Abstract
Therapeutic apheresis is one of the key perioperative strategies for reduction of antibodies directed against donor antigens such as anti-ABO blood type antibody or anti-donor specific human leukocyte antigen (HLA) antibody. Simultaneous therapeutic apheresis can improve kidney survival in antibody mediated rejection.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Main PointsTherapeutic apheresis is one of the key perioperative strategies for reduction of antibodies directed against donor antigens such as anti-ABO blood type antibody or anti-donor specific human leukocyte antigen (HLA) antibody. Simultaneous therapeutic apheresis can improve kidney survival in antibody mediated rejection.
1 Introduction
Japan has very few deceased kidney donors and therefore, as a means to increase the demands of kidney transplantation, ABO-incompatible transplantation (see Notes) was started since 1989. Now, ABO-incompatible kidney transplantation accounts for about 15 % of all living related kidney transplantation in Japan, which has become a leading country in the practice of ABO-incompatible kidney transplantation. Due to the innovation of therapeutic immunosuppression, kidney survival after ABO-incompatible kidney transplantation has favorable results similar to that for ABO-compatible kidney transplantation [1] (Fig. 37.1). In the ABO-incompatible recipient, anti-A or -B antibodies directed against the ABO antigen expressed in the intima of the donor renal artery are the cause of humoral rejection. Likewise, pre-formed or newly-formed donor specific HLA antibodies are the cause of humoral rejection in HLA-antibody related rejection. Elimination of such antibodies, called desensitization, is the purpose of therapeutic apheresis in kidney transplantation and has an essential role together with immunosuppressive therapy. Because ABO-incompatible kidney transplantation needs patient desensitization before transplantation, ABO-incompatible transplantation is not indicated for deceased donor kidney transplantation.
2 Apheresis Before Transplantation
2.1 ABO-Incompatible Transplantation
2.1.1 Target of Elimination and Pathophysiology
-
Target: Anti-A or -B IgG, IgM antibody.
-
Pathophysiology: Anti-A or -B antibodies directed against donor vascular endothelium.
2.1.2 Therapeutic Effect
Removing as many anti-A or -B antibodies as possible is favored. Many institutions aim to reduce antibody titer level below 16–32 after apheresis.
2.1.3 Treatment
-
Insurance restrictions: four timest before and two times after transplantation
-
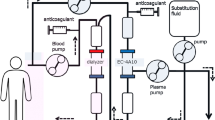
Modality: Plasma exchange (PE) and double filter plasmapheresis (DFPP) are indicated. Plasma adsorption is also possible but at the moment is not covered by insurance. The characteristics of PE and DFPP are shown in Table 37.1 [2], and the therapeutic plasma exchange volume is usually 1.2–1.5 times the plasma volume.
Table 37.1 Characteristics of therapeutic apheresis -
Actual frequency: Depending on the titer of the antibodies of the target, two to three times before transplantation is common. In our institution, DFPP is done between dialysis sessions. On the day before transplantation either PE, or DFPP with the addition of ten units of fresh frozen plasma to restore coagulation factors, is done followed by hemodialysis.
-
Therapeutic target: In most institutions, an anti-A or -B titer below 16–32 times is preferred. Due to innovations in immunosuppression, recipients with high titers can be desensitized and safely undergo transplantation treatment [3]. However, recipients showing high titer rebound after apheresis are at risk of acute antibody mediated rejection. In such patients, sufficient immunosuppression and frequent apheresis (as many as ten times) before transplantation may be indicated.
2.1.4 Points to Note
When DFPP is chosen, discarded plasma must be replaced by albumin and normal saline. When PE is chosen, plasma must be replaced by blood type AB plasma that contains no anti-A or -B antibodies.
2.2 Anti-Donor Specific HLA Antibody Positive Transplantation
2.2.1 Target of Elimination and Pathophysiology
-
Target: Mainly anti-donor specific HLA IgG antibodies.
-
Pathophysiology: Anti-donor specific HLA antibodies directed against donor kidney.
2.2.2 Therapeutic Effect
Recipients of positive direct T cell complement dependent cytotoxic (CDC) crossmatch tests are contraindicated for transplantation. However, a negative direct T cell CDC but a positive crossmatch test with flow cytometry, a highly sensitive marker, may enable transplantation (see Notes). The criteria for successful transplantation in such circumstances are not standardized and differ between institutions.
2.2.3 Treatment
-
Insurance restrictions: four times before and two times after transplantation
-
Modality: Same as in ABO incompatible transplantation
-
Actual frequency: Same as in ABO incompatible transplantation
-
Therapeutic target: Eliminating anti-donor specific HLA antibody to undetectable levels is ideal but no consensus exists on the target level.
3 Apheresis After Transplantation
3.1 Antibody Mediated Rejection
3.1.1 Target of Elimination and Pathophysiology
-
Target: Mainly IgG anti-donor specific HLA antibody or anti-A or -B IgG, IgM antibody
-
Pathophysiology: Antigen–antibody reaction due to anti donor specific HLA antibody or anti-A, -B antibody directed at donor kidney.
3.1.2 Therapeutic Effect
Treatment is based on intensifying immunosuppression by the elimination of antibodies with therapeutic apheresis. The simultaneous use of apheresis, immunosuppressants, and intravenous immunoglobulins (IVIG) have been shown to effectively treat antibody mediated rejection. However, renal survival after hyperacute rejection is extremely poor.
3.1.3 Treatment
-
Insurance restrictions: There is no indication for apheresis after transplantation for antibody mediated rejection.
