Abstract
Axonal degeneration often leads to the death of retinal ganglion cell (RGC) bodies. The pattern of localized retinal nerve fiber layer defects observed in glaucoma patients suggests that axonal degeneration may occur first, followed by sequential RGC body loss in this pathological condition. The molecular mechanism of axonal degeneration in the optic nerve is still unclear. The tumor necrosis factor injection model and the hypertensive glaucoma model may be useful in understanding the mechanism of axonal degeneration of RGCs because axon loss precedes RGC body loss in both models. There is a growing body of evidence that glaucoma may be correlated with Alzheimer’s disease. Autophagy impairment may be involved in neurodegenerative diseases including Alzheimer’s disease and glaucoma. Thus, the modulation of these signaling pathways will lead to a new concept of axonal protection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Amyloidogenic Pathway and Axonal Degeneration
Alzheimer’s disease (AD) is a neurodegenerative disorder in which axonal degeneration may precede cell body death [1]. The deposition of β-amyloid (Aβ) in neuronal cells is a hallmark of AD. The unfavorable metabolism of amyloid precursor protein (APP) leads to Aβ production. APP is proteolytically cleaved by β-secretase, generating a short C-terminal fragment (CTFβ) of 99 amino acids. The CTFβ fragment of APP is then cleaved by γ-secretase into an Aβ peptide and a cytosolic APP intracellular domain in the amyloidogenic pathway [2]. Although mutations in the APP or presenilin (PS) genes have been implicated in familial AD [3], the physiopathological events underlying chronic Aβ production/clearance imbalance may be different in sporadic AD and familial AD [4]. Nonetheless, an increase in PS1 and subsequent Aβ accumulation have been found in the hippocampus of senescence-accelerated mice (SAMP8), suggesting the involvement of PS1, one of the γ-secretase complexes, in sporadic AD [5]. It has also been suggested that overexpression of PS1 in vivo is sufficient to elevate γ-secretase activity and that upregulation of PS1/γ-secretase activity could contribute to increased risk for late-onset sporadic AD [6]. Thus, elucidating the mechanism of the amyloidogenic pathway remains a potential target for discovering a treatment for sporadic AD.
APP has been reported to accumulate in the optic nerve in rat and mouse glaucoma models [7, 8]. Aβ was also found to accumulate in retinal ganglion cells (RGCs) in a rat glaucoma model [9]. Although some studies found no positive association between glaucoma and AD [10, 11], other supported the correlation of these two neurodegenerative diseases [12, 13]. A very recent study has demonstrated that biomarkers of AD, such as apolipoprotein E and transthyretin, were increased in the aqueous humor in glaucoma patients compared with those in a control cataract patients group [14], suggesting that there is a linking pathophysiology in both diseases.
As another linking factor in the pathophysiology of AD and glaucoma, tumor necrosis factor (TNF), a cytokine that is synthesized and released from astrocytes and microglia, has been proposed. For example, TNF has been implicated in the pathogenesis and progression of AD [15, 16]. A meta-analysis demonstrated that AD is accompanied by an inflammatory response, particularly higher peripheral concentrations of TNF, interleukin (IL)-6, IL-1β, transforming growth factor-β, IL-12, and IL-18 [17]. Recent studies have demonstrated that the inhibition of TNF signaling reduces multiple hallmark of AD, including APP, Aβ peptide, and Aβ plaque [18], and prevents pre-plaque amyloid-associated pathology, cognitive deficits, and the loss of neurons in a mouse model of AD [19]. Similar to the findings of its crucial roles in AD, TNF has also been shown to have pivotal roles in the pathogenesis of glaucoma [20]. Glial production of TNF is increased in the glaucomatous optic nerve and TNF-mediated neurotoxicity is a component of neurodegeneration in glaucoma [20]. Increases in TNF have been demonstrated in the retina [21] and optic nerve [22] in hypertensive glaucoma models. A recent study of the aqueous humor has demonstrated that a significantly higher percentage of patients in the glaucoma group were positive for TNF compared with the cataract group [23]. A more recent study of the proteomic data from human glaucoma has shown a prominent upregulation of TNF/TNFR1 signaling in the glaucomatous retina [24]. A meta-analysis demonstrated that patients with open-angle glaucoma may have higher TNF levels in the aqueous humor compared with the control group, and the TNF-308G/A polymorphism is significantly associated with the risk of high-tension glaucoma [25]. Taken together with the finding that APP and Aβ accumulate in the optic nerve and RGCs in glaucoma models, it is possible that TNF signaling and the amyloidogenic pathway are involved in the pathophysiology of both AD and glaucoma.
