Abstract
Odor information is substantially modulated in the first relay of the central nervous system, the olfactory bulb (OB). This chapter focuses on how the large number of local inhibitory interneurons in the OB contribute to the structural and functional plasticity of the OB neuronal circuits, and odor information processing within them. The major OB interneurons, granule cells (GCs) and periglomerular cells (PGCs), form the characteristic synaptic structures, called dendrodendritic reciprocal synapses with excitatory projection neurons, the mitral and tufted cells. Dendrodendritic synaptic inhibition of OB interneurons induces feedback inhibition, lateral inhibition, and synchronization to mitral/tufted cells. Dendrodendritic synaptic inhibition is regulated by various signals, including olfactory sensory inputs, top-down inputs from the olfactory cortex (OC), and neuromodulatory signals. Outputs from mitral/tufted cells are in turn substantially influenced by these signals via the plasticity of dendrodendritic inhibition of OB interneurons. A second intriguing property of OB interneurons is that they are continually generated throughout life, conferring high plasticity on the OB neuronal circuits. The life-and-death decision of adult-born GCs is regulated by sensory experience and the behavioral state of the animal, indicating that the fate decision of new OB interneurons is also under the control of multiple signals. Further, behavioral analysis of mice with suppression of adult neurogenesis revealed abnormalities in many kinds of odor-guided behaviors. These observations collectively indicate that the structural and functional plasticity of OB interneurons plays crucial roles in odor information processing, and that this plasticity contributes to the expression of proper olfactory behaviors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adult neurogenesis
- Behavioral state
- Cell elimination
- Dendrodendritic synapse
- Interneuron
- Neuromodulatory input
- Sensory input
- Slow-wave sleep
- Top-down input
6.1 Introduction
The olfactory bulb (OB) is the first relay of odor information processing in the central nervous system. In rodents, the OB is located at the rostral end of the brain, where it forms part of the telencephalon. It receives sensory input from the olfactory sensory neurons (OSNs) in the olfactory epithelium and sends the information to the olfactory cortex (OC). Dendrites of excitatory projection neurons in the OB, the mitral cells and tufted cells, form synapses with OSN axons to receive odor inputs, and their axons form synapses with dendrites of pyramidal cells in the OC to send the odor information to higher cortical regions.
Odor information processing in the OB has been an attractive target of analysis. One reason for this is the rather simple structure of OB neuronal circuits: different types of neurons and their synaptic connections are well organized in a laminated fashion, which is advantageous in both the structural and functional analysis of the neuronal circuits. A second reason is that analysis is facilitated by the identification of odorant receptors and axonal convergence of OSNs on topographically fixed glomeruli.
A remarkable feature of OB neuronal circuits is that local inhibitory interneurons outnumber excitatory projection neurons. In contrast to most brain regions, where a smaller number of local interneurons regulate the activity of a larger number of projection neurons, the OB has a larger number of local interneurons participating in the regulation of mitral/tufted cells. This fact indicates that odor information received from the OSNs is substantially modulated in the OB neuronal circuits by local interneurons before it is transferred to the OC.
This chapter focuses on OB interneurons, with particular regard to the contribution of these interneurons to the structural and functional plasticity of OB neuronal circuits. The structural organization and activity of OB interneurons are plastically regulated by a variety of signal systems. These signal systems in turn plastically modulate the outputs from mitral and tufted cells. The first topic in this chapter is how OB interneurons regulate the activity of mitral and tufted cells via the characteristic synaptic structure, dendrodendritic reciprocal synapses. Dendrodendritic synaptic inhibition is regulated by various signals, including olfactory sensory input, top-down inputs from the OC, and neuromodulatory signals.
The next topic of this chapter describes the adult neurogenesis of OB interneurons. OB interneurons are generated throughout life, a characteristic that provides highly plastic features to the OB neuronal circuits. Utilization of adult-born OB interneurons is regulated by olfactory sensory inputs and also by the behavioral state of the animal. OB interneurons undergo extensive turnover and are considered to contribute to the maintenance and plastic reorganization of OB neuronal circuits. The contribution of adult-born OB interneurons to olfactory behaviors is also discussed.
Through a comprehensive discussion of these topics, this chapter states that odor information is substantially modulated as early as in the first relay, the OB, and that OB neuronal circuits work in concert with other cortical and subcortical brain regions. OB interneurons play central roles in the plastic odor information processing in the OB neuronal circuits.
6.2 Structure and Function of Interneurons in the OB
6.2.1 Local Interneurons in the OB Form Dendrodendritic Reciprocal Synapses with Projection Neurons
In most brain regions, a smaller number of local inhibitory neurons regulates the activity of a larger number of excitatory projection neurons. In the OB, in contrast, this relationship is reversed: local inhibitory neurons far outnumber excitatory projection neurons, mitral and tufted cells, by about two orders of magnitude (Kaplan et al. 1985; Parrish-Aungst et al. 2007). OB local interneurons consist of the two most abundant populations, granule cells (GCs) and periglomerular cells (PGCs) (Mori 1987; Shepherd et al. 2004). The number of GCs is one order larger than that of PGCs. The somata of GCs are densely packed in the granule cell layer (GCL) whereas those of PGCs are aligned around glomeruli in the glomerular layer (GL) (Fig. 6.1).
Neuronal circuits of the olfactory bulb (OB). Layer structure of the OB. A Nissl-stained coronal section is shown on the left. Names of individual layers and their abbreviations are shown on the right. Axons of olfactory sensory neurons (OSNs) in the olfactory epithelium expressing the same type of odorant receptors (light blue, blue, dark blue) converge onto the same glomeruli (yellow circles). Mitral cells (M) and tufted cells (T) extend primary dendrites to a single glomerulus. Lateral dendrites of mitral cells distribute in the deep sublamina of the EPL; those of tufted cells distribute in the superficial sublamina of the EPL. A mitral cell-targeting granule cell (GC) (GC (M)) forms dendrodendritic synaptic contacts with mitral cell lateral dendrites. A tufted cell-targeting GC (GC (T)) forms dendrodendritic synaptic contacts with tufted cell lateral dendrites. A periglomerular cell (PGC) makes dendrodendritic synaptic contacts with mitral/tufted cells in a single glomerulus
The characteristic synaptic structure in the OB is the dendrodendritic reciprocal synapse, between interneurons and projection neurons. In contrast to ordinary synapses between axons and dendrites, dendrites of OB interneurons make synapses with dendrites of mitral/tufted cells. The dendrodendritic synapse is reciprocal. In the case of GCs, the dendrodendritic synapse consists of a mitral/tufted-to-granule glutamatergic excitatory synapse and a granule-to-mitral/tufted GABAergic inhibitory synapse (Fig. 6.2). Historically, pioneering work by neuroanatomists Camillo Golgi and Santiago Ramón y Cajal suggested that GCs in the OB lack axons (Golgi 1875; Cajal 1890). Because of this peculiar morphology, the functions of GCs, including whether they are actually neurons, long remained obscure, although Cajal and his student T. Blanes raised the possibility that GC dendrites may emit signals similar to axons (Blanes 1897). Many decades later, advances in electrophysiological recordings and computational modeling of neuronal circuits led to the suggestion that GCs were the major source of inhibitory synaptic input to mitral/tufted cells, and the existence of dendrodendritic reciprocal synapses was proposed (Shepherd 1963; Rall et al. 1966). In fact, this unique synaptic structure was identified by electron microscopic analysis (Hirata 1964; Andres 1965; Price and Powell 1970a, b). Eventually, the concept of dendrodendritic reciprocal synapses was established both in structure and in function (Rall and Shepherd 1968; for review, Shepherd et al. 2007).
