Abstract
Neural circuit assembly requires the coordination of various developmental processes, including axon guidance and synapse formation. Growth cones, the leading edges of axons, navigate by interacting with different kinds of attractive and repulsive axon guidance cues along their trajectories and at target areas.
Spinal cords consist of different types of neural circuits, such as sensory-motor, commissural, and sympathetic circuits. Each neural circuit provides an unique model system to understand the cellular and molecular mechanisms underlying neural circuit formation. Studies of sensory-motor circuits have outlined the basic events leading to circuit formation. First, both sensory and motor axons project to the appropriate peripheral muscles. Sensory axons then form precise monosynaptic connections with a select group of motor neuron pools in the ventral spinal cord, and these pools of motor neurons ultimately develop their appropriate dendrite morphologies. Examination of commissural neurons and their axon trajectories in the spinal cord has contributed important data on how attractants and repellents influence the midline crossing of axons. Finally, studies of sympathetic neurons have led toward a greater overall understanding of the cellular and molecular mechanisms underlying neuronal survival, retrograde signaling, neuronal migration, and axon guidance.
Semaphorin signaling in neural circuit assembly has been studied extensively in the spinal cord. Importantly, the first mammalian semaphorin, Sema3A, was identified by monitoring growth cone collapse in sensory neurons in vitro. Here, we review the current knowledge on semaphorin signaling in various steps of vertebrate neural circuit assembly in the spinal cord, including axon guidance, synaptic specificity, synapse formation, and dendrite development, highlighting the different neural circuit systems that have helped to broaden our understanding of semaphorins and their functions in the developing nervous system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Identification of Semaphorins
The first semaphorin was originally cloned in 1992 as Fasciclin IV (Sema-I) in the grasshopper using monoclonal antibody (MAb) screens (Kolodkin et al. 1992). Subsequently, the Drosophila homologues D-Sema-I (Sema-1a) and D-Sema-II (Sema-1b) were identified a year later (Kolodkin et al. 1993). In vertebrates, Sema3A was first discovered as collapsin in chicks (Luo et al. 1993) using a functional cloning assay developed by the same group to detect growth cone collapse in sensory neurons in vitro (Raper and Kapfhammer 1990). When tested for activity, recombinant collapsin-1 (Sema3A) caused growth cones of sensory neurons to collapse but had no effect on retinal ganglion cell growth cones (Luo et al. 1993). Other collapsin members (collapsin-2 to -5; corresponding to Sema3C–Sema3E and Sema4D) were subsequently cloned in the chick (Luo et al. 1995).
Mammalian semaphorins were first cloned by two groups led by Messersmith et al. (1995) and Puschel et al. (1995), followed by other teams shortly thereafter (Inagaki et al. 1995; Wright et al. 1995; Zhou et al. 1997). Sema3A, the first mammalian semaphorin, was shown to be expressed in the ventral region of the developing spinal cord in rodents (Messersmith et al. 1995; Puschel et al. 1995). Because cutaneous and proprioceptive sensory neurons, whose cell bodies are both located in the dorsal root ganglia (DRG), project axons to the dorsal and ventral spinal cord regions, respectively (Fig. 3.1a) (Koerber and Mendell 1992; Brown 1981), the expression of Sema3A in the ventral region suggested that it may play a role in inhibiting cutaneous, but not proprioceptive, axons in the developing spinal cord. In fact, coculture assays using DRG neurons and COS cells expressing Sema3A in collagen gels showed that Sema3A repels nerve growth factor (NGF)-responsive axons, which are likely to be cutaneous axons, whereas it does not repel NT3-responsive axons, which are presumably proprioceptive axons (Fig. 3.1b) (Messersmith et al. 1995). This finding suggests that repellent Sema3A may inhibit specifically the ventral projections of cutaneous sensory axons in the spinal cord.
Sema3A repels cutaneous sensory axons. (a) Axons of Ia proprioceptive sensory neurons (red) penetrate the spinal cord, extend to the ventral spinal cord, and form synapses with motor neurons. Cutaneous axons (blue) form synapses with dorsal spinal cord neurons. Sema3A (green) is expressed in the ventral spinal cord. (b) Schematic drawing of an in vitro dorsal root ganglia (DRG) culture experiment. Exogenous Sema3A (green) acts as a repulsion cue for nerve growth factor (NGF)-induced cutaneous axons (blue, left) but not NT3-induced proprioceptive axons (red, right)
3.2 Identification of Semaphorin Receptors
The cloning of the first semaphorin receptors, plexin and neuropilin, was accomplished by Fujisawa’s group in Japan using MAb screens to search for molecules involved in axon interaction and growth cone navigation in Xenopus tadpoles. Two antibodies, MAb-A5 and MAb-B2, showed specific axon staining in their assay (Takagi et al. 1987). The protein recognized by the MAb-A5 antibody was identified (Takagi et al. 1991) and named neuropilin from the term neuropile (Satoda et al. 1995), whereas the MAb-B2 antibody was found to bind a different protein, identified as plexin-A1 from the term plexiform layer (Ohta et al. 1995). Although plexin-A1 and neuropilin were discovered concurrently by Fujisawa’s group, their interactions with one other as co-receptors for semaphorins were completely unknown at that time.
Human plexins were identified independently by molecular homology searches for the ectodomain of the Met receptor (Maestrini et al. 1996). Meanwhile, neuropilin-1 and neuropilin-2 (Npn1 and Npn2) were identified as class 3 semaphorin receptors by different groups using varying approaches: an expression cloning assay using COS cells (He and Tessier-Lavigne 1997; Kolodkin et al. 1997); a candidate molecule approach (Feiner et al. 1997); and RT-PCR cloning of Npn1-related molecules (Chen et al. 1997).
The first interactions between semaphorins and plexins were observed in the binding of viral semaphorin with a novel transmembrane protein that happened to be a virus-encoded semaphorin protein receptor (VESPR; later named plexin-C1) (Maestrini et al. 1996). Corroborating evidence for semaphorin–plexin interactions arose when plexin-A was shown to bind to Sema-1a and Sema-1b in the Drosophila nervous system (Winberg et al. 1998). The complex of class 3 semaphorin/neuropilin/plexin-A was ultimately demonstrated by three groups (Takahashi et al. 1999; Tamagnone et al. 1999; Rohm et al. 2000), who showed that plexin-As are required for Sema3A-mediated growth cone collapse of DRG or spinal neurons using dominant-negative forms of plexin-As (Takahashi et al. 1999; Tamagnone et al. 1999; Rohm et al. 2000).
3.3 Sensory and Motor Neuron Projections
As already mentioned, Sema3A was identified as a potential chemorepellent for cutaneous sensory axons (Luo et al. 1993). During development, cutaneous sensory neurons project axons to the dorsal root entry zone, then wait for several days before innervating the spinal cord. Once these axons reach the dorsal spinal cord region, they stop and do not extend to the ventral spinal cord. There are two regions where Sema3A is expressed in the spinal cord during development. First, Sema3A is expressed in the dorsal spinal cord before any afferents begin to grow into the spinal gray matter (Shepherd et al. 1997; Fu et al. 2000). Expression then declines (Fu et al. 2000), suggesting that Sema3A may inhibit sensory axon ingrowth to the spinal gray matter during the waiting period. Sema3A is also expressed in the embryonic ventral spinal cord (Wright et al. 1995; Puschel et al. 1996; Shepherd et al. 1997; Luo et al. 1995; Messersmith et al. 1995), suggesting that it may also prevent cutaneous axons from extending to the ventral region of the spinal cord during embryonic development.
Since the initial in vitro culture experiments by Messersmith et al. (1995), many in vitro and in vivo studies have corroborated the finding that Sema3A inhibits axons of cutaneous sensory neurons. For example, anti-Sema3A (collapsin-1) function-blocking antibodies neutralize repulsion of chick sensory axons by ventral spinal cord explants (Shepherd et al. 1997). In addition, recombinant Sema3A proteins injected into the explants of chick spinal cords with DRGs reduces the ingrowth of TrkA+ (presumably cutaneous) axons, whereas TrkC+ (presumably proprioceptive) axons, which do not express Npn1, are not affected (Fu et al. 2000). Adenoviral vector-mediated ectopic expression of Sema3A in rat dorsal spinal cords with DRGs also reveals that Sema3A strongly inhibits NGF-responsive afferent fibers, and these afferents fail to reach their appropriate target regions (Pasterkamp et al. 2000).
