrose decrease. It is suggested that longer photoperiods favor sorbitol over sucrose accumulation whereas shorter photoperiods favor sucrose over sorbitol synthesis (Wang et al. 1997). These changes affect other organs, including sink leaves, stems, and roots, as a result of the transport of translocated sugars from the source leaves (Wang et al. 1998). Sorbitol and starch content show diurnal changes at regular intervals throughout a natural day–night cycle. S6PDH activity also shows diurnal changes; these changes seem to be related to endogenous rhythms, although sucrose phosphate synthase (SPS) activity is not (Zhou et al. 2001).
3.2 Sugar Signaling in Rosaceae Fruit Trees
In plants, sugars not only are a carbon resource but also function as signal molecules; they modulate gene expression, in which way they could play roles in development, growth, and differentiation (Koch 1996; Rolland et al. 2006; Smeekens 2000). However, most research has focused on sucrose and hexoses, although research on polyols and the plants using them as translocated sugars is limited.
3.2.1 Regulation by Sorbitol
SDH activity is decreased by girdling treatment, which interrupts the assimilate supply in fruit (Berüter and Studer Feusi 1997; Morandi et al. 2008), whereas SDH activity of fruit cortex sections from the fruit treated with defoliation and girdling is increased by incubating in a sorbitol solution (Archbold 1999). In transgenic apple trees with decreased sorbitol synthesis, both SDH activity and transcripts are decreased in shoot tips and fruit (Teo et al. 2006; Zhou et al. 2006). Conversely, by exogenously feeding sorbitol to shoot tips, both SDH transcription and activity are stimulated (Zhou et al. 2006). Partial defoliation treatments, which cause more carbohydrate demand upon the remaining source leaves, increase S6PDH activity, although girdling treatment does not affect it (Zhou and Quebedeaux 2003). Furthermore, exogenously feeding sorbitol does not affect the activity and transcripts of sucrose synthase, a key enzyme of sucrose metabolism, in the shoot tips of apple trees (Zhou et al. 2006), whereas it decreases the transcript levels of S6PDH, SPS, and ADPGPPase large subunit in mature leaf-petiole cuttings of loquats (Suzuki and Dandekar 2014a). These facts indicate that sorbitol regulates gene expression as a signal molecule in Rosaceae fruit trees, and, as a result, sorbitol affects vegetative growth and fruit quality. In fact, the effects have been revealed with analyses on transgenic apple plants silenced or upregulated for S6PDH (Teo et al. 2006) and cDNA microarray analyses of fruit and leaves of transgenic apples; drastic changes in expression of various genes were shown (Dandekar et al. 2008; Suzuki and Dandekar 2014b). Alteration of the phenotype thus could result from regulation of gene expression by sorbitol.
A mechanism for sorbitol uptake of sorbitol transporter, isolated from the apple, being regulated with sorbitol level around cells, has been revealed: interaction of the sorbitol transporter, MdSOT6, with cytochrome b5, MdCYB5, in response to low sorbitol supply leads to enhancing the affinity of MdSOT6 to sorbitol, stimulating sorbitol uptake (Fan et al. 2009). Because the sugar regulation of sugar transporters has been reported to be at transcriptional level, this posttranslational regulation is proposed as a novel mechanism by Fan et al. (2009).
