Abstract
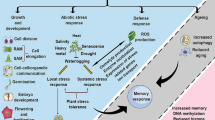
Polyamines (PAs) are low molecular weight aliphatic cations that are ubiquitous in all organisms, including plants. PA accumulation occurs under stress in plants, and modulation of the PA biosynthetic pathway confers tolerance to stresses. Over the past two decades, many reports have unraveled significant functions of PAs in the regulation of abiotic stress tolerance in plants. Here, we focused on the involvement of PA pathways in plants, including those of horticultural crops, in ameliorating abiotic stresses such as salt, drought, heat, chilling, and heavy metals. The possible mechanisms of PA functions on stress tolerance have also been summarized. In addition, the current research trends and future perspectives, especially in horticultural crops, are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Polyamines (PAs) are ubiquitous low molecular weight aliphatic cations found in all living organisms. Because of their cationic nature, they can readily bind to the negatively charged head groups of phospholipids or other anionic sites on membranes, thus affecting the stability and permeability of such membranes. PAs are also able to bind to cellular polyanions such as DNA, RNA, and protein, thereby affecting the synthesis, structure, and function of these macromolecules. Through such possible interactions with other cell components, the involvement of PAs in a wide range of plant growth and developmental processes, as well as responses to environmental stresses, has been demonstrated. Furthermore, PAs can function as direct or indirect free radical scavengers (Ha et al. 1998) and have an antioxidative property by limiting the accumulation of O2 −, probably through the inhibition of NADPH oxidase (Papadakis and Roubelakis-Angelakis 2005).
Previous studies have described the correlation between changes in PA levels and the protective effects of PAs from environmental stresses (Alcázar et al. 2006). However, the effect of PA concentration upon stress was known to show variations, and even sometimes to be contradictory, which was ascribed to the differences in the plant species/cultivars and treatment methods. The creation of several transgenic plants with modified PA biosynthetic genes and the utilization of mutants has been carried out to clearly demonstrate their functions on abiotic stresses; however, those of horticultural crops were quite limited (Wen et al. 2008, 2009, 2010, 2011; Hazarika and Rajam 2011). In this chapter, we intend to summarize the metabolism and functions of PAs on stresses in plants, including those in horticultural crops.
2 Polyamine Biosynthesis and Catabolism
With some variations, biosynthetic pathways for PAs are generally conserved from bacteria to animals and plants (Fig. 3.1). Putresine (Put) is synthesized via ornithine by arginase (EC 3.5.3.1) or ornithine decarboxylase (ODC, EC 4.1.1.17), which are known as the arginine and ornithine pathways, respectively. In the arginine pathway, Put is formed indirectly via the decarboxylation of arginine by arginine decarboxylase (ADC, EC 4.1.1.19), whereas in the ornithine pathway, Put is formed directly from the decarboxylation of ornithine by ODC. Spermidine (Spd) and spermine (Spm) are formed by the successive transfer of an aminopropyl moiety from decarboxylated S-adenosylmethionine (dcSAM) to Put and Spd, respectively; the processes are catalyzed by the aminopropyl transferases Spd synthase (SPDS, EC 2.5.1.16) and Spm synthase (SPMS, EC 2.5.1.22), respectively. In Arabidopsis, ACAULIS5 (ACL5), which is required for stem elongation, was also identified as spermine synthase (Hanzawa et al. 2000), but ACL5 synthesizes thermospermine, an isomer of spermine rather than spermine (Knott et al. 2007). dcSAM is produced from the decarboxylation of S-adenosylmethionine (SAM) by SAM decarboxylase (SAMDC, EC 4.1.1.50). SAM is also the precursor for ethylene biosynthesis; thus, PA and ethylene biosynthesis may compete for the utilization of SAM pools in the cell.
