Abstract
Progressive dysfunction and death of photoreceptors is the major cause of loss of vision in most retinal diseases. Many studies investigating photoreceptor protection have used animal models, and some of the results have been implemented clinically. These include responsible gene applications, applying neurotrophic factors or antioxidants and blocking or preventing specific death signal transduction. Retinal prosthesis or appropriate cell transplantations have also been reported at the end stage of photoreceptor death. Conventional strategies for neuroprotection using neurotrophic factors or antioxidants have attempted to strengthen the cellular metabolism against variable stresses. However, not every application is well tolerated, although some clinical applications are ongoing. Recent understanding of photoreceptor cell death has led to targeting cell death initiation or blocking its execution. These include correcting the amount of intracellular cGMP, modification of epigenetic processes, and prevention of some proteolytic enzyme activity, such as calpains. Further, another approach from the aspect of the drug delivery system has also been developed. An improved design of photoreceptor protection will require a better understanding of the photoreceptor neurodegenerative mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Retinal disease is the main cause for blindness. Retinal disease includes age-related macular degeneration (AMD), diabetic retinopathy, and retinitis pigmentosa (RP). In these diseases, photoreceptor degeneration is a hallmark of severity. Photoreceptor cell death has been studied extensively in retinal detachment [1] and inherited retinal disease by using suitable animal models. Retinitis pigmentosa (RP) is the leading cause of incurable inherited blindness and is caused by mutations in many different genes that cause the death of rod photoreceptors. More than 60 causative genes have been identified (http://www.sph.uth.tmc.edu/retnet/sum-dis.htm). During the course of rod photoreceptor cell death, a marked increase of reactive oxygen species (ROS) due to reduced oxygen utilization [2] or a decrease of neurotrophic factors from the rods [3] was reported to be the main cause of gradual cone cell death. In the retinal detachment animal model, photoreceptor cell death was reported to start at approximately 12 h after detachment, and this may cause a loss and/or delay in the recovery of visual acuity if the detachment covered the area of cone-rich macular region [4]. Because clinically significant vision loss is associated with this deterioration of cone function, the prevention of cone loss during retinal degeneration is one of the targets for retinal degeneration therapy [5].
2 Structure of Photoreceptors
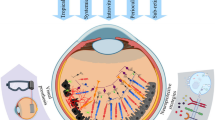
Human photoreceptors include rods and cones. Rods comprise 95 % of the photoreceptors in the humans and play role in scotopic vision, while cones are responsible for photopic vision and increase in cell density towards the center of the macula [6]. The photoreceptors have unique shapes, consisting of an outer segment (OS), an inner segment (IS), a cell body, and a synaptic terminal where neurotransmission occurs. Visual pigments called opsins are involved in the OS and participate as the trigger of phototransduction, namely conversion of light into electrochemical messages. Disorganization of this signal transduction results in a major impact on quality of life, and the prevention of this cell death has important implications. Recent advances in diagnostic methods, such as optical coherence tomography (OCT), improve the evaluation of these structures (Fig. 14.1). OCT has become the gold standard for assessing anatomical abnormalities in retinal diseases. In particular, a recent advanced model of adaptive optics-OCT images the cell density of the macula. RP patients, even with good visual acuity, may show a disrupted arrangement of cones when using this advanced method, while conventional OCT cannot distinguish the difference in macular structure [7]. Advances in clinical equipment may provide new insights into photoreceptor death.
Optical coherence tomography (OCT) has become the gold standard for assessing anatomical abnormalities in retinal diseases, especially for the macular area. RP patients with good visual acuity (18-year-old woman in the right column) show a relatively reserved macular structure, although the outer segment (OS)/inner segment (IS) line was disrupted and was not observed outside of the macula
3 Mechanism of Photoreceptor Cell Death
In the course of cell death in higher organisms, including humans, a variety of morphologies have been identified so far. In 1960, Lockshin and Williams [8] reported a series of cell death and proposed the term programmed cell death. From the difference in cell morphology at the time of cell death, this programmed cell death is classified as type 1 (apoptosis), type 2 (cell death with autophagy), or type 3 (necrosis).