3.2.2 Regulation of Sorbitol-Metabolizing Enzymes by Sugars
Sugars are metabolized to various sugars and their derivatives by enzymes in plants. For example, in sink organs of Rosaceae fruit trees, sorbitol could first be converted to fructose by SDH, fructose to fructose-6-phospahte (F6P) by fructokinase, and then F6P to G6P, sucrose-6-phosphate, and fructose-1,6-phosphate by phosphoglucoisomerase, SPS, and phosphofructokinase, respectively, and then further metabolized. Thus, gene expression regulated by sugars is complicated, and the regulation of sorbitol-metabolizing enzymes by various sugars has been researched to comprehend that. In apples, SDH transcripts in shoot tips are upregulated by sorbitol, downregulated by sucrose, and not affected by nonmetabolized sucrose analogues (palatinose and turanose), glucose, and fructose (Zhou et al. 2006), whereas those in sliced tissues of the fruit of Japanese pears are upregulated by sorbitol, glucose, sucrose, mannitol, and fructose (Iida et al. 2004). This inconsistency might be dependent on the differences of organ and physiological condition. In mature loquat leaves, S6PDH transcripts are, interestingly, increased by sucrose but decreased by sorbitol (Suzuki and Dandekar 2014a). These trees might have mechanisms to positively keep sorbitol as the dominant translocated sugar, suggesting that sorbitol has an important role in their survival strategy. In addition, S6PDH transcripts are increased by palatinose, a sucrose analogue, and mannose and 3-O-methylglucose, glucose analogues, but not by glucose, and are decreased by fructose. Understanding the function of sorbitol as a signal molecule and sugar-signaling system in Rosaceae fruit trees contributes to the improvement of fruit quality and stress tolerance.
4 Sorbitol and Stress Tolerance
Compatible solutes, which are of low molecular weight, highly soluble, and nontoxic at high concentrations, are accumulated in response to abiotic stress and include proline, betaine, and polyols (Chen and Murata 2002). A series of studies has revealed sorbitol accumulation in response to stress and the biochemical mechanism of its accumulation in Rosaceae fruit trees. Furthermore, recent molecular analyses are providing direct evidence of the correlation of proteins.
4.1 Drought Stress
In Rosaceae fruit trees, including apples, cherries, and peaches, sorbitol is the soluble carbohydrate primarily accumulated in response to drought stress to decrease osmotic potential and maintain turgor pressure (Arndt et al. 2000; Escobar-Gutierrez et al. 1998; Lo Bianco et al. 2000; Ranney et al. 1991; Wang et al. 1995; Wang and Stutte 1992). Additionally, in mature leaves, increase in sorbitol concentration is observed in young leaves, stems, and roots. Although the increase does not occur in some cases, such as peach seedlings and the root of potted apple trees based on glasshouse experiments (Escobar-Gutierrez et al. 1998; Wang et al. 1995), research on field-grown peach trees experiencing drought periods confirmed that sorbitol is significantly accumulated, resulting in active osmotic adjustment (Arndt et al. 2000). The contribution of sorbitol to osmotic adjustment is reported to be more than 50 % and from 60 % to 80 % in the mature leaves of apples and peaches, respectively (Lo Bianco et al. 2000; Wang and Stutte 1992). On the other hand, in response to drought stress, the roles of other sugars, including glucose, fructose, and sucrose, seem to be limited; increase of those concentrations is not necessarily observed, and the contribution to osmotic adjustment is small (Escobar-Gutierrez et al. 1998; Lo Bianco et al. 2000; Ranney et al. 1991). These results suggest that sorbitol has an important role in osmotic adjustment in Rosaceae fruit trees when they are exposed to drought stress.
It appears to be generally accepted that sorbitol accumulation in response to drought stress is principally caused by an increase in S6PDH activity because S6PDH is an essential enzyme of sorbitol synthesis. Sorbitol accumulation may result from the preferential conversion of glucose to sorbitol rather than to sucrose and starch when the apple is under osmotic stress (Wang et al. 1996), suggesting that the accumulation is caused by S6PDH. In peach seedlings in response to short-term drought stress, S6PDH activity in mature leaves significantly increases linearly with the severity of the stress and correlates with the increase in sorbitol content in the phloem sap (Escobar-Gutierrez et al. 1998). However, in micropropagated apple exposed to water stress and accumulating sorbitol in mature leaves, S6PDH activity increases; still, its positive effect on sorbitol accumulation is limited because the correlation between sorbitol content and the activity is not significant. SDH has a direct effect on sorbitol accumulation in response to water stress because the negative correlation between sorbitol content and SDH activity is significant (Li and Li 2005, 2007). In potted peaches exposed to drought stress, both S6PDH and SDH activities are reduced in both mature leaves and shoot tips, respectively, during drought, suggesting that osmotic adjustment via sorbitol accumulation results from the decrease in the metabolism of sorbitol by SDH in shoot tips, not the increase in sorbitol synthesis by S6PDH in the mature leaves (Lo Bianco et al. 2000). On the basis of these biochemical analyses, it is suggested that sorbitol accumulation is related not only to an increase in sorbitol synthesis by S6PDH but also to a decrease in sorbitol catabolism by SDH.
