Abstract
In this chapter, we describe culture methods to construct microvascular networks as well as approaches to integrating capillary networks with 3D epithelial tissue-engineered constructs. First, culture models of microvascular networks such as in vitro angiogenesis and vasculogenesis models are introduced. Using these culture models, the roles of endothelial cells (ECs), such as endothelial tip, stalk, and phalanx cells, are demonstrated. Additionally, regulatory factors, including both biochemical and biophysical factors, are discussed in the context of 3D capillary formation, including the process of vascular development, growth, and maturation. Next, we focus on the use of microfluidics technologies for investigating capillary morphogenesis. Examples of 3D capillary formation assays with growth factor gradients and different extracellular matrix materials are described. Cocultures of ECs and the other cell types in microfluidic devices are also introduced to show the potential of microfluidic vascular formation models. The vascularization of constructed tissues is discussed from the viewpoints of horizontal and vertical approaches for combining capillary structures and epithelial tissues in vitro. Finally, the concept of integrated vascular engineering and future perspectives are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 In Vitro Culture Models of Microvascular Networks
16.1.1 General Introduction for In Vitro Capillary Formation
Blood vessels are tubular structures through which blood passes to provide oxygen and nutrients to individual cells within tissues and organs. Because oxygen and nutrients are the most important fundamental factors for vital activity, blood vessels are essential tissues in the body. The luminal surface of blood vessels is covered by endothelial cells (ECs). Thus, we need to understand how ECs form blood vessels for the development of vascular tissue engineering. In particular, in the context of tissue engineering for three-dimensional (3D) tissues and organs, construction of microvascular networks, rather than large blood vessels, has a high priority.
There are two processes of microvessel formation in vivo, referred to as “angiogenesis ” and “vasculogenesis ” (Risau 1997). Angiogenesis is the formation of new capillaries branching from preexisting blood vessels. To investigate this angiogenic process, an in vitro 3D angiogenesis model has been developed. In this model, hydrogels, such as collagen gel and fibrin gel, are formed in a culture dish, and ECs are seeded on the surface of the 3D hydrogel scaffold. ECs grow on the gel surface and finally form a confluent monolayer. When the cells are cultured with no growth factors, they maintain a confluent monolayer, which is similar to the ECs covering the luminal surface of blood vessels in vivo (step 1, Fig. 16.1a). However, when ECs are cultured and supplemented with angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), some ECs penetrate into the underlying gel to form vascular sprouts, which is the beginning of vascular formation (step 2, Fig. 16.1a). These vascular sprouts gradually extend and branch into the gel and finally develop into 3D capillary-like structures (step 3, Fig. 16.1a).
The process of microvascular network formation in angiogenesis and vasculogenesis culture models. (a) In the angiogenesis model, ECs form a confluent monolayer covering the surface of the hydrogel. Some cells within the monolayer form vascular sprouts, which finally develop into three-dimensionally extending capillary-like networks. (b) In the vasculogenesis model, individual ECs are embedded in a hydrogel. These cells extend within the hydrogel and connect with each other. Some cells start to form vacuoles within their cytoplasm. These cells finally connect and develop into capillary-like structures with partial lumens
The other process of microvessel formation, vasculogenesis, is the process of vascular formation in the absence of preexisting blood vessels, which occurs in the developmental process and the process of adult vascular growth (Risau and Flamme 1995). First, cells from the mesoderm form aggregates that are called blood islands. The peripheral cells of the blood islands are angioblasts, which are precursors of ECs and have not yet formed a lumen. Then, angioblasts differentiate into ECs while fusion of blood islands results in the formation of a primary capillary plexus, which, subsequently, generates primitive blood vessels.
This vasculogenesis process can also be reproduced in an in vitro 3D vasculogenesis model. In this model, ECs are mixed in hydrogel prepolymer solution. The prepolymer solution is then poured into a culture dish and incubated for gelation, resulting in a hydrogel with embedded individual ECs (step 1, Fig. 16.1b). ECs embedded in the hydrogel, such as a collagen gel or fibrin gel, extend in 3D and connect with each other, while some cells form vacuoles within their cytoplasm (step 2, Fig. 16.1b). These vacuoles, formed in individual cells, subsequently fuse with each other, resulting in capillary-like structures with partial lumens (step 3, Fig. 16.1b).
Control of microvascular network formation is important not only in physiological but also in pathological conditions. For example, the formation of microvascular networks is required to promote 3D tissue formation in the field of tissue engineering and regenerative medicine. In contrast, it is also important to inhibit the formation of microvascular networks in terms of cancer therapy because inhibition of vascular formation is an important strategy to treat cancer. Thus, in vitro culture models of both angiogenesis and vasculogenesis are used to investigate how to control the formation of microvascular networks.
16.1.2 In Vitro Angiogenesis and Vasculogenesis Models
In vitro culture models of angiogenesis and vasculogenesis play important roles in investigating the mechanisms of vascular generation, growth, stabilization, and pathophysiology because cells in these models can be cultured under controlled conditions, which are independent from the complex cellular microenvironments in vivo.
Numerous in vitro angiogenesis and vasculogenesis models have been used to reveal molecular mechanisms and cell-to-cell interactions for constructing microvascular networks by ECs in 3D extracellular matrix (ECM) environments, focusing on biochemical factors (Davis et al. 2002; Newman et al. 2011). Another aspect to controlling vascular formation is that of biophysical factors, such as flow-induced mechanical signals and ECM stiffness. Ueda et al. (2004) investigated the effects of shear flow in a 3D angiogenesis model. They applied a laminar shear stress of 0.3 Pa to the surface of a confluent monolayer of ECs seeded on the surface of a collagen gel. Microvessel formation within the gel was promoted by shear flow application. The length of the capillary networks was significantly longer than that in static conditions. Microvessel formation was promoted in the gel whereas shear flow was applied to the cells on the surface of the gel, suggesting that the behavior of the cells on the gel is important to understand capillary morphogenesis of the cells in the gel.
A 3D vasculogenesis model can also be used to investigate biophysical factors, such as ECM stiffness, on capillary morphogenesis. For example, Sieminski et al. (2004) cultured ECs in floating or constrained collagen gels with different collagen concentrations to control matrix stiffness. EC morphogenesis differed depending on the matrix stiffness. Additionally, their results suggested that the relative magnitudes of cellular force generation due to the different matrix stiffness regulated capillary morphogenesis.
16.1.3 Endothelial Tip, Stalk, and Phalanx Cells
In the process of angiogenesis, ECs form vascular sprouts and gradually develop into branching networks with continuous lumens. Although vascular structures are formed by a single cell type, the EC, three different EC phenotypes are involved in the process of angiogenesis: endothelial tip, stalk , and phalanx cells (Hellström et al. 2007; Tammela et al. 2008; Carmeliet et al. 2009; Mazzone et al. 2009). Thus, we need to understand the characteristics of each EC phenotype and consider them in controlling the construction of microvascular networks in vitro.
Endothelial tip cells are the cells that locate at the tip of branching vascular networks. These cells extend filopodia to sense the surrounding microenvironment, such as growth factor gradients and ECM signaling molecules and stiffness, leading to the extension of vascular networks through tip cell migration. Thus, tip cells play an important role in determining the direction in which the capillary extends, while the cells rarely proliferate. In the context of vascular tissue engineering, the control of endothelial tip cells is important in guiding the direction of capillaries.
Endothelial stalk cells are the cells that follow the leading tip cells. Stalk cells proliferate to fill the elongating stalk in capillary networks. Although endothelial tip cells have no lumen, stalk cells create a lumen in the process of vascular formation. The assignment of ECs into the tip and stalk cells is regulated by endothelial cell-cell communication via Notch receptors and their transmembrane ligands, the delta-like ligand 4 (‘Dll4’) (Carmeliet et al. 2009). When ECs are activated by cytokines, such as VEGF, Dll4 expression is induced in the cells. The cells that strongly express Dll4 become endothelial tip cells. Dll4 expressed in tip cells activates Notch in neighboring cells, which induce stalk cells. This Dll4/Notch signaling from tip to stalk cells downregulates expression of VEGFR2 in stalk cells.
Endothelial phalanx cells are the third phenotype, which are located far from the tip cells. These ECs form a monolayer with a cobblestone appearance, which also resembles a phalanx, a Greek military formation. Phalanx cells form continuous and tightly packed monolayers as a quiescent endothelium (Mazzone et al. 2009).
16.1.4 Regulatory Factors in Vascular Formation
Advances in angiogenesis and vasculogenesis studies have revealed that both biochemical and biophysical factors regulate vascular formation. In vivo, ECs are exposed directly to blood flow, which supplies biophysical factors directly and indirectly. Blood flow delivers soluble factors, such as cytokines and growth factors, produced in different tissues. ECs are not only exposed to blood flow but also exposed to mural cells and ECM, such as basement membrane matrices, which also give rise to biophysical and biochemical factors.