3.1 Apoptosis, Autophagy, and Necrosis
Kerr and coworkers reported a specific form of cell death as apoptosis [9]. The cell death was controlled genetically, and proteins of the B-cell lymphoma 2 (BCL2) families are well-described key regulators involved in this mechanism. The caspase family of cysteine protease is regulated by these proteins [10], and they are divided into proapoptotic and antiapoptotic proteins, which, together, make the “life-or-death decision” for the cell [11]. However, recent studies showed that caspase activity is not the sole mediator of photoreceptor cell death, although it may be activated during photoreceptor degeneration [1].
Cell death with autophagy is Type 2 programmed cell death. Cells, when exposed to stress such as amino acid starvation or the accumulation of abnormal protein, show phospholipid gathered along with the abnormal protein and autophagy begins. The intracellular lysosomes and autophagosomes cause membrane fusion, followed by breakdown of the abnormal proteins. Thus, the autophagy phenomenon is believed to allow the temporary avoidance of cell damage against some stresses, such as starvation. However, the avoidance of cell damage by autophagy cannot be maintained during longer periods of starvation. Punzo and coworkers reported results of starvation experiments and showed that the nonautonomous cone death in retinitis pigmentosa may be a result of the starvation of cones [12]. Apoptotic and autophagic pathways have been reported to share common pathways [13] and may coexist in cells [14].
Traditionally, necrosis has been considered an uncontrolled process of cell death. However, this status has regulated components in certain circumstances. Recent studies revealed that death receptor-induced necrosis is mediated by the activation of receptor-interacting protein 1 (RIP1) [15], which is regulated by RIP3-dependent phosphorylation of RIP kinase [16]. In photoreceptor necrosis, tumor necrosis factor (TNF)-induced cellular necrosis was reported in interphotoreceptor retinoid-binding protein (IRBP) (−/−) mice [17]. The study also showed that, in addition to apoptosis, RIP kinase-mediated necrosis contributes strongly to cone and rod degeneration in Irbp −/−retina. Nakazawa and coworkers also reported that TNF-α plays a critical role in RD-induced photoreceptor degeneration [18].
3.2 Detection of Cell Death
These death features were reported originally from morphological analysis, and transmission electron microscopy (TEM) was best for the morphological detection of the cell death [19]. Biochemical analyses to detect cell death have also been developed [20]. However, each cell death type, either apoptosis, autophagy, or necrosis, has common and/or similar features, and some biological reactions show redundancy during the photoreceptor death process. Therefore, many authors have used these methods in combination and examined the results carefully.
4 Strategy for Photoreceptor Protection
There are several reasons for delay in the development of the photoreceptor cell death prevention. These are in part due to an incomplete understanding of the regulation of photoreceptor cell death as described above. Cell death is not only apoptosis, but necrosis and autophagy have been reported to be important factors, and these events may occur together. Another major reason for the delay in treatment options is the structure of the retina and its related tissues, including the blood-retinal barrier (BRB) (Fig. 14.2). The development of novel therapeutic strategies to overcome these challenges will require the use of suitable animal models [21].
Retina is protected by many tissue barriers (a), which is why development of retinal therapy has been difficult. Recently, we reported a novel trans-scleral drug delivery device placed on the sclera that consisted of a drug-releasing semipermeable membrane and impermeable membranes acting as the drug reservoir (b). The device facilitates a sustained one-way and multiple drug release (c)
4.1 Gene Therapy
Many methods have been reported to rescue photoreceptor cells from death, such as neurotrophic factor application, cell transplantation, and genetic replacement [22]. Since mutation in many different genes can generate inherited retinal degeneration and almost all patients lose rods, genetic replacement has been discussed as an option to only some patients [22]. Gene replacement therapy for Leber congenital amaurosis type 2 (LCA2) has been applied in five patients with LCA2 using adeno-associated virus type 2 (AAV2). Three-year follow-up results were reported recently, and the data showed a statistically significant improvement of best-corrected visual acuity between baseline and 3 years (maximum visual acuity was achieved around 6 months and persisted) [23]. Although the natural history of the disease may not be altered by the therapy [24], some gene applications may show promising results. Recently, an interesting application has reported by converting adult rods into cones via knockdown of the rod photoreceptor determinant Nrl [25]. The lineage made the cells resistant to the mutations in rod-specific genes and prevented photoreceptor loss in mice. New methods associated with genetic application may provide breakthroughs to the difficulties identified so far.