Dendrodendritic reciprocal synapses between OB interneurons and mitral/tufted cells. A schema of a dendrodendritic reciprocal synapse between the lateral dendrite of a mitral/tufted cell (M/T) and the gemmule of a GC apical dendrite. A mitral/tufted-to-granule glutamatergic excitatory synapse and a granule-to-mitral/tufted GABAergic inhibitory synapse are arranged side by side in the contact site. Synaptic densities and synaptic vesicles are illustrated
GCs are GABAergic local inhibitory interneurons that lack axons. They extend apical dendrites into the external plexiform layer (EPL) and basal dendrites within the GCL (Fig. 6.1). Apical dendrites of GCs form dendrodendritic reciprocal synapses with lateral dendrites of mitral/tufted cells in the EPL. A given GC makes such dendrodendritic reciprocal synapses with mitral/tufted cells belonging to the same glomerular unit, namely, those extending primary dendrites to the same glomerulus, and with mitral/tufted cells belonging to different glomerular units, namely, those extending primary dendrites to different glomeruli. Accordingly, GCs modulate the activity of mitral/tufted cells within and across glomerular units.
The second major type of interneuron, PGCs, typically extend dendrites into a single glomerulus (Fig. 6.1). Within the glomerulus, PGC dendrites receive excitatory inputs from the OSNs via axodendritic synapses and from mitral or tufted cell primary dendrites via dendrodendritic synapses. A PGC sends inhibitory output via dendrodendritic synapses to mitral/tufted cell primary dendrites and thereby modulates the activity of mitral/tufted cells in a given glomerular unit (Shepherd et al. 2004). PGCs also have axons that innervate mitral/tufted cells of different glomerular units and thereby modulate the activity of mitral/tufted cells across glomerular units. The OB also contains another type of inhibitory neuron called short axon cells, whose function has attracted recent interest but remains largely unknown (Schneider and Macrides 1978; Eyre et al. 2008; Boyd et al. 2012).
Although the dendrodendritic synapses are the sole route of output from GCs, the synapses are not the only route of excitatory inputs to GCs. GCs also receive glutamatergic synaptic input from pyramidal cells in the olfactory cortex (OC) via axodendritic synapses in the GCL (Fig. 6.3). Pyramidal cells in the OC project axon collaterals massively back to the OB. The top-down centrifugal axons distribute mostly to the GCL of the OB and terminate on inhibitory interneurons such as GCs and short axon cells (Luskin and Price 1983; Boyd et al. 2012; Markopoulos et al. 2012). Thus, GCs are excited both by olfactory sensory inputs transmitted from mitral/tufted cells via the dendrodendritic synapses and by the activities of the OC pyramidal cells via the top-down axodendritic synapses. PGCs are also considered to receive top-down inputs from the OC, albeit it to a lesser degree than that to GCs.
Signals to OB interneurons. GCs receive excitatory synaptic inputs from mitral/tufted cells (M/T) via dendrodendritic synapses. These inputs represent olfactory sensory inputs (red arrows). GCs also receive top-down excitatory synaptic inputs from pyramidal cells (Py) in the OC via axodendritic synapses (blue arrows). These inputs represent the firing activity of OC pyramidal cells. GCs receive neuromodulatory signals (black) including noradrenergic, cholinergic, and serotonergic inputs
6.2.2 Physiological Properties of OB Interneurons and Their Role in the Modulation of Mitral and Tufted Cell Activity
Dendritic spines of GCs that form reciprocal synapses in the EPL are larger in size than ordinary spines and are specifically called gemmules. At the site of contact of a mitral/tufted cell lateral dendrite and a GC gemmule, a mitral/tufted-to-granule excitatory synapse and a granule-to-mitral/tufted inhibitory synapse are positioned side by side in close proximity. This structure efficiently generates feedback inhibition on the lateral dendrite of the mitral/tufted cell (Fig. 6.4, left). This feedback inhibition can occur at local sites at dendrites without the firing activity of GCs (Jahr and Nicoll 1982; Schoppa et al. 1998; Isaacson and Strowbridge 1998). The activation of a mitral/tufted-to-granule excitatory synapse induces an increase in calcium in the GC gemmule, which then activates GABA release at the granule-to-mitral/tufted inhibitory synapse from the GC gemmule to the mitral/tufted cell lateral dendrite. Calcium influx in the GC gemmule through NMDAR crucially promotes GABA release (Schoppa et al. 1998; Isaacson and Strowbridge 1998; Chen et al. 2000; Halabisky et al. 2000).
Modulation of mitral/tufted cell activity by dendrodendritic synaptic inhibition from OB interneurons. Left: A mitral/tufted cell (M/T1) receives strong olfactory sensory input and another mitral/tufted cell (M/T2) receives weak olfactory sensory input. A GC forms dendrodendritic reciprocal synapses with these mitral/tufted cells and fires action potentials in response to the strong excitatory synaptic input via the dendrodendritic synapse with M/T1 (thick white arrow). The GC sends inhibition to M/T1 (feedback inhibition; thick black arrow). The GC sends inhibition also to M/T2 (lateral inhibition; thick black arrow), as a result of the propagation of action potentials to the dendrodendritic synapse with M/T2. This lateral inhibition suppresses the output from M/T2, which receives weak olfactory sensory input, and enhances the output contrast between M/T1 and M/T2. Right: Both mitral/tufted cells (M/T1 and M/T2) receive strong olfactory sensory input. A GC forming dendrodendritic reciprocal synapses with these mitral/tufted cells fires action potentials in response to the strong excitatory synaptic input via the dendrodendritic synapse with M/T1 and M/T2. Because of the propagation of action potentials to the entire dendritic tree of the GC, the GC sends simultaneous inhibition to M/T1 and M/T2 via the dendrodendritic synapses. The temporally coordinated inhibition of M/T1 and M/T2 leads to the synchronized firing of M/T1 and M/T2
Lateral dendrites of individual mitral or tufted cells extend as long as 1–1.5 mm, which covers almost half of the circumference of the OB (Mori et al. 1983; Orona et al. 1984). Because an action potential in a given mitral/tufted cell can propagate throughout the length of the lateral dendrites (Xiong and Chen 2002; Debarbieux et al. 2003), the firing activity of a mitral/tufted cell activates many dendrodendritic reciprocal synapses formed with many GCs along the lateral dendrites, by which individual dendrodendritic reciprocal synapses provide feedback inhibition to the mitral/tufted cell dendrites. One proposed role of this feedback inhibition is regulation of the spiking rate and timing of mitral/tufted cells. Pharmacological blockade of GABAergic inputs increases the firing rate of mitral/tufted cells during odor stimulation and also perturbs the prompt cessation of their firing at the end of odor stimulation (Margrie et al. 2001).