The effects of biophysical factors, such as shear flow, interstitial flow, and ECM stiffness, have been investigated using in vitro models. For example, shear stress has been recognized as a promoter of angiogenesis (Ueda et al. 2004; Kang et al. 2008; Kaunas et al. 2011). In addition to shear flow, interstitial flow also plays important roles in capillary morphogenesis (Ng et al. 2004; Helm et al. 2005; Semino et al. 2006; Hernández Vera et al. 2009). ECM stiffness is also important in the regulation of angiogenesis. Yamamura et al. (2007) revealed that ECs cultured in rigid and flexible gels formed 3D networks via different processes; cells formed dense, thin networks in a flexible gel, while thicker and deeper networks were formed in a rigid gel. Sieminski et al. (2004) also reported that the relative magnitudes of cellular force generation and ECM stiffness modulated capillary morphogenesis in vitro.
Biochemical factors, such as growth factors, cytokines, chemokines, and their concentration gradients, have also been investigated in the context of vascular formation using in vitro angiogenesis and vasculogenesis models. VEGF and bFGF are major growth factors promoting vascular formation (Montesano et al. 1986). Phorbol myristate acetate (PMA), a tumor promoter that increases the production of collagenase and plasminogen activator, is also a promoter of angiogenesis. Montesano and Orci (1985) revealed that bovine microvascular ECs grown on collagen gels invaded the underlying collagen gel and formed extensive capillary networks when they were treated with PMA, whereas control ECs were confined to the surface of the gels.
Not only proangiogenic factors but also antiangiogenic factors have been identified, such as endostatin, tissue inhibitor of matrix metalloproteinase 3 (TIMP-3; Anand-Apte et al. 1997), and leukemia inhibitory factor (LIF; Pepper et al. 1995). Some of these factors are summarized in Table 16.1. Transforming growth factor β (TGFβ) has been reported to have biphasic effects on vascular formation; low concentrations (0.1-1 ng/mL) promoted angiogenesis while higher concentrations (5-10 ng/mL) inhibited capillary lumen formation (Pepper et al. 1993). In addition to individual factors, their combined effects are also important. For example, Vernon and Sage (1999) demonstrated that VEGF and bFGF have synergistic proangiogenic effects using an in vitro culture model. Newman et al. (2011) also demonstrated the importance of growth factor combinations. They showed that a combination of angiopoietin-1 (Ang-1), angiogenin, hepatocyte growth factor (HGF), TGFα, and tumor necrosis factor (TNF) could substitute for the effects of fibroblast-derived factors on sprout formation whereas no protein alone could function in this way. They also identified fibroblast-derived factors required for lumen formation, which were collagen I, procollagen C endopeptidase enhancer 1, “secreted protein acidic and rich in cysteine” (SPARC), the TGFβ-induced protein “ig-h3,” and insulin growth factor-binding protein 7 (Newman et al. 2011). The combined effects of multiple biochemical and biophysical factors need further clarification in future studies.
16.2 Microfluidic Devices for the Culture of Microvascular Networks
16.2.1 Endothelial Monolayers in Microfluidic Devices
This section provides an introduction to the use of microfluidic devices to study microvessel formation, which deals with the culture of ECs or monolayers in microfluidic channels. After EC loading, the cells adhere to the channel surface and form a confluent endothelial monolayer. This mimics the intima of the endothelium, the blood contact surface formed by a single layer of ECs. Mechanotransduction is an important issue for such a monolayer; signal transduction is regulated by the shear stress of blood flow.
Many studies since the 1990s have reported remodeling and alignment of ECs in monolayers due to shear stress gradients (Tardy et al. 1997). Another role for shear stress, in changing endothelial barrier function and permeability, has also been reported (Seebach et al. 2000; Colgan et al. 2007). This process involves cytoskeletal remodeling and molecular expression within ECs. Precise regulation based on fluidic shear stress types – laminar, turbulent, or pulsatile – has been an important challenge, attracting many interesting microfluidic approaches.
16.2.1.1 Endothelial Monolayer on Microfluidic Channel Surface
A realistic in vivo-like microenvironment of the endothelial monolayer would include shear stress, physiological pressure, and stretch. A microfluidic device has been used to culture endothelial monolayers on a stretchable and thin planar membrane (Estrada et al. 2011) (Fig. 16.2a). The membrane was made on the bottom of a microfluidic channel, forming a concave shape under pressure, representing a segment of a blood vessel wall. Precision regarding the physical stimuli can be achieved by a computer-controlled microcirculatory support system integrated with microfluidic devices (Song et al. 2005). It has microfluidic valve and pump units, and the advantages achieved by the integration show a good correlation between levels of shear stress that should be applied to the ECs and pump capacity. The tiny volumes of microfluidic channels have made it challenging to connect “conventional” pumps and microscale channels in parallel. Fluctuation, even due to tube compliance between a pump and a microfluidic device, can make the precise regulation of microfluidic shear stress inefficient. Other types of microfluidic units have been reported enabling pulsatile or oscillatory regulation of shear stress to mimic the in vivo situation (Shao et al. 2009) (Fig. 16.2b). Another study included sensor integration in microfluidic channels to precisely measure flow rate, pressure, and shear stress around an endothelial monolayer (Liu et al. 2013). The studies showed an important advantage of the microfluidic strategy: the potential for parallelization and increased throughput over conventional assays. It is also clear that the endothelial monolayer can discriminate pulsatile environments from a static flow condition. However, as yet, the response and mechanism(s) involved cannot be investigated fully, even using microfluidic approaches. This is because of the complexity of pulsatile flow patterns and also the scale of microfluidic devices.
Formation of an endothelial monolayer and tube-like endothelium in the microfluidic channel. (a) EC culture model integrated with flow loop. (b) Integrated microfluidic chip exposed to a pulsatile and oscillatory shear stress. (c) Reconstituted functional endothelialized microvascular networks with circular cross sections
16.2.1.2 Endothelial Monolayer as Part of In Vitro Blood Vessels
The mechanotransduction studies with shear stress mentioned above revealed interesting features of ECs and/or endothelial monolayers as the interface of a blood vessel and the blood flow within. ECs are reorganized and remodeled actively by shear stress and circulating cytokines, and these effects can be explored precisely with microfluidic regulation. Other factors in recapitulating the functionality of blood vessels include the permeability of the endothelial monolayer, the tube-like morphological characteristics, and interactions between ECs and other neighboring cells. These factors characterize many functions of blood vessels as an important transport system of blood throughout the body (Srigunapalan et al. 2011). When an endothelial monolayer forms in microfluidic channels, the cells cover every surface inside the microfluidic channels, in general, and form a conformal tube-like endothelium. Microfabrication techniques can draw two-dimensional (2D) pipelines and complex networks, with square, rectangular, or hemi- or fully circular cross sections (Borenstein et al. 2010) (Fig. 16.2c). Cell-cell interactions and tube-like morphological characteristics can also be examined with various antibodies and signaling molecules to test the effects of drugs and to study the interaction between different human vascular cell types (ávan der Meer et al. 2013).
However, these models lack the possibility of exploring one of the most important features of blood vessels: the barrier function for oxygen, nutrients, molecules, and/or cells. The barrier is semiselective and can control the passage of many materials into or out of blood. However, molecules cannot pass through an endothelial monolayer cultured on a channel surface. Layers beyond the endothelium are needed to support molecular transport through the monolayer. In blood vessels in vivo, layers with smooth muscle cells (SMCs), fibroblasts, and ECM are located outside the endothelial monolayer. They support the monolayer, providing rigidity for the vascular tube structure.
Hydrogels mimicking ECM have been used as suitable supports for endothelial monolayers in microfluidic channels (Wong et al. 2012; Zheng C et al. 2012; Zheng Y et al. 2012; Bischel et al. 2013; Baker et al. 2013). With ECM, the endothelial monolayers finally form endothelial tubes mimicking endothelium in blood vessels. Type I collagen hydrogel, fibrin, and Matrigel have been used widely in many applications to form tubes attached to ECM. They are perfusable by medium containing nutrients, oxygen, proteins, chemical reagents, drugs, and cells. Microfabrication techniques can also make planar structures behave as complex 2D networks, with tube-like cross sections of ECM attached to the endothelial monolayer (Zheng C et al. 2012; Zheng Y et al. 2012; Bischel et al. 2013) (Fig. 16.3). Not only permeability but also other important factors regarding the physics of blood vessels, thrombosis and angiogenesis, can be analyzed in vitro. Compared with “traditional” in vitro assays with ECs, this is physically relevant to in vivo vascular networks, with liquid flow inside and ECM materials outside (ávan der Meer et al. 2013). A gradient of diffusive molecules three-dimensionally within ECM can be applied to the formed 3D endothelial tubes, to mimic the guidance provided by the molecules in many processes within development, angiogenesis, and cancer (Baker et al. 2013). The applied molecules, distributed spatiotemporally within 3D ECM, and their diffusion patterns, are affected by the tubes. At the same time, the diffusion patterns also affect the tubes, guiding locations and driving morphogenetic events, such as angiogenesis.