Interestingly, Sema3A can inhibit not only developing but also adult sensory afferents. Using in vitro culture systems, Sema3A has been shown to induce growth cone collapse of only small-diameter (likely cutaneous) sensory afferents from adult rats, and this effect is inhibited by anti-Npn1 function-blocking antibodies (Reza et al. 1999), suggesting that Sema3A inhibits adult sensory axons through Npn1. Moreover, gene gun-mediated ectopic expression of Sema3A in vivo repulses adult sensory afferents in adult rabbits (Tanelian et al. 1997). Sema3A was also found to inhibit NGF-induced sprouting of sensory afferents in adult rat spinal cords (Tang et al. 2004).
Many attempts to elucidate the physiological roles of semaphorins and their receptors in sensory and motor axons in vivo have been undertaken using loss-of-function approaches. Using Npn1 Sema- mice, which express a mutant Npn1 protein that fails to bind class 3 semaphorins, Gu et al. showed that TrkA+ cutaneous fibers enter the spinal cord gray matter earlier than those in wild-type mice (2003), indicating that Sema3A-Npn1 repulsive signaling is involved in eliciting the waiting period before sensory ingrowth into the spinal gray matter. Studies using single-knockout mice (mutant mice) have shown some surprising results. Despite the hypothesis that Sema3A inhibits ventral projections of cutaneous sensory neurons through the Npn1 receptor, Sema3A, Npn1, plexin-A3, and plexin-A4 mutant mice do not show strong defects in central projections of cutaneous sensory neurons in the spinal cord (Taniguchi et al. 1997; Kitsukawa et al. 1997; Cheng et al. 2001; Suto et al. 2005). Only a minor population of cutaneous axons reach the ventral spinal cord in Sema3A mutant mice (Behar et al. 1996) as well as Npn1 Sema- mice (Gu et al. 2003). Therefore, Sema3A-Npn1 signaling does not appear to have a major role in preventing cutaneous axons from entering the ventral spinal cord in vivo, even though Npn1 and co-receptors plexin-A3/A4 have been shown to be relevant receptors for mediating Sema3A-mediated growth cone collapse in vitro (Kitsukawa et al. 1997; Yaron et al. 2005; Suto et al. 2005).
In addition to showing temporally segregated expression in the dorsal and ventral spinal cord, Sema3A has also been shown to be expressed in the peripheral tissues where sensory and motor axons meet (Taniguchi et al. 1997), suggesting that Sema3A-Npn1 signaling through plexin-A3/A4 receptors may be important in regulating peripheral projections of both sensory and motor neurons. In fact, Sema3A null, Npn1 null, plexin-A3/A4 null, and Npn1 Sema- mice all showed defasciculation, disorganization, branching defects, and overshooting phenotypes of peripheral projections in both sensory and motor neurons during early embryogenesis [embryonic day (E) 10.5–12.5] (Kitsukawa et al. 1997; Yaron et al. 2005; Suto et al. 2005; Taniguchi et al. 1997; Cheng et al. 2001). Interestingly, the aberrant peripheral projections in these mutants are mostly eliminated by E15.5 (White and Behar 2000), indicating that axon pruning to remove aberrant axons occurs during this stage in embryonic development.
Motor neuron axonal trajectories have been extensively studied to understand the cellular and molecular mechanisms underlying the establishment of functional motor circuitry. Vertebrate motor neurons extend axons out of the spinal cord and innervate target skeletal muscles. Along these trajectories, motor axons encounter many axon guidance cues and ultimately find their appropriate muscle targets, including those that reside within the developing limb. In the ventral spinal cord, cohorts of motor neurons are arrayed in longitudinal columns and project their axons to distinct peripheral regions. For example, lateral motor column (LMC) neurons, which are generated only at limb levels, extend their axons into the limb mesenchyme, whereas median motor column (MMC) neurons, which are generated at all spinal cord levels, extend their axons specifically to axial muscles.
As already described, Sema3A-Npn1 signaling is required for peripheral nerve projections during mouse embryogenesis (Taniguchi et al. 1997; Kitsukawa et al. 1997; Yaron et al. 2005; Cheng et al. 2001; Suto et al. 2005). More recent detailed analyses of the roles of Sema3A-Npn1 and Sema3F-Npn2 signaling in motor axon pathfinding have shown how semaphorin signaling participates in motor axon targeting (Huber et al. 2005; Huettl et al. 2011). At E10.5, Npn1 mRNA is expressed by most LMC neurons, whereas Npn2 is expressed by only a subset of the medial LMC (LMCm) motor neurons but not by motor neurons in the lateral division of the LMC (LMCl) (Huber et al. 2005). Expression of Sema3A is detected in the limb at E10.5, when spinal nerves have extended into the plexus region but have not yet entered the limb (Huber et al. 2005), suggesting that Sema3A regulates the timing of motor axon ingrowth into the limb. Supporting this idea, precocious extensions of motor and sensory projections toward distal forelimb regions have been observed in Sema3A-mutant mice and in Npn1 Sema- mice (Gu et al. 2003; Huber et al. 2005). Sema3A is also expressed adjacent to peripheral nerve tracts within the forelimb at E11.5 (Huber et al. 2005), raising the possibility that Sema3A may regulate motor axon fasciculation at later embryonic stages. Peripheral nerves in Npn1 Sema- and Sema3A mutants also show marked defasciculation and aberrant growth when compared to wild-type mice (Fig. 3.2a, b) (Huber et al. 2005). Sema3F, which encodes a ligand for the Npn2 receptor, is expressed in the dorsal limb bud and participates in directing Npn2-expressing LMCm motor neuron axons along a ventral trajectory in the forelimb (Huber et al. 2005). Loss-of-function and gain-of-function experiments reveal that Sema3F-Npn2 signaling is required to direct LMCm motor neuron axons along their ventral trajectories (Fig. 3.2c, d; Huber et al. 2005).
Semaphorins and neuropilins are essential for motor axon innervation of the forelimb. (a) Sema3A is expressed in developing forelimbs (gray) and Npn1 is expressed by lateral motor column (LMC) motor neurons (red). LMCm (green) and LMCl (orange) axons innervate the ventral and dorsal forelimb regions, respectively. (b) Dorsoventral LMC axon innervation patterns were perturbed in Npn1 mutant and Sema3A mutant embryos. (c) Sema3F is expressed in the dorsal forelimb (gray) and Npn2 is expressed by only LMCm motor neurons (blue). (d) LMCm axon projections were affected in Npn2 mutant and Sema3F mutant embryos
A more recent study examined the reciprocal interactions between sensory and motor axons as they navigate along their trajectories and investigated the roles played by Npn1 signaling in these axon–axon interactions (Huettl et al. 2011). Deletion of Npn1 solely in motor neurons reveals that peripheral sensory projections are still established correctly, even though motor projections in the distal limbs may be severely defasciculated (Huettl et al. 2011). Genetic elimination of motor neurons demonstrates that sensory axons require only minimal motor axon scaffolding to establish their projections in the distal limb (Huettl et al. 2011). In contrast, defects in sensory axonal trajectories caused by sensory neuron-specific Npn1 deletion are accompanied by defasciculation of motor axons (Huettl et al. 2011). Thus, motor axons are dependent on sensory axons, and they interact, in part, through Npn1-mediated fasciculation in the developing limb.
In addition to peripheral tissues, Sema3A is also expressed by motor neurons. Because motor neurons also express the Sema3A receptor, Npn1, it was a mystery why both Npn1 and Sema3A are present on motor neurons. Using gain-of-function and knockdown approaches in chicks, Moret et al. (2007) found that coexpression of Npn1 and Sema3A in motor neurons regulates axon sensitivity to environmental Sema3A sources encountered along their motor axon trajectories. In chicks, Sema3A is expressed by MMC motor neurons at both early and late developmental stages, whereas Sema3A is not expressed by LMC motor neurons at early stages but is expressed when the axons reach the base of the limb (Moret et al. 2007). In contrast, Npn1 is expressed by both MMC and LMC motor neurons in the developing chick spinal cord (Moret et al. 2007). Premature expression of Sema3A in LMC motor neurons leads motor axons to defasciculate and invade territories they normally avoid, suggesting that Npn1 becomes insensitive to early ectopic environmental expression of Sema3A (Moret et al. 2007). Moreover, knockdown studies show that Sema3A expression in motor neurons is required for correct spinal nerve compaction and dorsal motor axon extension (Moret et al. 2007). Therefore, Sema3A in chick motor neurons sets the level of sensitivity of their growth cones to exogenous Sema3A exposure (Moret et al. 2007), and this regulation is associated with posttranslational and local control of Npn1 expression levels at the growth cone surface (Moret et al. 2007). This interplay between intrinsic and extrinsic Sema3A may represent a fundamental mechanism in the accurate specification of axon pathways. Future studies will explore this proposed role for nonneuronal Sema3A and the regulation of motor axon guidance.
