Abstract
Semaphorins, which are a large family of secreted and membrane-bound molecules, were initially identified as neuronal axon-guidance signaling molecules but are now known as important key regulators for cell adhesion and motility in a wide range of organ systems, such as angiogenesis and immune response. The semaphorin receptors, neuropilins and plexins, are expressed in a variety of cell types, including neurons, endothelial cells, and cancer cells. Plexins are primarily receptors responsible for intracellular semaphorin signalings. Plexins possess an intrinsic GAP (glyceraldehyde-3-phosphate) activity for R-Ras subfamily GTPases, and this GAP activity is one of the crucial signals of semaphorins. In addition, plexins associate with a variety of signaling molecules, such as Rho GEFs and Rho GAP, and these associated molecules determine the characters of semaphorin signals. On the other hand, their signalings are critically modulated by their associated co-receptor molecules, including tyrosine kinase receptors. Semaphorins provide attractive and repulsive responses in a variety of cells, but associated co-receptors of plexins frequently hold the key to conversion between attraction and repulsion. In this chapter, we focus attention on the molecular signaling systems of plexins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
The semaphorins were initially identified as axon-guidance factors that induce axonal growth cone collapses. However, it is now clear that semaphorins and their receptors are widely expressed in a variety of cells, including neurons, endothelial cells, and various tumor cells, and they are implicated in a variety of physiological functions, including control of nervous and endothelial development and immune systems. Semaphorins display their functions through direct binding to either their specific receptors, plexins, or their co-receptors, neuropilins, which form complexes with plexins. Plexins function as crucial receptors of semaphorins, and their intracellular domains are responsible for initiating cellular signal transduction inducing cellular functions of semaphorins. Initially, Plexin-B1, the receptor for Sema4D, has been shown to activate RhoA through binding to RGS-RhoGEF, but the plexin family has been identified to display GAP activity for R-Ras and Rap small GTPases through intrinsic GAP domains within the intracellular tails of plexins (Negishi et al. 2005; Zhou et al. 2008). In this chapter we focus attention on the molecular signaling systems of plexins.
1.2 Semaphorins and Their Receptors, Plexins and Neuropilins
Semaphorins are a large family of secreted or membrane-bound proteins. The semaphorin family contains 21 vertebrate genes and 8 additional genes found in invertebrates, and they are now classified into 8 subclasses on the basis of amino acid sequence similarity and distinctive structural features (Neufeld and Kessler 2008). Classes 1 and 2 are invertebrate semaphorins, classes 3 through 7 are vertebrate semaphorins, and class V are viral-encoded semaphorins, found in the genome of non-neurotrophic DNA viruses. Among these, classes 1, 4, 5, and 6 are transmembrane molecules, and class 7 is a membrane-associated form through a glycosylphosphatidylinositol-anchor motif. In the case of Sema4D, membrane-bound Sema4D is cleaved proteolytically, generating a biologically active soluble Sema4D fragment in some conditions (Zhu et al. 2007). On the other hand, classes 2, 3, and V are secreted proteins. Semaphorins are characterized by the sema domains and PSI (plexins, semaphorins, and integrins) domains, located in amino-terminal regions. In contrast, the carboxyl-terminal regions are more variable, and some of them have immunoglobulin-like loops. Class 3 and 4 semaphorins are homodimerized through cysteine disulfide bonds, and dimerization appears to be critical for their biological function. Semaphorins of classes 4 to 7 as well as Sema3E bind directly to specific plexins and produce plexin-mediated signal transductions. Other class 3 semaphorins do not bind to plexins but instead bind to neuropilins.
The function of semaphorins is mediated by their receptors, the plexins, and mammalian plexins are classified into four subfamilies: Plexin-A1-4, Plexin-B1-3, Plexin-C1, and Plexin-D1 (Tamagnone et al. 1999). Plexins are single-pass transmembrane receptors, and they contain sema domains, PSI domains, and G-P (glycine-proline)-rich motifs in the extracellular regions. On the other hand, in the cytoplasmic regions, plexins directly encode GAP domains for Ras family small GTPases split by the docking site for several Rho family small GTPases such as Rnds, RhoD, and Rac1. In addition, B-type plexins contain a C-terminal PDZ-binding motif for RGS-RhoGEFs. The neuropilins, neuropilin-1 and neuropilin-2, are another type of receptor and were initially identified as direct binding receptors for class 3 semaphorins. The B-type plexins also contain a conserved cleavage site in their extracellular domains for furin-like pro-protein convertases that is not found in other plexins. The two neuropilins are single-pass transmembrane receptors, and they have two CUB (complement-binding) domains, two FV/FVIII coagulation factor-like domains, and a MAM domain in their extracellular regions. However, neuropilins have very short cytoplasmic tails, and they alone have no ability to transduce the signals of Sema3. Neuropilins form complexes with Plexin-As, and associated Plexin-As are signaling moieties. Classification of semaphorins and their receptors are depicted in Fig. 1.1.
