Abstract
Arterial spin labeling (ASL) is a type of MR perfusion imaging which utilizes magnetically labeled arterial blood as an intrinsic tracer. Because ASL involves no radioactive tracers or contrast administration, it is considered to be completely noninvasive. Moreover, ASL theoretically allows for quantitative measurement of regional cerebral blood flow (rCBF). However, ASL suffers from technical limitations including low signal-to-noise ratio and high sensitivity to arterial transit time inhomogeneity. A variety of ASL pulse sequences have been proposed, which are categorized into pulsed ASL and continuous ASL sequences. Maps of rCBF obtained with ASL are typically analyzed quantitatively, often using image statistical methods. Typical ASL findings of Alzheimer’s disease include hypoperfusion in the cortical areas of the posterior cingulate and precuneus as well as the temporoparietal association cortex. Several studies have shown that ASL has high diagnostic performance in discriminating patients with Alzheimer’s disease and healthy elderly subjects, although its clinical roles in diagnosing dementia have not been established.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Since the 1980s, various techniques of MRI-based brain perfusion-weighted imaging techniques have been proposed. Today, the most widely available is the dynamic susceptibility contrast (DSC) technique, in which a bolus of paramagnetic contrast agent is intravenously injected [1]. With a strong susceptibility effect from the paramagnetic agent, this method provides a high contrast-to-noise ratio at both 1.5 T and 3 T, providing primarily regional blood volume. While the DSC method is very popular in imaging of brain tumors and acute ischemia, its application in dementia has been limited due to the necessity of contrast administration. Arterial spin labeling (ASL) is a type of MR perfusion imaging which involves no contrast administration. Due to its noninvasiveness, it has been attracting much attention in dementia imaging.
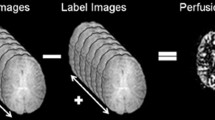
ASL was first proposed by Williams and Detre in 1992 [2, 3]. In ASL, magnetically “labeled” arterial blood is used as an intrinsic tracer. Labeling of arterial blood is achieved by inversion pulse, which inverts the magnetization of the spins in the blood, at the upstream location. Images of the brain are obtained when the arterial blood including the inverted spins flows in the brain tissue. These images are called “labeled images,” since they include labeled arterial blood. During the same imaging session, images without effective labeling, which are called “control images,” are also obtained. Simple subtraction of the two images (control images–labeled images) is used to cancel out the signals from the static brain tissues, thereby yielding a perfusion-related signal from the labeled arterial blood (Fig. 6.1). The signal intensity of the perfusion-related signal accounts for only a few percentage points of the total signal intensity. To increase the ratio of the perfusion-related signal to the noise, typically 30–50 sets of control and labeled scans are repeated, resulting in an imaging time of 4–5 min. For some time, the problem of the low signal-to-noise ratio hampered the clinical use of ASL. However, the recent spread of clinical 3 T MR systems, which theoretically doubles the signal-to-noise ratio compared to 1.5 T systems, has made the clinical use of ASL feasible.
Schematic description of ASL perfusion imaging. Labeled images are obtained when labeled arterial blood with inverted magnetization reaches the brain tissue in the imaging plane, while control images are obtained without effective labeling of blood. Perfusion-related signals can be obtained by subtracting the labeled images from the control images
6.2 Quantification of Regional Cerebral Blood Flow
Theoretically, ASL enables us to quantify regional cerebral blood flow (rCBF) [4]. In this quantification, the length of time after labeling needs to be taken into account, since inverted spins in the arterial blood will return to the steady state with a time constant of T1, which is approximately 1.4 s at 1.5 T. After a post-labeling delay much longer than the blood T1, the effect of inverted magnetization would be rapidly diminished. Higher magnetic field strength has advantages over conventional field strength in ASL not only due to its improved intrinsic signal-to-noise ratio but also its prolonged T1 of blood (approximately 1.6 s at 3 T), which in turn results in more durable inverted arterial blood spins as an intrinsic tracer.