3.4 Sensory-Motor Reflex Circuit in the Spinal Cord
In the mammalian somatosensory system, peripheral stimuli are conveyed by sensory neurons in the DRG. As mentioned earlier, DRG sensory neurons are subdivided into two major groups: those transducing proprioceptive stimuli and those transducing cutaneous sensory stimuli (Brown 1981; Koerber and Mendell 1992). Proprioceptive sensory neurons convey information about the state of muscle contraction and limb position, whereas cutaneous sensory neurons mediate a wide range of noxious and innocuous stimuli (Brown 1981; Koerber and Mendell 1992). The myelinated axons of proprioceptive sensory neurons avoid the superficial dorsal horn and project to the intermediate or ventral spinal cord, whereas the axons of cutaneous sensory neurons project directly into the superficial dorsal horn (Fig. 3.1a) (Brown 1981; Koerber and Mendell 1992). Signals mediated by Sema3A have been suggested to inhibit cutaneous axons in the ventral spinal cord (Messersmith et al. 1995; Fu et al. 2000). However, genetic inactivation of Sema3A in mice has yet to reveal a major role for these ligands in the patterning of these sensory axonal trajectories (Behar et al. 1996; Taniguchi et al. 1997; Gu et al. 2003). Nevertheless, other semaphorins are known to be expressed in the spinal cord (Cohen et al. 2005), and plexins are expressed by sensory and spinal neurons (Cheng et al. 2001; Cohen et al. 2005), raising the possibility that Sema–plexin signaling may play some role in establishing central projections of sensory afferents.
Two studies demonstrate the role of the plexin-A1 receptor and its ligand Sema6D in proprioceptive sensory axon positioning in the dorsal spinal cord and their effects on cutaneous sensory axons (Yoshida et al. 2006; Leslie et al. 2011). Plexin-A1 is exclusively expressed by proprioceptive sensory neurons in the DRG, whereas Sema6D is expressed in the dorsal spinal cord (Fig. 3.3a, left) (Yoshida et al. 2006). In plexin-A1 or Sema6D mutants, proprioceptive axons ectopically invade the dorsal horn (Fig. 3.3a, middle) (Yoshida et al. 2006; Leslie et al. 2011). Proprioceptive axons are heavily myelinated whereas most cutaneous axons either have no myelination or are only thinly myelinated (Fig. 3.3a, left and middle). Although the oligodendrocytes associated with the ectopic proprioceptive axons in plexin-A1 or Sema6D mutants synchronously invade the dorsal spinal cord (Fig. 3.3a, middle) (Yoshida et al. 2006; Leslie et al. 2011), the axonal trajectories of cutaneous sensory neurons are unaffected. However, these cutaneous sensory neurons are unable to form appropriate synapses with dorsal spinal cord neurons (Fig. 3.3a, middle). Genetic deletion of oligodendrocytes demonstrates that it is the ectopic oligodendrocytes, not the proprioceptive axons, in the dorsal spinal cord that inhibit synapse formation in the absence of Sema6D–plexin-A1 signaling (Fig. 3.3a, right) (Leslie et al. 2011). These studies provide new insights into the roles of oligodendrocytes in synapse formation in vivo, which may be an important element regulating the overall development of neural wiring in the central nervous system (CNS).
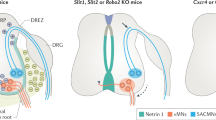
Roles of Sema–plexin signaling in sensory-motor circuitry. (a) Cross-sectional diagrams depict the spinal cords of wild-type (left), Sema6D and plexin-A1 mutant (Sema6D −/− or plexin-A1 −/−) (middle), and oligodendrocyte-deleted Sema6D mutant mice (Sema6D −/− ; ΔOligodendrocyte) (right). Plexin-A1 is expressed by proprioceptive axons (red lines; plexin-A1on) but not cutaneous axons (blue lines; plexin-A1off), and Sema6D is expressed in the dorsal spinal cord (green shaded area). Oligodendrocytes (yellow circles) and cutaneous synapses (blue dotted lines) are shown. (b, c) Cross-sectional diagrams depict the cervical (b) and lumbar (c) spinal cords of wild-type (left), motor neuron-specific Sema3E-expressing mice (middle; gain-of-function), and plexin-D1 and Sema3E mutant mice (right; loss-of-function). At cervical levels of the spinal cord, plexin-D1 is expressed by both cutaneous maximus (Cm) and triceps (Tri) sensory neurons (red and blue lines in b; plexin-D1on) and Sema3E is expressed by only Cm motor neurons (green areas in b). Cm sensory neurons project to Cm motor neurons through interneurons (shown in black in b). At spinal cord lumbar levels, plexin-D1 is expressed by many hamstring (Ham; red lines in c; plexin-D1on) but by few gluteus (Glu; blue lines in c; plexin-D1off) sensory neurons. Sema3E is only expressed by Glu motor neurons (green areas in c). lsl :lox-stop-lox
Once proprioceptive axons reach the ventral spinal cord, most of the axons make monosynaptic connections with specific motor neurons. However, in the case of cutaneous maximus (Cm) group Ia afferents that project to the Cm muscle, they form only indirect connections with Cm motor neurons through interneurons in wild-type mice (Fig. 3.3b, left) (Vrieseling and Arber 2006). This failure to develop monosynaptic inputs between Cm sensory and motor neurons was found to be regulated by plexin-D1 and its ligand Sema3E (Pecho-Vrieseling et al. 2009). Deletion of plexin-D1 or Sema3E in mice results in ectopic monosynaptic connections between Cm Ia afferents and Cm motor neurons (Fig. 3.3b, right) (Pecho-Vrieseling et al. 2009). Furthermore, ectopic expression of Sema3E in triceps (Tri) motor neurons similarly prevents monosynaptic connectivity between Tri sensory and motor neurons (Fig. 3.3b, middle) (Pecho-Vrieseling et al. 2009). Thus, repulsive Sema3E–plexin-D1 signaling controls the exclusion of sensory afferent inputs on Cm motor neuron pools at cervical levels.
With the exception of Cm motor neurons, most other motor neuron pools make monosynaptic connections with proprioceptive Ia afferents in the ventral spinal cord. Although anatomical and electrophysiological analyses have revealed many details of the specificity of monosynaptic connections between Ia afferents and motor neurons, the molecular mechanisms underlying this sensory-motor specificity remained largely unknown. Recently, the specificity of these connections has been shown to be regulated by the dorsoventral positions of motor neuron pools, independently of motor neuron-derived cues (Surmeli et al. 2011). Mice in which FoxP1 has been deleted in motor neurons exhibit scrambled motor neuron positions and defects in sensory-motor specificity (Surmeli et al. 2011). Interestingly, these FoxP1 mutants showed that the axon terminals of proprioceptive sensory neurons depend on the dorsoventral tier positions of motor neuron pools but not on the motor neurons themselves (Surmeli et al. 2011), indicating that the relevant cues are not derived from motor neurons. Although the initial patterns of sensory-motor connectivity seems to be independent of motor neurons, it is still unclear whether motor neuron-based recognition systems are involved in consolidating and reinforcing initial tier-based connectivity restrictions.
A recent study revealed that motor neuron-derived Sema3E controls pool-by-pool specificity of sensory-motor connections at lumbar levels (Fukuhara et al. 2013), in addition to inhibiting monosynaptic Cm sensory-motor connections at cervical levels (Pecho-Vrieseling et al. 2009). First, Sema3E expression was shown to be expressed by gluteus (Glu) motor neurons, but not by hamstring (Ham) motor neurons in the lumbar spinal cord (Fig. 3.3c, left) (Fukuhara et al. 2013). Conversely, plexin-D1 is expressed by many Ham proprioceptors but by few Glu proprioceptors. The authors focused on Glu and Ham motor neurons because these two motor neuron subtypes innervate muscles that control different leg joints and occupy overlapping rostrocaudal levels of the lumbar spinal cord. Both anatomical and electrophysiological analyses revealed that ectopic expression of Sema3E in Ham motor neurons reduces monosynaptic sensory-motor connections (Fig. 3.3c, middle) (Fukuhara et al. 2013). Furthermore, significant numbers of Ham sensory axons make strong aberrant monosynaptic connections with Glu motor neurons in the absence of Sema3E–plexin-D1 signaling (Fig. 3.3c, right) (Fukuhara et al. 2013). Taken together, these studies show that motor neuron-derived molecules can regulate the specificity of monosynaptic sensory-motor connections in the spinal cord. However, considering that there are approximately 50 different kinds of limb muscles, other motor neuron-derived molecules as well as motor neuron-independent cues are likely to be involved in regulating sensory-motor specificity. Future studies will identify and characterize these molecules.