4.2 Neuroprotection by Neurotrophic Factors
Neuroprotection is a mutation-independent approach to protect photoreceptor cells. Neurotrophic factors have been used to enhance cell survival in retinas undergoing cell death from a wide variety of insults. Neurotrophic factors that promote cell growth, differentiation, survival, and function of specific nerve cell populations were identified during neuroscience investigations. The discovery of nerve growth factor (NGF) by Rita Levi-Montalcini in the 1950s was an important event in modern neuronal cell biology [26]. Neurotrophic factors are families of proteins that promote neuron survival, growth, and development. These neurotrophic factors include neurotrophins (NGF, brain-derived neurotrophic factor (BDNF), NT-3, NT-4/5), the serine protease inhibitor (srpin) family (pigment epithelium-derived factor (PEDF)), and neuropoietic cytokines (ciliary neurotrophic factor (CNTF)) [27]. Continuous BDNF expression in double transgenic mice showed significant photoreceptor protection [28]. CNTF caused a delay of retinal degeneration in several retinal degeneration animal models [29]. Further, amyotrophic lateral sclerosis (ALS) trials using subcutaneous CNTF injection that showed no differences between the placebo and treatment groups and the delivery methods of CNTF were reconsidered and applied for patients with RP using CNTF-expressing intraocular implants for cone protection [30]. Although a phase III clinical trial has been reported for RP patients using this intraocular implant, evaluation of the clinical results has not been completed [31]. Both BDNF and CNTF execute its photoreceptor trophic effects via Müller glial cells, suggesting the presence of secondary effects from the glial cells [32]. Glial cell line-derived neurotrophic factor (GDNF) [3] was also considered and examined as a candidate for photoreceptor neuroprotection.
4.3 Neuroprotection by Antioxidants
The retina has the highest metabolic rate by weight in the body [33], and it is always exposed to ROS. Thus, the redox-regulating system is important for retinal survival [34]. Antioxidants have been used to increase the resistance of neurons to oxidative stress, and they are considered as another avenue for neuroprotection in animal models [35]. Increased stress is found not only in photoreceptor cell death but also in other diseases, such as glaucoma [36]. Animal experiments and clinical trials have been reported. These included vitamin A alone or in combination with vitamin E, docosahexaenoic acid (DHA), or lutein and were shown to be partially effective [37]. Berson and coworkers reported a slower decline of visual acuities in patients with RP who were taking vitamin A and consumed a diet rich in omega-3 fatty acids [38]. Nonetheless, only minor and highly variable protective effects have been observed in these patients [39]. This phenomenon may be due to the diversity of experimental designs in regard to treatment time, dosage, and the variable background of the patients. Recently, very interesting results was published by Jin and coworkers [40]. They constructed patient-specific rod photoreceptor cells using induced pluripotent stem (iPS) cells derived from each patient’s fibroblasts. Cells with specific mutations exhibited different responses to vitamin E. Their study may clarify the pathogenic mechanism induced by different gene mutations and suggest strategies of future treatment for patients with different genetic backgrounds.
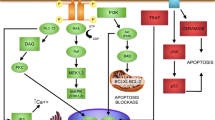
4.4 Blocking Cell Death by the Regulation of cGMP
Conventional strategies for neuroprotection using neurotrophic factors or antioxidants have sought to strengthen the cellular metabolism against variable stresses as determined by analysis of cell survival. However, neuroprotection using neurotrophic factors or antioxidants as described above is not sufficient to prevent photoreceptor cell death [41]. Therefore, recent approaches have attempted to prevent cell death by targeting the initiation of cell death or blocking its execution. These have been considered differentially from the stages of photoreceptor cell death: early stage for the accumulation of cGMP, intermediate stage for epigenetic processes, and end stage for excessive activation of proteolytic enzymes such as calpains [39].
cGMP, which is produced by retinal guanylate cyclase and hydrolyzed by phosphodiesterase-6 (PDE6), plays an important role in signal phototransduction [42]. High cGMP levels have been reported in a murine animal model of rd1, which showed an abnormal activity of phosphodiesterase (PDE) due to gene mutation. Evidence suggests that cGMP accumulation is not specific to rd1 and is observed in many other inherited retinal degeneration animal models during the course of retinal degeneration [43]. The correlation between very low levels of cGMP and chick photoreceptor cell death suggests that an inadequate level of cGMP may cause photoreceptor degeneration [44]. cGMP targets cyclic nucleotide-gated (CNG) ion channels, which mediate the influx of Ca++, and cGMP-dependent protein kinase (PKG). PKG activates the transcription factor cyclic AMP response element-binding protein (CREB), which has antiapoptotic pathway activity [45]. Targeting of these signals for photoreceptor protection may require more extensive studies.