16.2.2 3D Capillary Formation Assays Using Microfluidic Devices
16.2.2.1 3D Angiogenesis Assay
The microfluidic assays described can be applied to the basic morphogenetic analysis of blood vessels; however, they lack any ability to induce fully functional structures due to the high stiffness of the ECM used to support the fluidic channel structure (Borenstein et al. 2010) and limited ECM space around the endothelial tubes (ávan der Meer et al. 2013). Hydrogel-incorporating microfluidic devices have been developed to investigate the morphogenetic nature of blood vessels, angiogenesis (Chung et al. 2009b; Shin et al. 2011, 2012; Jeong et al. 2011a, b; Song and Munn 2011), and vasculogenesis (Kim et al. 2013; Whisler et al. 2014; Chen et al. 2013). Angiogenesis denotes the formation of new blood vessels from preexisting vessels, and vasculogenesis is the process that occurs when there are no preexisting vessels. Angiogenesis is a normal and vital process in growth and development. It is also a fundamental step in the transition of tumors (Penn 2008). Many mechanical and chemical factors regulating angiogenesis have been revealed, including VEGF and angiopoietins. For diseases due to poor or abnormal vascularization, such as cardiovascular diseases and tumors, angiogenesis has already emerged as an effective therapeutic target.
When ECs are seeded in a hydrogel incorporating microfluidic channels, the seeded cells attach to the hydrogel and form an endothelial monolayer. Type I collagen, Matrigel, and fibrin are used widely to mimic the ECM surrounding blood vessels. Chung et al. (2009b) developed an optimized protocol to induce sprouting angiogenesis into type I collagen hydrogel and confirmed its 3D tube-like structure by confocal microscopy (Fig. 16.4a). They found that a VEGF gradient induced sprouting angiogenesis formation but also that additional signals are probably necessary to determine which segments sprout, which dilate, and which remain quiescent. Combined treatment with angiogenic factors and VEGF can induce a complex and integrated network of blood vessels, angiopoietin (Shin et al. 2011), and sphingosine (Nguyen et al. 2013) (Fig. 16.4b, c). Microfluidic schemes enable in situ monitoring of collective and complex angiogenic procedures with enhanced views. Many interesting phenomena involved in 3D angiogenesis have been reported, including 3D cooperative migration of tip cells (with filopodia-like protrusions) and stalk cells (with apical-basal polarity), lumen formation, and the connection and division of capillary branches with perfusion inside the lumens. Anti- or proangiogenic drugs can be evaluated (Nguyen et al. 2013; Lee et al. 2014), as can angiogenic inhibitors and matrix metalloproteinase inhibitors.
Another study on the combined effects of growth factors and fluid shear stress also revealed the important nature of capillary angiogenesis (Shin et al. 2011). Endothelial monolayers are always under fluidic shear stress and interstitial (leaking) flow, and these effects, combined with various growth factors, are now under investigation. It also makes the assay more quantitative and robust if these issues are considered. Studies on local and global gradient formation, 3D diffusion, convection, and interactions with cells (barrier function and materials consumption) can provide a basis for understanding the true distribution of the many factors involved and how they interact with blood vessels (Jeong et al. 2011b; Song and Munn 2011; Helm et al. 2005). Local gradients of the factors regulate angiogenic structures but are also interrupted by endothelial monolayers. When the monolayer prohibits the molecule(s) from passing through, a rapid change in concentration occurs across the monolayer. When the cells in the monolayer take up the molecule(s), a local gradient forms around the cells. Studies should also consider the synergistic interaction of molecule distributions under convection by blood flow and interstitial flow and interactions with cells and endothelial monolayers. It is still a very challenging issue to understand the true mechanism of angiogenesis.
Coculturing with stromal fibroblast, pericytes, or SMCs (Kim et al. 2013; Mack et al. 2009) has also been investigated to confirm these cells’ roles in adaptive remodeling of capillary structures. Mack et al. (2009) found that KLF2-expressing ECs cocultured with SMCs showed significantly reduced SMC migration. The cells form vascular smooth muscle, the majority of the wall of blood vessels outside the endothelial monolayer, to support the lumen structure and stabilize ECs. Coculturing enables a deep investigation of complex interactions between blood vessels and neighboring cells in development and many diseases, which cannot be achieved readily with conventional cell culture assays.
16.2.2.2 3D Vasculogenesis Assay
The hydrogel-incorporating vasculogenesis assays developed have a similar structure to angiogenesis assays. However, vasculogenesis assays usually involve culturing ECs embedded in ECM hydrogel. Before the development of hydrogel-incorporating microfluidic devices, the differentiation of angiogenesis and vasculogenesis procedures in conventional in vitro assays was almost impossible, while current vasculogenesis assays can form very complex, highly connected, functional, and perfusable 3D microvascular networks (Kim et al. 2013) (Fig. 16.5a). The assays show strong barrier function and long-term stability of the networks. Precise control of growth factors and coculturing with stromal fibroblasts, SMCs, or pericytes help to form stable but complex vascular structures (Whisler et al. 2014) (Fig. 16.5b). These assays are also used for tumor transendothelial studies, intra- and extravasation (Chen et al. 2013) (Fig. 16.5c). This will be discussed in detail later.
16.2.3 Vascular Formation Under a Gradient of Growth Factors in Microfluidic Devices
The generation and precise control of biochemical factor distributions is one of the most useful features of microfluidic devices. Concentration gradients formed around cells induce 2D chemotactic responses of ECs. ECs exposed to growth factor gradients displayed an increased number of filopodia and an asymmetric distribution of filopodia, with enhanced numbers of protrusions present along the leading edge (Shamloo et al. 2008). The absolute concentration of VEGF enhanced the total number of filopodia, while the steepness of the VEGF gradient induced filopodia localization, cell polarization, and subsequent directed migration.
A study on chemotactic responses in three-dimensionally aligned endothelial monolayer-induced angiogenic responses of ECs, in ECMs with varying densities and stiffnesses, has been reported. The mechanical properties of ECMs could regulate angiogenic sprout and lumen formation when combined with gradients of vascular growth factors (Shamloo and Heilshorn 2010). Microfluidic chips enable the precise regulation of gradients and in situ investigations and monitoring of complex 3D cellular morphogenesis. They also enable quantitative studies on chemical release systems to deliver growth factors, therapeutic agents, and drugs. Kim et al. (2012) evaluated the proangiogenic potential of factors secreted from encapsulated fibroblast cells on an endothelial monolayer. An extensive network of functional lumen structures was made in vitro, perfused with blood cells.
The true distribution of a chemical gradient around an endothelial monolayer is not easy to define because many factors are involved synergistically, as described in Section 16.2.2 (Jeong et al. 2011b; Song and Munn 2011; Helm et al. 2005). Pioneering works to precisely estimate actual distributions of chemical gradients by computational modeling have been reported, with experimental verification by monitoring 3D sprouting angiogenesis from an endothelial monolayer (Jeong et al. 2011b). Moreover, molecular diffusion perturbed by the endothelium helps to regulate the degree of gradient around the endothelial monolayer. The orchestration of multiple growth factors (e.g., VEGF and angiopoietins) has also been simulated, with experimental confirmation by 3D cooperative migration of tip and stalk cells and the lumen structures formed (Shin et al. 2011).
16.2.4 ECM Materials for Microfluidic Angiogenic Studies
Various ECM materials, including type I collagen, fibrin, hyaluronic acids (HA), Matrigel, fibronectin, and gelatin, have been used in angiogenic studies as a base for ECs (Table 16.2). Matrigel has been widely accepted as an ECM in 2D angiogenic studies, because ECs form tube-like “2.5-dimensional” structures on Matrigel (Soriano et al. 2004; Kieda et al. 2006). However, the structure formed is far from that of the in vivo vasculature. It grows on the ECM (Matrigel), with biased polarity, of the top to the medium and bottom to the ECM, which is the opposite of the in vivo vasculature, which has ECM outside containing blood flow inside.
Pioneering microfluidic works incorporating ECM have enabled the study of angiogenic responses in ECM. The vasculature in the ECM was made by combined effects of collective and cooperative migration of ECs, with active remodeling of the ECMs by soluble proteases and matrix metalloproteinases (MMPs). Type I collagen and fibrin are the major ECMs used for microfluidic angiogenesis and vasculogenesis studies. In some studies, the ECMs have been modified with other ECM components, such as laminin and fibronectin, to mimic the ECM composition of specific target organs. For example, the ECM of adult brain tissue has a unique composition. It has lecticans, proteoglycans with a lectin domain and a hyaluronic acid-binding domain. Hyaluronic acid and tenascin family adhesive/antiadhesive proteins are also abundant (Ruoslahti 1996). Brain also has high concentrations of matrix proteins common in other tissues, such as laminin and collagen, but only near the brain vasculature. The unique composition of brain tissue has trophic effects on specific brain cells and in diseases. ECM-incorporating microfluidic studies enable the exploration of the unique effects of cellular microenvironment on specific cells, precisely mimicking in vivo tissue physiology (Shin et al. 2014).