In addition to feedback inhibition, the dendrodendritic synapses also coordinate the activity of individual populations of mitral/tufted cells, based on the connectivity provided by the formation of dendrodendritic synapses from a given GC with many mitral/tufted cells. One mode of this coordination is called lateral inhibition (Fig. 6.4). Strong excitatory inputs from a subset of mitral/tufted cells induce spiking activities in GCs. The spiking activity propagates in the entire dendritic tree of the GCs, which then activates many dendrodendritic synapses on the GC dendrites (Chen et al. 2000; Egger et al. 2003). The granule-to-mitral/tufted synaptic inhibition is targeted not only to mitral/tufted cells involved in the GC excitation (feedback inhibition), but also to mitral/tufted cells that do not contribute to the GC excitation (lateral inhibition). The lateral inhibition suppresses weakly activated or nonactivated mitral/tufted cells and contributes to the contrast enhancement of mitral/tufted cell activity (Yokoi et al. 1995), and is thereby considered to potentiate odor discrimination ability in mice (Abraham et al. 2010).
Activation of dendrodendritic synaptic inhibition by GC firing requires voltage-gated calcium channels (Halabisky et al. 2000; Chen et al. 2000), which is in striking contrast to the dispensability of these channels in the feedback activation of dendrodendritic synaptic inhibition (Schoppa et al. 1998; Isaacson and Strowbridge 1998; Chen et al. 2000; Halabisky et al. 2000). This observation suggests that feedback inhibition and lateral inhibition are differentially regulated and differentially contribute to odor information processing. Subthreshold activity of GC dendrites can also spread in entire dendritic trees without firing activity, in which the spread is dependent on low-threshold, voltage-gated calcium channels (Egger et al. 2005). Lateral inhibition without GC firing is also considered to play a role in odor information processing.
Another mode of coordination by dendrodendritic synapses is the synchronization of mitral/tufted cells (Fig. 6.4). In local field potential (LFP) recording, the OB shows oscillatory activity at high frequency (gamma range, 30–80 Hz) which is enhanced by odor stimulation (Adrian 1950; Rall and Shepherd 1968). Single-unit recordings of mitral/tufted cells showed that their firing activity is phase locked to the oscillatory LFP, and that the synchronous firing of different mitral/tufted cell firing becomes evident during odor stimulation (Kashiwadani et al. 1999). Pharmacological blockade of synaptic inhibition in the MCL and EPL significantly diminishes this gamma oscillation (Lagier et al. 2004). Further, patch clamp recordings from mitral cell pairs showed that their synchronous firing is dependent on synaptic inhibition from GCs, which occurs synchronously in different mitral cells (Schoppa 2006). It is likely that synchronous recovery of mitral/tufted cells from dendrodendritic synaptic inhibition-mediated hyperpolarization triggers synchronized firing of mitral/tufted cells (Schoppa 2006). Although the function of this synchronized firing of mitral/tufted cells is not yet known, it is assumed that it effectively activates pyramidal cells in the OC that receive converged projection from many mitral/tufted cells (Mori et al. 1999). Oscillatory strength in the OB correlates with the efficacy of olfactory learning (Martin et al. 2006; Beshel et al. 2007).
In contrast to GCs, a given PGC typically forms dendrodendritic synapses with primary dendrites of mitral/tufted cells in a single glomerulus. PGC dendrites also form synapses on the axon terminals of OSNs that mediate retrograde inhibition to OSNs (Murphy et al. 2005). PGCs are therefore considered to primarily regulate odor-induced activities in particular glomeruli. In summary, OB interneurons modulate the activity of mitral/tufted cells by inducing feedback inhibition, lateral inhibition, and synchronization through dendrodendritic synaptic inhibition. Thus, odor information received from OSNs is substantially modulated by OB local interneurons before it is transferred to OC pyramidal cells.
6.2.3 Mechanisms of the Control of OB Interneuron Activity
Because dendrodendritic synapses are the sole outputs of GCs, the contribution of GCs to odor information processing is ultimately attributable to their dendrodendritic synaptic inhibition of mitral/tufted cells. The activation of dendrodendritic synaptic output of GCs is regulated by a variety of signals. As discussed, one notable signal is glutamatergic input from mitral/tufted cells via dendrodendritic synapses in the EPL. This input reflects odor information from the external world, which is received by OSNs and transferred to GCs via mitral/tufted cells.
Another crucial signal is the glutamatergic top-down input from OC pyramidal cells to GC dendrites in the GCL (Fig. 6.3). Although GC dendrites in the GCL also receive axon collaterals of mitral/tufted cells, anatomical and functional studies indicate that top-down inputs from the OC are much more massive than these mitral/tufted cell collaterals (de Olmos et al. 1978; Haberly and Price 1978; Balu et al. 2007). Top-down inputs to GCs in the GCL release NMDAR at dendrodendritic synapses in the EPL from the magnesium blockade and facilitate the dendrodendritic synaptic inhibition of mitral cells, thereby providing a “gating” signal for the dendrodendritic inhibition (Halabisky and Strowbridge 2003). In addition, theta-burst stimulation of GC synapses in the GCL induces long-term potentiation of the dendrodendritic synaptic inhibition of mitral/tufted cells in the EPL (Gao and Strowbridge 2009). Recent in vivo studies have shown that optogenetic stimulation of OC pyramidal cells suppresses spontaneous and/or odor-induced firing activity of mitral cells (Boyd et al. 2012; Markopoulos et al. 2012). This effect is most likely mediated by the enhanced dendrodendritic synaptic inhibition of GCs that receive substantial top-down inputs from OC pyramidal cells. These results indicate that dendrodendritic synaptic inhibition in the OB is under the control of OC pyramidal cell activity.
In addition to the top-down glutamatergic synaptic inputs, the OB is targeted by subcortical neuromodulatory systems that include cholinergic input from the horizontal limb of the diagonal band of Broca, noradrenergic input from the locus ceruleus, and serotonergic input from the raphe nuclei (Fig. 6.3) (Shipley and Ennis 1996). Most of the neuromodulatory fibers to the OB terminate on local interneurons and directly regulate their activity (Shipley and Ennis 1996; Shepherd et al. 2004). Cholinergic fibers innervate all layers of the OB, from the GCL to the GL (Kasa et al. 1995). Effects of acetylcholine vary among different cell types expressing different types of receptors (Le Jeune et al. 1995). Acetylcholine increases the excitability of PGCs and mitral cells via nicotinic receptors (Castillo et al. 1999). In contrast, the effect of acetylcholine on GCs is mainly mediated via muscarinic receptors. Although its effect on GC excitability is controversial (Castillo et al. 1999; Pressler et al. 2007), muscarinic receptor activation partially suppresses the dendrodendritic inhibition from GCs to mitral cells in vivo (Tsuno et al. 2008). Given that cholinergic input is potentiated during waking periods (Brown et al. 2012), the cholinergic modulation of dendrodendritic synapses likely optimizes odor information processing during waking olfactory behavior. Partial suppression of dendrodendritic inhibition might facilitate the propagation of action potentials in lateral dendrites of mitral/tufted cells (Xiong and Chen 2002), and may allow an appropriate number of mitral/tufted cells to synchronize for proper odor information processing. Although the exact mechanisms are not known, the blockade of cholinergic signals in the OB perturbs olfactory learning and memory (Ravel et al. 1994; Devore et al. 2012).
