Abstract
The vertebrate retina is a light-sensitive layer of tissue that lines the inner surface of the eye. Light striking the retina initiates a cascade of chemical and electrical events that ultimately trigger nerve impulses, which are sent to various visual centers of the brain through the fibers of the optic nerve. Each axis of the retina is mapped independently using different mechanisms and sets of axon-guidance molecules, such as the semaphorins , which are expressed in gradients to achieve projections from points in the retina to points in the target regions of the brain. In animal models, mutations in several of the guidance molecules disrupt axonal projections at specific sites, whereas mutation of one of the semaphorins reduces photoreceptor survival. Understanding the molecular mechanisms of neural defects in a variety of animal models can provide valuable insights into the effects of each molecule in clinical disorders and may form the basis of future therapies to prevent retinal diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Retina
- Rhodopsin
- Phosphodiesterase (PDE)
- The visual cycle
- The retinal pigment epithelium (RPE)
- Visual map
- The immunoglobulin superfamily (IgSF)
- Plexin A4 (PlexA4)
- Sema5A/B
- Robo2
- EphB1 receptor
- Neuropilin 1 (Nrp1)
- VEGF
- Retinitis pigmentosa
- Reactive oxygen and nitrogen species (RONS)
- Sema4A
- FIP2
- Rab11
- 11-cis-retinal
- CRALBP
- CRBP1
11.1 Anatomy of the Retina
The retina is a layered structure containing several layers of neurons interconnected by synapses (Fig. 11.1). The only neurons that are directly sensitive to light are the photoreceptor cells, which are mainly of two types: rods and cones. Rods function mainly in dim light and provide black-and-white vision, whereas cones support daytime vision and the perception of color. Neural signals from the rods and cones undergo processing by other neurons in the retina, taking the form of action potentials in retinal ganglion cells, whose axons form the optic nerve. These processes can be simplified into four main processing stages: photoreception, transmission to bipolar cells, transmission to ganglion cells, and transmission along the optic nerve. At each synaptic stage, there are also laterally connecting horizontal and amacrine cells. The optic nerve, a central tract consisting of many ganglion cell axons, connects primarily to the lateral geniculate body, a visual relay station in the diencephalon (the rear of the forebrain). It also projects to the superior colliculus, the suprachiasmatic nucleus, and the nucleus of the optic tract.
11.2 Physiology of the Retina
11.2.1 Phototransduction
In the photoreceptors , exposure to light hyperpolarizes the membrane (Fig. 11.2). The outer segment of a photoreceptor cell contains the photopigment rhodopsin. Inside the cell, normal levels of cyclic guanosine monophosphate (cGMP) keep Na+ channels open; therefore, in the resting state, the cell is depolarized. Photons cause 11-cis-retinal bound to the receptor protein (opsin) to isomerize to all-trans-retinal; once this isomerization reaction has taken place, the receptor activates multiple G proteins; this in turn causes the Gα-subunit of the protein to activate a phosphodiesterase (PDE), which degrades cGMP, resulting in the closing of cyclic nucleotide-gated ion channels. As a consequence, the cell is hyperpolarized. The amount of neurotransmitter released is reduced in bright light and increases as light levels fall. The actual photopigment is bleached away in bright light and can only be replaced by a chemical process; therefore, in a transition from bright light to darkness, the eye can take up to 30 min to reach full sensitivity.