The structures of semaphorins and their receptors. The structural features of the various classes of semaphorins and their neuropilin and plexin receptors are depicted. Semaphorins exist as the secreted, transmembrane, or GPI-anchored form shown above. The two neuropilins are single-pass transmembrane receptors. The nine vertebrate plexins are subdivided into four type A and three type B plexins and plexins C1 and D1. Plexins are single-pass transmembrane receptors distinguished by the presence of a split cytoplasmic GAP domain. Domains: PSI plexin, semaphoring, and integrin, Sema semaphorin, Ig immunoglobulin-like, G-P glycine-proline-rich, CUB complement binding, FV/FVIII FV/FVIII coagulation factor-like, MAM Meprin, A5, Mu
1.3 Invertebrate Plexins
Invertebrate semaphorins have been characterized in Drosophila melanogaster. D. melanogaster semaphorins consist of Sema-1a, Sema-1b, and Sema-2a. Sema-1a and Sema-1b are transmembrane semaphorins and bind to PlexA, whereas Sema-2a is a secreted semaphorin and binds to PlexB, inducing axon repulsion, dendritic targeting, and synapse formation (Fig. 1.2). PlexA forms a complex with Off-track, a transmembrane-type putative receptor tyrosine kinase, and this association has been thought to be required for axon repulsion (Winberg et al. 2001). In addition, PlexA probably associates with Gyc76C, a transmembrane-type guanylate cyclase, and Gyc76C-mediated cyclic GMP production has been thought to function in PlexA signaling (Ayoob et al. 2004). Downstream signaling systems of PlexA have been studied and several associated molecules have been identified. PlexA associates with Nervy, a member of the A-kinase anchoring proteins, and this association links to A-kinase, suggesting the involvement of cyclic AMP-dependent phosphorylation in the functions of Sema-1a/PlexA (Terman and Kolodkin 2004). Another important association molecule of PlexA is MICAL (molecule interacting with CasL). MICAL is a flavoprotein monooxygenase, and this enzyme activity has been shown to be required for Sema-1a-mediated axon repulsion (Terman et al. 2002). Recently, it has been shown that MICAL oxidizes actin, inducing the severing of actin filaments, and decreasing polymerization (Hung et al. 2011), and this redox activity of MICAL links semaphorins to F-actin disassembly (Hung et al. 2010), proposing a striking novel mechanism for semaphorin-mediated cytoskeletal reorganization. Furthermore, methionine-R-sulfoxide reductase B1 (MsrB1) has been shown to reduce the methionine residues of actin oxidized by MICAL and support actin assembly, indicating that MICAL and MsrB1 regulate actin assembly via oxidation and reduction of the methionine residues of actin (Lee et al. 2013). In contrast to PlexA, the signaling systems of PlexB are not yet understood. It has been reported that PlexB associates with the Rho family small GTPases, Rac and Rho, but this signaling system is not known (Hu et al. 2001).
Model for signal transduction of invertebrate semaphorin receptors. Two Drosophila melanogaster plexins have been described, PlexA and PlexB. Sema-1 directly binds to PlexA. A novel protein, MICAL, directly associates with the C2 domain of PlexA, inducing repulsive response through actin cytoskeleton reorganization. MICAL and MsrB1 are an oxidase and a reductase, respectively, and promote depolymerization and polymerization of acin filament, respectively, through regulation of actin oxidation. PlexB is a functional Sema-2a receptor and interacts directly with both Rac and Rho
1.4 Plexin-As
The Sema3 subfamily does not directly bind to Plexin-As but does bind to neuropilins, and the Sema3-bound Plexin-A/neuropilin complex triggers downstream signaling. Among the Sema3 subfamily, Sema3A and Sema3F selectively associate with the neuropilin-1/Plexin-A4 complex and the neuropilin-2/Plexin-A3 complex, respectively.
Semaphorins induce guidance signaling through regulation of cytoskeletal reorganization. One of the key regulators of cytoskeletal reorganization is Rho family small GTPases (Burridge and Wennerberg 2004). The Rho family GTPases serve as molecular switches by cycling between an inactive GDP-bound state and an active GTP-bound state, and, once activated, they can interact with their specific effectors, leading to a variety of biological functions. Activation of the Rho family GTPases requires GDP–GTP exchange catalyzed by various guanine nucleotide exchange factors (GEFs) in response to a variety of extracellular stimuli, and their activation is turned off by GTPase-activating proteins (GAPs), which promote the intrinsic GTPase activities of the G proteins. Extracellular stimuli regulate the activities of Rho family GTPases through control of either GEFs or GAPs. Sema3A-induced growth cone collapse has been known to require Rac1 activity in dorsal root ganglion (DRG) neurons and spinal motoneurons (Jin and Strittmatter 1997). Plexin-A1 has been shown to associate with FARP2 (FERM, Rho GEF, and pleckstrin domain protein 2), a Rac GEF, and Sema3A binding to the receptor triggers the dissociation of FARP2 from Plexin-A1 and induces Rac1 activation (Toyofuku et al. 2005) (Fig. 1.3). Concerning the role of Rac1 activity in Sema3A signaling, it was reported that Rac1 is required for Sema3A-mediated endocytosis of the growth cone plasma membranes and reorganization of F-actin in DRG neurons (Jurney et al. 2002). Endocytosis of plasma membranes is supposed to be an important step for growth cone collapse. Sema3A-induced Rac1 activation may drive endocytosis of the plasma membranes instead of membrane protrusions, resulting in growth cone collapse. In addition, Rac1 activation transduces signaling through PAK (p21-activated kinase)-LIM kinase-cofilin to reorganization of actin filaments. Another role of Rac1 in the signaling of Plexin-A is the regulation of Plexin-A GAP activity. Plexins have a GAP domain in the intracellular tail, and the GAP domain is split by the Rho family GTPase-binding domain (Negishi et al. 2005; Zhou et al. 2008). Rac1 activated by FARP2 facilitates the binding of Rnd1, a member of Rho family GTPases, to the inserted region between the split GAP domains, inducing GAP activity for R-Ras (Toyofuku et al. 2005). Dissociated FERP2 also inhibits type 1 phosphatidylinositol phosphate kinase (PIPKIγ661), resulting in suppression of integrin activation.