Put is oxidatively deaminated by copper-containing diamine oxidases (DAO, EC 1.4.3.6), and Spd and Spm are oxidized by flavoprotein-containing PA oxidase (PAO, EC 1.5.3.11). DAO preferentially oxidize Put and other diamines. The function of DAO is to convert Put into 4-aminobutanal and hydrogen peroxide (H2O2). PAO oxidizes Spd to 4-aminobutanal, 1,3-diaminopropane (DAP), and H2O2, and Spm to N-(3-aminopropyl)-4-aminobutanal, DAP, and H2O2. DAP can be catabolized to alanine; 4-aminobutanal can be further converted into γ-aminobutyric acid (GABA). GABA is transaminated and oxidized to form succinic acid, which may then enter the Krebs cycle, ensuring the recycling of the carbon and nitrogen from Put. The model plant Arabidopsis contains five PAO-like genes, among which AtPAO1 prefers T-Spm and norspermine to Spm, but does not recognize Spd; AtPAO2 and AtPAO3 preferably recognize Spd; and AtPAO4 is likely to be Spm specific (Takahashi et al. 2010). PA catabolism is not simply a degradative process but also a contributing process for PA homeostasis.
Considering the PA concentrations in the transgenic plants with PA biosynthetic genes, the increase in the target PA concentration was not as much as expected, despite the usage of the constitutive 35S promoter. For example, Spd concentrations in SPDS-overexpressing European pears (Wen et al. 2008) and Arabidopsis (Kasukabe et al. 2004) were only 1.5- to 2.0 fold higher than the concentrations in the wild type. It could be that the transgenic plantlets with high PA concentrations cannot be regenerated in the regeneration medium, so only transgenic plants with mild PA concentrations successfully regenerated and developed into whole plants. This observation may indicate that the PA concentrations in the cells are kept under homeostasis by fine regulation.
3 PA and Salt and Drought Stresses
When plants are exposed to salt stress, such as high NaCl, ionic homeostasis is disturbed. High levels of ionic molecules cause hyperosmotic stress and activate the synthesis of abscisic acid (ABA), which can then upregulate the vacuolar Na+/H+ exchanger, NHX (Shi and Zhu 2002). To maintain a high concentration of K+ and a low concentration of Na+ in the cytosol on induction of salt stress, plants exert a transport system, including an ion exchanger and an ion sensor via Ca2+ signaling (Zhu 2003). So far, many reports have suggested the involvement of PAs in salt stress alleviation through comparisons of salt-tolerant and salt-sensitive cultivars exposed to the exogenous application of PA. However, reports on the relationship between PA and transporters were quite limited. Yamaguchi et al. (2006) reported that an Spm-deficient Arabidopsis mutant (spms) was sensitive to salt without affecting the transcriptions of salt-overly-sensitive (SOS), NHX, and high-affinity K+ transporter (HKT), but calcium channel inhibitors could reduce the salt sensitivity of this mutant, demonstrating the importance of calcium signaling modulated by Spm. Instead, there were several reports of the antioxidant aspect of salt stress alleviation. It was shown that transgenic European pears, which had an overexpression of an apple SPDS, showed salt and osmotic stress alleviation (Wen et al. 2008). The induction of Spd concentration and antioxidant enzyme activities such as superoxide dismutase (SOD), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), and glutathione reductase (GR) upon salt treatment resulted in salt stress alleviation (He et al. 2008). Conversely, antisense inhibition of an apple SPDS in European pears showed an increased sensitivity to salinity by the reduction of Spd concentration, as well as SOD and GR activities, which confirmed the crucial role of PAs in stress alleviation (Wen et al. 2011). In SPDS-overexpressing transgenic tomato plants, APX activity was significantly higher in transgenic plants compared to the wild type under saline conditions, which might contribute largely to the protection against oxidative stress generated by NaCl treatments, and thus confer salinity tolerance in the transgenic tomato plants (Neily et al. 2011). By contrast, a DS insertion mutant of Arabidopsis ADC2 indicated a reduction of Put concentration by about 25 % and was more sensitive to salt stress than the wild type, but the sensitivity was recovered by the addition of Put. This result showed the importance of Put to the ADC pathway for salt stress sensitivity (Urano et al. 2004). Taken together, these results demonstrated the importance of PA for salt stress alleviation, although the key PA molecule may vary depending on the plant species or experimental design.