Light-induced photoreceptor excitation and visual cycle. Phototransduction occurs in the outer segment of the photoreceptor, where the light-induced isomerization of rhodopsin triggers sequential chemical steps leading to membrane depolarization. The visual cycle is a pathway of enzymatic reactions that recycle the retinoids used during light detection by the photoreceptor
11.2.2 Visual Cycle
The visual cycle is a pathway of enzymatic reactions that recycle the retinoids that are used during light detection in photoreceptor cells (Lamb and Pugh 2004) (Fig. 11.2). The activation of the photoreceptor rhodopsin by light occurs through the isomerization of 11-cis retinal, bound to opsin, to all-trans retinal. All-trans retinal is released from rhodopsin, conjugated with the membrane lipid phosphatidylethanolamine, and transported to the cytoplasm by the ATP-binding cassette, subfamily A, member 4 (ABCA4) protein. After modification to all-trans retinol by a retinol dehydrogenase (RDH12), the molecule is transported to the retinal pigment epithelium (RPE), where it is esterified to a fatty acyl group (FA) by lecithin retinol acyltransferase (LRAT) to form all-trans retinyl ester. This compound is subject to trans-isomerization to 11-cis retinal through the activities of two additional enzymes (RPE65 and 11-cis retinol dehydrogenase). After transport back to the photoreceptor, 11-cis retinal binds rhodopsin, rendering it sensitive to light. Retinoid-binding proteins, such as interstitial retinol-binding protein (IRBP), cellular retinol-binding protein (CRBP1), and cellular retinaldehyde-binding protein (CRALBP), are involved in transport of the hydrophobic retinoids in the aqueous environment of the cytoplasm.
11.3 Visual Map Development
Visual information is transferred from the retina to multiple areas in the brain, with the spatial information of the visual field maintained in each target region. Two of the main targets of the retina are the superior colliculus [SC, analogous to the optic tectum (OT) in lower vertebrates], a midbrain structure used to control head and eye movements, and the dorsal lateral geniculate nucleus (dLGN) in the dorsal thalamus. The dLGN in turn projects to the primary visual cortex (V1) in the posterior cerebral cortex, which subsequently projects to several “higher” visual areas responsible for conscious vision. Each component of the visual system is mapped topographically (Lewin 1994; Udin and Fawcett 1988).
11.3.1 Lamina-Specific Targeting of Neurites in the Retina
Retinal circuits are activated by photoreceptors (rods or cones), which transform light energy into neural signals and make contacts with both excitatory interneurons (bipolar cells) and inhibitory interneurons (horizontal cells) that process information within the retina. Subsets of bipolar cells and another class of inhibitory interneurons (amacrine cells) converge selectively onto one ganglion cell. The circuit of each type of ganglion cell determines its specific sensitivity to visual input. A fundamental organizing principle of the retina is its division into ON and OFF pathways. Bipolar cells are excited by either increases (ON cells) or decreases (OFF cells) in light; the axons of ON and OFF bipolar cells terminate in different sublayers of the inner synaptic layer of the retina (Fig. 11.3a). At least two distinct mechanisms serve to target neurites to the appropriate laminae: homophilic cell–cell adhesion via IgSF molecules, and short-range guidance by classical axon-guidance cues.
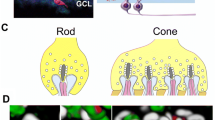
Schematic model of semaphorin-dependent neurite stratification in the retina. (a) The retina is divided into ON and OFF pathways. Bipolar cells are excited by either increases (ON cells) or decreases (OFF cells) in light; the axons of ON and OFF bipolar cells terminate in different sublayers of the inner synaptic layer of the retina, and connect the layer of ganglion cells. (b) Semaphorins regulate the formation of synapses between bipolar cells and ganglion cells
Intercellular (cell–cell) recognition is carried out by cell-surface adhesion molecules belonging to the immunoglobulin superfamily (IgSF) of transmembrane glycoproteins (Yamagata and Sanes 2008; Yamagata et al. 2002). These molecules often act in a homophilic fashion, that is, they function as reciprocal receptors and ligands on the surface of both axons and dendrites. The homophilic IgSF molecules Sidekick1 (Sdk1), Sdk2, Dscam, and DscamL are expressed in nonoverlapping subsets of bipolar cells, amacrine cells, and ganglion cells in the retina. For example, expression of Sdk1 on a neurite directs its stratification to a lamina containing high levels of Sdk1. Other classes of molecules, such as cadherins, have been implicated in cell–cell recognition (Inoue and Sanes 1997; Poskanzer et al. 2003).