16.3 Coculture of ECs and the Other Cell Types in Microfluidic Devices
16.3.1 Using ECs as a Vascular Microenvironment for Other Cell Types: Mesenchymal Cells
16.3.1.1 Proangiogenic Effects of Fibroblasts
Fibroblasts are a type of mesenchymal cell ; they play important roles in the synthesis and maintenance of ECM. Since it was first reported that factors produced by mouse embryo 3 T3 fibroblasts stimulated the proliferation of cultured ECs (Birdwell et al. 1977), fibroblasts have been cocultured with ECs to promote angiogenesis in 3D culture models. Montesano et al. (1993) investigated the effect of various fibroblasts, such as Swiss 3 T3, BALB 3 T3, NIH 3 T3 cells, and epithelial cells, such as MDCK and intestine 407 cells, on the angiogenic responses of bovine microvascular ECs. Their results revealed that only Swiss 3 T3 fibroblasts, among the various fibroblasts and epithelial cells tested, were able to induce an angiogenic response when cocultured with the ECs in their 3D angiogenesis model. This proangiogenic effect of fibroblast-derived factors was also used in recent in vitro angiogenesis models created in microfluidic devices (Yeon et al. 2012; Kim et al. 2012, 2013; Moya et al. 2013). Fibroblasts are known to produce various proangiogenic soluble factors, including VEGF, bFGF, IL-8, TGFα, TGFβ, HGF, angiogenin, Ang-1, TNF, and PDGF (Mansbridge et al. 1999; Hartlapp et al. 2001; Hurley et al. 2010; Newman et al. 2011). In addition to the growth factors secreted by fibroblasts, fibroblast-derived matrix proteins also play important roles in angiogenic responses (Berthod et al. 2006). Newman et al. (2011) identified collagen I, procollagen C endopeptidase enhancer 1, SPARC, ig-h3, and insulin growth factor-binding protein 7, which were expressed in fibroblasts and were essential for EC lumen formation.
16.3.1.2 Capillary Stabilization by Pericytes or SMCs
ECs have been cocultured with fibroblasts because of their ability to secrete various proangiogenic factors. In the context of vascular tissue engineering, not only the formation but also the stabilization of capillary networks is important to construct long-lasting capillary networks in vitro. Other types of mesenchymal cells, such as SMCs and pericytes , may be expected to interact with ECs because they localize around blood vessels in vivo. Pericytes wrap themselves around small vessels, such as capillaries <10 μm in diameter, as well as microvessels, 10-100 μm in diameter, while SMCs form many layers around larger blood vessels (Wanjare et al. 2013). These perivascular cells have different effects on angiogenesis compared with fibroblasts. SMCs and pericytes inhibit EC proliferation and angiogenic responses in cocultures as well as in vivo (Orlidge and D’Amore 1987; Wanjare et al. 2013; Crocker et al. 1970; Shepro and Morel 1993; Díaz-Flores et al. 2009). Activated TGFβ was the key factor for the inhibitory effect of pericytes on EC growth in EC-pericyte coculture (Antonelli-Orlidge et al. 1989). Saunders et al. (2006) found that EC-derived TIMP-2 and pericytes-derived TIMP-3 coregulated capillary tube stabilization. Pericytes-derived TIMP-3 inhibited vascular sprout formation by ECs in an in vitro angiogenesis model, while TIMP-3 contributed to capillary tube stabilization in an in vitro vasculogenesis model. Numerous studies suggest that Ang-1/Tie2 signaling between pericytes and ECs is required for vessel stabilization (Armulik et al. 2005).
16.3.1.3 Use of Stem Cell Source for EC-Pericyte Coculture
Mesenchymal stem cells (MSCs) have also been used for coculture with ECs. Because it is difficult to establish primary culture of pericytes, MSCs have been used to derive pericytes. Au et al. (2008) demonstrated that MSCs derived from human bone marrow differentiated into perivascular cells, which stained positively for α-smooth muscle actin (α-SMA), a pericyte marker, in vivo when coimplanted with ECs. Although capillary structures quickly regressed when only human umbilical vein endothelial cells (HUVECs) were implanted, pericytes differentiated from MSCs stabilized capillary structures when coimplanted with ECs, resulting in the maintenance of functional capillary structures for >130 days in vivo. Furthermore, these capillary structures constricted in response to stimulation by endothelin-1, an endogenous vasoconstrictive peptide.
Differentiation of MSCs into pericytes has also been confirmed in recent studies using in vitro culture models. Carrion et al. (2010) performed HUVEC-MSC coculture in an in vitro vasculogenesis model created in a microfluidic device and demonstrated that MSCs localized around the periphery of the vessel structure formed by HUVECs. These MSCs became positive for α-SMA and eventually wrapped themselves around the forming vessels. Yamamoto et al. (2013) reported that HUVEC-MSC direct contact was required for the differentiation of MSCs into pericytes in 2D culture. In this study, HUVEC-MSC coculture was also performed in a microfluidic device. Results showed that MSCs attenuated vascular sprout formation of HUVECs in the early stage of 3D angiogenesis as well as the extension of microvascular networks in the later stage, suggesting that MSCs have stabilization effects on the formation of microvascular networks. Jeon et al. (2014) also reported that HUVEC-MSC direct contact was required for the differentiation of MSCs into pericytes in 3D culture. In this study, HUVEC-MSC coculture was performed in an in vitro vasculogenesis model created in a microfluidic device. MSC-derived α-SMA-positive cells colocalized with HUVECs and wrapped around the newly formed microvascular networks, which resulted in a smaller vessel diameter versus HUVECs alone. According to the recent advances in in vitro culture systems mentioned here, there is increasing evidence that HUVEC-MSC coculture is promising for the development of functional, physiological, and long-lasting microvascular networks in vitro.
16.3.2 Using ECs to Create a Vascular Microenvironment for Other Cell Types: Neural Stem Cells
Cellular microenvironments include signaling molecules, small molecules, neighboring cells, ECMs, small organs, and mechanical stimuli. These provide structural and trophic support for cells, regulating their life cycle. The vascular microenvironment, one of the cellular microenvironments formed by blood vessels, supplies neighboring cells with nutrients, oxygen, and other soluble factors and actively regulates their life cycle. For example, neural stem cells (NSCs) reside in a brain vascular microenvironment. Brain vessels play an important role in maintaining an appropriate balance between NSC self-renewal and differentiation (Shin et al. 2014). NSCs in the subventricular zone maintain contact with the brain vasculature, maintaining its multipotency by self-renewal. Brain tissue damage by, for example, ischemic stroke, neurodegenerative diseases, and brain tumors, promotes homing and integration of NSCs. The NSCs integrated into the damaged brain tissue choose the proper differentiation lineage into neurons, astrocytes, or oligodendrocytes. In the process, the vascular microenvironment plays an essential role, and a study of the microenvironments may facilitate basic research on the development of potential therapies for degenerative disorders.
Shin et al. (2014) developed a microfluidic assay to reconstitute NSC-vascular microenvironments with a 3D brain vasculature and ECMs. The reconstituted vascular microenvironment regulated self-renewal, proliferation, and colony formation of NSCs and suppressed neuronal generation. It also promoted NSC differentiation into astrocytes and oligodendrocytes. Cell culture on a microfluidic scale was shown to emphasize cell-cell communications, by enrichment of secreted factors in the tiny microscale environment. It dramatically enhanced the proximity effect on NSCs from the brain vasculature, pushing NSCs to differentiate into astrocytes to form a brain-specific vascular structure, the blood-brain barrier (Shin et al. 2014).
Not only NSCs but also the other stem cells require an assay for assessing vascular microenvironments. Many in vivo models from mouse and Drosophila have helped to explore the role of stem cell microenvironments on stem cell maintenance and activity. However, details of morphogenetic processes of stem cells under specific microenvironments cannot be systematically monitored in vivo, raising the need for an in vitro assay regulating the microenvironmental factors. Another challenge involves demonstrating the combined effects of multiple microenvironmental factors. For example, low oxygen tension induces angiogenesis by increased expression of various angiogenic factors. The expressed angiogenic factors not only affect ECs but also stem cells and other stromal cells. Newly formed vasculature also affects the previously low oxygen tension and other stromal cells. These cell-cell and cell-microenvironment interactions are complex, affecting each other in a very complicated manner. Reconstitution of each microenvironmental factor and their combinations markedly increases the chances of developing new knowledge.
16.3.3 Using Other Cell Types to Interact with ECs: Immune and Cancer Cells
Cancer occurs by genetic damage to normal cells, leading to uncontrolled proliferation, resulting in a primary heterogenic tumor . The tumor induces angiogenesis and recruits new blood vessels to enhance its nutrient and oxygen supply. Some cancer cells acquire the ability to invade neighboring ECMs and/or tissues, and finally intravasate into lymphatic or blood vessels. They become circulating tumor cells and extravasate to secondary tumor sites. These extravasated tumor cells may die or become dormant due to the different microenvironment of the secondary site from that of the primary site, but sometimes proliferate and form micrometastases, resulting in secondary tumor site(s). Proliferation in the secondary tumor site also requires angiogenic responses from neighboring blood vessels. Angiogenesis is thus an essential process for a cancer to acquire malignancy by metastasis; it supplies the tumor site with nutrient and oxygen, and also works as a major route for tumor cells to escape from the original site.
Cancer angiogenesis is thus a promising drug target; suppressing it could starve tumor cells and minimize the chances of metastasis. The permeability and 3D structures of blood vessels are related to the metastasis procedure; they also work as a route for drug delivery, directly targeting tumor cells. The whole process of a cancer acquiring malignancy by metastasis has been addressed and defined as described above, but there is still a need for appropriate in vitro assays to investigate the individual steps involved in metastasis.