Noradrenergic fibers from the locus ceruleus are distributed predominantly in the GCL (McLean et al. 1989), and GCs express several subtypes of adrenergic receptors (McCune et al. 1993; Nai et al. 2009). The influence of noradrenergic signals in dendrodendritic inhibition has been closely discussed with regard to the accessory OB of pregnant rodents. Female mice form olfactory recognition memory to male mouse pheromones at mating, and noradrenergic input to the accessory OB is crucial to this memory formation. The memory trace for this is considered to be deposited as a plastic change in dendrodendritic reciprocal synapses in the accessory OB (Kaba and Nakanishi 1995). Based on the observation that noradrenalin reduces dendrodendritic synaptic inhibition via α2-receptors (Trombley and Shepherd 1992), a hypothesis was proposed that the mating-induced increase in noradrenalin transiently reduces granule-to-mitral dendrodendritic synaptic inhibition and that the resultant disinhibition of mitral-to-granule synaptic excitation leads to the long-term potentiation of granule-to-mitral dendrodendritic synaptic inhibition (Kaba and Nakanishi 1995). On the other hand, another study of the accessory OB showed that noradrenalin potentiates dendrodendritic synaptic inhibition via α1 receptors (Araneda and Firestein 2006). Although the results of these studies are contradictory, the effect of noradrenalin in the main OB was found to be concentration dependent: low doses of noradrenalin reduced dendrodendritic synaptic inhibition via α2-receptors whereas intermediate doses increased this inhibition via α1-receptors (Nai et al. 2009). The actual neuronal mechanisms of noradrenergic function awaits further study, but the importance of noradrenergic signals in olfactory behaviors has been widely documented. For example, activation of noradrenergic signals in the OB lowers the threshold for odor detection and discrimination (Escanilla et al. 2010), and blockade of signals in the OB perturbs olfactory learning (Sullivan et al. 1989; Veyrac et al. 2009).
Serotonergic fibers from the raphe nuclei predominantly innervate superficial layers of the OB, including GL (McLean and Shipley 1987). Serotonergic signals activate PGCs via serotonin 2C receptors, and the increased GABA release attenuates glutamate release from the OSNs (Petzold et al. 2009). Thus, serotonergic signals regulate odor inputs at a very early stage of information processing.
Taken together, these results indicate that the activity of OB interneurons and their dendrodendritic synaptic inhibition of mitral/tufted cells are regulated not only by odor inputs from the external world but also by the activity of the higher cortical region, the OC, and subcortical neuromodulatory centers. Indeed, the firing activity of mitral/tufted cells to odors is substantially modulated in a context- and behavioral state-dependent manner (Pager 1983; Kay and Laurent 1999; Doucette and Restrepo 2008; Doucette et al. 2011). Calcium imaging of the OB in vivo also showed that mitral/tufted cell activity is plastically modulated by odor experience (Kato et al. 2012). A large part of the plastic properties of mitral/tufted cell activity is considered to result from plasticity in the dendrodendritic synaptic inhibition of OB interneurons.
6.2.4 Subtypes of OB Interneurons
Although I have referred to GCs and PGCs as the two major populations of OB interneurons, they are in fact a heterogeneous population consisting of various subtypes. Knowledge of individual interneuron subtypes is important to understanding the function of OB neuronal circuits. Because the functions of individual subtypes are now little understood, the difference in OB interneuron subtypes in molecular expression and morphology is described here as a basis for the future understanding of their cooperative activity in odor information processing.
PGCs are subdivided into three nonoverlapping populations based on molecular expression, namely, the tyrosine hydroxylase (TH)-expressing, calretinin-expressing, and carbindin-expressing subtypes (Kosaka et al. 1995; Parrish-Aungst et al. 2007). A subset of TH-positive PGCs is thought to receive direct input from OSNs and send feed-forward inhibition to mitral/tufted cells via the dendrodendritic synapses, whereas carbindin-expressing PGCs are thought to not receive direct input from OSNs but rather to send dendrodendritic feedback inhibition to mitral/tufted cells in response to mitral/tufted-to-periglomerular excitatory inputs (Toida et al. 1998, 2000; Shao et al. 2009).
A small subset of GCs expresses calretinin, and their somata distribute to the MCL and the superficial sublamina of the GCL (Parrish-Aungst et al. 2007). A different subset of GCs express 5T4, a cell adhesion molecule with leucine-rich repeats in the extracellular domain, and their somata distribute mostly to the MCL (Imamura et al. 2006). Some GCs in the deep sublamina of the GCL express a calmodulin-binding protein neurogranin (Gribaudo et al. 2009). In contrast to PGCs, however, a majority of GCs do not express known protein markers for interneuron subtypes. Their molecular heterogeneity is therefore less understood.
The dendritic morphology of GCs differentiates GC subtypes in relationship to their connectivity to mitral/tufted cells. A subset of GCs ramifies apical dendrites preferentially in the deep sublamina of the EPL. They are presumed to form dendrodendritic synapses with mitral cells, whose lateral dendrites extend into the deep EPL (mitral cell-targeting GCs) (Fig. 6.1) (Mori 1987). Another GC subset ramifies apical dendrites preferentially in the superficial sublamina of the EPL. They are presumed to form dendrodendritic synapses with tufted cells, whose lateral dendrites extend into the superficial EPL (tufted cell-targeting GCs). 5T4-expressing GCs are considered to be a small subpopulation of tufted cell-targeting GCs (Imamura et al. 2006). The OB also contains a GC subtype whose apical dendrites do not extend into the EPL but form reciprocal synapses specifically with the somata of mitral cells (perisomatic-targeting GCs), although perisomatic-targeting synapses are also made by typical GCs that form dendrodendritic synapses in the EPL (Naritsuka et al. 2009). Somata of the mitral cell-targeting GCs tend to distribute to the deep sublamina of the GCL; those of the tufted cell-targeting GCs to the MCL and superficial sublamina of the GCL; and those of perisomatic-targeting GCs to the middle sublamina of the GCL (Mori 1987; Naritsuka et al. 2009).
It has been suggested that mitral cells and tufted cells constitute parallel pathways that handle different submodalities of odor information (Nagayama et al. 2004; Igarashi et al. 2012). It is possible that yet-unknown functional differences between mitral cell-targeting GCs and tufted cell-targeting GCs contribute to the different firing properties of mitral and tufted cells. In addition, subcellular targeting of interneurons is a crucial clue to the understanding of their function. In the hippocampus and neocortex, perisomatic-targeting interneurons play central roles in the synchronized firing of pyramidal cells (Sohal et al. 2009). In the OB, PGCs are targeted to the primary dendrites of mitral/tufted cells whereas GCs are targeted to the lateral dendrites and perisoma of mitral/tufted cells. Dendrodendritic synaptic inhibition of GCs to the proximal portion of mitral cell lateral dendrites inhibits the propagation of action potentials to the distal portion of the lateral dendrites (Xiong and Chen 2002), which presumably restricts the mitral/tufted cell population participating in lateral inhibition and synchronization. A closer understanding of the differential subcellular targeting of interneuron subtypes would reveal their differential roles in feedback inhibition, lateral inhibition, and synchronization of mitral/tufted cells.