On the other hand, the regulatory signaling cascade in drought stress responses has been well clarified, and both ABA-dependent and ABA-independent regulatory systems are involved in this stress response (Shinozaki and Yamaguchi-Shinozaki 2000). As mentioned previously, salt stress may induce osmosis, and lead to ABA synthesis, consequently resulting in the ABA-dependent signaling cascade. The integration of transcriptomic and metabolomic data sets has revealed that drought-induced ABA-dependent transcriptional regulation had a pivotal role in PA biosynthesis (Fujita et al. 2011). There were several reports on the relationships between stomata dynamics and PAs, possibly through ABA signaling. Alcázar et al. (2006) reported that transgenic Arabidopsis lines that constitutively expressed ADC2 showed different degrees of drought tolerance paralleled with Put concentrations, wherein these lines contain high levels of Put upon drought stress without obvious changes in Spd and Spm. Interestingly, there were no significant differences in the number of stomata between wild-type and transgenic plants, but a reduction in the transpiration rate and stomata conductance was observed in the transgenic lines. Thus, a mechanism underlying drought tolerance is controlled by a reduction in water loss modulated by Put (Alcázar et al. 2006). PAs, including Spm, inhibited stomatal opening and induced the closure of Vicia faba guard cells (Liu et al. 2000). In tomato seedlings, exogenous application of Spd enhanced the photosynthetic rate (Zhang et al. 2010). It was also documented that ABA was at least partially responsible for the induction of PA accumulation and exodus into the apoplast. There, they were oxidized by the apoplastic amine oxidases, producing H2O2, which signaled secondary stress responses in grapevines (Toumi et al. 2010).
4 PA and Temperature Stress
Throughout the world, many crops are frequently exposed to severe high temperatures during their life cycle, resulting in a reduction in quality and quantity of biomass. To protect themselves from severe damage and survive high-temperature stress, plants adopt a set of responsive mechanisms characterized by the elevated synthesis of heat-shock proteins (HSPs). It was postulated that PAs might directly affect HSP production at the level of protein synthesis. HSP synthesis was detected up to 46 °C in the cells of the heat-tolerant tobacco BY2, although it ceased at 40 °C in cells of a heat-susceptible alfalfa line. Higher leakage of soluble PAs was observed from the alfalfa cells than the tobacco cells at high temperatures (Königshofer and Lechner 2002). The inhibition of Put biosynthesis in alfalfa cells reduced PA leakage at high temperatures, and thus improved HSP synthesis, indicating that PA might influence the extent of HSP synthesis under heat stress (Königshofer and Lechner 2002). Transgenic tomato plants overexpressing yeast SAMDC, which produce 1.7- and 2.4-fold-higher levels of Spd and Spm, respectively, compared with the wild type, became thermotolerant by remarkable enhancement of the antioxidant enzyme activities and protecting membrane lipid peroxidation (Cheng et al. 2009). Recently, it was reported that Spm concentration positively correlated with the levels of heat stress tolerance accompanying an increase in the genes encoding HSPs and heat-shock transcription factors using transgenic Arabidopsis plants overexpressing SPMS and Spm-deficient Arabidopsis mutants, spms. Thus, Spm increases the heat-shock (HS) response at transcriptional and translational levels and protects host plants from HS-induced damage (Sagor et al. 2013).