16.3.3.1 Remote Interaction Between Tumor Cells and ECs: Angiogenic Response
A microfluidic platform was developed to evaluate and quantify capillary growth from an endothelial monolayer, when placed in coculture with physiologically relevant cell types, including cancer cells and SMCs (Chung et al. 2009a) (Fig. 16.6a). Three-dimensional capillary structures were grown into type I collagen ECM, under a precisely controlled coculture microenvironment. Breast cancer cells were shown to induce capillary formation, while SMCs suppressed it. A similar interaction between endothelial cells and tumor cells was also realized on the 2D surface of a microfluidic array (Zheng C et al. 2012; Zheng Y et al. 2012). The device offered 16 parallel arrays to study interactions between cells. It successfully provided an accurate spatiotemporal microenvironment in cocultures of multiple types of cells (Zheng C et al. 2012; Zheng Y et al. 2012) (Fig. 16.6b).
16.3.3.2 Remote Interaction Between Tumor Cells and ECs: Antiangiogenic Response
It is now widely recognized that cancer cells induce angiogenesis (Folkman 1971). However, antiangiogenic responses were also observed in in vitro coculture models. Kalchman et al. (2013) reported the coculture of HUVECs and HepG2, a hepatocellular carcinoma cell line. HUVECs and HepG2 cells were cultured in separate microfluidic channels. When HUVECs were cultured alone, they migrated into collagen gel and formed microvascular networks. However, when HUVECs were cultured with HepG2, the formation of vascular sprouts was completely inhibited and HUVECs formed monolayers (Fig. 16.7). Instead, HUVECs induced the formation of protrusions by HepG2, indicating that there are interactions between HUVEC and HepG2, although no angiogenic response was observed. These results suggest that tumor-induced angiogenesis can be confirmed in culture models, depending on the cell lines used. Further investigations are needed to clarify interactions between cancer cells and ECs in terms of angiogenesis.
16.3.3.3 Direct Interaction Between Tumor Cells and ECs: Intravasation and Extravasation
Many microfluidic devices have been used to investigate intravasation , entry into the bloodstream, or extravasation , movement out of the blood stream, of tumor cells. A microfluidic device reconstituting the 3D tumor-vascular interface, allowing real-time monitoring of cell-cell interactions and precise quantification of endothelial barrier function, such as endothelial permeability has been developed (Zervantonakis et al. 2012) (Fig. 16.8a). The endothelial monolayer was shown to pose a barrier to tumor cell intravasation that could be regulated by factors present in the tumor microenvironment. Another microfluidic device provided an endothelial monolayer culture in a microfluidic device to study the intravascular adhesion of circulating breast cancer cells on an endothelial monolayer at potential sites of metastasis (Song et al. 2009). Spatially restricted stimulation of an endothelial monolayer with the chemokine CXCL12 from the basal side mimicked the organ-specific localization and polarization of chemokines and other signaling molecules, and was shown to affect cancer cell adhesion on the endothelial monolayer.
Another study dealt with the effects of CXCL12 on tumor cell aggregates near the endothelial monolayer (Zhang et al. 2012) (Fig. 16.8b). CXCL12 stimulation damaged the integrity of the endothelial monolayer and attracted cancer cells transmigrating through the monolayer. Thus, the evaluation of antimetastasis agents by inhibiting transendothelial invasion or the adhesion of cancer cells on the endothelial monolayer can be performed with such microfluidic devices. A similar device was developed to mimic tumor cell extravasation, transmigrating across an endothelial monolayer and migrating into a hydrogel, to model the extracellular space (Jeon et al. 2013). Precise measurement of endothelial permeability and in situ monitoring of cancer cell transmigration can help in understanding permeability changes during tumor cell intra/extravasation events.
A tumor extravasation study in a microfluidic device was enhanced dramatically by reconstituting complex 3D vascular networks (Chen et al. 2013) (Fig. 16.8c). The network was made by vasculogenesis in a fibrin ECM, having tight endothelial junctions with low vessel permeability. Extravasation efficiency was increased by inflammatory cytokine stimulation and trapping cancer cells in complex junction structures. Dynamic interactions of tumor cells and ECs were monitored: protrusion formation, extrusion of the cell body through a very small opening in the endothelial barrier, and nuclei transmigration. These methods can be applied not only to tumor cell metastasis studies but also to the spatiotemporal analysis of the inflammatory response to characterize transendothelial migration behaviors of neutrophils under the influence of various inflammatory stimuli (Han et al. 2012).
16.4 Vascularization of Reconstructed Tissues
16.4.1 General Introduction to the Vascularization of Tissue-Engineered Constructs
Tissue engineering is an engineering technique to construct tissues and organs from individual cells (Langer and Vacanti 1993). Since the concept was first reported, many tissue engineering approaches have been studied with a view to producing various tissues and organs in vitro and in vivo (Khademhosseini et al. 2009; Griffith and Naughton 2002). The development of tissue engineering has allowed the construction of 2D tissues, such as skin substitutes and cornea, and 3D avascular tissues, such as cartilage. Although these engineered tissues have been assessed in clinical trials, construction of 3D vital organs, such as liver, heart, and pancreas, remains a key challenge in this field.
In contrast to 2D tissues, 3D organs include abundant microvascular networks, because cells in 3D tissues require oxygen and nutrients to be provided from blood flow. Because the diffusion distance of oxygen in a living tissue is limited to 100-200 μm (Carmeliet and Jain 2000), microvessels in 3D tissues have to be located within the interval of this critical distance. Thus, complex 3D tissue structures, including microvascular networks, make it difficult to construct 3D vital organs.
To construct 3D vital organs, formation of microvascular networks is important, as is constructing 3D epithelial tissues composed of parenchymal cells. In previous studies, the formation processes of the microvascular networks and the 3D epithelial tissues have been investigated individually. However, in recent studies, not only constructing the microvessels but also incorporating the microvessels within engineered 3D tissues, referred to as vascularization , has become increasingly important. Thus, this vascularization of tissue-engineered constructs is a key challenge in achieving 3D thick tissues in vitro (Rouwkema et al. 2008; Lovett et al. 2009; Auger et al. 2013).
16.4.2 Coculture of ECs and Hepatocytes in a Microfluidic Device: A Horizontal Approach
To achieve the vascularization of tissue-engineered constructs, it is essential to investigate the heterotypic interaction between microvascular ECs that form the microvascular networks and host organ cells (e.g., hepatocytes) used to construct the 3D tissues. Liver is one of the most highly vascularized organs and is composed mainly of functional epithelial cells, the hepatocytes. Because hepatocytes assemble into organized 3D tissues in vivo, various 3D hepatocyte culture models have been developed (Sudo et al. 2005; Sudo 2014; Hwa et al. 2007; Toh et al. 2007). Culturing hepatocytes in a 3D configuration is useful to maintain differentiated hepatic functions in vitro. However, the current techniques fail to incorporate microvascular networks within the 3D hepatocyte tissues. Vascular tissue engineering has developed separately from liver tissue engineering. The formation of microvascular networks has been investigated with various in vitro 3D angiogenesis models, and the molecular mechanism of capillary formation has been studied (Davis et al. 2002; Vailhé et al. 2001). However, little is known concerning the interaction between the resulting capillary structures and other epithelial cell types, such as hepatocytes. Thus, there is a need to establish a coculture system where microvascular ECs and the other parenchymal cell types can interact in a controlled microenvironment with physiological realistic topology and cell organization (e.g., capillary networks formed by ECs and 3D structures, composed of hepatocytes). Because 3D tissue formation and vascularization can be achieved in 3D tissues in vivo, there is an increasing demand for in vitro models that capture the physiological complexity such as controllable biochemical (e.g., local chemotactic gradient and ECM properties) and biophysical factors (e.g., interstitial fluid flow, shear flow, and mechanical properties of scaffold materials) (Griffith and Swartz 2006; Yamada and Cukierman 2007). Although in vitro models that incorporate these features are difficult to create, recent advances in microfluidic technologies (Kim et al. 2007; Khademhosseini et al. 2006) have allowed the development of such an in vitro culture model.
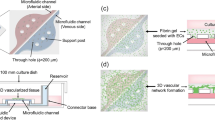
A microfluidic platform for the vascularization of 3D tissue-engineered constructs was reported, integrating knowledge from microfluidics, in vitro angiogenesis models, and liver tissue engineering (Sudo et al. 2009). The microfluidic device was made of polydimethylsiloxane (PDMS) and a cover glass. There were two microfluidic channels and an intervening collagen gel (Fig. 16.9a). To investigate the interaction between hepatocytes and ECs, hepatocytes were seeded into a microfluidic channel, while ECs were seeded into the channel of the other side. This microfluidic design allowed locating hepatocytes and ECs in close proximity, separated by collagen gel, the width of which was 750 μm (Fig. 16.9b).