3.5 Midline Crossing of Commissural Axons
Commissural neurons are located in the dorsal spinal cord and project their axons to the ventral spinal cord. After these axons reach the floor plate and cross the midline, they turn rostrally, extend longitudinally on the contralateral side of the ventral spinal cord, and eventually project to the brain. Importantly, once they cross the midline, they never re-cross it. Commissural axons are attracted by netrin-1 that is produced in the floor plate. However, after commissural axons cross the floor plate, they lose their responsiveness to chemoattractants such as netrin-1. In Drosophila, the interaction between a Slit repellent protein and its receptor Roundabout (Robo) prevent commissural axons from re-crossing the midline (Kidd et al. 1998a, b, 1999). Robo protein expression on the surface of commissural axons is low before midline crossing; however, after crossing, Robo expression is augmented, which prevents any subsequent re-crossing (Kidd et al. 1998a, b).
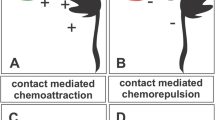
In vertebrates, Zou et al. (2000) tested whether mammalian Slit and semaphorins are involved in preventing the re-crossing of commissural axons at the midline. To understand the molecular mechanisms underlying commissural axon guidance in the spinal cord, Zou et al. (2000) developed a novel in vitro explant assay in which commissural axon extensions can be observed in vitro using an “open book” preparation where the spinal cord is opened at the dorsal midline (Fig. 3.4b) (Zou et al. 2000). This assay is very useful, because both pre- and post-midline crossing axons can be examined using explants of either the spinal cord plus the floor plate or just the dorsal spinal cord alone. Using these explant culture experiments, Slit-2, Sema3B, and Sema3F were shown to inhibit the extensions of post-crossing but not pre-crossing commissural axons (Fig. 3.4b; Zou et al. 2000).
Roles of Sema–Npn/plexin signaling in the midline. (a) Commissural neurons (red) express both Npn2 and plexin-A1. Sema3B (green) and Sema3F (cyan) are expressed in the floor plate and mantle layer, respectively, of the developing spinal cord. (b) An “open-book” view of the developing spinal cord. NrCAM (magenta circles), GDNF (gray circles), and Shh (blue circles) are expressed in the floor plate. Pre-crossing midline commissural axons (blue line) are not repulsed by Sema3B and Sema3F, but after crossing the midline these axons (red line) are actively repulsed by both Sema3B and Sema3F. (c) Molecular mechanism of calpain-mediated plexinA1 proteolysis in pre- (left; Npn2/plexin-A1inactive) and post-midline crossing commissural axons (right; Npn2/plexin-A1active). In the absence of NrCAM/GDNF, calpain (yellow) can cleave plexin-A1 (left). After crossing the midline, NrCAM and GDNF inhibit calpain-mediated plexin-A1 cleavage (right). Shh-mediated downregulation of PKA/cAMP signaling is essential for the response to Sema3B and Sema3B
As Sema3B and Sema3F are ligands for Npn2, the authors examined Npn2 mutant mice using their explant culture assay. Previous studies reported no defects in commissural axon trajectories during the period of initial axon growth to the floor plate in Npn2 mutant mice; however, the axons had not been examined within or around the floor plate (Chen et al. 2000; Giger et al. 2000). Using the open-book preparation with DiI injected into the dorsal spinal cord to visualize the commissural axons, an array of defects were detected in Npn2 mutant mice (Zou et al. 2000). Although there were no obvious defects observed in axon trajectories approaching the floor plate, some commissural axon growth cones stalled while crossing the midline in Npn2 mutant mice, and many axons within the floor plate area seemed less straight and more wavy in Npn2 mutant mice compared to controls. Moreover, many axons that did not cross the floor plate made aberrant turns causing axonal trajectories to be randomized along the anteroposterior axis. This observation led to the conclusion that Sema3B/3F–Npn2 interactions transduce repulsive signals for commissural axons in the floor plate (Fig. 3.4b).
The molecular mechanisms underlying this change of responsiveness to floor plate cues were largely unknown. Three recent studies revealed novel mechanisms that may explain how this switch occurs (Parra and Zou 2010; Nawabi et al. 2010; Charoy et al. 2012). First, the mechanisms by which semaphorin repulsion is switched on at the midline were investigated by Parra and Zou (2010). Using a collagen explant assay, sonic hedgehog (Shh) was shown to activate a novel repulsive response to Sema3B and Sema3F in pre-crossing commissural axons (Parra and Zou 2010). Furthermore, perturbing the Shh receptors Ptch1 and Smo, or blocking Shh activity, caused defects in midline guidance, indicating that post-crossing commissural axons are no longer repulsed by Sema3B or Sema3F (Parra and Zou 2010). The involvement of cyclic nucleotides in this switching event was also examined because they are known to influence signaling in response to general guidance cues, with the ratio of cAMP/cGMP being particularly important for regulating axonal attraction and repulsion in vitro (Song et al. 1998). Consistent with previous observations, enhancing protein kinase A (PKA) activity in pre-crossing axons diminishes Shh-induced semaphorin-mediated repulsion and causes profound midline stalling, along with overshooting and wandering of post-crossing axons (Parra and Zou 2010). Thus, this study showed that Shh can alter commissural axon guidance responses to semaphorins by reducing cAMP/PKA signaling (Fig. 3.3c).
The second study presented a different mechanism for altering the responsiveness to guidance cues by commissural axons (Nawabi et al. 2010). In this study, plexin-A1 was shown to bind to Npn2 to mediate repellency to Sema3B (Nawabi et al. 2010). Interestingly, plexin-A1 protein levels in commissural axons are upregulated by floor plate signals that prevent calpain 1 from cleaving and inactivating plexin-A1 (Nawabi et al. 2010). NrCAM was identified as the floor plate cue responsible for regulating plexin-A1 levels (Nawabi et al. 2010). NrCAM was a candidate signaling molecule because of its high and restricted expression in the floor plate and its ability to regulate axon growth and guidance during the formation of various commissural tracts (Falk et al. 2005; Williams et al. 2010). A variety of in vitro and in vivo experiments revealed that floor plate-derived NrCAM indeed inhibits plexin-A1 cleavage by calpain 1 and thus increases growth cone sensitization to the repellent signals of Sema3B (Fig. 3.4c) (Nawabi et al. 2010). These results demonstrate novel mechanisms for changing commissural axon responsiveness to semaphorin guidance cues.
In the third study, the same group that investigated NrCAM also showed that glial cell line-derived neurotrophic factor (GDNF) activates midline repulsion by Sema3B during commissural axon guidance (Charoy et al. 2012). The authors first found that GDNF is strongly expressed in the floor plate at E11.5–E12.5 during commissural axon crossing; however, they also showed that GDNF is not required for commissural axons to reach the floor plate and that it does not elicit commissural growth cone collapse (Charoy et al. 2012). As others have shown that (1) the sensitivity of commissural axons to floor plate repellents is switched on after midline crossing (Evans and Bashaw 2010; Chedotal 2011; Nawabi and Castellani 2011), and (2) the local floor plate cues trigger responsiveness of commissural axons to the midline repellent, Sema3B (Nawabi et al. 2010), the authors examined whether GDNF could be a local floor plate cue. When tested, GDNF induced Sema3B-mediated collapse of commissural growth cones (Charoy et al. 2012). Interestingly, GDNF also mediates suppression of calpain 1 activity and increases plexin-A1 protein levels in commissural neurons similar to floor plate-derived NrCAM (Charoy et al. 2012). Moreover, the analysis of GDNF/NrCAM double-mutant mice showed that neither cue is redundant: both are required to ensure appropriate axon guidance across the floor plate (Charoy et al. 2012). The major receptors for GDNF are tyrosine kinase RET and IgSFCAM NCAM, but only NCAM was found to be required for GDNF-induced regulation of plexin-A1 protein levels and capain 1 activity in commissural axons (Fig. 3.4c) (Charoy et al. 2012).