On the other hand, low temperature is also one of the most severe environmental stresses, which inhibits the growth and distribution of plants. Several scientists have reviewed the involvement of PAs in low-temperature stress (Theocharis et al. 2012). In general, differential accumulation of PAs during low-temperature stress in a number of plant species has been reported and seems to have potential for counteracting chill-induced injuries (Alcázar et al. 2011). Shen et al. (2000) reported that Spd prevented the chill-induced increases in H2O2 content in cucumber leaves, as well as activities of NADPH oxidases and NADPH-dependent superoxide generation in microsomes; thereby conferring chilling tolerance in cucumbers. It was also shown that exogenous application of Spd in cucumbers improved the chilling tolerance of the photosynthetic apparatus (He et al. 2002), with increases in SAMDC activity in the tolerant cultivar. The SPDS from Cucurbita ficifolia was introduced to Arabidopsis, and the transgenic lines exhibited a significant increase in SPDS activity and Spd content in leaves, together with enhanced tolerance to various stresses, including chilling and freezing, compared with the wild type (Kasukabe et al. 2004). Recently, transcriptome analysis suggested a positive feedback regulatory mechanism between ABA and Put, clearly suggesting that Put modulates ABA biosynthesis at the transcriptional level in response to low temperatures, thus uncovering a novel role for PA as a regulator of hormone biosynthesis (Cuevas et al. 2009).
5 PA and Heavy Metal Stress
Heavy metals, which are one of the major environmental pollutants, can cause serious problems for all organisms when present in the atmosphere, soil, and water, even in trace concentrations. Plants develop systems for metal tolerance, such as cell wall binding, chelation with phytochelatin, and compartmentalization (Zhang and Shu 2006). However, overaccumulation of heavy metals causes the generation of reactive oxygen species, which modifies the antioxidant defense and balance of redox status (Sharma and Dietz 2008), and elicits oxidative stress. Groppa et al. (2001, 2003) observed that Spm or Spd completely recovered the activity of GR, which had been impaired by copper (Cu) or cadmium (Cd) stress. Our previous work also revealed that Spd levels were implicated in the alleviation of heavy metal stresses such as Cd, lead (Pb), zinc (Zn), or a combination thereof in an MdSPDS1-overexpressing transgenic European pear by exerting SOD and GR activities (Wen et al. 2010). Thus, enhancement of antioxidant enzyme activities could be at least one reason for the mitigation of heavy metal stress. Indeed, overexpression of SOD and APX mitigated heavy metal stress, including Cu, Cd, and arsenic, in tall fescue transgenic plants (Lee et al. 2007). By contrast, Choudhary et al. (2012) provided the effects of exogenously applied brassinosteroids and PAs on radish plants exposed to toxic concentrations of Cu. The combined application of 24-epibrassinolide and Spd modulated the expression of genes encoding PA enzymes and genes that impact the metabolism of indole-3-acetic acid and ABA, resulting in enhanced tolerance to Cu stress.
In addition, several cases concerning the mitigation of other metal stress such as nickel (Ni) and aluminum (Al) have been also documented. Shevyakova et al. (2011) reported that Amaranthas leaves treated with Put or Spd did not show any signs of injury despite an increase in the amount of Ni; thus, they assumed that PAs manifested their protective action as Ni chelators and detoxicants. Wen et al. (2009) revealed that the activities of SOD and GR and the accumulation of proline or malondialdehyde acted upon Al stress, resulting in the SPDS-overexpressing European pear transgenic line toward a more favorable survival status than the wild type.