Molecular approaches provide evidence of the contribution of S6PDH to sorbitol accumulation in response to drought stress. In the leaves of micropropagated apples exposed to osmotic stress, the S6PDH gene is induced, and the level of expression of the S6PDH gene is positively correlated with the severity of the stress. The gene expression level almost coincides with the enzyme activity and sorbitol accumulation, suggesting that S6PDH has an important role in the response of the apple to osmotic stress and that the regulation is at gene level. Furthermore, promoter analysis of the S6PDH gene shows that a positive regulatory region is present between −361 and −221 and causes a key response to osmotic stress, which contains two ABA-responsive elements and a putative MYB-recognition sequence (Zhang et al. 2011). In addition, it is suggested that SOTs are related to the response to drought stress. In micropropagated apple exposed to water stress, mRNAs of SOTs in roots, phloem tissues, and leaves are upregulated, and the sorbitol content is increased in those organs. Increased SOTs contribute to loading more sorbitol into the phloem and roots. The apple adapts to drought stress via increasing in sorbitol transport as well as sorbitol synthesis (Li et al. 2012b). Truncation analysis reveals that MdSOT3 and MdSOT5 promoters contain a number of cis-acting elements related to drought stress (Li et al. 2012a), which also supports the contribution of the sorbitol transporter to the response to drought stress.
4.2 Salt Stress
The relationship between sorbitol accumulation and salt stress has been often documented using Plantago (Ahmad et al. 1979; Gorham et al. 1981; Lambers et al. 1981). In apples, it is shown that leaf disks treated with high-salinity stresses accumulate sorbitol and, concomitantly, the expression of the S6PDH gene increases (Kanayama et al. 2006). The Japanese persimmon, which does not have potential to produce sorbitol, when transformed with apple cDNA encoding S6PDH accumulates sorbitol and shows enhanced tolerance of salt stress (Deguchi et al. 2004; Gao et al. 2001). These results indicate that sorbitol enhances salt stress tolerance and that the accumulation of sorbitol in response to salt stress in Rosaceae fruit trees is the result of transcriptional regulation of S6PDH. In addition, although tobacco transformed with apple cDNA encoding S6PDH accumulating sorbitol is growth inhibited with necrosis (Sheveleva et al. 1998), transgenic persimmons show dwarfism; however, that is not severe, and has no necrosis, suggesting that persimmons seem to be tolerant to sorbitol and tolerance to sorbitol varied with plant species (Deguchi et al. 2004).
4.3 Cold Stress
Total soluble carbohydrates are associated with increased cold hardiness in fruit trees (Palonen and Buszard 1997). As temperature decreases, sorbitol content increases in apples (Raese et al. 1978; Williams and Raese 1974) and loquats (Hirai 1983). The increase in sorbitol content in mature leaves of the loquat, which is an evergreen tree, in an orchard in winter is correlated with an increase in S6PDH activity (Hirai 1983). The increased activity is caused by the induction of S6PDH expression by low temperatures (Kanayama et al. 2006). Low-temperature treatment of apple leaf disks induces S6PDH expression and ABA content, which means that expression of S6PDH is under the control of ABA when the apple responds to cold stress (Kanayama et al. 2006). Similar induction by low temperature is observed in leaf disks of peaches and Japanese pears; the response of apples as described here is one of the common mechanisms to achieve cold hardiness in Rosaceae fruit trees (Deguchi et al. 2002a, b). S6PDH induction by low temperature has been confirmed by promoter analyses; the promoter region of apple S6PDH can be induced by cold and abscisic acid treatment, and the abscisic acid-responsive cis-element has been identified in the gene promoter (Liang et al. 2012).