Model for Plexin-A signal transduction. Sema3A and Sema3F bind to neuropilin-1 and -2, respectively, which associate with Plexin-A, and Plexin-A transduces repulsive signals. Several tyrosine and serine/threonine protein kinases, including Fes/Fps, Fyn, Cdk5, and GSK3β, are activated by Sema3A, and eventual phosphorylation of CRMP2 is involved in Sema3A-induced repulsive response. Sema3A stimulates R-Ras GAP activity of Plexin-A, suppressing R-Ras activity, whereas it activates Rac through a Rac GEF, FARP2. Sema6A directly binds to Plexin-A2 and -A4, whereas Sema6C binds to Plexin-A1 to elicit axon repulsion
Tyrosine phosphorylation is involved in Sema3A/Plexin-A signaling, and Fes/Fps tyrosine kinase has been implicated in Sema3A-induced growth cone collapse (Mitsui et al. 2002). Fes/Fps is a non-receptor-type tyrosine kinase and directly binds to the cytoplasmic tail of Plexin-A1. In the unstimulated condition, neuropilin associates with Plexin-A1 and blocks the binding of Fes/Fps to Plexin-A1. Sema3A binding to neuropilin permits Fes/Fps to associate with and phosphorylate Plexin-A1. This tyrosine phosphorylation stimulates a repulsive response in the receptor. Collapsin response mediator protein 2 (CRMP2) was originally identified as an intracellular signaling molecule of Sema3A (Goshima et al. 1995). CRAM was identified as another member of CRMPs and was shown to associate with CRMP2, forming a CRMP2–CRAM complex (Inatomi et al. 2000). Fes/Fps tyrosine-phosphorylates the CRMP2/CRAM complex as well as Plexin-A1. In addition to Fes/Fps, another kinase signaling is involved in Sema3A-mediated repulsive response. Fyn, a member of the Src family of tyrosine kinases, associates with and phosphorylates Plexin-A2 in response to Sema3A (Sasaki et al. 2002). Furthermore, serine/threonine kinase Cdk5 associates with Plexin-A2 and is activated through Sema3A-mediated Fyn; activated Cdk5 initially phosphorylates CRMP2. Then, another Ser/Thr kinase, GSK3β, subsequently phosphorylates the Cdk5-phosphorylated CRMP2, and this dually phosphorylated CRMP2 is essential for the Sema3A-induced growth cone collapse (Brown et al. 2004). CRMP2 binds to tubulin heterodimers, promoting microtubule assembly (Fukata et al. 2002). It is thus plausible that Cdk5 and GSK3β participate in Sema3A signaling through regulation of microtubule dynamics by phosphorylation of CRMP2.
In addition to Sema3s, Sema6s are ligands for Plexin-As. In contrast to Sema3s, secreted semaphorins, membrane-associated Sema6s directly bind to Plexin-As and do not require neuropilins (Fig. 1.3). Sema6A binds to Plexin-A2, controlling cerebellar granule cell migration (Kerjan et al. 2005). Sema6A binds to both Plexin-A2 and Plexin-A4, controlling lamina-restricted projection of hippocampal mossy fibers (Suto et al. 2007). Sema6A has been shown to associate with Plexin-A4 and promote the dendritic growth of spinal motor neurons through FARP1, a member of the Rho GEFs (Zhuang et al. 2009). In optic chiasm retinal ganglion cells, Sema6D has been shown to regulate axon guidance through Plexin-A1, switching the responses between repulsion and attraction dependent on the combination with Nr-CAM (Kuwajima et al. 2012). Interestingly, it has been shown that cis interaction between Sema6A and Plexin-A4 modulates the repulsive response to Sema6A (Topper et al. 2010). Further, reverse signaling of Sema6D has been reported, meaning that Sema6D is a Plexin-A1 ligand and also a receptor for Plexin-A1 (Toyofuku et al. 2004). Sema6D/Plexin-A1 regulates ventricular expansion, whereas Sema6D reverse signaling induces the expansion of the outer myocardial layer and trabeculation through the recruitment of Abl to the C-terminal domain of Sema6D. Thus, Sema6s/Plexin-As functions are diverse and complicated, and their downstream signaling pathways are not well characterized.
In addition to the aforementioned molecular systems, several other signaling molecules have been reported to associate with Plexin-As, including RanBPM (Togashi et al. 2006), DAP12 (Takegahara et al. 2006), and p75 neurotrophin receptor (Zvi et al. 2007). Furthermore, it has been reported that Sema3A induces intra-axonal translation of RhoA mRNA, and this local translation of RhoA is necessary and sufficient for Sema3A-mediated axonal growth cone collapse, indicating regulation of the neuronal cytoskeleton by local RhoA translation (Wu et al. 2005). These signaling molecules may contribute to many functions of the Sema3 subfamily.