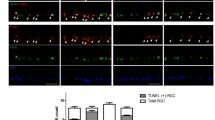
In longitudinal sections of normal rat optic nerve, myelinated axons can be recognized as myelin basic protein (MBP)-positive staining starting around the laminar portion (Fig. 9.1a, d). In myelinated areas, neurofilament is located inside (green dots) and MBP is located outside rings (red rings) in cross sections (Fig. 9.1b). In TNF-induced optic nerve degeneration, there is an increase in phosphorylated PS1 located in astroglial cells, thereby leading to a subsequent increase in γ-secretase activity in the optic nerve [26]. APP is also located in astroglial cells, because there is substantial colocalization of APP and glial fibrillary acidic protein in cross sections of the optic nerve (Fig. 9.1c, e). The cleavage of APP by the activation of γ-secretase occurs mostly in glial membranes in the optic nerve in this TNF-induced neurodegeneration model [26], which displays primary axonal degeneration with sequential RGC body death [27]. Taken together, the activation of the amyloidogenic pathway in glial cells may be involved in nearby axonal degeneration.
(a) Myelin basic protein (MBP) immunostaining in normal rat optic nerve. (b) Immunohistochemistry of neurofilament (NF) and MBP in cross sections of the optic nerve. (c) Immunohistochemistry of amyloid precursor protein (APP) and glial fibrillary acidic protein (GFAP) in cross sections of the optic nerve. (d) Schema of RGC axons in unmyelinated and myelinated areas. (e) High-magnification schema of a myelinated axon
Although several clinical trials of γ-secretase inhibitors (GSIs) for AD treatment have not achieved success so far, some clinical trials are still ongoing [28]. GSIs decreased Aβ production in the human central nervous system [29] and decreased the appearance of new amyloid plaque and the growth of preexisting plaque in APP/PS1 mice [30]. It has been suggested that synapses are the initial target in AD and that learning and memory deficits occur before the formation of plaque [31]. Instead of plaque, soluble Aβ impaired memory function [32], and the GSI LY-411575 reduced soluble Aβ and rescued the neuronal dysfunction in the hippocampus of a mouse model of AD [33]. Therefore, it is likely that GSIs have beneficial effects on neurons including synapses through the inhibition of both amyloid plaque and soluble Aβ. Moreover, the novel GSI ELN594 attenuated the formation and growth of new plaque and led to a normalization of the enhanced dynamics of synaptic structures close to plaque [34]. Furthermore, the GSI N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) induced neural differentiation of human Müller stem cells into RGC precursors and increased the number and length of neurites in cultured cells [35]. Since a recent study has suggested that RGC dendritic atrophy precedes cell loss in a mouse model of AD [36], preventing dendrite atrophy as well as axonal degeneration of RGCs before cell death is important in both AD and glaucoma. In axons, it was shown that DAPT injection immediately after axotomy increased axon regeneration in mature Caenorhabditis elegans neurons [37]. In optic nerve axons, treatment with the GSI BMS299897 significantly prevented axonal loss in TNF-induced optic nerve degeneration [26]. These findings suggest that GSIs can protect synapses and axons that are the early manifestation sites in AD and glaucoma. Further studies will be needed to clarify notch conditions after administration of GSIs to examine whether local applications for optic nerve diseases may be feasible.
2 Autophagy and Axonal Degeneration
Autophagy is a self-digestion system that is a cellular pathway involved in protein and organelle degradation. Alteration of autophagy is associated with several conditions, including cancer, infectious and immunity disease, liver disease, heart disease, myopathy, and neurodegenerative disease [38]. In the central nervous system (CNS), a previous study suggested that the induction of autophagy serves as an early stress response in axonal dystrophy and may participate in the remodeling of axon structures in Purkinje cells [39]. It was also suggested that autophagy is required for normal axon terminal membrane trafficking and turnover, and an essential role of local autophagy in the maintenance of axonal homeostasis and prevention of axonal degeneration in Purkinje cells was demonstrated [40]. In contrast, in superior cervical ganglion neurons, the autophagy inhibitor 3-methyladenine (3-MA) efficiently suppressed neurite degeneration by protecting neurites from the loss of viability and mitochondrial function [41], suggesting that autophagy may play several distinct roles in axons depending on different types of neuron or different types of injury. Autophagosomes inside axons can move toward cell bodies, and this movement is dependent on a dynein motor [42]. Therefore, it is possible that axonal transport may affect autophagosome clearance.