3.6 Segregation Between the Central and Peripheral Nervous System
The physical separation between the CNS and the peripheral nervous system (PNS) is evident at the ventral and dorsal root transitional zones in the spinal cord where two boundaries are observed. The first boundary exists at the motor exit point (MEP) where motor axons leave the spinal cord, and the second boundary appears at the dorsal root entry zone (DREZ) where sensory axons enter the spinal cord. Boundary cap (BC) cells at the MEP inhibit the emigration of motor neurons from the ventral spinal cord (Vermeren et al. 2003), and deletion of these BC cells leads to the ectopic positioning of motor neuron cell bodies along their axons and into the ventral nerve roots (Vermeren et al. 2003).
Semaphorin–plexin signaling aids in establishing the division between CNS and PNS (Bron et al. 2007; Mauti et al. 2007). Sema6A is expressed in BC cells, and deletion of Sema6A in mice (Bron et al. 2007) or downregulation of Sema6A (Mauti et al. 2007) in chicks causes ectopic motor neuron cell bodies to be distributed along the ventral roots. Because class 6 semaphorins bind to plexin-A family members (Toyofuku et al. 2004; Suto et al. 2005; Yoshida et al. 2006), plexin-A1 or plexin-A2 knockdown chick embryos or Npn2 mouse mutant embryos have been studied and noted to exhibit similar defects (Mauti et al. 2007; Bron et al. 2007). It remains unclear which plexin-A is required for Sema6A signaling in mice, but these knockdown studies suggest that Sema6A/plexin-A/Npn2 signaling is involved in constraining spinal motor neuron migration at the MEP. Furthermore, knockdown of Sema6A, plexin-A1, plexin-A4, or Sema6D causes improper formation and segregation of dorsal roots (Mauti et al. 2007), indicating that semaphorin–plexin signaling functions in the DREZ as well as in the MEP.
3.7 Dendrite Development in the Spinal Cord
The development of dendrites, including growth, targeted extension, and branching, must be precisely regulated (Jan and Jan 2003). Sema3A has been shown to control dendritic growth and orientation of cortical pyramidal neuron subtypes in the developing brain (Polleux et al. 2000; Fenstermaker et al. 2004), and recently Sema signaling has been implicated in dendrite development in the spinal cord (Zhuang et al. 2009).
Specific motor neuron pools exhibit distinct dendritic arbor morphologies that are critical for their ability to receive and process various types of synaptic inputs (Landmesser 1978; Okado et al. 1990; Vrieseling and Arber 2006). Although the molecular mechanisms underlying motor pool-specific dendrite development are not well understood, it is known that the Ets class transcription factor, Pea3, is expressed by only a few motor neuron pools, and in Pea3 mutants, aberrant dendrite morphology is detected in Pea3on motor neuron pools (Vrieseling and Arber 2006). Interestingly, Sema3E mRNA expression is absent in these Pea3on motor neuron pools (Livet et al. 2002), raising the possibility that semaphorin signaling may function in regulating dendrite morphologies of motor neurons. In fact, Sema6A–plexin-A4 signaling controls dendritic growth of motor neuron subtypes in the spinal cord by modulating a downstream signaling molecule, FARP1, which is a FERM Rho-GEF protein (Zhuang et al. 2009). FARP1 proteins are expressed on dendrites of LMC, but not MMC, motor neurons in chicks (Zhuang et al. 2009). Ectopic expression studies as well as knockdown approaches demonstrate that FARP1 is sufficient and essential for regulating the dendrite process length of LMC motor neurons (Zhuang et al. 2009). FARP2, a protein related to FARP1, interacts with all plexin-A family members (Toyofuku et al. 2005), whereas FARP1 strongly binds to plexin-A4 but associates only very weakly with plexin-A1 (Zhuang et al. 2009). Gain-of-function and knockdown experiments for Sema6A and plexin-A4 show results similar to those observed following similar manipulations of FARP1, suggesting that FARP1 is a downstream molecule of Sema6A–plexin-A4 signaling (Zhuang et al. 2009). Sema6A has thus been shown to regulate motor neuron subtype-specific dendritic growth through the action of FARP1. Future studies will explore other potential roles of semaphorin–plexin signaling in the regulation of dendrite morphology in motor neuron pools and other CNS neurons.
3.8 Sympathetic Nervous System
The sympathetic nervous system, a major component of the autonomic nervous system, is required for organ homeostasis. Postganglionic sympathetic neurons arise from neural crest cells and eventually establish the superior cervical ganglion (SCG), whereas preganglionic neurons are generated and located within the spinal cord. Sympathetic neurons, particularly those from the SCG, have been used widely to understand the cellular and molecular mechanisms governing neuronal survival, retrograde signaling, neuronal migration, and axon guidance (Glebova and Ginty 2005).
Sema3A, Sema3C, Sema3D, and Sema3F have been shown to induce growth cone collapse or axonal repulsion of SCG neurons in vitro (Koppel et al. 1997; Adams et al. 1997; Chen et al. 1998), indicating that Npns and plexins are likely expressed by SCG neurons. In fact, Npn1, Npn2, plexin-A3, and plexin-A4 have all been shown to be expressed in the SCG (Cheng et al. 2001; Chen et al. 1998). SCG explants with COS cell aggregates expressing different class 3 semaphorins revealed that Sema3A and Sema3F signals are mediated by Npn1 and Npn2, respectively, and both Npn1 and Npn2 can transduce Sema3C signals (Chen et al. 1998). Conversely, loss-of-function together with SCG explant culture experiments using COS cells expressing class 3 semaphorins showed that Npn2 is, indeed, required for axon repulsion of sympathetic neurons by Sema3F but not Sema3A (Chen et al. 2000).
Interestingly, Npn1 and Sema3A mutant embryos showed displacement of both sympathetic precursors and neurons in vitro (Kawasaki et al. 2002), suggesting that Sema3A–Npn1 signaling controls cell migration. In fact, Sema3A suppressed neuronal migration activity of sympathetic neurons (Kawasaki et al. 2002). Further studies in which Sema3A or Npn1 are deleted after the completion of neuronal migration will have to be conducted to determine whether Sema3A–Npn1 signaling is also involved in axon guidance of SCG neurons in vivo.
Because both plexin-A3 and plexin-A4 are strongly expressed by SCG neurons (Cheng et al. 2001), SCG axons were analyzed in plexin-A3 single, plexin-A4 single, and plexin-A3/A4 double mutants (Cheng et al. 2001; Yaron et al. 2005; Suto et al. 2005). Sema3F-mediated SCG axon repulsion was completely abolished in plexin-A3 single-mutant mice, but plexin-A4-deficient SCG axons responded to Sema3F in a manner similar to wild-type neurons (Cheng et al. 2001; Yaron et al. 2005; Suto et al. 2005). Thus, the Npn2–plexin-A3 receptor complex mediates Sema3F-induced SCG axonal repulsion. In contrast, both plexin-A3 and plexin-A4 transduce Sema3A signals to induce SCG axon repulsion (Cheng et al. 2001; Yaron et al. 2005). In vivo, aberrant axonal projections of SCG are observed in plexin-A4 mutant mice (Suto et al. 2005). In contrast to Npn1 mutant mice, plexin-A4 mutant mice do not show displacement of SCG neurons (Suto et al. 2005). Future studies, particularly conditional targeting approaches, will reveal further roles for semaphorin signaling in the different steps of sympathetic nervous system development.
3.9 Motor Axon Guidance in Zebrafish
Three types of primary motor neurons are located in each trunk segment of zebrafish: caudal primary (CaP), middle primary (MiP), and rostral primary (RoP) motor neurons (Fig. 3.5a). Half the segments additionally contain a fourth type of motor neuron called variable primary (VaP); however, only a few VaP motor neurons survive until 36 h postfertilization (Beattie 2000). Axons of CaP, MiP, and RoP motor neurons exit the spinal cord from a defined exit point and innervate their specific targets on myotomes (Fig. 3.5a). Using transplant experiments, the myotome has been shown to be essential for motor axon targeting, suggesting that the myotome or myotome-derived factors regulate motor axon targeting (Beattie and Eisen 1997).