4.5 Blocking Cell Death by Epigenetic Modification
Epigenetic factors, such as methylation, acetylation, deacetylation, and poly-ADP-ribosylation, influence histone modification and translation and have been recognized as important events for cell death and survival [46]. Typical enzymes that control these activities are DNA methyltransferases, histone acetyltransferases (HATs), histone deacetylases (HDACs), and poly-ADP-ribose polymerase (PARP). Inhibition of HDAC activity increases the cell survival rate and is suspected to be an upstream molecule that reduces PARP activation [47]. Thus, a PARP inhibitor also showed reduced photoreceptor cell death in some animal model [48]. Interestingly, related studies also revealed epigenetic modification of DNA repair, termed abortive mitosis [49]. The degenerating photoreceptors incorporate bromodeoxyuridine, which is usually observed in DNA replication. The antioxidant molecules described above may reduce the DNA oxidation and show reduced epigenetic effects.
4.6 Blocking Cell Death by Blocking Proteolytic Activity
At the end stage of photoreceptor cell death, proteolytic degradation of the cellular components has been investigated. Well-established components of proteolytic degradation are calpains and calpastains. Calpains are a family of Ca++-activated cysteine proteases and execute their activity by dimers of an 80 kDa catalytic subunit and a 30 kDa regulatory subunit. Elevated levels of cellular Ca++ activate calpain activity and lead to cell death of not only photoreceptor cells [50] but also ganglion cells [51]. Calpastain activity correlates with the reduced neuronal cell death [52]. However, contradictory results have been reported [53], and the elucidation of the precise mechanism for cell survival and cell death related with these molecules is required for clinical application.
Increased of calcium ion concentrations inside neural cells results in the activation of Ca++-dependent proteolysis, hydrolysis, lipid peroxidation, and ROS production [54]. These results lead to the neuronal cell death and prevention of these events has been considered. The activation of K+ channels in nerve cells effectively regulates cell membrane potentials. Large-conductance voltage- and Ca++-dependent K+ (max-K or BK) channels react to increased intracellular Ca++ and membrane depolarization and show rapid hyperpolarization of the membrane and reduce voltage-dependent Ca++ influx [55]. Activation of this channel has been reported to rescue many neuronal cells [56, 57]. Isopropyl unoprostone, a prostaglandin metabolite analog that has been used clinically as an anti-glaucomatous agent, has been shown to protect photoreceptors against oxidative stress- and light-induced retinal damage in rats [58] through BK channel regulation [59]. A pilot study using topical unoprostone twice a day in patients with retinitis pigmentosa demonstrated significant improvement in central 2° retinal sensitivity [60].
4.7 Advanced Stages of Retinal Prosthesis and Cell Transplantations
Retinal prosthesis and cell transplantations may be an option for the restoration of vision, because once photoreceptor cell loss occurs, there are no effective treatments to restore sight. However, electronic devices have been used for the replacement of dead photoreceptor cells. The devices were placed in the cortex, suprachoroidal space, and epiretinal and subretinal spaces. These devices convert images into electric signals using the remaining neuronal cells [61–63].
Cell transplantation offers a promising approach for treatment at the end stages of retinal degenerative diseases. The techniques of transplanting different types of cells have advanced rapidly in the past 30 years, and the results have suggested that transplantation may be a useful approach to treat some retinal diseases. Some of the transplanted cells produce neurotrophic factors to support photoreceptor cells [64]. Photoreceptor cell generation and transplantation from both mouse and human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have been reported. Thus, cell transplantation provides another promising strategy for photoreceptor functional rescue [65, 66].