Microfluidic device for coculture of hepatocytes and microvascular ECs. (a) A schematic image of a microfluidic device made of PDMS and a cover glass. There are two microfluidic channels and an intervening collagen gel. Hepatocytes were seeded in the microfluidic channel while ECs were seeded on the other side for a coculture. (b) Enlarged image of collagen gel scaffold in a microfluidic device
First, hepatocytes were seeded in one of the two microfluidic channels and cultured in interstitial flow conditions (day 0, Fig. 16.10). This interstitial flow application during day 0-1 in the beginning of the culture induced 3D tissue formation by the hepatocytes. Hepatocytes maintained a 3D tissue structure even when cultured in static conditions going forward from day 1. ECs were then added to the other side to start the coculture (day 2–0, Fig. 16.10). Although rat ECs failed to form capillary-like structures in the absence of hepatocytes, they formed vascular sprouts extending in the collagen gel only in the case of coculture (day 6–4, Fig. 16.10). Vascular sprouts subsequently extended toward 3D hepatocyte tissues and developed into capillary-like structures (day 8–6, Fig. 16.10). Phase-contrast images of the capillary-like structures formed in the coculture suggested luminal spaces (day 10–8, Fig. 16.11), which was investigated further by confocal microscopy. Cells were fixed and stained for actin to visualize 3D structures. Confocal images revealed that rat ECs formed capillary-like structures with continuous lumens (day 12–10, Fig. 16.11). In summary, hepatocytes formed 3D tissue structures while ECs formed 3D capillary-like structures, penetrating into the collagen gel adjacent to the 3D hepatocyte tissue. Both cell types were initially cultured in separate microfluidic channels but could communicate directly with each other via the gel scaffold located between the channels. The microfluidic platform allowed monitoring of cell behavior and heterotypic interactions. This experimental approach showed the potential for constructing vascularized organs in vitro. Further investigations are needed to achieve vascularized liver tissues.
Phase-contrast images from cocultured hepatocytes and microvascular ECs in a microfluidic device. Day 0: hepatocytes were seeded into a microfluidic channel and cultured in interstitial flow conditions for 1 day. Day 2–0: Hepatocytes form 3D tissues. ECs were added to the other side of the microfluidic channel, and coculture was performed in static conditions to enhance interactions between hepatocytes and ECs. Day 6–4: ECs formed vascular sprouts in the collagen gel. Day 8–6: Vascular sprouts extended toward the 3D hepatocyte tissues and developed into capillary-like structures
Capillary-like structures formed in hepatocyte-EC coculture. Phase-contrast images taken on day 10–8 showed that ECs formed capillary-like structures extending toward the 3D hepatocyte tissues (arrowheads). Day 12–10: Cells were fixed on day 12–10 and stained for actin. A projection image was reconstructed from z-stack confocal images. Images #1-5 are cross section images corresponding to the dotted lines #1-5
16.4.3 Coculture of ECs and Hepatocytes Using a Microporous Membrane: A Vertical Approach
Another approach for constructing vascularized tissues is a method using microporous membranes. The microfluidic approach mentioned in Section 16.4.2 is a method to combine liver and vascular tissues horizontally. However, liver and vascular tissues can also be combined vertically, by stacking microporous membranes.
Heterotypic interactions between hepatocytes and ECs were investigated in a coculture model using microporous membranes. First, small hepatocytes (SHs) , hepatic progenitor cells, were cultured on microporous membranes. SHs grew on the membranes, and the membranes were stacked onto other membranes to construct 3D stacked structures (Sudo et al. 2005). This 3D culture model was extended to coculture with ECs and hepatic stellate cells (HSCs), resulting in a 3D triculture model, established using microporous membranes, to create the functional unit of proximal layers of hepatocytes, HSCs, and ECs (Kasuya et al. 2011) (Fig. 16.12). HSC behavior was controlled by the membrane pore size, which was key for achieving proximal cell layers. With a specific pore size, the HSCs intercalated between layers of hepatocytes and ECs. When only cytoplasmic processes of quiescent HSCs were adjacent to ECs, while the HSC bodies remained on the side of the hepatocytes, the ECs changed morphologically and were capable of long-term survival. HSCs mediated communication between hepatocytes and ECs in terms of EC morphogenesis. This triculture model allowed investigating the roles of HSCs as both facilitators and integrators of cell-cell communication between hepatocytes and ECs and is useful for investigating heterotypic cellular communication in vitro. However, in terms of vascularization of the hepatocyte tissues, ECs in the triculture model formed monolayers rather than capillaries. Thus, next, this triculture model was modified to have ECs forming capillary-like structures.
A triculture model using a microporous membrane. First, SHs and HSCs were seeded on the bottom surface of a microporous membrane. The membrane was turned over in the holding well after 2 h. The SHs proliferated and formed colonies within 2 weeks in culture, while HSCs located between the SH colonies and the membrane. ECs were seeded onto the top surface of the membrane on day 14, resulting in the HSC-mediated layered architecture of SHs and ECs
To modify the hepatocyte-HSC-EC triculture model, the effects of direct HSC-EC contacts on EC capillary morphogenesis were investigated using the triculture model where HSC behavior was controlled spatially to achieve HSC-mediated proximal layers of hepatocytes and ECs. EC capillary morphogenesis was induced by overlaying Matrigel on an EC layer (Fig. 16.13). Direct HSC-EC contacts inhibited EC capillary morphogenesis, suggesting that the HSC-EC contacts may be an important factor in capillary formation (Kasuya et al. 2012a). Next, the hypothesis that, in addition to spatial control, temporal control of HSC behavior is also important in achieving capillary morphogenesis in the triculture was tested. ECs responded to the induction of capillary morphogenesis before the formation of direct HSC-EC contacts, while the ECs remained to form monolayers when capillary morphogenesis was induced after the HSC-EC contacts were established (Kasuya et al. 2012a) (Fig. 16.13). When capillary morphogenesis was achieved in the triculture, HSCs tended to preferably localize near the preformed capillary-like structures, resulting in reconstruction of liver sinusoid-like structures. In these structures, maturation of the hepatocytes was induced. These findings indicated that control, both spatially and temporally, of HSC behavior is a key engineering strategy for the vascularization of engineered liver tissue in vitro.
A triculture model with modification of capillary morphogenesis. First, a SH-HSC-EC triculture model was constructed, in which ECs formed a confluent monolayer. Then, capillary morphogenesis was induced by overlaying Matrigel on the confluent EC monolayer. HSCs were recruited by the capillary-like structures, resulting in a triculture model where HSCs mediated an SH layer and capillary-like structures
In liver sinusoids, HSCs line the outer surface of microvessels to form a functional unit with endothelia and hepatocytes. To reconstruct a functional liver tissue in vitro, recapturing the HSC-lined sinusoidal structures is essential. We previously demonstrated capillary formation in triculture where a Matrigel angiogenesis assay and SH-HSC coculture were combined using polymeric microporous membranes. However, the large thickness and low porosity of the membranes limited the heterotypic cell-cell interactions that are essential in forming HSC-lined structures. In addition to the achievement of capillary formation in the triculture, we previously established a stack-up culture method for reconstructing 3D hepatic tissues using thin and highly porous poly (D,L-lactide-co-glycolide acids) (PLGA) membranes (Kasuya et al. 2012b). We thus focused on the effective use of the PLGA microporous membranes in a hepatocyte-HSC-EC triculture to reconstruct the HSC-lined liver sinusoidal structures in vitro. First, the formation of EC capillary-like structures was induced on Matrigel-coated microporous PLGA membranes (Fig. 16.14). Then, the membranes were stacked on hepatic organoids composed of SHs and HSCs. When membranes with optimized configurations were used, HSCs selectively migrated to the EC capillary-like structures. This process was mediated, in part, by platelet-derived growth factor (PDGF) signaling. In addition, these HSCs firmly lined the outer surface of the EC capillary-like structures with long cytoplasmic processes (Kasuya et al. 2015) (Fig. 16.14). In the HSC-lined sinusoidal tissues, SHs retained higher levels of differentiated functions compared with those without ECs. This model provides a basis for the construction of functional, thick, vascularized liver tissues in vitro.
A triculture model using a biodegradable, PLGA microporous membrane. SHs, including the HSC fraction, were cultured on a culture dish, while ECs were cultured on a Matrigel-coated microporous PLGA membrane to allow them to form capillary-like structures. Next, the membrane was stacked on the top of SH colonies grown in a culture dish, resulting in a 3D stacked-up triculture. Initially, HSCs were located between the SH layer and culture dish. However, HSCs passed through the microporous membrane and finally covered the outer surface of the capillary-like structures
16.5 Summary
16.5.1 Integrated Vascular Engineering
In this chapter, we introduced in vitro culture models for constructing microvascular networks, which are angiogenesis and vasculogenesis models. Because the control of microvascular formation is important both in the context of tissue engineering and cancer therapy, numerous studies have been performed to clarify the mechanism as to how we can construct or regress the microvasculature using these in vitro models. Although “conventional” culture models are still useful in investigating vascular formation, recent advances in microfluidic technologies have allowed studying vascular formation under more controlled culture conditions. Because microfluidic devices have advantages in regulating the spatial and temporal control of cellular distributions, we can create increasingly physiological in vitro culture models that more precisely mimic capillary morphogenesis in vivo. In this context, the integration of culture microenvironments, such as biochemical and biophysical factors and cell-to-cell interactions both with temporal and spatial variations, is important for achieving more functional vascular formation.