6.3 Adult Neurogenesis of OB Interneurons
6.3.1 Adult OB Neurogenesis Provides Remarkable Plasticity in the OB Neuronal Circuit
The plasticity of OB neuronal circuits is further potentiated by an intriguing property of OB interneurons. Although the production of new neurons occurs only during the embryonic and neonatal periods in most brain regions, interneurons in the OB are continually generated even in adulthood (Lledo et al. 2006). In the olfactory system, OSNs in the olfactory epithelium turn over throughout life, with loss of old and incorporation of new cells. In spite of this turnover, the axonal targeting of OSNs expressing a given type of olfactory receptor to topographically fixed glomeruli is maintained (Gogos et al. 2000). OSNs are continually exposed to noxious stimuli from the external environment, such as viral infection and chemicals, and the continual generation of OSNs is thus considered to aid the maintenance of neuronal circuits despite the frequent loss of damaged OSNs. OB interneurons also turn over throughout life. In contrast to OSNs, however, turnover of OB interneurons provides a rich opportunity for extensive remodeling of the neuronal connectivity of OB neuronal circuits, which is likely not achievable by preexisting interneurons alone. As is explained in the following sections, the fact that utilization of new OB interneurons is plastically regulated by olfactory sensory experience and the behavioral state of the animal supports the notion that OB interneuron turnover contributes to the plasticity of neuronal circuits.
6.3.2 Generation and Synaptic Integration of Adult-Born OB Interneurons
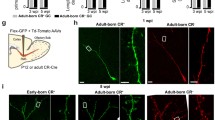
Precursors of OB interneurons are produced in the subventricular zone (SVZ) of the lateral ventricle (Fig. 6.5). Embryonic precursors of interneurons in the ganglionic eminence and neocortex migrate to and settle down in the SVZ and continue to generate OB interneurons throughout life (Young et al. 2007). The newly generated neuronal precursors in the SVZ migrate along a specific route called the rostral migratory stream (RMS) to the OB, where they differentiate into GCs and PGCs (Fig. 6.5). Interneuron precursors in the SVZ are heterogeneous and differentiate into distinct subtypes of OB interneurons depending on their position in the SVZ (Merkle et al. 2007). Although generation of GCs and PGCs peaks during the late embryonic and early neonatal periods, it continues substantially in adulthood. The number of adult-born OB interneurons is indeed large: in rodents, at least several tens of thousands of neurons enter the OB each day (Alvarez-Buylla et al. 2001; Winner et al. 2002; Lledo et al. 2006), corresponding to roughly 1 % of the total number of OB interneurons.
Adult neurogenesis in the OB. (a) Neuronal precursors are generated in the subventricular zone (SVZ) around the lateral ventricle and migrate to the OB via a specific route called the rostral migratory stream (RMS). (b) Adult-born GCs are visualized by retrovirus-mediated green fluorescent protein (GFP) expression. GFP-expressing retrovirus was injected in the SVZ and the OB was analyzed 28 days after the injection
Similar to embryonic and neonatal-born GCs, adult-born GCs receive glutamatergic synaptic contact from the same two major sources, mitral or tufted cells via dendrodendritic synapses in the EPL and pyramidal cells in the OC via axodendritic synapses in the GCL. Synaptic incorporation of adult-born GCs occurs roughly within 1 month after their generation (Petreanu and Alvarez-Buylla 2002; Carleton et al. 2003; Whitman and Greer 2007; Kelsch et al. 2008, 2010; Katagiri et al. 2011): axodendritic synaptic contacts from OC pyramidal cells occurs earlier, at around day 14, synaptic contacts from mitral/tufted cell dendrites become apparent later, at around day 21, and all synaptic structures become indistinguishable from those of preexisting mature GCs by day 28.
6.3.3 Selection of New GCs for Incorporation and Elimination
Not all new interneurons in the OB are stably incorporated into the neuronal circuit. Under normal conditions only half of new GCs succeed in living longer than 1 month after generation: the other half are eliminated by apoptosis (Petreanu and Alvarez-Buylla 2002; Winner et al. 2002; Yamaguchi and Mori 2005). Although such a large loss of neurons seems to be a wasteful process, initial excess neurogenesis and subsequent elimination commonly occur in both embryonic and adult neurogenesis. Neuronal selection during embryonic development is crucial to refining the neuronal circuitry for proper information processing (Buss et al. 2006), and this seems to similarly apply to neuronal selection in adult neurogenesis. When apoptotic elimination of adult-born OB interneurons is suppressed by a caspase inhibitor, odor discrimination ability is disturbed (Mouret et al. 2009), possibly because inappropriately incorporated adult-born interneurons disturb proper information processing.
The life and death of new OB interneurons are not predetermined. The fate decision of new OB interneurons is regulated by the olfactory sensory experience and the behavioral state of the animal. Revealing the regulatory mechanisms of the life-and-death decision of new OB interneurons is important to understanding how adult-born OB interneurons contribute to the plasticity of olfactory information processing.
6.3.4 Sensory Experience-Dependent Life-and-Death Decision of New GCs
The life and death of adult-born GCs depends on olfactory sensory experience. An increased survival rate of adult-born GCs was observed in mice that were repeatedly exposed to novel odors (odor-enriched environment) (Rochefort et al. 2002). Conversely, the survival rate of adult-born GCs was decreased in anosmic mice lacking a cyclic nucleotide-gated channel in OSNs (Petreanu and Alvarez-Buylla 2002) and in the ipsilateral OB of mice with unilateral sensory input deprivation (Corotto et al. 1994; Saghatelyan et al. 2005; Yamaguchi and Mori 2005; Mandairon et al. 2006). Olfactory sensory deprivation by nostril occlusion remarkably increases the apoptosis of new GCs, which can be detected immunohistochemically by the activation of caspase-3 (Fig. 6.6a,b) (Yamaguchi and Mori 2005).