In the eyes, the existence of microtubule-associated protein light chain 3 (LC3)-II, an autophagic marker, in RGCs and its transient upregulation after optic nerve transection have been demonstrated [43]. That study showed that the inhibition of autophagy with bafilomycin A1, 3-MA, and wortmannin in RGC-5 cells under serum-deprived conditions decreased cell viability by approximately 40 %, suggesting the activation of autophagy in RGCs after optic nerve transection and its protective role in RGC-5 cells maintained under conditions of serum deprivation [43]. Those findings are consistent with the results of a recent study demonstrating that decreased Brn-3a-immunopositive RGCs in flat-mounted retinas after optic nerve transection were significantly increased by rapamycin, an autophagy inducer [44]. In addition, rapamycin decreased intracellular reactive oxygen species (ROS) production and increased cell viability in RGC-5 cells with the ROS-inducing agent paraquat [44]. That study also showed that the autophagy inhibitor 3-MA increased ROS production and reduced cell viability in RGC-5 cells, implying that autophagy induction protects RGC-5 cells from mitochondrial damage and cell death, whereas autophagy inhibition promotes ROS production and cell death [44]. In contrast, another study demonstrated that decreased 4′,6-diamidino-2-phenylindole (DAPI)-positive cells after intraocular pressure (IOP) elevation in the RGC layer were significantly increased by 3-MA [45]. Thus, the role of autophagy in RGC death is still controversial, although the fact that about 50 % of DAPI-positive cells in the RGC layer are displaced amacrine cells in rats and mice may affect the RGC survival estimation. Nonetheless, all of the above studies and a very recent study in a nonhuman primate chronic hypertensive glaucoma model [46] support the concept that autophagy is activated in RGCs in response to damage such as glaucoma and other optic nerve injuries.
In optic nerve axons, a previous study showed an increase in autophagosomes inside axons until 6 h after optic nerve crush [47]. That electron microscopy finding is consistent with the electron microscopy findings of a recent study demonstrating that abnormal mitochondria and autophagic vacuoles were noted inside axons 3 weeks after glaucoma induction [48]. Thus, autophagosomes and mitochondria move inside axons (Fig. 9.1d, e). Similar to the findings in RGC body death, the role of autophagy in optic nerve axonal degeneration is still controversial. For example, it was shown that the application of 3-MA, an autophagy inhibitor, resulted in a significant delay in axonal degeneration during the acute phase after optic nerve crush [47], while the application of 3-MA exaggerated axonal degeneration induced by IOP elevation [48].
It is interesting to note that the upregulation of autophagy may aid in oligodendrocyte survival in the Long-Evans shaker (les) rat, which has a mutation in MBP that results in severe CNS dysmyelination and subsequent demyelination during development [49]. Because oligodendrocyte loss was observed in the optic nerve after IOP elevation [21], how autophagy in oligodendrocytes can alter axon survival may be particularly interesting. It was shown that the neuroprotective effect of brain-derived neurotrophic factor (BDNF) was mediated by autophagy through the PI3K/Akt/mTOR pathway, although in cortical neurons rather than oligodendrocytes [50]. Moreover, exogenous BDNF can protect optic nerve axons by recruiting endogenous BDNF located in oligodendrocytes [51]. Oligodendrocytes seem to be sources of BDNF for nearby axons. One hypothesis posits that autophagy induction in oligodendrocytes with the possible involvement of BDNF has beneficial effects on nearby optic nerve axons.
p62, which is also called sequestosome 1, is a multifunctional protein that acts as a critical ubiquitin chain-targeting factor shuttling substrates for proteasomal degradation [52] and interacts with LC3-II, an autophagic marker. p62 is normally degraded by the lysosomal proteases through the interaction with LC3-II [53]. A recent study has shown the accumulation of p62 and LC3-II in the chronically compressed spinal cord, and the forced expression of p62 and the inhibition of autophagy decreased the number of neuronal cells [54]. It has been demonstrated that the inhibition of autophagy is correlated with increased levels of p62 in neuronal cells [39] and that autophagy deficiency leads to the abnormal accumulation of p62 and neurodegenerative changes in the cerebellum [55]. Thus, increased p62 protein levels including autophagic flux impairment may lead to neurodegeneration. An increased p62 protein level was observed in the optic nerve in a hypertensive glaucoma model [48]. Under pathological conditions with impaired autophagy, there is a constitutively high level of p62, thereby leading to the accumulation of damaged mitochondria and subsequent ROS production [56].