1.5 Plexin-Bs
Plexin-Bs are responsible for a wide range of Sema4 subfamily functions, including axon guidance, dendrite remodeling, and angiogenesis, and Sema4s directly bind to Plexin-Bs, transducing signals. Plexin-B1 is the receptor for Sema4D, and it has been well characterized in its signaling pathways. Rho family small GTPases are also thought to be required for Plexin-B1 signaling. Active Rac1 was first reported to directly bind to the CRIB-like domain located between two GAP domains in the cytoplasmic tail of Plexin-B1 (Driessens et al. 2001). Plexin-B suppresses Rac activity by sequestering active Rac from its downstream effector PAK, inducing repulsive response in both Drosophila and mammalian cells (Hu et al. 2001; Vikis et al. 2002). Inhibition of Rac-mediated PAK activation by Plexin-B may suppress Rac-induced membrane protrusions, supporting the repulsive response. Furthermore, active Rac was shown to stimulate the localization of Plexin-B1 to the cell surface, enhancing Sema4D binding to the receptor (Vikis et al. 2002). Thus, signaling of Rac and Plexin-B1 appears to be bidirectional; Rac modulates Plexin-B1 activity, and Plexin-B1 modulates Rac function.
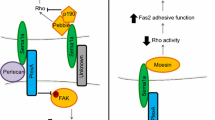
Among plexin families, the Plexin-B subfamily has a PSD-95/Dlg/ZO-1 (PDZ) domain-binding motif at the C-terminus (Fig. 1.4). Plexin-B1 directly interacts with two Rho GEFs containing PDZ domains, PDZ-RhoGEF and leukemia-associated Rho GEF (LARG), through the C-terminal PDZ binding motif (Swiercz et al. 2002; Aurandt et al. 2002; Perrot et al. 2002). They have PDZ domains at the N-terminal region and are members of the RGS-RhoGEF family, which has G12-family binding domains and specifically activates RhoA through G12-family binding. Sema4D binding to Plexin-B1 regulates the activity of PDZ-RhoGEF/LARG, leading to activation of RhoA, and this RhoA activation is involved in the Plexin-B1-induced repulsive response. It was shown that Sema4D/Plexin-B1-induced repulsive response is inhibited by an inhibitor of Rho-kinase, a well-studied Rho effector (Swiercz et al. 2002). Rho-kinase is known to phosphorylate the myosin-binding subunit of myosin phosphatase and myosin light chain, causing contraction of actomyosin (Fukata et al. 2001). The activation of myosin by Rho-kinase and the resultant contraction of actomyosin trigger cellular contraction and neurite retraction. In angiogenic systems, Sema4D/Plexin-B1 promotes endothelial cell motility through RhoA/Rho-kinase activation, and this action is mediated by integrin-dependent Pyk2 activation and the resultant stimulation of PI3kinase, Akt, and ERK through RhoA/Rho-kinase activation (Basile et al. 2007). Plexin-B1 constitutively interacts with PDZ-RhoGEF through its C-terminal PDZ domain-binding motif. How does Sema4D trigger the activation of associated PDZ-RhoGEF? It is known that ErbB-2, a tyrosine kinase receptor, associates with Plexin-B1 and tyrosine-phosphorylates Plexin-B1 in response to Sema4D (Swiercz et al. 2004). It has been shown that this ErbB-2-mediated tyrosine phosphorylation of Plexin-B1 promotes the interaction of phosphorylated Plexin-B1 with phospholipase Cγ through its SH2 domain and that phospholipase Cγ activates PDZ-RhoGEF via its SH3 domain (Swiercz et al. 2009). This phospholipase Cγ-mediated activation of PDZ-RhoGEF is independent of its lipase activity.
Model for signal transduction of regulation of Rho activity by Plexin-B1. The C-terminal tail of Plexin-B1 has a PDZ-binding motif and stably associates with Rho-specific GEF, PDZ-RhoGEF, and LARG through this motif. Tyrosine phosphorylation of Plexin-B1 by ErbB2 in response to Sema4D induces phospholipase Cγ association with Plexin-B1, inducing RGS-RhoGEF and resultant RhoA activation. On the other hand, tyrosine phosphorylation of Plexin-B1 by Met triggers the association of Grb2/p190RhoGAP with Plexin-B1, activating p190RhoGAP and inhibition of RhoA activity
Sema4D/Plexin-B1 stimulation frequently evokes different and sometimes opposing cellular responses depending on the cellular context; Sema4D/Plexin-B1 inhibits integrin-mediated cell attachment and cell migration in NIH-3T3 cells, whereas Sema4D/Plexin-B1 stimulates migration of SK-BR3 cells and endothelial cells through RhoA activation (Zhou et al. 2008). In contrast to RhoA activation by Plexin-B1 through PDZ-RhoGEF, Plexin-B1 has been shown to associate with p190 RhoGAP, leading to inhibition of cell migration (Barberis et al. 2005). Furthermore, it has been shown that interaction of Plexin-B1 with Met, another tyrosine kinase receptor, promotes the association of Plexin-B1 with p190 RhoGAP and subsequent RhoA inactivation, leading to Sema4D-mediated inhibition of cell migration (Swiercz et al. 2008). The activation of Plexin-B1 by Sema4D induces tyrosine phosphorylation of Plexin-B1 by Met, creating a docking site for the SH2 domain of Grb2, and associated Grb2 recruits p190 RhoGAP into the Plexin-B1/Grb2 complex through its SH3 domain, inducing RhoA inactivation (Sun et al. 2012). Therefore, Plexin-B1 associates with two types of tyrosine kinase receptors, ErbB-2 and Met, and reciprocally regulates RhoA activity; the Plexin-B1/ErbB-2 complex activates RhoA through PDZ-RhoGEF, whereas Plexin-B1/Met complex inactivates RhoA through p190 RhoGAP, explaining how Sema4D exerts differential activities through governing different signaling pathways.