Sensory experience-dependent life-and-death decision of adult-born GCs. (a) Deprivation of olfactory sensory input by single-nostril occlusion. Olfactory sensory input is conducted from the open nostril (left) to the same side of the olfactory epithelium (OE) and OB but is not conducted to the occluded side (right). (b) Caspase-3-activated apoptotic GCs in the OB (white spots). Compared to the sensory input-intact OB, a large number of apoptotic GCs is seen in the sensory-deprived OB. (c) Effect of sensory deprivation for 14 days at various periods after BrdU labeling on the survival of BrdU-labeled new GCs. Sensory deprivation during day 14–28 decreased survival; deprivation before or after this period showed no significant effect on survival. (d) A schema of the life-and-death decision of adult-born GCs. The sensory experience-dependent life-and-death decision of adult-born GCs is considered to occur during or after they form synaptic contacts with preexisting mitral/tufted cells (M/T) and pyramidal cells in the OC (Py). (Modified from Yamaguchi and Mori 2005)
In general, sensory experience-dependent plastic change in the central nervous system is subject to the influence of time windows. For example, deprivation of visual input from one eye shifts the response property of binocular zone neurons in the visual cortex preferentially toward the nondeprived eye input (Hensch 2005). This ocular dominance plasticity occurs during a specific period after birth, called the critical period. Whether there is a critical period for adult-born GCs when their life and death is strongly influenced by olfactory sensory experience was examined. Newly generated GCs in adult mice were labeled by intraperitoneal BrdU injection, and the mice were then deprived of olfactory sensory input by nostril occlusion at various time periods after labeling. The results showed that sensory deprivation during days 14–28 after GC generation greatly reduced the survival of GCs, whereas deprivation before or after this period had no significant effect (Fig. 6.6c) (Yamaguchi and Mori 2005). Consistent with this, most apoptotic GCs were aged 14–28 days. These observations indicate that the sensory experience-dependent life-and-death decision of new GCs occurs during a critical time window at days 14–28 after their generation.
This time window corresponds to the period when adult-born GCs make extensive synaptic contacts with preexisting neurons (Petreanu and Alvarez-Buylla 2002; Carleton et al. 2003; Whitman and Greer 2007; Kelsch et al. 2008, 2010; Katagiri et al. 2011), suggesting that synaptic input plays a crucial role in the selection of adult-born GCs. Morphological examination of adult-born GCs in anosmic mice showed that young GCs destined for later elimination had already developed many synaptic structures in the EPL (Petreanu and Alvarez-Buylla 2002). Thus, it appears that the life-and-death decision of new GCs is conducted after they form synaptic contacts with preexisting neurons (Fig. 6.6d). The usability of individual new GCs may be determined with respect to their interaction with preexisting neurons in the neuronal circuit.
6.3.5 Behavioral State-Dependent Life-and-Death Decision of New GCs
Olfactory sensory experience is tightly linked to behavioral outputs of animals, such as food searching/eating, mating with partners, and escaping from predators, all of which are critical to the maintenance of life (Doty 1986). Because many odor-guided behaviors have to be newly acquired or updated to cope with a changing odor world, it is likely that neuronal circuits in the central olfactory system, including the OB, are reorganized on a daily basis during the odor experience and the acquisition/improvement of odor-guided behaviors. Thus, the life-and-death decision of new GCs may occur in association with olfaction-related behaviors, which consist of a sequence of different behavioral states. A remarkable alteration in behavioral state is the wake–rest/sleep cycle: after extensive waking olfactory behaviors, animals then take a rest or sleep. In the hippocampus and neocortex, reorganization of the neural circuits that accompanies the consolidation of spatial memory is considered to occur during the rest/sleep period that follows the spatial learning (Buzsaki 1989; Diekelmann and Born 2010).
On the supposition that the life-and-death decision of new GCs may occur during the time course of olfactory sensory experience and rest/sleep, we examined the fate decision of new GCs during feeding behavior, a typical olfactory behavior that is often followed by rest/sleep. Given that under ad libitum feeding conditions mice show sporadic and fragmented eating behavior, which is not suitable for efficient analysis, feeding behavior was controlled using a restricted feeding paradigm. Food pellets were made available only during a fixed 4-h time window (11:00–15:00) each day (Fig. 6.7a). After habituation to this schedule, all mice showed extensive eating behavior during the first hour of food availability (11:00–12:00), and then showed grooming, resting, and sleeping, which are typical postprandial (after-meal) behaviors, during the subsequent hour (12:00–13:00). In the first 1 h of the eating period, no increase in apoptotic GCs was observed (Fig. 6.7b). Interestingly, however, apoptotic GCs increased approximately twofold during the next 1 h of postprandial behaviors. Perturbation of these postprandial behaviors remarkably suppressed this increase in GC apoptotic elimination, suggesting that they are important to it (Fig. 6.7b). Most of the apoptotic GCs were newly generated GCs aged 14–28 days.
Behavioral state-dependent life-and-death decision of adult-born GCs. (a) Apoptotic GCs increase during the feeding and postprandial periods. Mice were under restricted feeding in which food is supplied for only 4 h (11:00–15:00; gray bar) per day. On day 10 of restricted feeding, mice were analyzed at various circadian time points for caspase-3-activated apoptotic GCs in the OB. (b) Postprandial behaviors including sleep are crucial to the increase in GC elimination. After food delivery, mice were allowed to behave freely (filled black dots) for 1 or 2 h and then analyzed for caspase-3-activated apoptotic GCs. At 1 h after food presentation (No disturb: 1 h), the number of apoptotic GCs was not increased compared to that just before food delivery (pre). In contrast, at 2 h after food presentation (No disturb: 2 h), the apoptotic GC number was considerably increased. When postprandial behaviors, including rest, grooming, and sleep, were disturbed during the postprandial period (between 1 and 2 h after food delivery) (Disturb: 2 h), the apoptotic GC number was significantly suppressed. (c) In food-restricted mice, olfactory sensory input was deprived unilaterally by chronic occlusion of one nostril. The number of caspase-3-activated apoptotic GCs was examined at various circadian time points in the sensory input-intact (left) and sensory input-deprived (right) OB. The number of apoptotic GCs increased dramatically in the sensory-deprived OB during the postprandial period (13:00) compared to the sensory input-intact OB. In contrast, the apoptotic GC number just before the feeding time (11:00) and at any time period outside the feeding time were comparable between sensory-deprived and sensory input-intact OB. In a–c, each dot represents the number of caspase-3-activated GCs in one animal (average of left and right OBs for a and b; either side of OB for c). Bars represent the average. *p < 0.05; **p < 0.01; ***p < 0.001; n.s. not significant; one-way ANOVA with post hoc Bonferroni test. (Modified from Yokoyama et al. 2011) with permission
Sleep is the most characteristic behavior during the postprandial period. Among various stages of sleep (light sleep, slow-wave sleep, and REM sleep), slow-wave sleep plays an important role in promoting GC elimination. The length of the slow-wave sleep roughly correlated with the number of apoptotic GCs. With regard to REM sleep, in contrast, this was rarely observed during this period; and when it did occur, its length showed no significant correlation with the magnitude of GC apoptosis. Interestingly, the length of slow-wave sleep during the period was only 10–30 min in total, indicating that short time periods of slow-wave sleep in the range of a “nap” can nevertheless strongly promote GC elimination. It should be emphasized that, although food-restricted mice sleep without preceding feeding, GC apoptosis did not increase during sleep periods outside the feeding time. The increased GC apoptosis is therefore not dependent on sleep alone, but on the combination of feeding and subsequent sleep episodes.