A recent study has revealed a novel role for transmembrane semaphorin 6A (Sema6A) signaling through plexin A4 (PlexA4) in the regulation of retinal neurite stratification (Matsuoka et al. 2011b; Sun et al. 2013) (Fig. 11.3b). Restricted neurite stratification in OFF layers is controlled, at least in part, by semaphorin-mediated repulsion away from ON layers. Sema6A, which is concentrated within the ON layers of the inner plexiform layer (IPL), repels PlexA4-expressing dopaminergic amacrine cells (DACs) in the OFF region of the IPL, whereas M1-melanopsin-positive intrinsically photosensitive retinal ganglion cells (RGCs) (M1-ipRGCs) that do not express PlexA4 stratify to the OFF layer through the Sema6A region (Matsuoka et al. 2011b). Sema5A/B signaling, mediated by the PlexA1 and PlexA3 receptors (which are functionally redundant for Sema5 signaling in the retina), repels initial neurite extension of bipolar cells away from the INL (Matsuoka et al. 2011a). Stardust amacrine cells (SACs) are divided into ON and OFF subtypes, which co-stratify with distinct direction-selective ganglion cells (DSGCs) at the ON and OFF layers. This laminification is important for direction-selective responses to visual cues. The arborization of ON and OFF SACs is controlled by Sema6A (Sun et al. 2013); arborization of OFF SACs is restricted to the OFF layer through the action of Plex-A2, whereas arborization of ON SACs is restricted to the ON layer through the action of Sema6D in the absence of PlexA2. These findings provide an example of multilevel control of synaptic specificity. In the case of DACs, Sema5 signaling repels initial neurite extension away from the inner nuclear layer. Subsequently, Sema6A directs terminal arborization of DACs to OFF strata of the IPL by repelling their neurites away from ON layers; homophilic adhesion may function to specify cell type-specific synapse formation within these layers. Furthermore, Sema6A directs arborization of ON and OFF cells to their respective layers.
11.3.2 Retinal Axon Guidance at the Optic Chiasm
The optic chiasm is the structure where partial contralateral crossover of RGC axons occurs. Netrin-1 likely exerts its attractant influence on RGC axons after they exit the eye (Fig. 11.4). However, netrin-1 is not present around the chiasm midline, where RGC axons come under the influence of repulsive molecules such as Sema5A and Slit/Robo. Sema5A is expressed at the optic disc and along the optic nerve, and blockade of Sema5A function causes retinal axons to stray out of the optic nerve bundle (Oster et al. 2003). It is possible that Slit/Robo signaling defines the site of optic chiasm formation. RGCs express Robo2, a receptor for Slit, and Slit1 and Slit2 are present in the ventral diencephalon (Plump et al. 2002; Long et al. 2004). Thus, Sema5A and Slit proteins act as a repulsive “guardrail,” establishing a corridor through which RGC axons are channeled. Ephrin-B2 and EphB1 control axon divergence at the optic chiasm (Williams et al. 2003). Ephrin-B2 is expressed in radial glial cells at the optic chiasm concurrent with the development of the ipsilateral projections; in mice, blockade of ephrin-B2 eliminates ipsilateral projections. The EphB1 receptor is specifically expressed in RGCs in the mouse ventrotemporal (VT) retina that give rise to the ipsilateral projections. By contrast, Sema6D, which is expressed in radial glial cells at the optic chiasm, regulates the contralateral projection of PlexA1-expressing RGCs in combination with coexpressed Nr-CAM (Kuwajima et al. 2012).