16.5.2 Future Perspectives
Because previous studies have focused on individual factors, such as one biochemical or biophysical factor, combinations of these factors need to be investigated further, which may result in the discovery of nonlinear, synergistic, and system-level effects. Heterotypic cellular interactions are also important aspects. Because microvessels always interact with other tissues, ECs interact with other epithelial tissues and cells. Such interactions will be characterized once vascularized 3D tissues are constructed in vitro. Recent advances in microfluidic technologies have opened a new field exploring multiple organ-level functions, often referred to as “human-on-a-chip ” or “organs-on-a-chip ” (Zhang et al. 2009; Luni et al. 2014; Bhatia and Ingber 2014; Huh et al. 2011). Because organs are connected with the vascular networks of our body, methods for vascular formation described in this chapter also have the potential to be integrated into human-on-a-chip or organs-on-a-chip devices.
References
Anand-Apte B, Pepper MS, Voest E, Montesano R, Olsen B, Murphy G, Apte SS, Zetter B (1997) Inhibition of angiogenesis by tissue inhibitor of metalloproteinase-3. Invest Ophthalmol Vis Sci 38(5):817–823
Antonelli-Orlidge A, Saunders KB, Smith SR, D’Amore PA (1989) An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A 86(12):4544–4548
Armulik A, Abramsson A, Betsholtz C (2005) Endothelial/pericyte interactions. Circ Res 97(6):512–23
Au P, Tam J, Fukumura D, Jain RK (2008) Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111(9):4551–4558
Auger FA, Gibot L, Lacroix D (2013) The pivotal role of vascularization in tissue engineering. Annu Rev Biomed Eng 15:177–200
ávan der Meer AD, Dijke P, den Berg A (2013) Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 13:3562–3568
Baker B, Trappmann B, Stapleton SC, Toro E, Chen CS (2013) Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip 13:3246–3252
Bayless KJ, Davis GE (2003) Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun 312(4):903–913
Berthod F, Germain L, Tremblay N, Auger FA (2006) Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol 207(2):491–498
Bhatia SN, Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32(8):760–772
Bikfalvi A, Sauzeau C, Moukadiri H, Maclouf J, Busso N, Bryckaert M, Plouet J, Tobelem G (1991) Interaction of vasculotropin/vascular endothelial cell growth factor with human umbilical vein endothelial cells: binding, internalization, degradation, and biological effects. J Cell Physiol 149(1):50–59
Birdwell CR, Gospodarowicz D, Nicholson GL (1977) Factors from 3T3 cells stimulate proliferation of cultured vascular endothelial cells. Nature 268(5620):528–531
Bischel LL, Young EW, Mader BR, Beebe DJ (2013) Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials 34(5):1471–1477
Borenstein JT et al (2010) Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed Microdevices 12:71–79
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257
Carmeliet P, De Smet F, Loges S, Mazzone M (2009) Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 6(6):315–326
Carrion B et al (2010) Recreating the perivascular niche ex vivo using a microfluidic approach. Biotechnol Bioeng 107:1020–1028
Chaw K, Manimaran M, Tay E, Swaminathan S (2007) Multi-step microfluidic device for studying cancer metastasis. Lab Chip 7:1041–1047
Chen MB, Whisler JA, Jeon JS, Kamm RD (2013) Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr Biol 5:1262–1271
Chrobak KM, Potter DR, Tien J (2006) Formation of perfused, functional microvascular tubes in vitro. Microvasc Res 71:185–196
Chung S et al (2009a) Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9:269–275
Chung S, Sudo R, Zervantonakis IK, Rimchala T, Kamm RD (2009b) Surface‐treatment‐induced three‐dimensional capillary morphogenesis in a microfluidic platform. Adv Mater 21:4863–4867
Chung S, Sudo R, Vickerman V, Zervantonakis IK, Kamm RD (2010) Microfluidic platforms for studies of angiogenesis, cell migration, and cell–cell interactions. Ann Biomed Eng 38:1164–1177
Colgan OC et al (2007) Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physioly-Heart Circ Physiol 292:H3190–H3197
Crocker DJ, Murad TM, Geer JC (1970) Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol 13(1):51–65
Dai X et al (2011) A novel in vitro angiogenesis model based on a microfluidic device. Chin Sci Bull 56:3301–3309
Davis GE, Bayless KJ, Mavila A (2002) Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec 268(3):252–275
Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L Jr (2009) Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24(7):909–969
Estrada R et al (2011) Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Anal Chem 83:3170–3177
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Golden AP, Tien J (2007) Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 7:720–725
Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP (1990) A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A 87(17):6624–6628
Griffith LG, Naughton G (2002) Tissue engineering – current challenges and expanding opportunities. Science 295(5557):1009–1014
Griffith LG, Swartz MA (2006) Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7(3):211–24
Han S, Yan JJ, Shin Y, Jeon JJ, Won J, Jeong HE, Kamm RD, Kim YJ, Chung S (2012) A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab Chip 12:3861–3865
Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN (2001) Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15(12):2215–2224
Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445(7129):776–780
Helm CL, Fleury ME, Zisch AH, Boschetti F, Swartz MA (2005) Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci U S A 102(44):15779–15784
Hernández Vera R, Genové E, Alvarez L, Borrós S, Kamm R, Lauffenburger D, Semino CE (2009) Interstitial fluid flow intensity modulates endothelial sprouting in restricted Src-activated cell clusters during capillary morphogenesis. Tissue Eng A 15(1):175–185
Hiraki Y, Inoue H, Iyama K, Kamizono A, Ochiai M, Shukunami C, Iijima S, Suzuki F, Kondo J (1997) Identification of chondromodulin I as a novel endothelial cell growth inhibitor. Purification and its localization in the avascular zone of epiphyseal cartilage. J Biol Chem 272(51):32419–32426
Huh D, Hamilton GA, Ingber DE (2011) From 3D cell culture to organs-on-chips. Trends Cell Biol 21(12):745–754
Hurley JR, Balaji S, Narmoneva DA (2010) Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol 299(2):C444–C453
Hwa AJ, Fry RC, Sivaraman A, So PT, Samson LD, Stolz DB, Griffith LG (2007) Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J 21:2564–2579
Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL (2013) In vitro model of tumor cell extravasation. PLoS One 8:e56910
Jeon JS, Bersini S, Whisler JA, Chen MB, Dubini G, Charest JL, Moretti M, Kamm RD (2014) Generation of 3D functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr Biol (Camb) 6(5):555–63
Jeong GS, Kwon GH, Kang AR, Jung BY, Park Y, Chung S, Lee SH (2011a) Microfluidic assay of endothelial cell migration in 3D interpenetrating polymer semi-network HA-Collagen hydrogel. Biomed Microdevices 13(4):717–723
Jeong GS, Han S, Shin Y, Kwon GH, Kamm RD, Lee SH, Chung S (2011b) Sprouting angiogenesis under a chemical gradient regulated by interactions with an endothelial monolayer in a microfluidic platform. Anal Chem 83(22):8454–8459
Kalchman J, Fujioka S, Chung S, Kikkawa Y, Mitaka T, Kamm RD, Tanishita K, Sudo R (2013) A three-dimensional microfluidic tumor cell migration assay to screen the effect of anti-migratory drugs and interstitial flow. Microfluid Nanofluid 14:969–981
Kang H, Bayless KJ, Kaunas R (2008) Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiol Heart Circ Physiol 295(5):H2087–H2097
Kasuya J, Sudo R, Mitaka T, Ikeda M, Tanishita K (2011) Hepatic stellate cell-mediated three-dimensional hepatocyte and endothelial cell triculture model. Tissue Eng A 17(3–4):361–370
Kasuya J, Sudo R, Mitaka T, Ikeda M, Tanishita K (2012a) Spatio-temporal control of hepatic stellate cell-endothelial cell interactions for reconstruction of liver sinusoids in vitro. Tissue Eng A 18(9–10):1045–1056
Kasuya J, Sudo R, Tamogami R, Masuda G, Mitaka T, Ikeda M, Tanishita K (2012b) Reconstruction of 3D stacked hepatocyte tissues using degradable, microporous poly(d, l-lactide-co-glycolide) membranes. Biomaterials 33(9):2693–2700
Kasuya J, Sudo R, Masuda G, Mitaka T, Ikeda M, Tanishita K (2015) Reconstruction of hepatic stellate cell-incorporated liver capillary structures in small hepatocyte tri-culture using microporous membranes. J Tissue Eng Regen Med 9(3):247–56
Kaunas R, Kang H, Bayless KJ (2011) Synergistic regulation of angiogenic sprouting by biochemical factors and wall shear stress. Cell Mol Bioeng 4(4):547–559
Khademhosseini A, Langer R, Borenstein J, Vacanti JP (2006) Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A 103:2480–2487
Khademhosseini A, Vacanti JP, Langer R (2009) Progress in tissue engineering. Sci Am 300(5):64–71
Kieda C et al (2006) Suppression of hypoxia-induced HIF-1α and of angiogenesis in endothelial cells by myo-inositol trispyrophosphate-treated erythrocytes. Proc Natl Acad Sci 103:15576–15581
Kim L, Toh YC, Voldman J, Yu H (2007) A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 7:681–694
Kim C, Chung S, Yuchun L, Kim MC, Chan JK, Asada HH, Kamm RD (2012) In vitro angiogenesis assay for the study of cell-encapsulation therapy. Lab Chip 12(16):2942–2950
Kim S, Lee H, Chung M, Jeon NL (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13:1489–1500