Plexin-B1 activates RhoA through association of PDZ-RhoGEF with the C-terminal PDZ-binding motif of Plexin-B1. However, among the entire plexin family, the C-terminal PDZ-binding motif is found only in the Plexin-B subfamily, and it is not found in invertebrate Plexin-B. Thus, it is unlikely that PDZ-RhoGEF-mediated RhoA activation is a common signaling pathway for the plexin family. The cytoplasmic tails of plexins have GAP domains. Plexin-B1 was first identified to display GAP activity for R-Ras (Oinuma et al. 2004a, b), and Plexin-A1, -D1, and -C1 have been shown to have R-Ras GAP activity in succession (Uesugi et al. 2009) (Fig. 1.5). Plexin-B1 has two subdomains, C1 and C2, showing GAP activity within the C-terminal tail, and these domains contain primary and secondary arginine motifs, respectively, which are critical motifs for GAP activity for small GTPases. Rnd1, a constitutively active atypical Rho family GTPase, binds to the region between C1 and C2, and this Rnd1 binding is essential for the expression of R-Ras GAP activity. Both Rnd1 binding to Plexin-B1 and Sema4D stimulation are indispensable for this activity (Oinuma et al. 2004a, b). In the absence of Rnd1, C1 and C2 domains interact intramolecularly, rendering the receptor inactive for R-Ras GAP activity. Rnd1 binding to the region between C1 and C2 domains disrupts the interaction of C1 and C2 domains, indicating that Rnd1 relieves the closed conformation of the C-terminal tail of Plexin-B1. This Rnd1-bound open conformation acquires an ability to associate with GTP-bound R-Ras. The Rnd1–Plexin-B1 complex can hold the GTP-bound R-Ras but not promote GTPase activity. Sema4D is homodimerized through cysteine disulfide bonds. Sema4D ligand binding to Plexin-B1 induces clustering of the Rnd1-bound monomeric receptor, and this clustering triggers the hydrolysis of GTP on R-Ras. Therefore, GAP activation of Plexin-B1 consists of two steps, interaction with GTP-bound R-Ras and promotion of GTP hydrolysis by R-Ras; the former is stimulated by Rnd1, and the latter is a process induced by the clustering by Sema4D. In addition to Plexin-B1, interaction of C1 and C2 domains has also been reported in Plexin-A1 and Plexin-D1, suggesting that this activation mechanism is a common system for the plexin family (Uesugi et al. 2009). The expression of R-Ras GAP activity of Plexin-B1 in response to Sema4D is required for the Sema4D/Plexin-B1-mediated axonal growth cone collapse. Sema4D/Plexin-B1 inhibits integrin-mediated cell migration in a variety of cells through R-Ras GAP activity (Oinuma et al. 2006). R-Ras has been shown to play a key role in cell adhesion and its activation is known to promote cell adhesion and neurite outgrowth through integrin activation (Kinbara et al. 2003). Therefore, downregulation of R-Ras activity by Sema4D/Plexin-B1 suppresses integrin activation and thereby reduces cell adhesiveness, leading to growth cone collapse and inhibition of cell migration.
Model for signal transduction of R-Ras GAP activity of Plexin-B1. The C1 and C2 domains of the cytoplasmic tail of Plexin-B1 encode R-Ras GAP. The C1 and C2 domains interact with each other, and Rnd1 binding to the region between C1 and C2 domains disrupts this interaction, allowing the receptor to associate with GTP-bound R-Ras. Sema4D-induced clustering of the Plexin-B1/Rnd1 complex promotes the hydrolysis of GTP by R-Ras. Downregulation of R-Ras activity inhibits integrin activity, reducing cell adhesion. Sema4D/Plexin-B1 suppresses the PI3-kinase pathway through both inhibition of R-Ras activity and PTEN activation, leading to CRMP2 phosphorylation and resultant microtubule depolymerization. R-Ras/M-Ras GAP activity suppresses R-Ras/M-Ras-mediated lamellipodin activation, leading to inhibition of actin filament polymerization
Downstream signaling pathways of R-Ras GAP activity of Plexin-B1 were characterized. PI3-kinase is one of the prominent downstream effectors of R-Ras. It has been shown that Sema4D stimulation of Plexin-B1 inactivates PI3-kinase and Akt and activation of GSK3β through R-Ras GAP activity, and that active GSK3β phosphorylates and inactivates CRMP2, inducing axonal growth cone collapse (Ito et al. 2006). On the other hand, the phosphatidylinositol-3-phosphate level is critically regulated by PI3-kinase and PTEN (phosphatase and tensin homologue deleted chromosome 10). Phosphorylation of PTEN is known to suppress its phosphatase activity. It has been shown that Sema4D stimulation of Plexin-B1 induces dephosphorylation and activation of PTEN through R-Ras GAP activity and that this PTEN activation is also involved in Sema4D/Plexin-B1-mediated axonal growth cone collapse (Oinuma et al. 2010). Furthermore, Sema4D-induced PTEN activation via R-Ras GAP activity is mediated by the inhibition of casein kinase 2α. Thus, R-Ras GAP activity of Plexin-B1 dually regulates the phosphatidylinositol-3-phosphate level, inhibition of PI3-kinase activity, and stimulation of PTEN activity, leading to Akt activity suppression, GSK3β activation, and CRMP2 phosphorylation and then induces repulsive response, probably through suppression of microtubule polymerization mediated by CRMP2.