6 PAs and Other Molecules Related to Abiotic Stress
As mentioned previously, increased ABA concentrations brought on by abiotic stress could trigger the expression of multiple abiotic stress-adaptive genes in plants, possibly including PA biosynthesis genes (Hussain et al. 2011). PA catabolism produces H2O2 (Fig. 3.1), which has often been considered a toxic molecule. Recently, there has been a surge of reports highlighting the nature of H2O2 as a signal molecule, capable of diffusing into neighboring cells and tissues from the site of its production, and activating responses against stresses (Rhee 2006). Tun et al. (2006) reported on PA-induced nitric oxide (NO) biosynthesis in Arabidopsis. Subsequently, Palavan-Unsal and Arisan (2009) further confirmed that in addition to H2O2, PAs induced the production of NO in various tissues in seedlings of Arabidopsis. Wang et al. (2012) have recently elucidated the involvement of Put and NO in Al tolerance by modulating citrate secretion from the roots of red kidney beans. The evidence obtained so far strongly indicates the involvement and interaction of PA, ABA, H2O2, and NO during stress responses to be a complex network. Additionally, the interplay among PAs, ABA, H2O2, and NO in plant stomatal regulation is worth mentioning (Yamasaki and Cohen 2006). Cona et al. (2006) reported that the generation of H2O2 by amino oxidases was tightly linked to PA catabolism and was associated with plant abiotic stress responses. The regulation of stomatal movements in response to ABA was documented to be dependent on both H2O2 and NO, in that NO generation depends on H2O2 production (Paschalidis et al. 2010). Therefore, PAs may regulate stomatal closure by being directly involved in the biosynthesis of signaling molecules such as ABA, H2O2, and NO. Conclusively, plant responses to unfavorable environmental conditions, and the crosstalk between these signaling molecules, have a vital role in its defense against harsh environments (Bitrián et al. 2012).
7 Perspectives
During the past decade, analyses of metabolic adjustments of plants with different stress tolerances and transgenic research have provided important evidence for better understanding the role of PAs in response to abiotic environments; thus, our knowledge of the involvement of PA on tolerance to environmental stresses has increased considerably. Overexpressing PA biosynthetic genes confers enhanced abiotic stress tolerance in many transgenic plants. It is worthy to note that transgenic plants overexpressing one PA biosynthetic gene showed tolerance to a wide range of abiotic stresses such as salt, osmosis, cold, freezing, and even heavy metals (Kasukabe et al. 2004; Wen et al. 2008). It was apparent that wild-type European pear showed some wilting of leaves and necrosis of apical shoots even after a short period of stress treatment, although these phenotypic features were alleviated in the transgenic line (Fig. 3.2). One of the important PA functions could be the alleviation of oxidative stress through the enhancing of antioxidant molecules and antioxidant enzyme activities, possibly protecting cell membranes, proteins, and DNA/RNA from degradation, because all abiotic stresses eventually cause oxidative stress. At present, the detailed molecular mechanism of PA in these processes remains enigmatic, but intensive research using modern biological technologies, such as genetics, molecular biology, proteomics, and metabolomics, could facilitate the clarification of detailed PA functions in plant development, including abiotic stress response.
Morphological features of the wild type (WT) and transgenic line after 10 days of mannitol (300 mM) treatment (a) and after 15 days of CuSO4 (500 μM) treatment (b). (Modified from Wen et al. 2008)
Horticultural crops are of high importance among the agronomic crops. Unfortunately, information regarding the involvement of PAs in the abiotic stress tolerance of horticultural crops, as well as improvements in survival rates in harsh environments via transformation approaches with PA biosynthetic genes, have thus far been very limited. Abiotic stress tolerance is especially important with respect to fruit trees, which cannot be easily rotated as can annual crops to avoid stress, because they are generally exposed to environmental stress over several years, once planted. In addition to abiotic stress, damage to plants and loss of crop production caused by biotic stresses such as plant disease and insects should be also considered. Interestingly, overexpression of an apple SPDS in a sweet orange reduced canker susceptibility (Fig. 3.3), indicating the possibility for both abiotic and biotic stress alleviation with PAs. Thus, the use of PA should be explored for stable and sustainable production of horticultural crops toward global warming conditions.