Retinal ganglion cell (RGC) axon guidance. At the optic disc, RGC axons exit the retina into the optic nerve because of an attractive effect mediated by netrin/DCC. Within the optic nerve, RGC axons are kept within the pathway through Sema5A and by inhibitory Slit/Robo interaction. At the optic chiasm, ephrin-B2 repels EphB1-expressing axons and terminates at ipsilateral targets, whereas Sema6D in combination with Nr-CAM regulates the contralateral projection of axons
11.3.3 Retinal Axon Guidance at SC and dLGN
Upon crossing the midline, RGC axons project to their major targets, the SC and dLGN. Topographic mapping of RGC axons occurs along two sets of orthogonally oriented axes. The nasal–temporal (NT) axis of the retina maps along the posteroanterior (PA) axis of the SC, and the dorsoventral (DV) retinal axis maps along the lateromedial (LM) SC axis. Accumulating evidence has revealed that ephrin-As and their EphA receptors are required for proper retinal NT mapping along the SC PA axis (Brown et al. 2000; Feldheim et al. 2000; Rashid et al. 2005). In addition, an external gradient of Engrailed-2, a homeodomain transcription factor, may also participate in the formation of the PA axis in the vertebrate SC, possibly by regulating the expression of Eph family members (Brunet et al. 2005; Itasaki and Nakamura 1996; Logan et al. 1996). By contrast, Ephrin-Bs in the SC, and their EphB receptors in the retina, are also required for proper DV mapping along the LM axis (Hindges et al. 2002).
11.3.4 Retinal Axon Guidance at Thalamocortical (TC) Projections
During development, TC axons grow into the subcortical telencephalon (ST); postnatally, they continue from the ST along their paths to the cortex. Recent studies have suggested that some guidance cues may be required in the ST, and that such cues may play a key role in controlling the initial topography of thalamic projections to the neocortex. EphAs in the thalamus and ephrin-As in the ST are involved in the regulation of TC projections in the somatosensory area in the frontal cortex (Dufour et al. 2003). Slit/Robo signaling is involved in the lamination of TC projections (Xiao et al. 2011). The optic tectum is pre-patterned by specific ECM molecules such as large glycoproteins and proteoglycans including Tenascin, Versican, and Nel; each has been shown to influence neurite growth in vitro (Yamagata and Sanes 2005; Yamagata et al. 1995; Jiang et al. 2009). Slit and Col4a5 bind directly in vitro, suggesting that Slit influences TC projections through direct binding to the ECM.
11.4 Vascular Development
The multilayered retina is initially supplied by a combination of two extraretinal vascular systems, the choroidal vasculature that supplies the outer retina and the hyaloid arteries that supply the inner retina and lens. The choroidal vasculature persists throughout the lifespan; by contrast, late in mammalian development, the hyaloid arteries are replaced with a dedicated intraretinal vascular system. In rodents, the retina is vascularized after birth to give rise to a system of three interconnected vascular plexi (Fruttiger 2007). Vascularization begins on the day of birth, when vessel sprouts emerge from the optic nerve head and spread radially over the retina, guided by a template of astrocytes, with blood vessels and astrocytes forming copatterned networks (Fruttiger 2007; West et al. 2005). During the process of radial expansion, the primary plexus undergoes arteriovenous differentiation. After the first week of life, vessel sprouts emerge from this primary retinal vessel plexus to dive into the inner retinal layers at near right angles and form the deep plexus (during week 2 after birth) and then the intermediate plexus (during week 3).
In the neonatal mouse retina, the three vascular endothelial growth factor (VEGF) isoforms are produced and displayed by an astrocytic network located beneath the expanding vascular plexus. Neuropilin 1 (Nrp1) is a noncatalytic transmembrane protein whose genetic loss, either globally or specifically in endothelial cells, severely inhibits central nervous system (CNS) vascularization. Nrp1 serves as a receptor for VEGF165 and a member of the structurally unrelated class 3 semaphorin family, Sema3A (Schwarz and Ruhrberg 2010). Tumor studies have implicated Sema3A as a modulator of pathological angiogenesis. Thus, Sema3A reduces the overall vascularity of tumors and “normalizes” tumor vessels, in part by recruiting myeloid cells that stimulate vessel maturation (Maione et al. 2009).