4.4 Micronutrient Deficiency Stress
In peaches, iron (Fe)-deficiency chlorosis largely reduces fruit yields and leads to firmer fruits with higher acidity, total phenolics, and carboxylates. In such a situation, the sorbitol content in the fruit increases, although the content of other major sugars, including sucrose, fructose, and glucose, does not change; this might be an adaptive response to Fe-deficiency stress (Álvarez-Fernández et al. 2011). Sorbitol contributes to boron transport through the phloem in Rosaceae fruit trees by the formation of boron–sorbitol complexes (Brown and Hu 1996, 1998; Hu et al. 1997). In transgenic apple silenced for the S6PDH gene, because of a lack of sorbitol, cracking and necrotic spot occurred in fruit and shoot growth was inhibited (Suzuki and Dandekar 2014b). These facts suggest that sorbitol could potentially cause boron-deficiency stress, if boron is inadequate.
References
Ahmad I, Larher F, Stewart GR (1979) Sorbitol, a compatible osmotic solute in Plantago maritima. New Phytol 82:671–678
Álvarez-Fernández A, Melgar JC, Abadía J, Abadía A (2011) Effects of moderate and severe iron deficiency chlorosis on fruit yield, appearance and composition in pear (Pyrus communis L.) and peach (Prunus persica (L.) Batsch). Environ Exp Bot 71:280–286
Archbold DD (1999) Carbohydrate availability modifies sorbitol dehydrogenase activity of apple fruit. Physiol Plant 105:391–395
Arndt SK, Wanek W, Clifford SC, Popp M (2000) Contrasting adaptations to drought stress in field-grown Ziziphus mauritiana and Prunus persica trees: water relations, osmotic adjustment and carbon isotope composition. Aust J Plant Physiol 27:985–996
Bantog NA, Yamada K, Niwa N, Shiratake K, Yamaki S (2000) Gene expression of NAD+-dependent sorbitol dehydrogenase and NADP+-dependent sorbitol-6-phosphate dehydrogenase during development of loquat (Eriobotrya japonica Lindl.) fruit. J Jpn Soc Hortic Sci 69:231–236
Berüter J, Studer Feusi ME (1997) The effect of girdling on carbohydrate partitioning in the growing apple fruit. J Plant Physiol 151:277–285
Brown PH, Hu H (1996) Phloem mobility of boron is species dependent: evidence for phloem mobility in sorbitol-rich species. Ann Bot 77:497–505
Brown PH, Hu H (1998) Phloem boron mobility in diverse plant species. Bot Acta 111:331–335
Chen THH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5:250–257
Cheng L, Zhou R, Reidel EJ, Sharkey TD, Dandekar AM (2005) Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta (Berl) 220:767–776
Dandekar AM, Arokiasamy S, Ibáñez AM, Phu ML, Reagan RL, Suzuki Y (2008) Reverse genomic analysis of ethylene and sorbitol regulation in apple fruit tissues. In: Abstracts of 19th New Phytologist Symposium, Timberline Lodge, Mount Hood, 17–20 September 2008
Deguchi M, Saeki H, Ohkawa W, Kanahama K, Kanayama Y (2002a) Effects of low temperature on sorbitol biosynthesis in peach leaves. J Jpn Soc Hortic Sci 71:446–448 (in Japanese with English summary)
Deguchi M, Watanabe M, Kanayama Y (2002b) Increase in sorbitol biosynthesis in stressed Japanese pear leaves. Acta Hortic 587:511–518
Deguchi M, Koshita Y, Gao M, Tao R, Tetsumura T, Yamaki S, Kanayama Y (2004) Engineered sorbitol accumulation induces dwarfism in Japanese persimmon. J Plant Physiol 161:1177–1184
Escobar-Gutiérrez AJ, Gaudillèr JP (1997) Carbon partitioning in source leaves of peach, a sorbitol-synthesizing species, is modified by photosynthetic rate. Physiol Plant 100:353–360