Figure 6.2 shows a typical rCBF maps obtained using ASL. Previous studies have demonstrated that quantitative in vivo measurement of rCBF by ASL is feasible in healthy subjects, based on a comparison of this approach with the gold standard method of positron emission tomography (PET) using 15O-labeled tracers [5, 6]. ASL rCBF measurement is prone to failure in subjects with pathological conditions, especially those with steno-occlusive lesions of major cerebral arteries [7]. Major arterial steno-occlusive lesions result in a delayed arrival of the labeled blood at the brain tissue due to both lowered perfusion pressure and blood supply via collateral circulation. The transit time for the labeled blood to reach the imaging plane is called the arterial transit time (ATT). The prolonged ATT can profoundly affect the accuracy in rCBF measurement. In ASL, the labeled arterial blood must reach the brain tissue before a labeled image can be obtained. To meet this requirement, the labeled images are obtained after a certain post-labeling delay. Since the post-labeling delay cannot be much longer than the T1 of the arterial blood, the typical delay setting for clinical 3 T scanners ranges from 1.5 to 2 s. These post-labeling times are long enough for healthy young individuals in whom ATT is relatively short. However, in patients with steno-occlusive lesions in the major cerebral arteries, in whom ATT will be much longer, a post-labeling delay of 1.5–2 s would be inadequate. Consequently, the labeled images are scanned before the labeled blood reaches the brain tissue, which in turn results in underestimation of rCBF. Under these conditions, the labeled blood stays in the arterial vessels at the time of imaging. This labeled blood within the arteries can create spotty areas of high blood flow in the final blood flow maps, and these artifactual hyperperfused areas may coexist with the underestimated rCBF, completely degrading the rCBF mapping. Figure 6.3 shows a typical example. To minimize the effect of residual intra-arterial-labeled blood, ASL pulse sequences are often implemented with crusher gradients, which selectively attenuate signals from flowing blood [8]. The normal range of ATT is known to be longer in the elderly population. Since dementia is a geriatric disease, it should be noted that underestimation of rCBF due to prolonged ATT can occur among healthy elderly individuals, especially in the vascular border zones [9], where ATT is always longer than the other regions (Fig. 6.4).
Effect of ATT prolongation on ASL images. A patient with bilateral middle cerebral artery occlusion on MR angiography (a) but without obvious infarction on a T2-weighted image (b) underwent pulsed ASL imaging and 15O PET. PET (c) reveals preserved blood flow, presumably due to collateral blood supply. In the ASL image (d), a complete deficit of blood flow is observed in the bilateral middle cerebral artery territories, likely due to ATT prolongation. Spotty hyperperfused areas (arrows) are seen in the periphery of the perfusion deficits, indicating labeled blood in the arteries
In clinical imaging, ASL measurement can be deteriorated by several other factors. Patients with cognitive disorders often find it difficult to stay still during the scan. As ASL rCBF measurement includes subtraction between the labeled and control images, the patients’ head motion can seriously degrade the rCBF measurement. Magnetic field inhomogeneity due to air-filled paranasal sinuses and mastoid air cells, skull base bones, or implanted metallic devices may result in failure of ASL, especially when echo planar imaging sequence is employed. A metallic stent in the neck where labeling inversion pulses are applied can also cause problems.
The reproducibility of ASL rCBF measurement has been demonstrated using healthy volunteers [10,11,12], but it has not been fully established in clinical settings.
6.3 ASL Pulse Sequences
There are two distinct types of ASL pulse sequences, pulsed ASL (PASL) and continuous ASL (CASL). In PASL, labeling of arterial blood is carried out in short duration over a relatively thick labeling slab, whereas in CASL a continuous labeling pulse is applied over a thinner labeling slab. Compared to CASL, PCASL is easier to implement in clinical MR scanners. However, PASL sequences generally suffer from lower SNR and higher sensitivity to ATT prolongation. To mitigate the latter issue, sophisticated PASL sequences with multiple inversion time sampling have been proposed [13, 14]. On the other hand, the longer labeling in CASL results in a larger number of labeled spins, thereby improving the signal-to-noise ratio in rCBF measurement. Moreover, CASL has an advantage over PCASL in terms of its lower sensitivity to ATT prolongation. However, due to the longer labeling pulse, CASL tends to deposit higher amounts of energy, often exceeding the regulatory limit on the specific absorption rate. This issue of CASL has been solved by using a series of short labeling pulses in a scheme called pseudo-continuous ASL or pulsed continuous ASL (PCASL) [15, 16]. PCASL has been combined with 2-D and 3-D acquisition and is fastly becoming the standard ASL method [17].