Sema3E is the only class 3 semaphorin that does not bind to a neuropilin receptor, but instead binds directly to PlexD1 (Gu et al. 2005). In the developing retinal vasculature, high VEGF levels emanating from the avascular retinal periphery induce PlexD1 expression in endothelial cells at the vascular front; this phenomenon is dependent on VEGFR2 (Kim et al. 2011). Furthermore, loss-of-function studies have demonstrated that Sema3E, derived from the neural layers of the retina, signals through endothelial PlexD1 to upregulate DLL4 at the vascular front. This, in turn, increases endothelial Notch signaling, resulting in a loss of tip cells and tip-cell filopodia. Consequently, normal vascular expansion into the retinal periphery is disrupted in mice lacking Sema3E. Remarkably, Sema3E normalizes VEGF-A-induced pathological vessel growth in a mouse model of oxygen-induced retinopathy, in which retinal vessels grow abnormally into the vitreous humor (Fukushima et al. 2011). In that study, intravitreal administration of Sema3E protein prevented this abnormal vessel growth. This observation suggests that Sema3E could serve as a therapeutic tool for fine-tuning VEGF-A signaling and vascular growth in the ischemic nervous system.
11.5 Semaphorin in Retinal Diseases
11.5.1 Photoreceptor Degeneration
Retinitis pigmentosa (RP) is an inherited, degenerative eye disease that causes severe vision impairment and often blindness. A form of retinal dystrophy, RP is caused by abnormalities of the photoreceptors (rods and cones) or the retinal pigment epithelium (RPE) of the retina leading to progressive sight loss. More than 100 mutations have been found in this gene, accounting for 15 % of all types of retinal degeneration. Most of those mutations are missense mutations, and the disease is mostly inherited in a dominant manner.
The most obvious factor that makes photoreceptors (PRs) vulnerable to degeneration is light exposure. Visible and ultraviolet light are insufficiently energetic to ionize most biomolecules, but oxygen enhances the ionizing effect of light. Consequently, damage can occur when reactive oxygen and nitrogen species (RONS) are generated by light acting on photosensitizing molecules such as retinoids. Light damage to PRs requires the release of all-trans retinal from light-activated rhodopsin (Sun and Nathans 2001; Travis et al. 2007). Photo-excitation of all-trans retinal generates singlet oxygen and can cause photo-oxidative damage. If mutations affecting the visual cycle block the recycling of all-trans retinal to 11-cis retinal, toxic bis-retinoids (such as the all-trans retinal dimer) and adducts [such as N-retinylidene-N-retinyl-ethanolamine (A2E)] build up during the course of aging.
Some types of PR degeneration are accelerated by light (Hartong et al. 2006; Cideciyan et al. 2005) (Fig. 11.5). Defects that are potentially exacerbated by light exposure are as follows. (1) Visual cycle defects, for example, mutations in ABCA4 or retinol dehydrogenase 12 (RDH12) (Radu et al. 2005); ABCA4 transports toxic all-trans-retinal to the cytoplasm, and RDH12 dehydrogenates it. Defects in these enzymes increase the levels of toxic retinoids in photoreceptor cells. (2) RPE phagocytosis defects, for example, mutations in c-Mer proto-oncogene tyrosine kinase (MERTK) (Tschernutter et al. 2006); MERTK in retinal pigment epithelial cells is required to clear the light-damaged outer segments of photoreceptors. (3) PR cilia defects that slow outer segment turnover, for example, mutations in RPGR (Robson et al. 2008). PR cilia connect the outer and inner segments of the photoreceptor cell and transport the components required for phototransduction to the outer segment; defects in PR cilia compromise all outer-segment functions. (4) Defects in the stability of outer-segment discs, such as mutations in peripherin 2 (PRPH2) (Renner et al. 2009).