The R-Ras subfamily consists of three G proteins: R-Ras, M-Ras, and TC21. Among these, Plexin-B1 displays GAP activity for R-Ras and M-Ras but not for TC21 (Saito et al. 2009). During neuronal development, R-Ras is expressed in the stage of axon specification and elongation, whereas M-Ras expression is upregulated during dendritic development, and R-Ras and M-Ras localize at the axon and dendrite, respectively (Saito et al. 2009). Sema4D/Plexin-B1 induces axonal growth cone collapse through inhibition of R-Ras activity, whereas Sema4D/Plexin-B1 induces reduction of dendrite growth through inhibition of M-Ras activity (Saito et al. 2009). Thus, Plexin-B1 is a dual functional GAP for R-Ras and M-Ras, remodeling axon and dendrite morphology, respectively. Concerning downstream signaling of M-Ras, lamellipodin was identified to be a novel effector of M-Ras (Tasaka et al. 2012). Lamellipodin is a ligand for Ena/VASP, which is an actin polymerization-promoting factor (Legg and Machesky 2004). Furthermore, Sema4D/Plexin-B1 has been shown to induce inhibition of lamellipodin action and the resultant disappearance of F-actin from distal dendrites through M-Ras GAP activity, reducing dendrite outgrowth (Tasaka et al. 2012). Thus, the M-Ras GAP activity of Plexin-B1 induces repulsive response through suppression of actin polymerization mediated by lamellipodin. Recently, plexins, including Plexin-B1, have been reported to display GAP activity for Rap1, and Rnd1 binding to plexins does not contribute to the activation of Rap GAP activity (Wang et al. 2012). However, it is not well characterized how both GAP activities, Rap GAP and R-Ras GAP, coordinately contribute to functions of plexins.
In contrast to Plexin-B1, signaling systems of the other Plexin-B subfamily receptors, Plexin-B2 and -B3, are not well characterized. Sema5A is a ligand for Plexin-B3, and Sema5A induces cell collapse of NIH3T3 fibroblasts through Plexin-B3, whereas Sema5A elicits attractive responses for epithelial and endothelial cells through the Plexin-B3/Met receptor complex (Artigiani et al. 2004). Thus, Sema5A/Plexin-B3 appears to provide reciprocal responses in cell contexts and Met may convert the Sema5A signaling.
1.6 Plexin-C1 and -D1
Plexin-D1 is a receptor for Sema3E. In contrast to the other Sema3s, Sema3E directly binds to Plexin-D1. Plexin-D1 is expressed in vascular endothelium, and Sema3E acts as a repulsive cue for the endothelial cells (Gu et al. 2005). Plexin-C1, also named VESPR for its viral origin, serves as a receptor for the virally encoded SemaVA, and Plexin-C1 stimulation induces inhibition of integrin-mediated adhesion and the chemokine-induced migration of dendritic cells (Walzer et al. 2005). Plexin-D1 and -C1 also display GAP activity for R-Ras and M-Ras, but differ from Plexin-A and -B subfamilies in the requirement for Rnd GTPases of R-Ras GAP activity (Fig. 1.6). Although Plexin-A and -B subfamilies require Rnd1 for displaying R-Ras GAP activity among Rnd subfamily GTPases, Plexin-C1 exhibits R-Ras GAP activity without the Rnd subfamily, whereas Plexin-D1 requires Rnd2 for displaying R-Ras GAP activity (Uesugi et al. 2009). Therefore, R-Ras GAP activity is a common signaling of plexin subfamilies, but the regulation of R-Ras GAP activity of plexins by Rnd proteins differs among plexin subfamilies.
Models for signal transduction of Plexin-C1 and D1. Sema3E directly binds to Plexin-D1 to either induce repulsive response through R-Ras GAP activity or attract response through the Plexin-D1/neuropilin/VEGF receptor complex. Plexin-C1 is a receptor for viral SemaVA or Sema7A. Plexin-C1 encodes R-Ras GAP, inhibiting R-Ras activity. Sema7A also binds to and activates integrins
Concerning antiangiogenic signaling through Plexin-D1, Sema3E/Plexin-D1 has been reported to initiate a two-pronged mechanism, R-Ras inactivation and Arf6 stimulation, which affect the status of activation of integrins and their intracellular trafficking, respectively (Sakurai et al. 2010). Furthermore, Sema3E stimulation has been shown to recruit phosphatidylinositol-4-phosphate-5-kinase to Plexin-D1, and its product, phosphatidylinositol-4,5-bis-phosphate, binds to GEP100/Brag2, a GEF for Arf6, stimulating Arf6 activity and inducing endothelial cell collapse (Sakurai et al. 2011). Sema3E/Plexin-D1 acts as the critical regulator for angiogenesis through cooperative linkage of two small GTPase signaling pathways. In contrast, Sema3E/Plexin-D1 provides reciprocal responses; Sema3E acts as a repellent for corticofugal and striatonigral neurons, expressing Plexin-D1 but not neuropilin-1, and Sema3E acts as an attractant for subiculomammillary neurons, coexpressing Plexin-D1 and neuropilin-1 (Chauvet et al. 2007). Furthermore, the latter neurons express VEGFR2 (vascular endothelial growth factor receptor type 2), and VEGFR2 associates with the Plexin-D1/neuropilin-1 receptor complex (Bellon et al. 2010). Sema3E triggers VEGFR2-dependent activation of the PI3-kinase/Akt signaling pathway, leading to axonal growth; this activation is independent of VEGF, a ligand for VEGFR2. Thus, gating of Sema3E/Plexin-D1 signaling by neuropilin-1 switches axonal repulsion to attraction, controlling the formation of select forebrain projections.