6.4 Analysis of ASL rCBF Maps
Imaging of rCBF during the resting state is known to be useful in diagnosing neurodegenerative dementias such as Alzheimer’s disease. It is conceivable that rCBF parallels the functional activity of the neural tissue. Thus a deficit in rCBF could reflect a functional failure representing either a degeneration of local brain tissue or its transneuronal effects in remote regions. The rCBF in dementia has been evaluated using nuclear medicine techniques such as PET utilizing 15O-labeled water and single-photon emission tomography (SPECT). In clinical practice, SPECT has been the most widely available modality (see Chap. 11: Brain perfusion SPECT in Alzheimer’s disease). Although SPECT is an established technique, ASL has several key advantages over SPECT. Most importantly, ASL does not use radiation or contrast administration and is completely noninvasive. In addition, ASL is superior to SPECT in terms of spatial resolution. Finally, ASL can be easily incorporated into a clinical MR imaging protocol, thereby allowing simultaneous morphological evaluation. This could lead to improved cost-effectiveness.
With these theoretical strengths in mind, researchers have investigated the feasibility of using ASL in the diagnosis of dementia, especially Alzheimer’s disease [18, 19]. The rCBF maps obtained with ASL can be evaluated both qualitatively and quantitatively. Quantitative evaluation is popular among the research community, since it provides more objective information. In the past reports, ASL rCBF maps have been analyzed with almost the same technique as used for SPECT rCBF maps. The quantitative analysis of ASL rCBF maps may include statistical methods, such as statistical parametric mapping (SPM). In these analyses, the rCBF map of each individual patient is normalized according to a common template of a standard anatomical space (a predefined “average brain”) so that the rCBF of different individuals can be compared on a voxel-by-voxel basis. Alternatively, mean rCBF values are measured in anatomically labeled brain regions on each patient’s native rCBF map using region-of-interest (ROI) templates defined in the standard space which are subsequently warped according to the native space.
Since ASL theoretically allows for quantitative rCBF measurement, it is possible that analysis of absolute rCBF maps is meaningful. However, rCBF values are often normalized, especially for the diagnosis of dementia. Normalization of rCBF could effectively eliminate variations in absolute rCBF measurement, which may originate from both physiological variability of global CBF and scaling error in absolute rCBF measurement. The normalization results are critically dependent on the choice of reference region: the reference regions should be among those least affected by the disease. In the past literature, localized reference regions such as the sensorimotor (or motor) and calcarine cortices were used.
ASL has better spatial resolution than SPECT. Nevertheless, it is still not high enough to clearly separate the white matter (WM), cortical gray matter (GM), and surrounding cerebrospinal fluid (CSF), and thus a substantial partial volume effect (PVE) in voxels involving the cortical GM is unavoidable. Many dementing diseases are associated with cortical volume loss, which can result in a decreased GM volume fraction in an imaging voxel. This in turn results in underestimation of the CBF of local GM. Correction for the PVE has been often implemented in the generation of rCBF maps in which corresponding high-resolution 3D anatomical MR images, which can distinguish the three compartments, are coregistered to the rCBF maps and used as a reference [20].