Escobar-Gutierrez AJ, Zipperlin B, Carbonne F, Moing A, Gaudillere JP (1998) Photosynthesis, carbon partitioning and metabolite content during drought stress in peach seedlings. Aust J Plant Physiol 25:197–205
Fan RC, Peng CC, Xu YH, Wang XF, Li Y, Shang Y, Du SY, Zhao R, Zhang XY, Zhang LY, Zhang DP (2009) Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol 150:1880–1901
Gao M, Tao R, Miura K, Dandekar AM, Sugiura A (2001) Transformation of Japanese persimmon (Diospyros kaki Thunb.) with apple cDNA encoding NADP-dependent sorbitol-6-phosphate dehydrogenase. Plant Sci 160:837–845
Gao Z, Maurousset L, Lemoine R, Yoo SD, van Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131:1566–1575
Gao Z, Jayanty S, Beaudry R, Loescher W (2005) Sorbitol transporter expression in apple sink tissues: implications for fruit sugar accumulation and watercore development. J Am Soc Hortic Sci 130:261–268
Gorham J, Hughes L, Wyn Jones RG (1981) Low-molecular-weight carbohydrates in some salt-stressed plants. Physiol Plant 53:27–33
Grant CR, ap Rees T (1981) Sorbitol metabolism by apple seedlings. Phytochemistry 20:1505–1511
Guo ZX, Pan TF, Li KT, Zhong FL, Lin L, Pan DM, Lu LX (2012) Cloning of NAD-SDH cDNA from plum fruit and its expression and characterization. Plant Physiol Biochem 57:175–180
Hirai M (1981) Purification and characteristics of sorbitol-6-phosphate dehydrogenase from loquat leaves. Plant Physiol 67:221–224
Hirai M (1983) Seasonal changes in sorbitol-6-phosphate dehydrogenase in loquat leaf. Plant Cell Physiol 24:925–931
Hu H, Penn SG, Lebrilla CB, Brown PH (1997) Isolation and characterization of soluble boron complexes in higher plants: the mechanism of phloem mobility of boron. Plant Physiol 113:649–655
Iida M, Bantog NA, Yamada K, Shiratake K, Yamaki S (2004) Sorbitol- and other sugar-induced expressions of the NAD+-dependent sorbitol dehydrogenase gene in Japanese pear fruit. J Am Soc Hortic Sci 129:870–875
International Peach Genome Initiative, Verde I, Abbott AG, Scalabrin S, Jung S, Shu S, Marroni F, Zhebentyayeva T, Dettori MT, Grimwood J, Cattonaro F, Zuccolo A, Rossini L, Jenkins J, Vendramin E, Meisel LA, Decroocq V, Sosinski B, Prochnik S, Mitros T, Policriti A, Cipriani G, Dondini L, Ficklin S, Goodstein DM, Xuan P, Del Fabbro C, Aramini V, Copetti D, Gonzalez S, Horner DS, Falchi R, Lucas S, Mica E, Maldonado J, Lazzari B, Bielenberg D, Pirona R, Miculan M, Barakat A, Testolin R, Stella A, Tartarini S, Tonutti P, Arús P, Orellana A, Wells C, Main D, Vizzotto G, Silva H, Salamini F, Schmutz J, Morgante M, Rokhsar DS (2013) The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat Genet 45:487–94
Kanamaru N, Ito Y, Komori S, Saito M, Kato H, Takahashi S, Omura M, Soejima J, Shiratake K, Yamada K, Yamaki S (2004) Transgenic apple transformed by sorbitol-6-phosphate dehydrogenase cDNA switch between sorbitol and sucrose supply due to its gene expression. Plant Sci 167:55–61
Kanayama Y, Yamaki S (1993) Purification and properties of NADP-dependent sorbitol-6-phosphate dehydrogenase from apple seedlings. Plant Cell Physiol 34:819–823
Kanayama Y, Mori H, Imaseki H, Yamaki S (1992) Nucleotide sequence of a cDNA encoding NADP-sorbitol-6-phosphate dehydrogenase from apple. Plant Physiol 100:1607–1608
Kanayama Y, Watanabe M, Moriguchi R, Deguchi M, Kanahama K, Yamaki S (2006) Effects of low temperature and abscisic acid on the expression of the sorbitol-6-phosphate dehydrogenase gene in apple leaves. J Jpn Soc Hortic Sci 75:20–25
Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47:509–540