Although LC3-II is known as an autophagic marker, it increases not only under autophagy activation but also under autophagy flux impairment [57]. Therefore, increases in both p62 and LC3-II observed in the optic nerve after IOP elevation imply that autophagy flux impairment may be involved in axonal degeneration in glaucoma models [48]. In addition, rapamycin, an autophagy inducer, increased LC3-II further and decreased p62 levels in the optic nerve and exerted axonal protection in a glaucoma model [48]. These findings are in agreement with those of a previous study demonstrating that LiCl, an autophagy inducer, increased the expression of LC3-II under hypoxic stress and decreased the expression of p62 under normoxia and hypoxic stress in a neuronal cell culture [54]. Furthermore, these findings are also supported by a recent study demonstrating that the activation of autophagy increased protein levels of LC3-II and Beclin1 and decreased p62 in neuroblastoma SH-SY5Y cells [58]. Thus, the modulation of autophagy may be a potential strategy against degenerative optic nerve disease.
References
Raff MC, Whitmore AV, Finn JT (2002) Axon self-destruction and neurodegeneration. Science 296:868–871
Jakob-Roetne R, Jacobsen H (2009) Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed Engl 48:3030–3059
Bekris LM, Yu CE, Bird TD et al (2010) Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol 23:213–227
Pera M, Alcolea D, Sánches-Valle R et al (2013) Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease. Acta Neuropathol 125:201–213
Kumar VB, Franko M, Banks WA et al (2009) Increase in presenilin 1 (PS1) levels in senescence-accelerated mice (SAMP8) may indirectly impair memory by affecting amyloid precursor protein (APP) processing. J Exp Biol 212:494–498
Li T, Li YM, Ahn K et al (2011) Increased expression of PS1 is sufficient to elevate the level and activity of γ-secretase in vivo. PLoS One 6:e28179
Childlow G, Ebneter A, Wood JP et al (2011) The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol 121:737–751
Goldblum D, Kipfer-Kauer A, Sarra GM et al (2007) Distribution of amyloid precursor protein and amyloid- beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Invest Ophthalmol Vis Sci 48:5085–5090
Guo L, Salt TE, Luong V et al (2007) Targeting amyloid-β in glaucoma treatment. Proc Natl Acad Sci U S A 104:13444–13449
Bach-Holm D, Kessing SV, Mogensen U et al (2012) Normal tension glaucoma and Alzheimer disease: comorbidity? Acta Ophthalmol 90:683–685
Ou Y, Grossman DS, Lee PP et al (2012) Glaucoma, Alzheimer disease and other dementia: a longitudinal analysis. Ophthalmic Epidemiol 19:285–292
Wostyn P, Audenaert K, De Deyn PP (2009) Alzheimer’s disease and glaucoma: is there a causal relationship? Br J Ophthalmol 93:1557–1559
Liu YH, Tian T (2011) Hypothesis of optineurin as a new common risk factor in normal-tension glaucoma and Alzheimer’s disease. Med Hypotheses 77:591–592
Inoue T, Kawaji T, Tanihara H (2013) Elevated levels of multiple biomarkers of Alzheimer’s disease in the aqueous humor of eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci 54:5353–5358
Paganelli R, Di Iorio A, Patricelli L et al (2002) Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp Gerontol 37:257–263
Alvarez A, Cacabelos R, Sanpedro C et al (2007) Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging 28:533–536
Swardfager W, Lanctôt K, Rothenburg L et al (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68:930–941
Tweedie D, Ferguson RA, Fishman K et al (2012) Tumor necrosis factor-α synthesis inhibitor 3,6′-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer’s disease. J Neuroinflammation 9:106
McAlpine FE, Lee JK, Harms AS et al (2009) Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis 34:163–177
Tezel G (2008) TNF-α signaling in glaucomatous neurodegeneration. Prog Brain Res 173:409–421
Nakazawa T, Nakazawa C, Matsubara A et al (2006) Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci 26:12633–12641
Munemasa Y, Kitaoka Y, Kuribayashi J et al (2010) Modulation of mitochondria in the axon and soma of retinal ganglion cells in a rat glaucoma model. J Neurochem 115:1508–1519
Sawada H, Fukuchi T, Tanaka T et al (2010) Tumor necrosis factor-α concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci 51:903–906
Yang X, Luo C, Cai J et al (2011) Neurodegenerative and inflammatory pathway components linked to TNF-α/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci 52:8422–8454
Xin X, Gao L, Wu T et al (2013) Roles of tumor necrosis factor alpha gene polymorphisms, tumor necrosis factor alpha level in aqueous humor, and the risks of open angle glaucoma: a meta-analysis. Mol Vis 19:526–535