Motor and sensory neuron development in zebrafish. (a) Caudal primary (CaP), middle primary (MiP), and rostral primary (RoP) motor neurons are located in the ventral spinal cord. Their axons exit the spinal cord from the exit point (black dotted circle) and innervate the myotome (orange shaded area). RoP, MiP, and CaP primary motor neurons all express plexin-A3. Npn1a is expressed by only CaP motor neurons before axons cross the horizontal myoseptal region (gray dotted line; left diagram). Expression patterns of Sema3As (magenta shaded area) also change from the posterior half of the myotome (left diagram) to the dorsal and ventral myotome regions with the exception of the horizontal myoseptal region (right diagram). At this stage CaP neurons do not express Npn1a, and CaP axons no longer respond to the repellent signals of Sema3A (black line in right diagram). (b) RB sensory neurons (green) project both centrally and peripherally. Sema3D (magenta shaded area) is expressed in the roof plate
Zebrafish contain two Npn1s (Npn1a and Npn1b) and two Sema3As (Sema3A1 and Sema3A2). Npn1a, which is expressed in CaP motor neurons, and its ligands Sema3A1 and Sema3A2, which are expressed in the myotome, all exhibit dynamic expression patterns (Feldner et al. 2005; Sato-Maeda et al. 2006; Shoji et al. 2003). Npn1a expression in motor neurons is only observed while their axons are extending to the horizontal myoseptal region (Fig. 3.5a, left). Once this region is crossed, Npn1a expression is no longer detected (Fig. 3.5a, right). In contrast, Sema3A1 and Sema3A2 are initially expressed in the posterior half of the myotome; then, in later stages, only Sema3A1 is expressed in the myotome dorsal region with the exception of the horizontal myoseptal region (Fig. 3.5a). At these later stages, CaP neurons no longer respond to Sema3A1 as a result of diminished expression of Npn1a (Fig. 3.5a).
Npn1a morphants show aberrant axon growth and multiple nerve exit points (Feldner et al. 2005; Sato-Maeda et al. 2006, 2008). Although Npn1a–Sema3A1 signaling is essential for axon growth, Npn1a–Sema3A2 signaling has been shown to be important for CaP positioning (Feldner et al. 2005; Sato-Maeda et al. 2006, 2008), which affects the location of CaP axons at the exit point (Sato-Maeda et al. 2008).
Another Sema3A receptor, plexin-A3, is expressed by primary motor neurons, and the plexin-A3 mutants sidetracked and vermicelli both show aberrant motor neuron exit points and axonal trajectories (Tanaka et al. 2007; Palaisa and Granato 2007). Double knockdown of plexin-A3 and Sema3As causes synergistic effects, but double knockdown of plexin-A3 and Npn1a does not affect the branching or exit points of motor axons (Feldner et al. 2007), suggesting that plexin-A3 acts as the receptor for Sema3As rather than as a co-receptor for Npn1a. Thus, both the Sema3A–Npn1a and Sema3A–plexin-A3 signaling cascades likely act independently in the guidance of primary motor axons. Another class 3 semaphorin, Sema3F, is also expressed in the myotome, raising the possibility that it may serve as an additional plexin-A3 ligand along with Sema3A (Palaisa and Granato 2007). Plexin-A3 together with plexin-A1 also acts as a receptor for class 5 semaphorins, and this semaphorin–plexin signaling is essential for retinal lamination and function (Matsuoka et al. 2011). The myotome also expresses a class 5 semaphorin, Sema5A, which, similar to plexin-A3, is involved in determining exit points and axon trajectories of motor neurons (Hilario et al. 2009). In fact, double knockdown of Sema5A and plexin-A3 produces synergistic exit point defects, suggesting that Sema5A binds to plexin-A3 to regulate exit pathways. Although plexin-B1 is another potential ligand for Sema5A, the double-knockdown phenotype of plexin-B1 and Sema5A does not exhibit any synergistic effects. These data suggest that other plexins, such as plexin-A1, could potentially be involved as Sema5A receptor(s) in regulating axon branching in zebrafish. In the zebrafish cranium, trigeminal motor neurons and facial motor neurons that originate from rhombomeres project into jaw muscles. Plexin-A3 is expressed in these cranial motor neurons, whereas Sema3A1 is expressed in the brachial region. In fact, both plexin-A3 mutants and Sema3A1 morphants show defects in facial motor axon guidance (Tanaka et al. 2007). Another semaphorin family member that is also expressed in the brachial region, Sema4E (Xiao et al. 2003), has been shown to inhibit motor axon growth. Conversely, Sema4E morphants show defasciculation of motor axons. These data demonstrate that spatial expression patterns of Sema3A1 and Sema4E are essential for proper motor axon guidance in the cranial region. Further studies will identify the relevant receptors for Sema4E in these processes.
3.10 Sensory Axon Guidance in Zebrafish
Rohon-Beard (RB) sensory neurons are the primary sensory neurons in zebrafish and provide a great model system for understanding sensory development in vertebrates. Central projections of RB sensory neurons innervate the spinal cord whereas peripheral projections extend into the myotome (Fig. 3.5b). The transcription factors, Islet 1 and 2a (Isl1 and Isl2a), are expressed by RB sensory neurons to regulate their peripheral projections. Isl2a is essential for plexin-A4 expression in RB sensory neurons (Miyashita et al. 2004). In addition, in embryos that overexpress the LIM domain of Isl1, plexin-A4 expression was reduced and peripheral axon projections were eliminated. Plexin-A4 knockdown also shows reduction in axon branching, suggesting that plexin-A4 is involved in regulating branching specifically, rather than affecting axon guidance. Interestingly, excessive branching of peripheral axons caused by Slit2 overexpression is rescued by plexin-A4 knockdown (Miyashita et al. 2004). These data suggest that plexin-A4 acts as a downstream target of Slit signaling to regulate peripheral axon branching.
Isl1 is also important for regulating the expression of collapsin-response mediator protein 4 (Crmp4), which is involved in the semaphorin-mediated signaling pathway controlling cytoskeleton and endocytosis regulation in RB neurons (Schmidt and Strittmatter 2007; Tanaka et al. 2011). Knockdown of Crmp4 affects peripheral axon growth of RB neurons, and this phenotype is similar to Sema3D morphants (Liu and Halloran 2005; Tanaka et al. 2011). Additionally, double knockdown of Sema3D and Crmp4 shows synergistic effects on peripheral axon growth. These data suggest that Sema3D and Crmp4 act on the same signaling cascade. Interestingly, both Sema3D and Crmp4 knockdown do not affect central projections of RB neurons. Only peripheral axons were shown to respond to ectopic Sema3D (Liu and Halloran 2005; Tanaka et al. 2011), indicating that Sema3D–Crmp4 signaling primarily affects the growth of peripheral axons. It has been hypothesized that the expression of Sema3D in the midline roof plate might repel peripheral axons from the spinal cord (Fig. 3.5b) (Liu and Halloran 2005; Tanaka et al. 2011). Alternatively, Sema3D might act as an initial stimulator for axon branching (Liu and Halloran 2005). In fact, Sema–plexin signaling is involved in axon branching in the fly and vertebrate retina (Campbell and Holt 2001; Winberg et al. 1998). Further studies seeking functional Sema3D receptor(s) such as plexins and neuropilins, and on the relationships between Sema3D signaling and their potential downstream targets such as Crmp4, will provide new insights into the molecular mechanisms of Sema–plexin-mediated sensory axon growth in zebrafish.
3.11 Summary
Semaphorins play various roles in neural development, such as in axon guidance, dendrite development, and synapse formation in the spinal cord through their plexin and neuropilin receptors. This chapter highlights the various combinations of semaphorins and their receptors that control different steps of neural circuit development within the spinal cord. Similar molecular mechanisms are observed in the invertebrate nervous system and the vertebrate developing brain (see earlier chapter by Kolodkin). Future studies will reveal how semaphorin signaling controls not only embryonic but also adult neural circuitry, whether it regulates neuronal degeneration/regeneration, and what upstream and downstream molecules are involved in different semaphorin signaling cascades. Furthering our knowledge of the roles of semaphorin signaling in neural circuit assembly and regeneration will indicate some of the fundamental mechanisms involved in establishing functional neural circuits, and may one day serve as a new paradigm for understanding how signaling pathways shape and influence the developing and adult nervous system.