Protection of light-induced photoreceptor degeneration. Photoreceptor (PR) homeostasis is functionally and mechanically supported by retinal pigment epithelium (RPE) cells. (1) RPE cells regenerate 11-cis-retinal from toxic all-trans retinal via the retinoid cycle. (2) RPE cells phagocytose the shed distal end of the outer segment of PR, which is constantly adding newly generated discs at its base. (3) The connecting cilium of the PR provides a pathway for transport of proteins and membranes from the inner segment to the outer segment
11.5.2 Sema4A in Retinal Degeneration
Rice et al. reported that insertion of a gene-trap vector into intron 11 of the mouse Sema4A gene results in the loss of retinal PRs (Rice et al. 2004). A subsequent study identified mutations in the human Sema4A gene in patients with retinal degeneration (Abid et al. 2006). In Sema4A-deficient (Sema4A−/−) mice, normal retinal development was observed at postnatal day 0 (P0), but at P14 the outer segments of PRs were disrupted, followed by a complete loss of PRs by P28 (Fig. 11.6a). In response to illumination, Sema4A−/− retinas exhibited a dramatic increase in the number of apoptotic cells in the outer nuclear layer before recovering to basal levels (Fig. 11.6b). Sema4A is expressed in RPE and bound to prosaposin. Prosaposin is synthesized and associated with procathepsin D in the Golgi membrane (Gopalakrishnan et al. 2004), and can be targeted to lysosomes (Kishimoto et al. 1992; Benes et al. 2008) or secreted into the extracellular space. Previous studies demonstrated that such secreted lysosomal precursor proteins are antiapoptotic for various neuronal populations (O’Brien et al. 1994; Benes et al. 2008). In those studies, oxidative stress caused by H2O2 treatment resulted in prosaposin transport to the cell periphery in Sema4A+/+ RPE cells, but not in Sema4A−/− RPE cells. Via its intercellular region, Sema4A bound to a complex of Rab11 and the adaptor protein FIP2 more effectively than to FIP2 alone; Rab11 is involved in Sema4A-mediated prosaposin transport to the cell periphery under oxidative stress. In response to H2O2, prosaposin-containing vesicles were transported to the cell periphery via Sema4A/Rab11-mediated transport machinery (Fig. 11.7).
Schematic model of Sema4A-mediated endosomal sorting in photoreceptor epithelium (PRE) after exposure to light. Sorting of prosaposin to the exosomal pathway is dependent on preferential binding of prosaposin to Sema4A and the Rab11/FIP2 endosomal sorting machinery. During dark adaptation, the recycling of retinoid-binding proteins is dependent on Sema4A-mediated transport
Levels of 11-cis-retinal are significantly increased in Sema4A+/+ retinas at P14 and P28, whereas these levels remain low in Sema4A−/− retinas. Sema4A participates in the retinoid cycle by regulating the transport of retinoid-binding proteins in RPE cells. At least two proteins that bind water-insoluble retinoids in RPE cells are involved in the retinoid cycle (Lem and Fain 2004): CRALBP and CRBP1, which transport 11-cis-retinal and all-trans-retinol, respectively. These proteins are transported via the Sema4A-mediated endosomal sorting machinery; in the absence of Sema4A, they are mistargeted to different compartments. CRALBP is mistargeted to the cell periphery where it is likely unable to interact with 11-cis-retinal, which is generated in the endoplasmic reticulum. CRBP1 is mistargeted to the endoplasmic reticulum where it cannot interact with all-trans-retinol, which is imported from the extracellular space. Thus, Sema4A regulates intracellular sorting of retinoid-binding proteins to regenerate retinoids for phototransduction, an essential process in the retinoid cycle during dark adaptation (Fig. 11.7). The finding that Sema4A functions as an intracellular guide for specific molecules complements the previously known functions of semaphorins as extracellular guidance molecules (Kolodkin and Tessier-Lavigne 2011).