5 Development of Delivery Tools
Recent progress in the treatment for some retinal diseases, such as AMD, allows administration of some drugs by intravitreal injection [67]. Intravitreal injections of neurotrophic factors have also been shown to rescue degenerating photoreceptor cells in animals [68]. However, several problems limit their clinical usefulness, such as delivering the neurotrophic factor to the appropriate site and the short half-life of the neurotrophic factors. Intravitreal sustained delivery of some neurotrophic factors has been shown to rescue retinal cells [27]. Although the surgical procedure was reported to be tolerable, the implants were set in the vitreous. Treatments inside the eyeball may induce adverse side effects, such as retinal detachment and infection [69]. In contrast, we reported our novel trans-scleral drug delivery device placed on the sclera that consisted of a drug-releasing semipermeable membrane and impermeable membranes acting as the drug reservoir. Because of the nonbiodegradable and one-way release nature of the device, we were able to achieve sustained release of the drug to the retina. This type of drug delivery system can also be designed to release multiple drugs [70] (Fig. 14.2).
6 Conclusion
Photoreceptor cell death is the major cause of loss of vision in most retinal diseases. So far, many studies have attempted to achieve photoreceptor cell protection by various molecules. However, not every application was well tolerated, although some types of applications (phase 2 or 3) are ongoing. Improved photoreceptor protective treatments require a better understanding of photoreceptor neurodegenerative mechanisms. In addition, improvement of molecule delivery systems (not only drugs, but also genes) may allow new insights into strategies for photoreceptor protection.
References
Murakami Y, Notomi S, Hisatomi T, Nakazawa T, Ishibashi T, Miller JW, Vavvas DG (2013) Photoreceptor cell death and rescue in retinal detachment and degenerations. Prog Retin Eye Res 37:114–140. doi:10.1016/j.preteyeres.2013.08.001
Komeima K, Rogers BS, Lu L, Campochiaro PA (2006) Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci USA 103(30):11300–11305. doi:10.1073/pnas.0604056103
Ohnaka M, Miki K, Gong YY, Stevens R, Iwase T, Hackett SF, Campochiaro PA (2012) Long-term expression of glial cell line-derived neurotrophic factor slows, but does not stop retinal degeneration in a model of retinitis pigmentosa. J Neurochem 122(5):1047–1053. doi:10.1111/j.1471-4159.2012.07842.x
Hisatomi T, Sakamoto T, Murata T, Yamanaka I, Oshima Y, Hata Y, Ishibashi T, Inomata H, Susin SA, Kroemer G (2001) Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol 158(4):1271–1278. doi:10.1016/S0002-9440(10)64078-3
Piano I, Novelli E, Gasco P, Ghidoni R, Strettoi E, Gargini C (2013) Cone survival and preservation of visual acuity in an animal model of retinal degeneration. Eur J Neurosci 37(11):1853–1862. doi:10.1111/ejn.12196
van Soest S, Westerveld A, de Jong PT, Bleeker-Wagemakers EM, Bergen AA (1999) Retinitis pigmentosa: defined from a molecular point of view. Surv Ophthalmol 43(4):321–334
Makiyama Y, Ooto S, Hangai M, Takayama K, Uji A, Oishi A, Ogino K, Nakagawa S, Yoshimura N (2013) Macular cone abnormalities in retinitis pigmentosa with preserved central vision using adaptive optics scanning laser ophthalmoscopy. PLoS One 8(11):e79447. doi:10.1371/journal.pone.0079447
Lockshin RA, Williams CM (1965) Programmed cell death–I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J Insect Physiol 11:123–133
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J (1993) Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75(4):653–660
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2(9):647–656. doi:10.1038/nrc883
Punzo C, Kornacker K, Cepko CL (2009) Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci 12(1):44–52. doi:10.1038/nn.2234