References
Adams RH, Lohrum M, Klostermann A, Betz H, Puschel AW (1997) The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J 16(20):6077–6086. doi:10.1093/emboj/16.20.6077
Beattie CE (2000) Control of motor axon guidance in the zebrafish embryo. Brain Res Bull 53(5):489–500. doi:S0361-9230(00)00382-8 [pii]
Beattie CE, Eisen JS (1997) Notochord alters the permissiveness of myotome for pathfinding by an identified motoneuron in embryonic zebrafish. Development 124(3):713–720
Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC (1996) Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383(6600):525–528. doi:10.1038/383525a0
Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J (2007) Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev 2:21. doi:1749-8104-2-21 [pii] 10.1186/1749-8104-2-21
Brown AG (1981) Organization in the spinal cord. Springer, New York
Campbell DS, Holt CE (2001) Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32:1013–1026
Charoy C, Nawabi H, Reynaud F, Derrington E, Bozon M, Wright K, Falk J, Helmbacher F, Kindbeiter K, Castellani V (2012) gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron 75(6):1051–1066. doi:10.1016/j.neuron.2012.08.021 S0896-6273(12)00758-1 [pii]
Chedotal A (2011) Further tales of the midline. Curr Opin Neurobiol 21(1):68–75. doi:S0959-4388(10)00116-9 [pii] 10.1016/j.conb.2010.07.008
Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M (1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19(3):547–559. doi:S0896-6273(00)80371-2 [pii]
Chen H, He Z, Bagri A, Tessier-Lavigne M (1998) Semaphorin–neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21(6):1283–1290. doi:S0896-6273(00)80648-0 [pii]
Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, Tessier-Lavigne M (2000) Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 25(1):43–56. doi:S0896-6273(00)80648-0 [pii]
Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M (2001) Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron 32(2):249–263. doi:S0896-6273(01)00478-0 [pii]
Cohen S, Funkelstein L, Livet J, Rougon G, Henderson CE, Castellani V, Mann F (2005) A semaphorin code defines subpopulations of spinal motor neurons during mouse development. Eur J Neurosci 21(7):1767–1776. doi:EJN4021 [pii] 10.1111/j.1460-9568.2005.04021.x
Evans TA, Bashaw GJ (2010) Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol 20(1):79–85. doi:S0959-4388(09)00196-2 [pii] 10.1016/j.conb.2009.12.006
Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, Sanes JR, Castellani V (2005) Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 48(1):63–75. doi:S0896-6273(05)00737-3 [pii] 10.1016/j.neuron.2005.08.033
Feiner L, Koppel AM, Kobayashi H, Raper JA (1997) Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron 19(3):539–545. doi:S0896-6273(00)80370-0 [pii]
Feldner J, Becker T, Goishi K, Schweitzer J, Lee P, Schachner M, Klagsbrun M, Becker CG (2005) Neuropilin-1a is involved in trunk motor axon outgrowth in embryonic zebrafish. Dev Dyn 234(3):535–549. doi:10.1002/dvdy.20520
Feldner J, Reimer MM, Schweitzer J, Wendik B, Meyer D, Becker T, Becker CG (2007) PlexinA3 restricts spinal exit points and branching of trunk motor nerves in embryonic zebrafish. J Neurosci 27(18):4978–4983. doi:27/18/4978 [pii] 10.1523/JNEUROSCI.1132-07.2007
Fenstermaker V, Chen Y, Ghosh A, Yuste R (2004) Regulation of dendritic length and branching by semaphorin 3A. J Neurobiol 58(3):403–412. doi:10.1002/neu.10304
Fu SY, Sharma K, Luo Y, Raper JA, Frank E (2000) SEMA3A regulates developing sensory projections in the chicken spinal cord. J Neurobiol 45(4):227–236. doi:10.1002/1097-4695(200012)45:4<227::AID-NEU4>3.0.CO;2-N [pii]
Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR et al (2013) Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. Cell Rep 5:748–758
Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, Kolodkin AL, Ginty DD, Geppert M (2000) Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 25(1):29–41. doi:S0896-6273(00)80869-7 [pii]
Glebova NO, Ginty DD (2005) Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28:191–222. doi:10.1146/annurev.neuro.28.061604.135659
Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD (2003) Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 5(1):45–57
He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90(4):739–751. doi:S0092-8674(00)80534-6 [pii]
Hilario JD, Rodino-Klapac LR, Wang C, Beattie CE (2009) Semaphorin 5A is a bifunctional axon guidance cue for axial motoneurons in vivo. Dev Biol 326(1):190–200. doi:10.1016/j.ydbio.2008.11.007 S0012-1606(08)01351-1 [pii]
Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL (2005) Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron 48(6):949–964. doi:S0896-6273(05)01049-4 [pii] 10.1016/j.neuron.2005.12.003
Huettl RE, Soellner H, Bianchi E, Novitch BG, Huber AB (2011) Npn-1 contributes to axon–axon interactions that differentially control sensory and motor innervation of the limb. PLoS Biol 9(2):e1001020. doi:10.1371/journal.pbio.1001020
Inagaki S, Furuyama T, Iwahashi Y (1995) Identification of a member of mouse semaphorin family. FEBS Lett 370(3):269–272. doi:0014579395008509 [pii]
Jan YN, Jan LY (2003) The control of dendrite development. Neuron 40(2):229–242. doi:S0896627303006317 [pii]
Kawasaki T, Bekku Y, Suto F, Kitsukawa T, Taniguchi M, Nagatsu I, Nagatsu T, Itoh K, Yagi T, Fujisawa H (2002) Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development 129(3):671–680
Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G (1998a) Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92(2):205–215. doi:S0092-8674(00)80915-0 [pii]
Kidd T, Russell C, Goodman CS, Tear G (1998b) Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron 20(1):25–33. doi:S0896-6273(00)80431-6 [pii]
Kidd T, Bland KS, Goodman CS (1999) Slit is the midline repellent for the robo receptor in Drosophila. Cell 96(6):785–794. doi:S0092-8674(00)80589-9 [pii]
Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H (1997) Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19(5):995–1005. doi:S0896-6273(00)80392-X [pii]
Koerber HR, Mendell LM (1992) Functional heterogeneity of dorsal root ganglion cells. In: Scott SA (ed) Sensory neurons: diversity, development and plasticity. Oxford University Press, New York
Kolodkin AL, Matthes DJ, O’Connor TP, Patel NH, Admon A, Bentley D, Goodman CS (1992) Fasciclin IV: sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 9(5):831–845. doi:0896-6273(92)90237-8 [pii]
Kolodkin AL, Matthes DJ, Goodman CS (1993) The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75(7):1389–1399. doi:0092-8674(93)90625-Z [pii]
Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD (1997) Neuropilin is a semaphorin III receptor. Cell 90(4):753–762. doi:S0092-8674(00)80535-8 [pii]
Koppel AM, Feiner L, Kobayashi H, Raper JA (1997) A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron 19(3):531–537. doi:S0896-6273(00)80369-4 [pii]
Landmesser L (1978) The development of motor projection patterns in the chick hind limb. J Physiol 284:391–414
Leslie JR, Imai F, Fukuhara K, Takegahara N, Rizvi TA, Friedel RH, Wang F, Kumanogoh A, Yoshida Y (2011) Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation. Development 138(18):4085–4095. doi:dev.066076 [pii] 10.1242/dev.066076
Liu Y, Halloran MC (2005) Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci 25(45):10556–10563. doi:25/45/10556 [pii] 10.1523/JNEUROSCI.2710-05.2005
Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S (2002) ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 35(5):877–892. doi:S0896627302008632 [pii]
Luo Y, Raible D, Raper JA (1993) Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75(2):217–227. doi:0092-8674(93)80064-L [pii]
Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper JA (1995) A family of molecules related to collapsin in the embryonic chick nervous system. Neuron 14(6):1131–1140. doi:0896-6273(95)90261-9 [pii]
Maestrini E, Tamagnone L, Longati P, Cremona O, Gulisano M, Bione S, Tamanini F, Neel BG, Toniolo D, Comoglio PM (1996) A family of transmembrane proteins with homology to the MET-hepatocyte growth factor receptor. Proc Natl Acad Sci U S A 93(2):674–678
Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H et al (2011) Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron 71:460–473
Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET (2007) Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Dev 2:28. doi:1749-8104-2-28 [pii] 10.1186/1749-8104-2-28
Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL (1995) Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron 14(5):949–959. doi:0896-6273(95)90333-X [pii]
Miyashita T, Yeo SY, Hirate Y, Segawa H, Wada H, Little MH, Yamada T, Takahashi N, Okamoto H (2004) PlexinA4 is necessary as a downstream target of Islet2 to mediate Slit signaling for promotion of sensory axon branching. Development 131(15):3705–3715. doi:10.1242/dev.01228 dev.01228 [pii]