In patients with retinal degenerative diseases, three mutations, D345H, F350C, and R713Q, in Sema4A have been reported (Abid et al. 2006). An analysis of a series of knock-in mouse lines carrying mutated alleles of Sema4A demonstrated that expression of Sema4A(F350C) caused severe retinal degeneration (Nojima et al. 2013). In the RPE, Sema4A(F350C) tends to aggregate, and the resultant mislocalization of Sema4A protein may lead to the impaired endosomal sorting of molecules such as prosaposin and retinoid-binding proteins. Notably, virus-mediated gene transfer of Sema4A into RPE in neonatal Sema4A-deficient mice successfully prevents retinal degeneration for at least 4 months after injection. Considering the importance of the endosomal sorting function of Sema4A in maintaining retinal homeostasis, it is possible that Sema4A replacement gene therapy might be efficacious in wider subsets of patients with retinal degenerative diseases.
References
Abid A, Ismail M, Mehdi SQ, Khaliq S (2006) Identification of novel mutations in the SEMA4A gene associated with retinal degenerative diseases. J Med Genet 43:378–381
Benes P, Vetvicka V, Fusek M (2008) Cathepsin D: many functions of one aspartic protease. Crit Rev Oncol Hematol 68:12–28
Brown A, Yates PA, Burrola P, Ortuno D, Vaidya A, Jessell TM, Pfaff SL, O’Leary DD, Lemke G (2000) Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell 102:77–88
Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C (2005) The transcription factor Engrailed-2 guides retinal axons. Nature (Lond) 438:94–98
Cideciyan AV, Jacobson SG, Aleman TS, Gu D, Pearce-Kelling SE, Sumaroka A, Acland GM, Aguirre GD (2005) In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc Natl Acad Sci U S A 102:5233–5238
Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P (2003) Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron 39:453–465
Feldheim DA, Kim YI, Bergemann AD, Frisen J, Barbacid M, Flanagan JG (2000) Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron 25:563–574
Fruttiger M (2007) Development of the retinal vasculature. Angiogenesis 10:77–88
Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A (2011) Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest 121:1974–1985
Gopalakrishnan MM, Grosch HW, Locatelli-Hoops S, Werth N, Smolenova E, Nettersheim M, Sandhoff K, Hasilik A (2004) Purified recombinant human prosaposin forms oligomers that bind procathepsin D and affect its autoactivation. Biochem J 383:507–515
Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD (2005) Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 307:265–268, Epub 2004 Nov 2018
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary D (2002) EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron 35:475–487
Inoue A, Sanes JR (1997) Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science 276:1428–1431
Itasaki N, Nakamura H (1996) A role for gradient en expression in positional specification on the optic tectum. Neuron 16:55–62
Jiang Y, Obama H, Kuan SL, Nakamura R, Nakamoto C, Ouyang Z, Nakamoto M (2009) In vitro guidance of retinal axons by a tectal lamina-specific glycoprotein Nel. Mol Cell Neurosci 41:113–119
Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C (2011) Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev 25:1399–1411
Kishimoto Y, Hiraiwa M, O’Brien JS (1992) Saposins: structure, function, distribution, and molecular genetics. J Lipid Res 33:1255–1267
Kolodkin AL, Tessier-Lavigne M (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3:1–14
Kuwajima T, Yoshida Y, Takegahara N, Petros TJ, Kumanogoh A, Jessell TM, Sakurai T, Mason C (2012) Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 74:676–690
Lamb TD, Pugh EN Jr (2004) Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 23:307–380
Lem J, Fain GL (2004) Constitutive opsin signaling: night blindness or retinal degeneration? Trends Mol Med 10:150–157
Lewin B (1994) On neuronal specificity and the molecular basis of perception. Cell 79:935–943
Logan C, Wizenmann A, Drescher U, Monschau B, Bonhoeffer F, Lumsden A (1996) Rostral optic tectum acquires caudal characteristics following ectopic engrailed expression. Curr Biol 6:1006–1014
Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M (2004) Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42:213–223
Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E (2009) Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest 119:3356–3372
Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS et al (2011a) Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron 71:460–473
Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL (2011b) Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature (Lond) 470:259–263
Nojima S, Toyofuku T, Kamao H, Ishigami C, Kaneko J, Okuno T, Takamatsu H, Ito D, Kang S, Kimura T et al (2013) A point mutation in Semaphorin 4A associates with defective endosomal sorting and causes retinal degeneration. Nat Commun 4:1406
O’Brien JS, Carson GS, Seo HC, Hiraiwa M, Kishimoto Y (1994) Identification of prosaposin as a neurotrophic factor. Proc Natl Acad Sci U S A 91:9593–9596
Oster SF, Bodeker MO, He F, Sretavan DW (2003) Invariant Sema5A inhibition serves an ensheathing function during optic nerve development. Development (Camb) 130:775–784
Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M (2002) Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron 33:219–232
Poskanzer K, Needleman LA, Bozdagi O, Huntley GW (2003) N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci 23:2294–2305
Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL (2005) Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci 46:4393–4401
Rashid T, Upton AL, Blentic A, Ciossek T, Knoll B, Thompson ID, Drescher U (2005) Opposing gradients of ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron 47:57–69
Renner AB, Fiebig BS, Weber BH, Wissinger B, Andreasson S, Gal A, Cropp E, Kohl S, Kellner U (2009) Phenotypic variability and long-term follow-up of patients with known and novel PRPH2/RDS gene mutations. Am J Ophthalmol 147:518–530
Rice DS, Huang W, Jones HA, Hansen G, Ye GL, Xu N, Wilson EA, Troughton K, Vaddi K, Newton RC et al (2004) Severe retinal degeneration associated with disruption of semaphorin 4A. Invest Ophthalmol Vis Sci 45:2767–2777
Robson AG, Michaelides M, Saihan Z, Bird AC, Webster AR, Moore AT, Fitzke FW, Holder GE (2008) Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc Ophthalmol 116:79–89
Schwarz Q, Ruhrberg C (2010) Neuropilin, you gotta let me know: should I stay or should I go? Cell Adhes Migr 4:61–66
Sun H, Nathans J (2001) ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem 276:11766–11774
Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Feller MB, Kolodkin AL (2013) On and off retinal circuit assembly by divergent molecular mechanisms. Science 342:1241974
Travis GH, Golczak M, Moise AR, Palczewski K (2007) Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol 47:469–512
Tschernutter M, Jenkins SA, Waseem NH, Saihan Z, Holder GE, Bird AC, Bhattacharya SS, Ali RR, Webster AR (2006) Clinical characterisation of a family with retinal dystrophy caused by mutation in the Mertk gene. Br J Ophthalmol 90:718–723
Udin SB, Fawcett JW (1988) Formation of topographic maps. Annu Rev Neurosci 11:289–327
West H, Richardson WD, Fruttiger M (2005) Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development (Camb) 132:1855–1862
Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M (2003) Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39:919–935
Xiao T, Staub W, Robles E, Gosse NJ, Cole GJ, Baier H (2011) Assembly of lamina-specific neuronal connections by slit bound to type IV collagen. Cell 146:164–176
Yamagata M, Sanes JR (2005) Versican in the developing brain: lamina-specific expression in interneuronal subsets and role in presynaptic maturation. J Neurosci 25:8457–8467
Yamagata M, Sanes JR (2008) Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature (Lond) 451:465–469
Yamagata M, Herman JP, Sanes JR (1995) Lamina-specific expression of adhesion molecules in developing chick optic tectum. J Neurosci 15:4556–4571
Yamagata M, Weiner JA, Sanes JR (2002) Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell 110:649–660
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Toyofuku, T. (2015). Semaphorin in the Retinal System. In: Kumanogoh, A. (eds) Semaphorins. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54385-5_11
Download citation
DOI: https://doi.org/10.1007/978-4-431-54385-5_11
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54384-8
Online ISBN: 978-4-431-54385-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)