Lambers H, Blacquière T, Stuiver BEE (1981) Interactions between osmoregulation and the alternative respiratory pathway in Plantago coronopus as affected by salinity. Physiol Plant 51:63–68
Li TH, Li SH (2005) Leaf responses of micropropagated apple plants to water stress: nonstructural carbohydrate composition and regulatory role of metabolic enzymes. Tree Physiol 25:495–504
Li TH, Li SH (2007) Enzymatic regulation of sorbitol metabolism in micropropagated apple plants in response to water stress. Eur J Hortic Sci 72:12–19
Li F, Lei HJ, Zhao XJ, Shen XJ, Liu AL, Li TH (2012a) Isolation and characterization of two sorbitol transporter gene promoters in micropropagated apple plants (Malus × domestica) regulated by drought stress. Plant Growth Regul 68:475–482
Li F, Lei HJ, Zhao XJ, Tian RR, Li TH (2012b) Characterization of three sorbitol transporter genes in micropropagated apple plants grown under drought stress. Plant Mol Biol Rep 30:123–130
Li M, Feng F, Cheng L (2012c) Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS One 7:e33055
Liang D, Cui M, Wu S, Ma FW (2012) Genomic structure, sub-cellular localization, and promoter analysis of the gene encoding sorbitol-6-phosphate dehydrogenase from apple. Plant Mol Biol Rep 30:904–914
Lo Bianco R, Rieger M (2002) Roles of sorbitol and sucrose in growth and respiration of ‘Encore’ peaches at the tree developmental stages. J Am Soc Hortic Sci 127:297–302
Lo Bianco R, Rieger M, Sung SJS (2000) Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiol Plant 108:71–78
Loescher WH (1987) Physiology and metabolism of sugar alcohols in higher plants. Physiol Plant 70:553–557
Loescher WH, Everard JD (1996) Sugar alcohol metabolism in sinks and sources. In: Zamski E, Schaffer A (eds) Photoassimilate distribution in plants and crops: source–sink relationships. Dekker, New York, pp 185–207
Loescher WH, Marlow GC, Kennedy RA (1982) Sorbitol metabolism and sink–source interconversions in developing apple leaves. Plant Physiol 70:335–339
Martinelli F, Teo G, Uratsu SL, Podishetty NK, Dandekar AM (2011) Effects of the silencing of sorbitol dehydrogenase on sugar partitioning in vegetative sinks in apple. Eur J Hortic Sci 76:56–62
Moing A, Carbonne F, Zipperlin F, Svanella L, Gaudillere JP (1997) Phloem loading in peach: symplastic or apoplastic? Physiol Plant 101:489–496
Morandi B, Corelli Grappadelli L, Rieger M, Lo Bianco R (2008) Carbohydrate availability affects growth and metabolism in peach fruit. Physiol Plant 133:229–241
Nosarzewski M, Archbold DD (2007) Tissue-specific expression of SORBITOL DEHYDROGENASE in apple fruit during early development. J Exp Bot 58:1863–1872
Nosarzewski M, Clements AM, Downie AB, Archbold DD (2004) Sorbitol dehydrogenase expression and activity during apple fruit set and early development. Physiol Plant 121:391–398
Oura Y, Yamada K, Shiratake K, Yamaki S (2000) Purification and characterization of a NAD+-dependent sorbitol dehydrogenase from Japanese pear fruit. Phytochemistry 54:567–572
Palonen P, Buszard D (1997) Current state of cold hardiness research on fruit crops. Can J Plant Sci 77:399–420
Pan Q, Wang Z, Quebedeaux B (1998) Responses of the apple plant to CO2 enrichment: changes in photosynthesis, sorbitol, other soluble sugars, and starch. Aust J Plant Physiol 25:293–297
Park SW, Song KJ, Kim MY, Hwang JH, Shin YU, Kim WC, Chung WI (2002) Molecular cloning and characterization of four cDNAs encoding the isoforms of NAD-dependent sorbitol dehydrogenase from the Fuji apple. Plant Sci 162:513–519
Raese JT, Williams MW, Billingsley HD (1978) Cold hardiness, sorbitol, and sugar levels of apple shoots as influenced by controlled temperature and season. J Am Soc Hortic Sci 103:796–801