6.3.6 Integration of Sensory Experience and Behavioral States in the Life–and-Death Decision of New GCs: Two-Stage Model for Sensory Experience-Dependent GC Selection
The extent of apoptotic GC elimination during the postprandial period is regulated by olfactory sensory input. In mice that received unilateral sensory deprivation by chronic occlusion of one nostril and were then subjected to restricted feeding, the number of apoptotic GCs increased dramatically in the sensory-deprived OB during the postprandial period (Fig. 6.7c). Intriguingly, the number of apoptotic GCs at any period outside the feeding time did not differ from that in the OB without sensory deprivation, in spite of the fact that sensory deprivation was continuously maintained by chronic occlusion of the nostril. Thus, in food-restricted mice, the sensory experience-dependent life-and-death decision of new GCs occurred specifically during the postprandial period, indicating that this process is tightly linked to behavioral states with the sequence of olfactory sensory experience during feeding followed by sleep.
These observations led us to propose a “two-stage model” for the sensory experience-dependent selection of new GCs, in which the two stages represent olfactory sensory experience during food search and eating (sensory experience stage) followed by postprandial sleep (sleep stage) (Fig. 6.8) (Yokoyama et al. 2011; Yamaguchi et al. 2013). During the waking period, when mice show food-searching and eating behaviors, a subset of newly generated adult-born GCs receives olfactory sensory inputs from mitral/tufted cells via dendrodendritic synapses in the EPL, while the remaining subset does not (Fig. 6.8). We assume that new GCs that are activated by these olfactory sensory inputs receive a kind of synaptic tagging that works as a substrate for subsequent plastic change (Frey and Morris 1997; Redondo and Morris 2011). These GCs may be “tagged” in the dendrodendritic synapses or the cells themselves, whereas other GCs that are not activated by olfactory sensory input remain “non-tagged.” Alternatively, activated new GCs might receive a “survival tag” while nonactivated new GCs receive a “death tag.” Top-down axodendritic synapses from the OC pyramidal cells to new GCs might also be candidates for tagging, although whether and how a subset of new GCs receives olfactory sensory experience-dependent top-down inputs remains unknown at present.
Two-stage model for the sensory experience- and behavioral state-dependent life-and-death decision of adult-born GCs. Adult-born GCs (green) make dendrodendritic reciprocal synapses with mitral/tufted cells (yellow, M/T) and receive top-down synaptic contacts from pyramidal cells in the OC (blue, Py). Left: During the waking period of olfactory behavior (sensory experience stage), local sensory input from the OSNs (red arrows) activates a subset of mitral/tufted cells. Activated mitral/tufted cells activate a subset of adult-born GCs. The activated GCs might deposit “sensory experience-dependent tags” in the dendrodendritic reciprocal synapses or the cells themselves (red marks). Other adult-born GCs lacking activation by sensory experience are left “non-tagged” or “death-tagged.” Activated mitral/tufted cells further activate pyramidal cells in the OC. The memory trace of the odor experience is deposited in the association fiber synapses among pyramidal cells in the OC (red marks). Right: During the subsequent sleep period (sleep-stage), association fiber synapses among pyramidal cells in the OC are reactivated and induce synchronized firing of the pyramidal cells. This self-organized internal activity of OC pyramidal cells (blue oval) is transferred to the adult-born GCs as synchronized top-down synaptic inputs (thick blue arrow). The synchronized top-down synaptic inputs may contribute to the putative “reorganizing signal” that promotes GC elimination during the postbehavioral sleep period. Adult-born GCs that are tagged by sensory experience during the preceding waking period survive; adult-born GCs that are not tagged (or death-tagged) are eliminated by the “reorganizing signal”
Importantly, although differential tagging of new GCs might occur during feeding behavior, the life-and-death decision of GCs is not made during feeding. During the subsequent postprandial period, the cell selection process is triggered and the increased GC apoptosis occurs. We hypothesized that some sort of “reorganizing signal” enters the OB during the postprandial period, typically during the postprandial slow-wave sleep period, and promotes GC selection according to the presence or absence or type of tag that the GCs received during the preceding waking period (Fig. 6.8). Adult-born GCs “tagged” or “survival-tagged” by sensory experience might be selected to survive by this “reorganizing signal” whereas other “non-tagged” or “death-tagged” adult-born GCs might be eliminated by it. The fate of individual adult-born GCs might be determined by the interplay between tagging that reflect the olfactory sensory experience during the waking period and the reorganizing signal that enters the OB during the subsequent sleep period.
This idea of a two-stage model of GC elimination is analogous to the two-stage model of memory formation and consolidation in the hippocampus. This model states that experience-dependent input induces memory trace formation during awake learning and that replay of the experience occurs for the reorganization and consolidation of neuronal circuits during subsequent sleep or rest (Buzsaki 1989; Diekelmann and Born 2010). Enhanced GC elimination during the postprandial period also resembles homeostatic synaptic downscaling during sleep (Tononi and Cirelli 2006; Vyazovskiy et al. 2008). It has been shown in the rodent neocortex and hippocampus that behavioral state modulates synaptic strength, with a net increase during waking and a reduction during sleep. Because a large number of adult-born GCs are recruited in the OB every day, elimination of adult-born GCs is necessary to maintaining the overall number of GCs in the entire OB within an appropriate range. This kind of downscaling may increase the ratio of useful versus useless GCs and thereby improve the signal-to-noise ratio for olfactory information processing, as has been proposed for the role of synaptic downscaling (Tononi and Cirelli 2006), and may make room for a successive cohort of new GCs to be integrated in preparation for the next round of new olfactory experience.
6.3.7 Possible Neuronal Mechanisms Underlying Sensory Experience- and Behavioral State-Dependent GC Selection
What are the neuronal mechanisms for the hypothetical reorganizing signal during postbehavioral sleep? One possibility is that the signal is contributed to by glutamatergic top-down inputs from the OC. Manabe et al. recorded single unit activities in the anterior piriform cortex (APC) in freely behaving rats and showed that numerous APC neurons fire synchronously during the slow-wave sleep state (Manabe et al. 2011). LFP recordings further showed that the deep layer of the APC generates repetitive sharp negative potentials during the slow-wave sleep state that resemble hippocampal sharp waves in both shape and duration. These “olfactory cortex sharp waves” (OC-SPWs) in the APC are associated with synchronized spike discharges of APC neurons. Importantly, simultaneous recording of LFP in the APC and GCL of the OB revealed that sharp wave-like potentials in the OB occurred in close temporal proximity to OC-SPWs, indicating that the repetitive synchronous discharge activity of APC neurons during the slow-wave sleep state is transferred to the OB GCL as the synchronized top-down synaptic inputs. Given that the synchronized top-down inputs occur repeatedly during slow-wave sleep but not during waking or REM sleep (Manabe et al. 2011), these inputs are a plausible candidate for the slow-wave sleep state-specific reorganizing signal. The synchronous discharge of APC neurons during the slow-wave sleep is a self-organized internal activity, and might be a replay of the memory trace of odor experience formed during the waking period, which is deposited in the association fibers of the APC neurons (Fig. 6.8) (for details please refer to Manabe et al. 2011 and Yamaguchi et al. 2013). Further examination will clarify the causal relationship between top-down inputs and GC elimination.