Sema7A was reported to associate with Plexin-C1, influencing immune cell functions, such as cytokine production. However, it was clearly shown that Sema7A promotes axon outgrowth through integrin activation and initiates T-cell-mediated inflammatory responses through the integrin receptor, indicating that integrin is a receptor for Sema7A (Pasterkamp et al. 2003; Suzuki et al. 2007). On the other hand, Sema7A has been shown to suppress melanoma progression through activation of Plexin-C1-mediated R-Ras GAP activity (Scott et al. 2009). Further work is needed for characterization of the endogenous ligands of Plexin-C1.
1.7 Concluding Remarks
Here we have summarized great advances in our understanding of the molecular mechanisms for intracellular signalings of semaphorin receptors. The main receptors for semaphorins are plexins, which directly encode R-Ras GAP and exert guidance signaling through R-Ras GAP activity and the resultant regulation of integrin activity, and this GAP activity is probably a common feature of semaphorin signaling. In addition, plexins differentially regulate Rho family GTPase activities through associating with either Rho GEF or Rho GAP. Identifying various molecules involved in semaphorin signal transduction pathways provides a good understanding of the diverse molecular mechanisms for the intricate guidance functions of semaphorins. In addition to semaphorins, many guidance molecules, such as netrins, ephrins, and slits, have been identified, and their signaling systems are extensively studied. Navigation of many cells is frequently determined by the integration of various guidance cues, and the combined actions of guidance molecules influence the outcome of guidance (Dudanova and Klein 2013). To unravel the complicated signaling systems, studies of the signaling by each guidance molecule and their combined actions are required. It seems certain that semaphorins are important guidance molecules in a wide range of cellular functions. Future research will undoubtedly reveal the entire picture of the signaling cascades of semaphorins and plexins for their diverse functions.
References
Artigiani S, Conrotto P, Fazzari P et al (2004) Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep 5:710–714
Aurandt J, Vikis HG, Gutkind JS et al (2002) The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci U S A 99:12085–12090
Ayoob J, Yu H-H, Terman JR et al (2004) The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a-plexin A-mediated axonal repulsion. J Neurosci 24:6639–6649
Barberis D, Casazza A, Sordella R et al (2005) p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signaling. J Cell Sci 118:4689–4700
Basile JR, Gavard J, Gutkind JS (2007) Plexin-B1 utilizes RhoA and Rho kinase to promote the integrin-dependent activation of Akt and ERK and endothelial cell motility. J Biol Chem 282:34888–34895
Bellon A, Luchino J, Haigh K et al (2010) VEGFR2 (KDR/FIk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron 66:205–219
Brown M, Jacobs T, Eickholt B et al (2004) a2-Chimaerin, cyclin-dependent kinase 5/p35 and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth cone collapse. J Neurosci 24:8994–9004
Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116:167–179
Chauvet S, Cohen S, Yoshida Y et al (2007) Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 56:807–822
Driessens MHE, Hu H, Nobes CD et al (2001) Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol 11:339–344
Dudanova I, Klein R (2013) Integration of guidance cues: parallel signaling and crosstalk. Trends Neurosci 36:295–304
Fukata Y, Amano M, Kaibuchi K (2001) Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22:32–39
Fukata Y, Ito T, Kimura T et al (2002) CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 4:583–591
Goshima Y, Nakamura F, Strittmatter P et al (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature (Lond) 276:509–514
Gu C, Yoshida Y, Livet J et al (2005) Semaphorin 3E and Plexin-D1 control vascular pattern independently of neuropilins. Science 307:265–268
Hu H, Marton TF, Goodman CS (2001) Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron 32:39–51
Hung RJ, Yazdani U, Yoon J et al (2010) Mical links semaphorins to F-actin disassembly. Nature (Lond) 463:823–827
Hung RJ, Pak CW, Terman JR (2011) Direct redox regulation of F-actin assembly and disassembly by Mical. Science 334:1710–1713
Inatomi R, Tsujimura T, Hitomi T et al (2000) Identification of CRAM, a novel unc-33 gene family protein that associates with CRMP3 and protein-tyrosine kinase(s) in the developing rat brain. J Biol Chem 275:27291–27302
Ito Y, Oinuma I, Katoh H et al (2006) Sema4D/Plexin-B1 activates GSK-3β through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep 7:704–709
Jin Z, Strittmatter SM (1997) Rac1 mediates collapsing-1-induced growth cone collapse. J Neurosci 17:6256–6263
Jurney WM, Gallo G, Letourneau PC et al (2002) Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J Neurosci 22:6019–6028
Kerjan G, Dolan J, Haunaitre C et al (2005) The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci 8:1516–1524
Kinbara K, Goldfinger LE, Hansen M et al (2003) Ras GTPases: integrins friends or foes? Nat Rev Mol Cell Biol 4:767–776
Kuwajima T, Yoshida Y, Takegahara N et al (2012) Optic chiasm presentation of semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 74:676–690
Lee BC, Peterfi Z, Hoffmann FW et al (2013) MsrB1 and MICALs regulates actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol Cell 51:397–404
Legg JA, Machesky LM (2004) MRL proteins: leading Ena/VASP to Ras GTPases. Nat Cell Biol 6:1015–1017
Mitsui N, Inatomi R, Takahashi S et al (2002) Involvement of Fes/Fps tyrosine kinase in semaphorin 3A signaling. EMBO J 21:3274–3285