6.5 ASL of Alzheimer’s Disease and Other Dementias
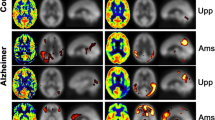
Previous nuclear medicine studies reported that the hypoperfusion related to Alzheimer’s disease typically involves several brain regions, including the posterior cingulate gyrus and precuneus, as well as the association cortex in the parietal and temporal lobes [21, 22]. Perfusion abnormality on ASL is seen in essentially the same brain regions as those on PET and SPECT [19, 20, 23,24,25] (Fig. 6.5), as shown in a few previous studies that compared ASL hypoperfusion with results of PET studies using the same subjects for validation [26,27,28].
Averaged ASL rCBF maps of healthy elderly subjects (n = 23) and patients with Alzheimer’s disease (n = 20) (a). Note the rCBF reduction in patients, especially in the posterior cingulate gyrus and precuneus. The SPM analysis (b) reveals significant rCBF reduction (p < 0.001, uncorrected for multiple comparisons)
The hippocampus is one of the brain regions that are affected by Alzheimer’s disease in its early stage. Previous ASL studies have shown an unexpected increase in PVE-corrected rCBF in the hippocampus of patients with early Alzheimer’s disease [29], which was attributed to the reorganization of local neuronal tissue, although this finding is still controversial.
Several studies have evaluated the diagnostic performance of ASL in Alzheimer’s disease [23, 24, 30, 31]. These studies have shown that ASL can discriminate between patients with Alzheimer’s disease and age-matched healthy subjects with relatively high accuracy, demonstrating the clinical feasibility of ASL as a tool for screening. Nevertheless, the diagnostic value of ASL in dementia has not been established. This is due not only to the small size of the patient populations in these studies but also to the divergent methodologies: they used different magnetic field strengths (1.5 T vs. 3 T), different ASL pulse sequences, and different methods of image analysis. Future studies with consistent methodologies are needed.
Mild cognitive impairment (MCI) includes a prodromal stage of Alzheimer’s disease. Discrimination of patients with MCI from healthy subjects using ASL appears to be more challenging than detection of Alzheimer’s disease, since the decrease in rCBF is more subtle [20, 25]. A previous study attempted to predict the conversion from MCI into Alzheimer’s disease based on ASL findings and found that hypoperfusion in several regions in the right cerebral hemisphere was predictive of subsequent conversion [32].
ASL may also be useful for differential diagnosis of dementia. Alzheimer’s disease and frontotemporal lobar degeneration displayed distinct spatial patterns of hypoperfusion on ASL rCBF maps [33, 34], suggesting that ASL is useful in distinguishing the two diseases.
As mentioned earlier, ASL can be obtained simultaneously with morphological imaging in the clinical protocol for dementia. It has been hypothesized that the combination of ASL rCBF measurement and morphological assessment would increase the diagnostic accuracy for dementia compared to either approach used singly. This hypothesis has been supported by a few studies [30, 35], although it is still controversial. Thus the relative roles of ASL and MR morphological assessment are subject to future research.
Conclusion
ASL is an emerging MR-based perfusion imaging technique which is most characterized by its noninvasiveness and convenience. Previous studies have found that it is a promising tool for diagnosing dementia, but further studies will be needed to establish its clinical values.
References
Rosen BR, Belliveau JW, Aronen HJ, et al. Susceptibility contrast imaging of cerebral blood volume: human experience. Magn Reson Med. 1991;22:293–9.
Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–6.
Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45.
Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–96.
Ye FQ, Berman KF, Ellmore T, et al. H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44:450–6.
Heijtel DF, Mutsaerts HJ, Bakker E, et al. Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with 15O H2O positron emission tomography. NeuroImage. 2014;92:182–92.
Kimura H, Kado H, Koshimoto Y, Tsuchida T, Yonekura Y, Itoh H. Multislice continuous arterial spin-labeled perfusion MRI in patients with chronic occlusive cerebrovascular disease: a correlative study with CO2 PET validation. J Magn Reson Imaging. 2005;22:189–98.
Ye FQ, Mattay VS, Jezzard P, et al. Correction for vascular artifacts in cerebral blood flow values measured by using arterial spin tagging techniques. Magn Reson Med. 1997;37:226–35.
Hendrikse J, Petersen ET, van Laar PJ, Golay X. Cerebral border zones between distal end branches of intracranial arteries: MR imaging. Radiology. 2008;246:572–80.