Kunchithapautham K, Rohrer B (2007) Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy 3(5):433–441
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752. doi:10.1038/nrm2239
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495. doi:10.1038/82732
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123. doi:10.1016/j.cell.2009.05.037
Sato K, Li S, Gordon WC, He J, Liou GI, Hill JM, Travis GH, Bazan NG, Jin M (2013) Receptor interacting protein kinase-mediated necrosis contributes to cone and rod photoreceptor degeneration in the retina lacking interphotoreceptor retinoid-binding protein. J Neurosci Official J Soc Neurosci 33(44):17458–17468. doi:10.1523/JNEUROSCI.1380-13.2013
Nakazawa T, Kayama M, Ryu M, Kunikata H, Watanabe R, Yasuda M, Kinugawa J, Vavvas D, Miller JW (2011) Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci 52(3):1384–1391. doi:10.1167/iovs.10-6509
Eskelinen EL (2008) To be or not to be? Examples of incorrect identification of autophagic compartments in conventional transmission electron microscopy of mammalian cells. Autophagy 4(2):257–260
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119(3):493–501
Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR (2002) Retinal degeneration mutants in the mouse. Vision Res 42(4):517–525
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368(9549):1795–1809. doi:10.1016/S0140-6736(06)69740-7
Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F (2013) Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 120(6):1283–1291. doi:10.1016/j.ophtha.2012.11.048
Cideciyan AV, Jacobson SG, Beltran WA, Hauswirth WW, Aguirre GD (2013) Reply to Townes-Anderson: RPE65 gene therapy does not alter the natural history of retinal degeneration. Proc Natl Acad Sci USA 110(19):E1706
Montana CL, Kolesnikov AV, Shen SQ, Myers CA, Kefalov VJ, Corbo JC (2013) Reprogramming of adult rod photoreceptors prevents retinal degeneration. Proc Natl Acad Sci USA 110(5):1732–1737. doi:10.1073/pnas.1214387110
Aloe L (2004) Rita Levi-Montalcini: the discovery of nerve growth factor and modern neurobiology. Trends Cell Biol 14(7):395–399. doi:10.1016/j.tcb.2004.05.011
Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, Stabila PF, Bell WJ, Dean BJ, Kauper KA, Budz VA, Tsiaras WG, Acland GM, Pearce-Kelling S, Laties AM, Aguirre GD (2002) Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci 43(10):3292–3298
Okoye G, Zimmer J, Sung J, Gehlbach P, Deering T, Nambu H, Hackett S, Melia M, Esumi N, Zack DJ, Campochiaro PA (2003) Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci Official J Soc Neurosci 23(10):4164–4172
Lavail MM (2005) Survival factors for treatment of retinal degenerative disorders: preclinical gains and issues for translation into clinical studies. Retina 25(8 Suppl):S25–S26
MacDonald IM, Sauve Y, Sieving PA (2007) Preventing blindness in retinal disease: ciliary neurotrophic factor intraocular implants. Can J Ophthalmol 42(3):399–402. doi:10.3129/can j ophthalmol.i07-039
Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W (2013) Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol 156(2):283–292. doi:10.1016/j.ajo.2013.03.021, e281
Rhee KD, Nusinowitz S, Chao K, Yu F, Bok D, Yang XJ (2013) CNTF-mediated protection of photoreceptors requires initial activation of the cytokine receptor gp130 in Muller glial cells. Proc Natl Acad Sci USA 110(47):E4520–E4529. doi:10.1073/pnas.1303604110
Yu DY, Cringle SJ (2005) Retinal degeneration and local oxygen metabolism. Exp Eye Res 80(6):745–751. doi:10.1016/j.exer.2005.01.018
Ahuja-Jensen P, Johnsen-Soriano S, Ahuja S, Bosch-Morell F, Sancho-Tello M, Romero FJ, Abrahamson M, van Veen T (2007) Low glutathione peroxidase in rd1 mouse retina increases oxidative stress and proteases. Neuroreport 18(8):797–801. doi:10.1097/WNR.0b013e3280c1e344
Bresnick GH (1970) Oxygen-induced visual cell degeneration in the rabbit. Invest Ophthalmol 9(5):372–387