Canker disease tolerance of the wild type (WT) and the transgenic line (TG9). Photographs show symptoms on the adaxial (a) and abaxial (b) sides of the leaves from WT and TG9. Selected inoculation sites of the leaves were zoomed in and shown below the corresponding photographs. (Photographs kindly provided by Drs. Xing-Zheng Fu and Ji-Hong Liu)
References
Alcázar R, Marco F, Cuevas JC, Patrón M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28:1867–1876
Alcázar R, Cuevas JC, Planas J, Zarza X, Bortolotti C, Carrasco P, Salinas J, Tiburcio AF, Altabella T (2011) Integration of polyamines in the cold acclimation response. Plant Sci 180:31–38
Bitrián M, Zarza X, Altabella T, Tiburcio AF, Alcázar R (2012) Polyamines under abiotic stress: metabolic crossroads and hormonal crosstalks in plants. Metabolites 2:516–528
Cheng L, Zou YJ, Ding SL, Zhang JJ, Yu XL, Cao JS, Lu G (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Int Plant Biol 51:489–499
Choudhary SP, Oral HV, Bhardwaj R, Yu JQ, Tran LSP (2012) Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J Exp Bot 63:5659–5675
Cona A, Rea G, Angelini R, Fererico R, Tavladoraki P (2006) Functions of amine oxidases in plant development and defence. Trends Plant Sci 11:80–88
Cuevas JC, Lopez-Cobollo R, Alcazar R, Zarza X, Koncz C, Altabella T, Salinas J, Tiburcio AF, Ferrando A (2009) Putrescine as a signal to modulate the indispensable ABA increase under cold stress. Plant Signal Behav 4:219–220
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Groppa MD, Tomaro ML, Benavides MP (2001) Polyamines as protectors against cadmium or copper-induced oxidative damage in sunflower leaf discs. Plant Sci 161:481–488
Groppa MD, Benavides MP, Tomaro ML (2003) Polyamine metabolism in sunflower and wheat leaf discs under cadmium or copper stress. Plant Sci 164:293–299
Ha HC, Sirosoma NS, Kuppusamy P, Zweiler JL, Woster PM, Casero RA (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95:11140–11145
Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G, Komeda Y (2000) ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J 19:4248–4256
Hazarika P, Rajam MV (2011) Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol Mol Biol Plants 17:115–128
He LX, Nada K, Tachibana S (2002) Effects of spermidine pretreatment through the roots on growth and photosynthesis of chilled cucumber plants (Cucumis sativus L.). J Jpn Soc Hortic Sci 71:490–498
He L, Ban Y, Inoue H, Matsuda N, Liu J, Moriguchi T (2008) Enhancement of spermidine content and antioxidant capacity in transgenic pear shoots overexpressing apple spermidine synthase in response to salinity and hyperosmosis. Phytochemistry 69:2133–2141
Hussain SS, Ali M, Ahmad M, Siddique KH (2011) Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol Adv 29:300–311
Kasukabe Y, He LX, Nada K, Misawa S, Ihara I, Tachibana S (2004) Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes in transgenic Arabidopsis thaliana. Plant Cell Physiol 45:712–722
Knott JM, Römer P, Sumper M (2007) Putative spermine synthases from Thalassinosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett 581:3081–3086
Königshofer H, Lechner S (2002) Are polyamines involved in the synthesis of heat-shock proteins in cell suspension cultures of tobacco and alfalfa in response to high-temperature stress? Plant Physiol Biochem 40:51–59
Lee S-H, Ahsan N, Lee K-W, Kim D-H, Lee D-G, Kwak S-S, Kwon S-Y, Kim T-H, Lee B-H (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164:1626–1638
Liu K, Fu H, Bei Q, Luan S (2000) Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol 124:1315–1326
Neily HM, Baldet P, Arfaoui I, Saito T, Li Q, Asamizu E, Matsukura C, Moriguchi T, Hirsohi E (2011) Overexpression of apple spermidine synthase 1 (MdSPDS1) leads to significant salt tolerance in tomato plants. Plant Biotechnol 28:33–42
Palavan-Unsal N, Arisan D (2009) Nitric oxide signalling in plants. Bot Rev 75:203–229
Papadakis AK, Roubelakis-Angelakis KA (2005) Polyamines inhibit NADPH oxidase-mediated superoxides generation and putrescine prevents programmed cell death syndrome induced by the polyamine oxidase generated hydrogen peroxide. Planta (Berl) 220:826–837