Jahng G-H, Song E, Zhu X-P, Matson GB, Weiner MW, Schuff N. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234:909–16.
Petersen ET, Mouridsen K, Golay X, et al. The QUASAR reproducibility study, part II: results from a multi-center arterial spin labeling test-retest study. NeuroImage. 2010;49:104–13.
Chen Y, Wang DJJ, Detre JA. Test-retest reliability of arterial spin labeling with common strategies. J Magn Reson Imaging. 2011;33:940–9.
Günther M, Bock M, Schad LR. Arterial spin labeling in combination with a look-locker sampling strategy: inflow turbo-sampling EPI-FAIR (ITS-FAIR). Magn Reson Med. 2001;46:974–84.
Petersen ET, Lim T, Golay X. Model-free arterial spin labeling quantification approach for perfusion MRI. Magn Reson Med. 2006;55:219–32.
Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97.
WC W, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–7.
Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2014;73(1):102–16. doi:10.1002/mrm.25197.
Sandson TA, O’Connor M, Sperling RA, et al. Noninvasive perfusion MRI in Alzheimer’s disease: a preliminary report. Neurology. 1996;47:1339–42.
Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100.
Johnson NA, Jahng G-H, Welner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–9.
Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–62.
Ishii K, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, Mori E. Demonstration of decreased posterior cingulate perfusion in mild Alzheimer’s disease by means of H2 15O positron emission tomography. Eur J Nucl Med. 1997;24:670–3.
Asllani I, Habeck C, Scarmeas N, Borogovac A, Brown TR, Stern Y. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. J Cereb Blood Flow Metab. 2008;28:725–36.
Yoshiura T, Hiwatashi A, Noguchi T, et al. Arterial spin labelling at 3-T MR imaging for detection of individuals with Alzheimer’s disease. Eur Radiol. 2009;19:2819–25.
Binnewijzend MA, Kuijer JP, Benedictus MR, et al. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology. 2013;267:221–30.
Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed. 2010;23:286–93.
Chen Y, Wolk DA, Reddin JS, et al. Voxel-based comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–85.
Musiek ES, Chen Y, Korczykowski M, et al. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer’s disease. Alzheimers Dement. 2012;8:51–9.
Alsop DC, Casement M, de Bazelaire C, Fong T, Press DZ. Hippocampal hyperperfusion in Alzheimer’s disease. NeuroImage. 2008;42:1267–74.
Mak HK, Qian W, Ng KS, et al. Combination of MRI hippocampal volumetry and arterial spin labeling MR perfusion at 3-Tesla improves the efficacy in discriminating Alzheimer’s disease from cognitively normal elderly adults. J Alzheimers Dis. 2014;41:749–58.
Bron EE, Steketee RM, Houston GC, et al. Alzheimer’s disease neuroimaging initiative. Diagnostic classification of arterial spin labeling and structural MRI in presenile early stage dementia. Hum Brain Mapp. 2014;35:4916–31.
Chao LL, Buckley ST, Kornak J, et al. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disorder. 2010;24:19–27.
Du AT, Jahng GH, Hayasaka S, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–20.
WT H, Wang Z, Lee VM, Trojanowski JQ, Detre JA, Grossman M. Distinct cerebral perfusion patterns in FLD and AD. Neurology. 2010;75:881–8.
Dashjamts T, Yoshiura T, Hiwatashi A, et al. Simultaneous arterial spin labeling cerebral blood flow and morphological assessments for detection of Alzheimer’s disease. Acad Radiol. 2011;18:1492–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan
About this chapter
Cite this chapter
Yoshiura, T. (2017). Arterial Spin Labeling in Dementia. In: Matsuda, H., Asada, T., Tokumaru, A. (eds) Neuroimaging Diagnosis for Alzheimer's Disease and Other Dementias. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55133-1_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-55133-1_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55132-4
Online ISBN: 978-4-431-55133-1
eBook Packages: MedicineMedicine (R0)