Tezel G (2006) Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 25(5):490–513. doi:10.1016/j.preteyeres.2006.07.003
Berson EL (1993) Retinitis pigmentosa. The Friedenwald lecture. Invest Ophthalmol Vis Sci 34(5):1659–1676
Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Willett WC (2012) Omega-3 intake and visual acuity in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol 130(6):707–711. doi:10.1001/archophthalmol.2011.2580
Trifunovic D, Sahaboglu A, Kaur J, Mencl S, Zrenner E, Ueffing M, Arango-Gonzalez B, Paquet-Durand F (2012) Neuroprotective strategies for the treatment of inherited photoreceptor degeneration. Curr Mol Med 12(5):598–612
Jin ZB, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y, Iwata T, Takahashi M (2011) Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One 6(2):e17084. doi:10.1371/journal.pone.0017084
Rhee KD, Ruiz A, Duncan JL, Hauswirth WW, Lavail MM, Bok D, Yang XJ (2007) Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 48(3):1389–1400. doi:10.1167/iovs.06-0677
Arshavsky VY, Wensel TG (2013) Timing is everything: GTPase regulation in phototransduction. Invest Ophthalmol Vis Sci 54(12):7725–7733. doi:10.1167/iovs.13-13281
Mendes HF, van der Spuy J, Chapple JP, Cheetham ME (2005) Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol Med 11(4):177–185. doi:10.1016/j.molmed.2005.02.007
Semple-Rowland SL, Cheng KM (1999) rd and rc chickens carry the same GC1 null allele (GUCY1*). Exp Eye Res 69(5):579–581. doi:10.1006/exer.1999.0743
Fabian E, Reglodi D, Mester L, Szabo A, Szabadfi K, Tamas A, Toth G, Kovacs K (2012) Effects of PACAP on intracellular signaling pathways in human retinal pigment epithelial cells exposed to oxidative stress. J Mol Neurosci 48(3):493–500. doi:10.1007/s12031-012-9812-7
Delcuve GP, Rastegar M, Davie JR (2009) Epigenetic control. J Cell Physiol 219(2):243–250. doi:10.1002/jcp.21678
Sancho-Pelluz J, Alavi MV, Sahaboglu A, Kustermann S, Farinelli P, Azadi S, van Veen T, Romero FJ, Paquet-Durand F, Ekstrom P (2010) Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. Cell Death Disease 1:e24. doi:10.1038/cddis.2010.4
Uehara N, Miki K, Tsukamoto R, Matsuoka Y, Tsubura A (2006) Nicotinamide blocks N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in rats through poly (ADP-ribose) polymerase activity and Jun N-terminal kinase/activator protein-1 pathway inhibition. Exp Eye Res 82(3):488–495. doi:10.1016/j.exer.2005.08.006
Herrup K, Yang Y (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8(5):368–378. doi:10.1038/nrn2124
Paquet-Durand F, Johnson L, Ekstrom P (2007) Calpain activity in retinal degeneration. J Neurosci Res 85(4):693–702. doi:10.1002/jnr.21151
Oka T, Walkup RD, Tamada Y, Nakajima E, Tochigi A, Shearer TR, Azuma M (2006) Amelioration of retinal degeneration and proteolysis in acute ocular hypertensive rats by calpain inhibitor ((1S)-1-((((1S)-1-benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-me thylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester. Neuroscience 141(4):2139–2145. doi:10.1016/j.neuroscience.2006.05.060
Wingrave JM, Sribnick EA, Wilford GG, Matzelle DD, Mou JA, Ray SK, Hogan EL, Banik NL (2004) Higher calpastatin levels correlate with resistance to calpain-mediated proteolysis and neuronal apoptosis in juvenile rats after spinal cord injury. J Neurotrauma 21(9):1240–1254. doi:10.1089/neu.2004.21.1240
Paquet-Durand F, Sanges D, McCall J, Silva J, van Veen T, Marigo V, Ekstrom P (2010) Photoreceptor rescue and toxicity induced by different calpain inhibitors. J Neurochem 115(4):930–940. doi:10.1111/j.1471-4159.2010.06983.x
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1(8):623–634
Gribkoff VK, Starrett JE Jr, Dworetzky SI (1997) The pharmacology and molecular biology of large-conductance calcium-activated (BK) potassium channels. Adv Pharmacol 37:319–348