Kojima K, Kitaoka Y, Munemasa Y et al (2012) Axonal protection via modulation of the amyloidogenic pathway in tumor necrosis factor-induced optic neuropathy. Invest Ophthalmol Vis Sci 53:7675–7683
Kitaoka Y, Kitaoka Y, Kwong JM et al (2006) TNF-alpha-induced optic nerve degeneration and nuclear factor-kappaB p65. Invest Ophthalmol Vis Sci 47:1448–1457
Mikulca JA, Nguyen V, Gajdosik DA et al (2014) Potential novel targets for Alzheimer pharmacotherapy: II. Update on secretase inhibitors and related approaches. J Clin Pharm Ther 39:25–37
Bateman RJ, Siemers ER, Mawuenyega KG et al (2009) A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol 66:48–54
Yan P, Bero AW, Cirrito JR et al (2009) Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci 29:10706–10714
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791
Lesné S, Koh MT, Kotilinek L et al (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Busche MA, Chen X, Henning HA et al (2012) Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 109:8740–8745
Liebscher S, Page RM, Käfer K et al (2013) Chronic γ-secretase inhibition reduces amyloid plaque-associated instability of pre- and postsynaptic structures. Mol Psychiatry 1–10 doi:10.1038/mp.2013.122
Singhal S, Bhatia B, Jayaram H et al (2012) Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transpl Med 1:188–199
Williams PA, Thirgood RA, Oliphant H et al (2013) Retinal ganglion cell dendritic degeneration in a mouse model of Alzheimer’s disease. Neurobiol Aging 34:1799–1806
El Bejjani R, Hammarlund M (2012) Notch signaling inhibits axon regeneration. Neuron 73:268–278
Mizushima N, Levine B, Cuervo AM et al (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Wang QJ, Ding Y, Kohtz DS et al (2006) Induction of autophagy in axonal dystrophy and degeneration. J Neurosci 26:8057–8068
Komatsu M, Wang QJ, Holstein GR et al (2007) Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A 104:14489–14494
Yang Y, Fukui K, Koike T et al (2007) Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci 26:2979–2988
Katsumata K, Nishiyama J, Inoue T et al (2010) Dynein- and activity-dependent retrograde transport of autophagosomes in neuronal axons. Autophagy 6:378–385
Kim SH, Munemasa Y, Kwong JM et al (2008) Activation of autophagy in retinal ganglion cells. J Neurosci Res 86:2943–2951
Rodríguez-Muela N, Germain F, Mariño G et al (2012) Autophagy promotes survival of retinal ganglion cells after optic nerve axotomy in mice. Cell Death Differ 19:162–169
Park HYL, Kim JH, Park CK (2012) Activation of autophagy induces retinal ganglion cell death in a chronic hypertensive glaucoma model. Cell Death Dis 3:e290
Deng S, Wang M, Yan Z et al (2013) Autophagy in retinal ganglion cells in a rhesus monkey chronic hypertensive glaucoma model. PLoS One 8:e77100
Knöferle J, Koch JC, Ostendorf T et al (2010) Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A 107:6064–6069
Kitaoka Y, Munemasa Y, Kojima K et al (2013) Axonal protection by Nmnat3 overexpression with involvement of autophagy in optic nerve degeneration. Cell Death Dis 4:e860
Smith CM, Mayer JA, Duncan ID (2013) Autophagy promotes oligodendrocyte survival and function following dysmyelination in a long-lived myelin mutant. J Neurosci 33:8088–8100
Chen A, Xiong LJ, Tong Y et al (2013) Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep 8:1011–1016
Fujino H, Kitaoka Y, Hayashi Y et al (2009) Axonal protection by brain-derived neurotrophic factor associated with CREB phosphorylation in tumor necrosis factor-alpha-induced optic nerve degeneration. Acta Neuropathol 117:75–84
Seibenhener ML, Babu JR, Geetha T et al (2004) Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol Cell Biol 24:8055–8068
Ichimura Y, Komatsu M (2010) Selective degradation of p62 by autophagy. Semin Immunopathol 32:431–436
Tanabe F, Yone K, Kawabata N et al (2011) Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 7:1462–1471
Liang CC, Wang C, Peng X et al (2010) Neural-specific deletion of FIP200 leads to cerebellar degeneration caused by increased neuronal death and axon degeneration. J Biol Chem 285:3499–3509
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Xu HD, Wu D, Gu JH et al (2013) The pro-survival role of autophagy depends on Bcl-2 under nutrition stress conditions. PLoS One 8:e63232
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Kitaoka, Y. (2014). Axonal Degeneration. In: Nakazawa, T., Kitaoka, Y., Harada, T. (eds) Neuroprotection and Neuroregeneration for Retinal Diseases. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54965-9_9
Download citation
DOI: https://doi.org/10.1007/978-4-431-54965-9_9
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54964-2
Online ISBN: 978-4-431-54965-9
eBook Packages: MedicineMedicine (R0)