-
Modality: Same as in ABO-incompatible transplantation
-
Actual frequency: Varies on the response and severity but many institutions implement two three sessions or more.
-
Therapeutic target: Anti-donor specific HLA antibody and anti-A or -B antibody may be absorbed by the donated kidney and show fairly low levels of antibody titers shortly after transplantation. Therefore, antibody titers may not reflect the activity of rejection. Furthermore, antibodies other than anti-donor specific HLA antibodies have been reported to cause antibody related rejection, which further complicates the diagnosis. The diagnosis of antibody related rejection therefore may rely on the synthetic judgment of urinary output, rising serum creatinine levels, and pre-transplant antibody titer shortly after transplantation. A biopsy is normally undertaken to confirm the diagnosis. In cases of ABO-incompatible transplantation, anti-A or -B titers increasing 2 weeks after transplantation may not reflect or induce antibody mediated rejection due to the mechanism of accommodation. However, anti-A or -B titers increasing rapidly within 2 weeks may be treated with apheresis as prophylaxis for rejection. Recent reports show poor long-term renal prognosis in recipients with high anti-HLA antibodies after transplantation [4], which suggests the need for further studies on the role of immunosuppression and apheresis.
3.2 Renal Disease Other than Antibody Mediated Rejection
Recurrence of focal segmental glomerulosclerosis (FSGS) after kidney transplantation is quite frequent (20–50 %) and treatment with apheresis, a strategy not unique to transplant patients, may be suggested. Recurrence of FSGS is known to affect renal survival (renal loss after FSGS recurrence is 13–20 %) [5]. Apheresis treatment of FSGS recurrence in kidney transplant patients is done in the same way as in non-transplant FSGS.
4 Therapies Other than Apheresis
4.1 Immunosuppression Before Kidney Transplantation
Immunosuppression directed against T cells and B cells is initiated before transplantation in living related kidney transplantation. Sufficient immunosuppression is especially needed in patients with high anti-A or -B titers in ABO incompatible transplantation, high anti-donor specific HLA antibody titers, and/or high rebound in titers after apheresis. The most frequently used immunosuppression regimen consists of calcineurin inhibitors, anti-metabolites, steroids, and anti-CD20 monoclonal antibodies (basiliximab). In ABO-incompatible transplantation, splenectomy is done adjunctively for additional B cell depletion. However, recent studies show favorable outcome with rituximab (anti-CD20 antibody) use as an alternative to splenectomy [6] and is now widely used as a standard protocol in most institutes. Unfortunately, use of rituximab in ABO-incompatible transplantation is still not covered by insurance in Japan which limits its use in some institutions.
4.2 Immunosuppression After Kidney Transplantation
In cases of uncontrollable antibody mediated rejection even after the sufficient use of immunosuppressants, splenectomy, and rituximab, IVIG administration may be required. If kidney function is unfortunately lost, transplant nephrectomy may be necessary in cases of poor general status (such as disseminated intravascular coagulation) and enlarged kidney, leading to risk of kidney rupture.
Notes:
ABO-Incompatible Transplantation
-
Specific examples are (1) type A to B or O; (2) type B to A or O; (3) type AB to A, B, or O.
-
Evaluation of anti-donor specific HLA antibody
-
Tissue typing tests are obligatory. A commonly used technique is the complement dependent cytotoxicity (CDC) test in which complement activation triggered by the recipient antibody binding on the surface of the donor antigen-expressing lymphocytes may lyse the cells. The advantage of this test is that it can detect the strength of cytotoxic effect, but its sensitivity is low. Recently, the more sensitive flow cytometric crossmatch (FCMX) test is used to detect antigen–antibody reactions between the donor lymphocytes and recipient serum. Other tests using flow cytometry and Luminex technology are available for detecting specific HLAs. Secondary transplantation, history of blood transfusion, and pregnancy (especially when the recipient has borne the child of the donor) are well known risks of anti-HLA antibody production.
Definition of Antibody Mediated Rejection
-
Pathological definitions of T cell or antibody mediated rejection are defined in the Banff 07 classification of renal allograft pathology. Antibody mediated rejection is defined as the existence of anti-donor specific HLA antibodies and C4d staining at the peritubular capillaries and morphologic evidence of tissue injury in the tubules (ATN-like inflammation), glomerulus (inflammation and/or thrombosis), or arteries (arteritis) [7].
References
Takahashi K (2008) ABO-incompatible kidney transplantation: update. Nihon Jinzo Gakkaishi 50(7):880–886
Nishi S (2009) Jin-ishoku no subete. Medical View co Ltd, Tokyo, pp 80–81
Tanabe K (2006) Jin-ishoku no shinnpo wagakunino genjyou to kongo no tenbou. Tokyo igakusha, Tokyo, pp 101–110
Lee P, Zhu L, Terasaki PI et al (2009) Transplantation 88(4):568–574
Choy B, Chan T, Lai K (2006) Am J Transplant 6(11):2535–2542
Saito K, Nakagawa Y, Suwa M et al (2006) Xenotransplantation 13(2):111–117
Solez K, Colvin R, Racusen L et al (2007) Am J Transplant 7(3):518–526
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Kawarazaki, H. (2014). Living Related Kidney Transplantation. In: Noiri, E., Hanafusa, N. (eds) The Concise Manual of Apheresis Therapy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54412-8_37
Download citation
DOI: https://doi.org/10.1007/978-4-431-54412-8_37
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54411-1
Online ISBN: 978-4-431-54412-8
eBook Packages: MedicineMedicine (R0)