Paschalidis KA, Toumi I, Moschou PN, Roubelakis-Angelakis KA (2010) ABA-dependent amine oxidases-derived H2O2 affects stomata conductance. Plant Signal Behav 5:1153–1156
Rhee SG (2006) H2O2, a necessary evil for cell signaling. Science 312:1882–1883
Sagor GHM, Berberich T, Takahashi Y, Niitsu M, Kusano T (2013) The polyamine spermine protects Arabidopsis from heat stress-induced damage by increasing expression of heat shock-related genes. Transgenic Res 22:595–605
Sharma SS, Dietz K-J (2008) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Shen WY, Nada K, Tachibana S (2000) Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol 124:431–439
Shevyakova NI, Cheremisina AI, Kuznetsov VV (2011) Phytoremediation potential of Amaranthus hybrids: antagonism between nickel and iron and chelating role of polyamines. Russ J Plant Physiol 58:634–642
Shi H, Zhu J-K (2002) Regulation of expression of the vacuolar Na+/H+ antiporter gene AtNHX1 by salt stress and abscisic acid. Plant Mol Biol 50:543–550
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Takahashi Y, Cong R, Sagor GHM, Niitsu M, Berberich T, Kusano T (2010) Characterization of five polyamine oxidase isoforms in Arabidopsis thaliana. Plant Cell Rep 29:955–965
Theocharis A, Clément EA, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta (Berl) 235:1091–1105
Toumi I, Moschou PN, Paschalidis KA, Bouamama B, Salem-fnayou AB, Ghorbel AW, Mliki A, Roubelakis-Angelakis KA (2010) Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J Plant Physiol 167:519–525
Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EIS, Scherer GFE (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol 47:346–354
Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313:369–375
Wang H, Huang J, Liang W, Liang X, Bi Y (2012) Involvement of putrescine and nitric oxide in aluminum tolerance by modulating citrate secretion from roots of red kidney bean. Plant Soil 366:479–490
Wen XP, Pang XM, Matsuda N, Kita M, Hao YJ, Kitashiba H, Honda C, Moriguchi T (2008) Overexpression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res 17:251–263
Wen XP, Ban Y, Inoue H, Matsuda N, Moriguchi T (2009) Aluminum tolerance in a spermidine synthase-overexpressing transgenic European pear is correlated with the enhanced level of spermidine via alleviating oxidative status. Environ Exp Bot 66:471–478
Wen XP, Ban Y, Inoue H, Matsuda N, Moriguchi T (2010) Spermidine levels are implicated in heavy metal tolerance in a spermidine synthase overexpressing transgenic European pear by exerting antioxidant activities. Transgenic Res 19:91–103
Wen XP, Ban Y, Inoue H, Matsuda N, Kita M, Moriguchi T (2011) Antisense inhibition of a spermidine synthase gene highlights the role of polyamines for stress alleviation in pear shoots subjected to salinity and cadmium. Environ Exp Bot 72:157–166
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Miyazaki A, Takahashi T, Michael A, Kusano T (2006) The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett 580:6783–6788
Yamasaki H, Cohen MF (2006) NO signal at the crossroads: polyamine induced nitric oxide synthesis in plants. Trends Plant Sci 11:522–524
Zhang J, Shu W-S (2006) Mechanisms of heavy metal cadmium tolerance in plants. J Plant Physiol Mol Biol 32:1–8
Zhang CM, Zou ZR, Huang Z, Zhang ZX (2010) Effects of exogenous spermidine on photosynthesis of tomato seedlings under drought stress. Agric Res Arid Areas 3:182–187
Zhu J-K (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Wen, X., Moriguchi, T. (2015). Role of Polyamines in Stress Response in Horticultural Crops. In: Kanayama, Y., Kochetov, A. (eds) Abiotic Stress Biology in Horticultural Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55251-2_3
Download citation
DOI: https://doi.org/10.1007/978-4-431-55251-2_3
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55250-5
Online ISBN: 978-4-431-55251-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)