Ranney TG, Bassuk NL, Whitlow TH (1991) Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry trees. J Am Soc Hortic Sci 116:684–688
Reidel EJ, Rennie EA, Amiard V, Cheng L, Turgeon R (2009) Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol 149:1601–1608
Rennie EA, Turgeon R (2009) A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA 106:14162–14167
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Sakanishi K, Kanayama Y, Mori H, Yamada K, Yamaki S (1998) Expression of the gene for NADP-dependent sorbtiol-6-phosphate dehydrogenase in peach leaves of various developmental stages. Plant Cell Physiol 39:1372–1374
Sheveleva EV, Marquez S, Chmara W, Zegeer A, Jensen RG, Bohnert HJ (1998) Sorbitol-6-phosphate dehydrogenase expression in transgenic tobacco: high amounts of sorbitol lead to necrotic lesions. Plant Physiol 117:831–839
Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51:49–81
Suzue Y, Tsukuda M, Hatano S, Kanayama Y, Yamada K, Shiratake K, Yamaki S (2006) Changes in the activity and gene expression of sorbitol- and sucrose-related enzymes with leaf development of ‘La France’ pear. J Jpn Soc Hortic Sci 75:45–50
Suzuki Y, Dandekar AM (2014a) Sucrose induces expression of the sorbitol-6-phosphate dehydrogenase gene in source leaves of loquat. Physiol Plant 150:355–362
Suzuki Y, Dandekar AM (2014b) Transcriptomic analysis of leaves of transgenic apple silenced for sorbitol-6-phosphate dehydrogenase gene. Acta Hortic (in press)
Tao R, Uratsu SL, Dandekar AM (1995) Sorbitol synthesis in transgenic tobacco with apple cDNA encoding NADP-dependent sorbitol-6-phosphate dehydrogenase. Plant Cell Physiol 36:525–532
Teo G, Suzuki Y, Uratsu SL, Lampinen B, Ormonde N, Hu WK, DeJong TM, Dandekar AM (2006) Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality. Proc Natl Acad Sci USA 103:18842–18847
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Dal Ri A, Goremykin V, Komjanc M, Longhi S, Magnago P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S, Zini E, Eldredge G, Fitzgerald LM, Gutin N, Lanchbury J, Macalma T, Mitchell JT, Reid J, Wardell B, Kodira C, Chen Z, Desany B, Niazi F, Palmer M, Koepke T, Jiwan D, Schaeffer S, Krishnan V, Wu C, Chu VT, King ST, Vick J, Tao Q, Mraz A, Stormo A, Stormo K, Bogden R, Ederle D, Stella A, Vecchietti A, Kater MM, Masiero S, Lasserre P, Lespinasse Y, Allan AC, Bus V, Chagné D, Crowhurst RN, Gleave AP, Lavezzo E, Fawcett JA, Proost S, Rouzé P, Sterck L, Toppo S, Lazzari B, Hellens RP, Durel CE, Gutin A, Bumgarner RE, Gardiner SE, Skolnick M, Egholm M, Van de Peer Y, Salamini F, Viola R (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet 42:833–839
Wang Z, Stutte GW (1992) The role of carbohydrates in active osmotic adjustment in apple under water stress. J Am Soc Hortic Sci 117:816–823
Wang Z, Quebedeaux B, Stutte GW (1995) Osmotic adjustment: effect of water stress on carbohydrates in leaves, stems and roots of apple. Aust J Plant Physiol 22:747–754
Wang Z, Quebedeaux B, Stutte GW (1996) Partitioning of [14C]-glucose into sorbitol and other carbohydrates in apple under water stress. Aust J Plant Physiol 23:245–251
Wang Z, Yuan Z, Quebedeaux B (1997) Photoperiod alters diurnal carbon partitioning into sorbitol and other carbohydrates in apple. Aust J Plant Physiol 24:587–597
Wang Z, Yuan Z, Quebedeaux B (1998) Photoperiod alters partitioning of newly-fixed 14C and reserve carbon into sorbitol, sucrose and starch in apple leaves, stems, and roots. Aust J Plant Physiol 25:503–506