Gribkoff VK, Starrett JE Jr, Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, Frantz SW, Heman K, Hibbard JR, Huston K, Johnson G, Krishnan BS, Kinney GG, Lombardo LA, Meanwell NA, Molinoff PB, Myers RA, Moon SL, Ortiz A, Pajor L, Pieschl RL, Post-Munson DJ, Signor LJ, Srinivas N, Taber MT, Thalody G, Trojnacki JT, Wiener H, Yeleswaram K, Yeola SW (2001) Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat Med 7(4):471–477. doi:10.1038/86546
Dal-Cim T, Martins WC, Santos AR, Tasca CI (2011) Guanosine is neuroprotective against oxygen/glucose deprivation in hippocampal slices via large conductance Ca(2)+-activated K+ channels, phosphatidilinositol-3 kinase/protein kinase B pathway activation and glutamate uptake. Neuroscience 183:212–220. doi:10.1016/j.neuroscience.2011.03.022
Tsuruma K, Tanaka Y, Shimazawa M, Mashima Y, Hara H (2011) Unoprostone reduces oxidative stress- and light-induced retinal cell death, and phagocytotic dysfunction, by activating BK channels. Mol Vis 17:3556–3565
Cuppoletti J, Malinowska DH, Tewari KP, Chakrabarti J, Ueno R (2007) Cellular and molecular effects of unoprostone as a BK channel activator. Biochim Biophys Acta 1768(5):1083–1092. doi:10.1016/j.bbamem.2006.12.015
Tawada A, Sugawara T, Ogata K, Hagiwara A, Yamamoto S (2013) Improvement of central retinal sensitivity 6 months after topical isopropyl unoprostone in patients with retinitis pigmentosa. Indian J Ophthalmol 61(3):95–99. doi:10.4103/0301-4738.109377
Tokuda T, Asano R, Sugitani S, Terasawa Y, Nunoshita M, Nakauchi K, Fujikado T, Tano Y, Ohta J (2007) In vivo stimulation on rabbit retina using CMOS LSI-based multi-chip flexible stimulator for retinal prosthesis. Conf Proc IEEE Eng Med Biol 2007:5791–5794. doi:10.1109/IEMBS.2007.4353663
Feucht M, Laube T, Bornfeld N, Walter P, Velikay-Parel M, Hornig R, Richard G (2005) Development of an epiretinal prosthesis for stimulation of the human retina. Der Ophthalmologe (Zeitschrift der Deutschen Ophthalmol Gesellschaft) 102(7):688–691. doi:10.1007/s00347-005-1186-6
Sahni JN, Angi M, Irigoyen C, Semeraro F, Romano MR, Parmeggiani F (2011) Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Curr Genom 12(4):276–284. doi:10.2174/138920211795860062
Abe T, Yoshida M, Yoshioka Y, Wakusawa R, Tokita-Ishikawa Y, Seto H, Tamai M, Nishida K (2007) Iris pigment epithelial cell transplantation for degenerative retinal diseases. Prog Retin Eye Res 26(3):302–321. doi:10.1016/j.preteyeres.2007.01.003
Jin ZB, Takahashi M (2012) Generation of retinal cells from pluripotent stem cells. Prog Brain Res 201:171–181. doi:10.1016/B978-0-444-59544-7.00008-1
West EL, Gonzalez-Cordero A, Hippert C, Osakada F, Martinez-Barbera JP, Pearson RA, Sowden JC, Takahashi M, Ali RR (2012) Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells 30(7):1424–1435. doi:10.1002/stem.1123
Grisanti S, Tatar O (2008) The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res 27(4):372–390. doi:10.1016/j.preteyeres.2008.05.002
Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM (1990) Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347(6288):83–86. doi:10.1038/347083a0
Pilli S, Kotsolis A, Spaide RF, Slakter J, Freund KB, Sorenson J, Klancnik J, Cooney M (2008) Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy injections in an office setting. Am J Ophthalmol 145(5):879–882. doi:10.1016/j.ajo.2007.12.036
Nagai N, Kaji H, Onami H, Ishikawa Y, Nishizawa M, Osumi N, Nakazawa T, Abe T (2013) A polymeric device for controlled trans-scleral multi-drug delivery to the posterior segment of the eye. Acta Biomater 10:680–687. doi:10.1016/j.actbio.2013.11.004
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Abe, T., Nagai, N. (2014). Neuroprotection for Photoreceptors. In: Nakazawa, T., Kitaoka, Y., Harada, T. (eds) Neuroprotection and Neuroregeneration for Retinal Diseases. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54965-9_14
Download citation
DOI: https://doi.org/10.1007/978-4-431-54965-9_14
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54964-2
Online ISBN: 978-4-431-54965-9
eBook Packages: MedicineMedicine (R0)