Negishi M, Oinuma I, Katoh H (2005) Plexin: axon guidance and signal transduction. Cell Mol Life Sci 62:1363–1371
Neufeld G, Kessler O (2008) The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 8:632–643
Oinuma I, Ishikawa Y, Katoh H et al (2004a) The semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305:862–865
Oinuma I, Katoh H, Negishi M (2004b) Molecular dissection of the semaphorin 4D receptor Plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J Neurosci 24:11473–11480
Oinuma I, Katoh H, Negishi M (2006) Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating β1 integrin activity. J Cell Biol 173:601–613
Oinuma I, Ito Y, Katoh H et al (2010) Semaphorin 4D/Plexin-B1 stimulates PTEN activity through R-Ras GTPase-activating protein activity, inducing growth cone collapse in hippocampal neurons. J Biol Chem 285:28200–28209
Pasterkamp RJ, Peschon JJ, Spriggs MK et al (2003) Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature (Lond) 424:398–405
Perrot V, Prado J, Gutkind JS (2002) Plexin-B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J Biol Chem 277:43115–43120
Saito Y, Oinuma I, Fujimoto S et al (2009) Plexin-B1 is a GTPase activating protein for M-Ras, remodeling dendrite morphology. EMBO Rep 10:614–621
Sakurai A, Gavard J, Linhares Y et al (2010) Semaphorin 3E initiates antiangiogenic signaling through Plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol 30:3086–3098
Sakurai A, Jian X, Lee CJ et al (2011) Phosphatidylinositol-4-phosphate 5-kinase and GEP100/Brag2 protein mediate antiangiogenic signaling by semaphorin 3E-Plexin-D1 through Arf6 protein. J Biol Chem 286:34335–34345
Sasaki Y, Cheng C, Uchida Y et al (2002) Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35:907–920
Scott GA, McClelland LA, Fricke AF et al (2009) Plexin C1, a receptor for semaphorin 7A, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol 129:954–963
Sun T, Krishnan R, Swiercz JM (2012) Grb2 mediates semaphorin-4D-dependent RhoA inactivation. J Cell Sci 125:3557–3567
Suto F, Tsuboi M, Kamiya H et al (2007) Interactions between Plexin-A2, Plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53:535–547
Suzuki K, Okuno T, Yamamoto M et al (2007) Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1integrin. Nature (Lond) 446:680–684
Swiercz JM, Kuner R, Behrens J et al (2002) Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35:51–63
Swiercz JM, Kuner R, Offermanns S (2004) Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol 165:869–880
Swiercz JM, Worzfeld T, Offermanns S (2008) ErbB-2 and Met reciprocally regulate cellular signaling via Plexin-B1. J Biol Chem 283:1893–1901
Swiercz JM, Worzfeld T, Offermanns S (2009) Semaphorin 4D signaling requires the recruitment of phospholipase Cγ into the plexin-B1 receptor complex. Mol Cell Biol 29:6321–6334
Takegahara N, Takamatsu H, Toyofuku T et al (2006) Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 8:615–622
Tamagnone L, Artigiani S, Chen H et al (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99:71–80
Tasaka G, Negishi M, Oinuma I (2012) Semaphorin 4D/Plexin-B1-mediated M-Ras GAP activity regulates actin-based dendrite remodeling through lamellipodin. J Neurosci 32:8293–8305
Terman JR, Kolodkin AL (2004) Nervy links protein kinase A to plexin-mediated semaphorin repulsion. Science 303:1204–1207
Terman JR, Mao T, Pasterkamp RJ (2002) MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell 209:887–900
Togashi H, Schmidt E, Strettmatter SM (2006) RanBPM contributes to semaphorin3A signaling through Plexin-A receptors. J Neurosci 26:4961–4969
Topper L, Mlechkovich G, Savariego D et al (2010) Cis interaction between semaphorin 6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J 29:2635–2645
Toyofuku T, Zhang H, Kumanogoh A et al (2004) Guidance of myocardial patterning in cardiac development by Sema6D reverse signaling. Nat Cell Biol 6:1204–1211
Toyofuku T, Yoshida J, Sugimoto T et al (2005) FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci 8:1712–1719
Uesugi K, Oinuma I, Katoh H et al (2009) Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. J Biol Chem 284:6743–6751
Vikis HG, Li W, Guan K (2002) The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Gene Dev 16:836–845
Walzer T, Galibert L, Comeau MR et al (2005) Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol 174:51–59
Wang Y, He H, Srivastava S et al (2012) Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal 5:1–12
Winberg M, Tamagnone L, Bai J (2001) The transmembrane protein off-track associates with plexins and functions downstream of semaphorin signaling during axon guidance. Neuron 32:53–62
Wu KY, Hengst U, Cox LJ et al (2005) Local translation of RhoA regulates growth cone collapse. Nature (Lond) 436:1020–1024
Zhou Y, Gunput RF, Pasterkamp RJ (2008) Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci 33:161–170
Zhu L et al (2007) Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A 104:1621–1626
Zhuang B, Su YS, Sockanathan S (2009) FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane semaphorin6A and PlexinA4 signaling. Neuron 61:359–372
Zvi A, Gigi L, Klein H et al (2007) Modulation of semaphorin 3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J Neurosci 27:13000–13011
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Negishi, M., Oinuma, I. (2015). Semaphorin Receptors and Their Signaling. In: Kumanogoh, A. (eds) Semaphorins. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54385-5_1
Download citation
DOI: https://doi.org/10.1007/978-4-431-54385-5_1
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54384-8
Online ISBN: 978-4-431-54385-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)