Wang Z, Pan Q, Quebedeaux B (1999) Carbon partitioning into sorbitol, sucrose, and starch in source and sink apple leaves as affected by elevated CO2. Environ Exp Bot 41:39–46
Wang XL, Xu YH, Peng CC, Fan RC, Gao XQ (2009) Ubiquitous distribution and different subcellular localization of sorbitol dehydrogenase in fruit and leaf of apple. J Exp Bot 60:1025–1034
Watari J, Kobae Y, Yamaki S, Yamada K, Toyofuku K, Tabuchi T, Shiratake K (2004) Identification of sorbitol transporters expressed in the phloem of apple source leaves. Plant Cell Physiol 45:1032–1041
Williams MW, Raese JT (1974) Sorbitol in tracheal sap of apple as related to temperature. Physiol Plant 30:49–52
Yamada K, Oura Y, Mori H, Yamaki S (1998) Cloning of NAD-dependent sorbitol dehydrogenase from apple fruit and gene expression. Plant Cell Physiol 39:1375–1379
Yamada K, Niwa N, Shiratake K, Yamaki S (2001) cDNA cloning of NAD-dependent sorbitol dehydrogenase from peach fruit and its expression during fruit development. J Hortic Sci Biotechnol 76:581–587
Yamada K, Suzue Y, Hatano S, Tsukuda M, Kanayama Y, Shiratake K, Yamaki S (2006) Changes in the activity and gene expression of sorbitol- and sucrose-related enzymes associated with development of ‘La France’ pear fruit. J Jpn Soc Hortic Sci 75:38–44
Yamaki S (1981) Subcellular localization of sorbitol-6-phosphate dehydrogenase in protoplast from apple cotyledons. Plant Cell Physiol 22:359–367
Yamaki S, Ishikawa K (1986) Role of four sorbitol-related enzymes and invertase in the seasonal alteration of sugar metabolism in apple tissue. J Am Soc Hortic Sci 111:134–137
Yamaki S, Moriguchi T (1989) Seasonal fluctuation of sorbitol related enzymes and invertase activities accompanying maturation of Japanese pear (Pyrus serotina Rehder var. culta Rehder) fruit. J Jpn Soc Hortic Sci 57:602–607
Zhang LY, Peng YB, Pelleschi-Travier S, Fan Y, Lu YF, Lu YM, Gao XP, Shen YY, Delrot S, Zhang DP (2004) Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol 135:574–586
Zhang JY, Tian RR, Dong JL, Zhao K, Li TH, Wang T (2011) Response and regulation of the S6PDH gene in apple leaves under osmotic stress. J Hortic Sci Biotechnol 86:563–568
Zhou R, Quebedeaux B (2003) Changes in photosynthesis and carbohydrate metabolism in mature apple leaves in response to whole plant source–sink manipulation. J Am Soc Hortic Sci 128:113–119
Zhou R, Sicher R, Quebedeaux B (2001) Diurnal changes in carbohydrate metabolism in mature apple leaves. Aust J Plant Physiol 28:1143–1150
Zhou R, Cheng LL, Wayne R (2003) Purification and characterization of sorbitol-6-phosphate phosphatase from apple leaves. Plant Sci 165:227–232
Zhou R, Cheng L, Dandekar AM (2006) Down-regulation of sorbitol dehydrogenase and up-regulation of sucrose synthase in shoot tips of the transgenic apple trees with decreased sorbitol synthesis. J Exp Bot 57:3647–3657
Zimmermann MH, Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In: Zimmermann MH, Milburn JA (eds) Transport in plants. I. Phloem transport. Springer-Verlag, Berlin, pp 480–503
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Suzuki, Y. (2015). Polyol Metabolism and Stress Tolerance in Horticultural Plants. In: Kanayama, Y., Kochetov, A. (eds) Abiotic Stress Biology in Horticultural Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55251-2_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55251-2_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55250-5
Online ISBN: 978-4-431-55251-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)