Moret F, Renaudot C, Bozon M, Castellani V (2007) Semaphorin and neuropilin co-expression in motoneurons sets axon sensitivity to environmental semaphorin sources during motor axon pathfinding. Development 134(24):4491–4501. doi:134/24/4491 [pii] 10.1242/dev.011452
Nawabi H, Castellani V (2011) Axonal commissures in the central nervous system: how to cross the midline? Cell Mol Life Sci 68(15):2539–2553. doi:10.1007/s00018-011-0691-9
Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, Moret F, Abouzid K, Castellani V (2010) A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev 24(4):396–410. doi:10.1101/gad.542510 24/4/396 [pii]
Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, Takagi S, Tanaka H, Fujisawa H (1995) Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron 14(6):1189–1199. doi:0896-6273(95)90266-X [pii]
Okado N, Homma S, Ishihara R, Kohno K (1990) Distribution patterns of dendrites in motor neuron pools of lumbosacral spinal cord of the chicken. Anat Embryol (Berl) 182(2):113–121
Palaisa KA, Granato M (2007) Analysis of zebrafish sidetracked mutants reveals a novel role for Plexin A3 in intraspinal motor axon guidance. Development 134(18):3251–3257. doi:dev.007112 [pii] 10.1242/dev.007112
Parra LM, Zou Y (2010) Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci 13(1):29–35. doi:10.1038/nn.2457 nn.2457 [pii]
Pasterkamp RJ, Giger RJ, Baker RE, Hermens WT, Verhaagen J (2000) Ectopic adenoviral vector-directed expression of Sema3A in organotypic spinal cord explants inhibits growth of primary sensory afferents. Dev Biol 2:129–141. doi:10.1006/dbio.2000.9627 S0012-1606(00)99627-1 [pii]
Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S (2009) Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature 459(7248):842–846. doi:10.1038/nature08000 nature08000 [pii]
Polleux F, Morrow T, Ghosh A (2000) Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404(6778):567–573. doi:10.1038/35007001
Puschel AW, Adams RH, Betz H (1995) Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron 14(5):941–948. doi:0896-6273(95)90332-1 [pii]
Puschel AW, Adams RH, Betz H (1996) The sensory innervation of the mouse spinal cord may be patterned by differential expression of and differential responsiveness to semaphorins. Mol Cell Neurosci 7(5):419–431. doi:S1044-7431(96)90030-5 [pii] 10.1006/mcne.1996.0030
Raper JA, Kapfhammer JP (1990) The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron 4(1):21–29. doi:0896-6273(90)90440-Q [pii]
Reza JN, Gavazzi I, Cohen J (1999) Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol Cell Neurosci 14(4-5):317–326. doi:S1044-7431(99)90786-8 [pii] 10.1006/mcne.1999.0786
Rohm B, Ottemeyer A, Lohrum M, Puschel AW (2000) Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev 93(1-2):95–104. doi:S0925477300002690 [pii]
Satoda M, Takagi S, Ohta K, Hirata T, Fujisawa H (1995) Differential expression of two cell surface proteins, neuropilin and plexin, in Xenopus olfactory axon subclasses. J Neurosci 15(1 pt 2):942–955
Sato-Maeda M, Tawarayama H, Obinata M, Kuwada JY, Shoji W (2006) Sema3a1 guides spinal motor axons in a cell- and stage-specific manner in zebrafish. Development 133(5):937–947. doi:dev.02268 [pii] 10.1242/dev.02268
Sato-Maeda M, Obinata M, Shoji W (2008) Position fine-tuning of caudal primary motoneurons in the zebrafish spinal cord. Development 135(2):323–332. doi:dev.007559 [pii] 10.1242/dev.007559
Schmidt EF, Strittmatter SM (2007) The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol 600:1–11. doi:10.1007/978-0-387-70956-7_1
Shepherd IT, Luo Y, Lefcort F, Reichardt LF, Raper JA (1997) A sensory axon repellent secreted from ventral spinal cord explants is neutralized by antibodies raised against collapsin-1. Development 124(7):1377–1385
Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY (2003) Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development 130:3227–3236
Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M (1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science 281(5382):1515–1518
Surmeli G, Akay T, Ippolito GC, Tucker PW, Jessell TM (2011) Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell 147(3):653–665. doi:10.1016/j.cell.2011.10.012 S0092-8674(11)01210-4 [pii]
Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H (2005) Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci 25(14):3628–3637. doi:25/14/3628 [pii] 10.1523/JNEUROSCI.4480-04.2005
Takagi S, Tsuji T, Amagai T, Takamatsu T, Fujisawa H (1987) Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev Biol 122(1):90–100. doi:0012-1606(87)90335-6 [pii]
Takagi S, Hirata T, Agata K, Mochii M, Eguchi G, Fujisawa H (1991) The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron 7(2):295–307. doi:0896-6273(91)90268-5 [pii]
Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM (1999) Plexin–neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99(1):59–69. doi:S0092-8674(00)80062-8 [pii]
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99(1):71–80. doi:S0092-8674(00)80063-X [pii]
Tanaka H, Maeda R, Shoji W, Wada H, Masai I, Shiraki T, Kobayashi M, Nakayama R, Okamoto H (2007) Novel mutations affecting axon guidance in zebrafish and a role for plexin signalling in the guidance of trigeminal and facial nerve axons. Development 134(18):3259–3269. doi:dev.004267 [pii] 10.1242/dev.004267
Tanaka H, Nojima Y, Shoji W, Sato M, Nakayama R, Ohshima T, Okamoto H (2011) Islet1 selectively promotes peripheral axon outgrowth in Rohon-Beard primary sensory neurons. Dev Dyn 240(1):9–22. doi:10.1002/dvdy.22499
Tanelian DL, Barry MA, Johnston SA, Le T, Smith GM (1997) Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat Med 3(12):1398–1401
Tang XQ, Tanelian DL, Smith GM (2004) Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci 24(4):819–827. doi:10.1523/JNEUROSCI.1263-03.2004 24/4/819 [pii]
Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T (1997) Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19(3):519–530. doi:S0896-6273(00)80368-2 [pii]
Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H (2004) Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18(4):435–447. doi:10.1101/gad.11673041167304 [pii]
Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H (2005) FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci 8(12):1712–1719. doi:nn1596 [pii] 10.1038/nn1596
Vermeren M, Maro GS, Bron R, McGonnell IM, Charnay P, Topilko P, Cohen J (2003) Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron 37(3):403–415. doi:S0896627302011881 [pii]
Vrieseling E, Arber S (2006) Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell 127(7):1439–1452. doi:S0092-8674(06)01522-4 [pii] 10.1016/j.cell.2006.10.042
White FA, Behar O (2000) The development and subsequent elimination of aberrant peripheral axon projections in Semaphorin3A null mutant mice. Dev Biol 225(1):79–86. doi:10.1006/dbio.2000.9822 S0012-1606(00)99822-1 [pii]
Williams ME, de Wit J, Ghosh A (2010) Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron 68(1):9–18. doi:10.1016/j.neuron.2010.09.007 S0896-6273(10)00724-5 [pii]
Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS (1998) Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 95(7):903–916. doi:S0092-8674(00)81715-8 [pii]
Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD (1995) The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J Comp Neurol 361(2):321–333. doi:10.1002/cne.903610209
Xiao T, Shoji W, Zhou W, Su F, Kuwada JY (2003) Transmembrane sema4E guides branchiomotor axons to their targets in zebrafish. J Neurosci 23(10):4190–4198. doi:23/10/4190 [pii]
Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M (2005) Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 45(4):513–523. doi:S0896-6273(05)00024-3 [pii] 10.1016/j.neuron.2005.01.013
Yoshida Y, Han B, Mendelsohn M, Jessell TM (2006) PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 52:775–788. doi:S0896-6273(06)00832-4 [pii] 10.1016/j.neuron.2006.10.032
Zhou L, White FA, Lentz SI, Wright DE, Fisher DA, Snider WD (1997) Cloning and expression of a novel murine semaphorin with structural similarity to insect semaphorin I. Mol Cell Neurosci 9(1):26–41. doi:S1044-7431(97)90607-2 [pii] 10.1006/mcne.1997.0607
Zhuang B, Su YS, Sockanathan S (2009) FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron 61(3):359–372. doi:10.1016/j.neuron.2008.12.022 S0896-6273(08)01094-5 [pii]
Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M (2000) Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102(3):363–375. doi:S0092-8674(00)00041-6 [pii]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Imai, F., Yoshida, Y. (2015). Axon Guidance in the Spinal Cord. In: Kumanogoh, A. (eds) Semaphorins. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54385-5_3
Download citation
DOI: https://doi.org/10.1007/978-4-431-54385-5_3
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54384-8
Online ISBN: 978-4-431-54385-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)