Lafleur MA, Handsley MM, Knäuper V, Murphy G, Edwards DR (2002) Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115(Pt 17):3427–3438
Langer R, Vacanti JP (1993) Tissue engineering. Science 260(5110):920–926
Lee H, Kim S, Chung M, Kim JH, Jeon NL (2014) A bioengineered array of 3D microvessels for vascular permeability assay. Microvasc Res 91:90–98
Liu M-C et al (2013) Electrofluidic pressure sensor embedded microfluidic device: a study of endothelial cells under hydrostatic pressure and shear stress combinations. Lab Chip 13:1743–1753
Lovett M, Lee K, Edwards A, Kaplan DL (2009) Vascularization strategies for tissue engineering. Tissue Eng B Rev 15(3):353–370
Luni C, Serena E, Elvassore N (2014) Human-on-chip for therapy development and fundamental science. Curr Opin Biotechnol 25:45–50
Mack PJ et al (2009) Biomechanical regulation of endothelium-dependent events critical for adaptive remodeling. J Biol Chem 284:8412–8420
Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ (1990) Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 247(4938):77–79
Mansbridge JN, Liu K, Pinney RE, Patch R, Ratcliffe A, Naughton GK (1999) Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab 1(5):265–279
Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragonés J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P (2009) Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136:839–851
Montesano R, Orci L (1985) Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell 42(2):469–477
Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L (1986) Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A 83(19):7297–7301
Montesano R, Pepper MS, Belin D, Vassalli JD, Orci L (1988) Induction of angiogenesis in vitro by vanadate, an inhibitor of phosphotyrosine phosphatases. J Cell Physiol 134(3):460–6
Montesano R, Pepper MS, Orci L (1993) Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. J Cell Sci 105(Pt 4):1013–1024
Moya ML, Hsu YH, Lee AP, Hughes CC, George SC (2013) In vitro perfused human capillary networks. Tissue Eng C Methods 19(9):730–737
Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130(4):601–10
Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CC (2011) The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol Biol Cell 22(20):3791–3800
Ng CP, Helm CL, Swartz MA (2004) Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res 68(3):258–264
Nguyen D-HT et al (2013) Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci 110:6712–6717
O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79(2):315–328
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88(2):277–285
Orlidge A, D’Amore PA (1987) Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol 105(3):1455–1462
Penn JS (2008) Retinal and choroidal angiogenesis. Springer Netherlands
Pepper MS, Ferrara N, Orci L, Montesano R (1992) Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun 189(2):824–831
Pepper MS, Vassalli JD, Orci L, Montesano R (1993) Biphasic effect of transforming growth factor-beta 1 on in vitro angiogenesis. Exp Cell Res 204(2):356–363
Pepper MS, Ferrara N, Orci L, Montesano R (1995) Leukemia inhibitory factor (LIF) inhibits angiogenesis in vitro. J Cell Sci 108(Pt 1):73–83
Risau W (1997) Mechanisms of angiogenesis. Nature 386(6626):671–674
Risau W, Flamme I (1995) Vasculogenesis. Annu Rev Cell Dev Biol 11:73–91
Rouwkema J, Rivron NC, van Blitterswijk CA (2008) Vascularization in tissue engineering. Trends Biotechnol 26(8):434–441
Ruoslahti E (1996) Brain extracellular matrix. Glycobiology 6:489–492
Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE (2006) Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol 175(1):179–191
Seebach J et al (2000) Endothelial barrier function under laminar fluid shear stress. Lab Investig 80:1819–1831
Semino CE, Kamm RD, Lauffenburger DA (2006) Autocrine EGF receptor activation mediates endothelial cell migration and vascular morphogenesis induced by VEGF under interstitial flow. Exp Cell Res 312(3):289–298
Shamloo A, Heilshorn SC (2010) Matrix density mediates polarization and lumen formation of endothelial sprouts in VEGF gradients. Lab Chip 10:3061–3068
Shamloo A, Ma N, Poo M-M, Sohn LL, Heilshorn SC (2008) Endothelial cell polarization and chemotaxis in a microfluidic device. Lab Chip 8:1292–1299
Shamloo A, Xu H, Heilshorn S (2011) Mechanisms of vascular endothelial growth factor-induced pathfinding by endothelial sprouts in biomaterials. Tissue Eng A 18:320–330
Shao J et al (2009) Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress. Lab Chip 9:3118–3125
Shepro D, Morel NM (1993) Pericyte physiology. FASEB J 7(11):1031–1038
Shin Y et al (2011) In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip 11:2175–2181
Shin Y, Han S, Jeon JS, Yamamoto K, Zervantonakis IK, Sudo R, Kamm RD, Chung S (2012) Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat Protoc 7(7):1247–1259
Shin Y et al (2014) Reconstituting vascular microenvironment of neural stem cell niche in three‐dimensional extracellular matrix. Adv Healthcare Mater 3:1457–1464
Sieminski AL, Hebbel RP, Gooch KJ (2004) The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res 297(2):574–584
Song JW, Munn LL (2011) Fluid forces control endothelial sprouting. Proc Natl Acad Sci 108:15342–15347
Song JW et al (2005) Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal Chem 77:3993–3999
Song JW et al (2009) Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One 4:e5756
Soriano JV et al (2004) Inhibition of angiogenesis by growth factor receptor bound protein 2-Src homology 2 domain bound antagonists. Mol Cancer Ther 3:1289–1299
Srigunapalan S, Lam C, Wheeler AR, Simmons CA (2011) A microfluidic membrane device to mimic critical components of the vascular microenvironment. Biomicrofluidics 5:013409
Sudo R (2014) Multiscale tissue engineering for liver reconstruction. Organogenesis 10(2):216–224
Sudo R, Mitaka T, Ikeda M, Tanishita K (2005) Reconstruction of 3D stacked-up structures by rat small hepatocytes on microporous membranes. FASEB J 19:1695–1717
Sudo R, Chung S, Zervantonakis IK, Vickerman V, Toshimitsu Y, Griffith LG, Kamm RD (2009) Transport-mediated angiogenesis in 3D epithelial coculture. FASEB J 23(7):2155–2164
Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Ylä-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K (2008) Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454(7204):656–660
Tardy Y, Resnick N, Nagel T, Gimbrone M, Dewey C (1997) Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler Thromb Vasc Biol 17:3102–3106
Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H (2007) A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 7:302–309
Ueda A, Koga M, Ikeda M, Kudo S, Tanishita K (2004) Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am J Physiol Heart Circ Physiol 287(3):H994–H1002
Vailhé B, Vittet D, Feige JJ (2001) In vitro models of vasculogenesis and angiogenesis. Lab Investig 81:439–452
Vernon RB, Sage EH (1999) A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res 57(2):118–133
Wanjare M, Kusuma S, Gerecht S (2013) Perivascular cells in blood vessel regeneration. Biotechnol J 8(4):434–447
Whisler JA, Chen MB, Kamm RD (2014) Control of perfusable microvascular network morphology using a multiculture microfluidic system. Tissue Eng C Methods 20(7):543–552
Wong KH, Truslow JG, Khankhel AH, Chan KL, Tien J (2012) Artificial lymphatic drainage systems for vascularized microfluidic scaffolds. J Biomed Mater Res A 101:2181–2190
Yamamoto K, Tanimura K, Mabuchi Y, Matsuzaki Y, Chung S, Kamm RD, Ikeda M, Tanishita K, Sudo R (2013) The stabilization effect of mesenchymal stem cells on the formation of microvascular networks in a microfluidic device. J Biomech Sci Eng 8(2):114–128
Yamamura N, Sudo R, Ikeda M, Tanishita K (2007) Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng 13(7):1443–1453
Yeon JH, Ryu HR, Chung M, Hu QP, Jeon NL (2012) In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip 12:2815–2822
Young EW, Wheeler AR, Simmons CA (2007) Matrix-dependent adhesion of vascular and valvular endothelial cells in microfluidic channels. Lab Chip 7:1759–1766
Zervantonakis IK et al (2012) Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci 109:13515–13520
Zhang C, Zhao Z, Abdul Rahim NA, van Noort D, Yu H (2009) Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 9(22):3185–3392
Zhang Q, Liu T, Qin J (2012) A microfluidic-based device for study of transendothelial invasion of tumor aggregates in realtime. Lab Chip 12:2837–2842
Zheng C et al (2012) Quantitative study of the dynamic tumor–endothelial cell interactions through an integrated microfluidic coculture system. Anal Chem 84:2088–2093
Zheng Y et al (2012) In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci 109:9342–9347
Acknowledgements
This work was partially supported by Grants-in-Aid for Scientific Research (25282135, 25560208, 25249018) from the Japan Society for Promotions of Science, and by the Human Resources Program in Energy Technology of the KETEP grant from the Ministry of Trade, Industry & Energy, Republic of Korea. (No. 20124010203250).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Sudo, R., Chung, S., Shin, Y., Tanishita, K. (2016). Integrated Vascular Engineering: Vascularization of Reconstructed Tissue. In: Tanishita, K., Yamamoto, K. (eds) Vascular Engineering. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54801-0_16
Download citation
DOI: https://doi.org/10.1007/978-4-431-54801-0_16
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54800-3
Online ISBN: 978-4-431-54801-0
eBook Packages: MedicineMedicine (R0)