GC elimination is promoted during postprandial sleep but not during sleep without preceding eating in food-restricted mice. However, OC-SPW-associated synchronized top-down inputs to GCs always occur during slow-wave sleep regardless of the presence or absence of preceding eating (Manabe et al. 2011; our unpublished observation). Synchronized top-down inputs from the OC alone may thus not be sufficient to trigger GC elimination. Deposition of putative tag signals during preceding waking may be prerequisite, and the life-and-death decision of new GCs might be determined after collation of the top-down reorganization signal with the putative deposited tag signals. Further, other behavioral state-dependent signals might also be involved. Neuromodulatory signals are activated during attentive behaviors and decline during slow-wave sleep (Brown et al. 2012). Noradrenergic input to the OB is potentiated in many olfactory behaviors (Brennan et al. 1990; Wellman 2000). Enhanced neuromodulatory signals during waking olfactory behavior might potentiate subsequent GC elimination during slow-wave sleep. The effects of noradrenergic or cholinergic signals on the survival of new GCs have been well documented (Cooper-Kuhn et al. 2004; Kaneko et al. 2006; Veyrac et al. 2009; Moreno et al. 2012).
The behavioral state-dependent life-and-death decision of adult-born GCs indicates that the fate of adult-born GCs is determined by the integration of various behavioral state-dependent signals. It is worth noting that the candidate signals, namely olfactory sensory inputs, top-down inputs from the OC, and neuromodulatory signals, overlap with those signals that regulate the activity of OB interneurons and their dendrodendritic synaptic inhibition. This observation implies that the function of OB interneurons and the life-and-death decision of adult-born OB interneurons are regulated by shared neuronal mechanisms, both of which would help the animal behave properly in response to ever-changing odor circumstances by utilizing the highly plastic properties of OB interneurons.
6.4 Coordination of Preexisting Interneurons and Newly Generated Interneurons in the OB
6.4.1 Relationship Between Preexisting and Newly Generated Interneurons for Constitution of the OB Neuronal Circuit
A large number of OB interneurons are continually generated in the adult. In rodents, roughly 1% of total OB interneurons are generated each day (Alvarez-Buylla et al. 2001; Winner et al. 2002; Lledo et al. 2006). Although the number declines with age, neurogenesis continues even in 2-year-old mice (Enwere et al. 2004). One question is to what extent do adult-born interneurons contribute to the overall constitution of the OB neuronal circuits in the adult. Although traditional techniques of BrdU- or retrovirus-mediated labeling of new neurons identify only a small fraction of adult-born neurons, recent advances in molecular and developmental biology have enabled the labeling of a much larger proportion and provided an estimation of the quantitative contribution of adult-born interneurons. A number of studies have used a tamoxifen-mediated Cre recombination system to permanently label adult-born OB interneurons by marker protein expression (Lagace et al. 2007; Ninkovic et al. 2007; Imayoshi et al. 2008). The proportion of labeled cells among total OB interneurons differed among studies, but all showed that the labeled cells gradually accumulate and increase in number for at least several months after the initiation of cell labeling. In one study, labeled adult-born GCs accounted for nearly 20 % of the total number of GCs, a proportion that remained stable between 4 and 9 months (Ninkovic et al. 2007). In another study, the proportion of labeled adult-born GCs continued to increase up to age 12 months to 60–70 % of GCs (Imayoshi et al. 2008). The former observation suggests the possibility that adult-born GCs may constitute a specific cohort of cells that are repeatedly replaced by much younger adult-born GCs, and the latter observation suggests that adult-born GCs may continue to accumulate and finally account for the majority of all GCs in the adult OB. Although the actual proportion of adult-born GCs awaits further confirmation, the two modes of contribution of adult-born GCs, repeated replacement and continual addition, are not mutually exclusive but rather may work together to differing extents under different conditions to maintain and modulate the OB neuronal circuits.
Besides the continual accumulation of adult-born GCs, preexisting old GCs are gradually lost from the neuronal circuits during adulthood. BrdU-labeled neonatal-born GCs decrease in number with age, by nearly half at 6 months old (Imayoshi et al. 2008). In the case of PGCs, an in vivo time-lapse imaging study showed the disappearance of old PGCs and appearance of new PGCs in the same observation field, with a turnover rate of approximately 3 % per month (Mizrahi et al. 2006). These observations indicate that OB interneurons are turning over, with the elimination of old cells and incorporation of new ones. What then is the relationship between old and new interneurons in their turnover? Interestingly, genetic ablation of adult-born GCs did not influence the rate of neonate-born GC loss, suggesting that old GCs are lost irrespective of the supply of new neurons (Imayoshi et al. 2008). This observation in turn suggests that continuous neurogenesis is required to compensate for the loss of old interneurons. Consistent with this, enhanced elimination of preexisting GCs in a local area of the OB by local injection of immunotoxin facilitates the incorporation of newly generated GCs in the local OB area (Fig. 6.9a) (Murata et al. 2011). This observation further supports the idea that the elimination of old GCs and incorporation of new GCs are coordinated, such that new GCs compensate for the loss of old GCs and contribute to the maintenance of the GC population in local neuronal circuits of the OB. In addition to the plastic modulation of OB neuronal circuits, adult-born interneurons play fundamental roles in the maintenance of OB neuronal circuits.
Turnover of preexisting old GCs and newly generated GCs. (a) Newly generated GCs compensate for the loss of preexisting GCs. Preexisting old GCs were ablated in the local area of the OB by the local injection of immunotoxin. At 2 weeks after immunotoxin injection, preexisting GCs (left panel, green; labeled by BrdU analogue CldU) decreased in the local area. In the same local area, newly generated adult-born GCs (middle panel, magenta; labeled by another BrdU analogue, IdU) increased. Right panel: Merged view. (b) Ablation and recovery of a GC subtype. Calretinin-expressing GCs (white) are a subpopulation of mGluR2-expressing GCs. At 2 weeks after immunotoxin injection, calretinin-expressing GCs decreased from mGluR2-expressing GC-specific ablation (middle panel). At 4 weeks, calretinin-expressing GCs showed recovery in density in the ablated area (right panel). (c) In the immunotoxin (Itx)-injected area, incorporation of calretinin-expressing new GCs increased (left panel). This effect was not seen in the noninjected area of the same OB (right panel). (d) Conceptual schema of subtype-specific turnover of GCs and maintenance of OB neuronal circuits. Following the loss of a specific subtype of GC in an OB neuronal circuit (middle panel, loss of a GC with dark green), an adult-born GC (magenta) compensates for the lost subtype of GC (right panel, a GC with magenta). This subtype-specific turnover maintains the constitution of OB neuronal circuits during the continual loss and incorporation of GCs. M mitral cell, T tufted cell. (Modified from Murata et al. 2011)
6.4.2 Generation of Different Subtypes at Different Ages and Subtype-Specific Replacement of OB Interneurons
OB neuronal circuits contain various subtypes of interneurons. Recent studies have revealed that the turnover of OB interneurons is conducted in a subtype-specific manner (Fig. 6.9b). In the experiment of immunotoxin-mediated GC ablation, a subset of preexisting GCs that expresses metabotropic glutamate receptor type II (mGluR2) was specifically ablated (Murata et al. 2011). Following this subtype-specific ablation, incorporation of new GCs was preferentially promoted for the mGluR2-expressing subtype over the mGluR2-negative subtype. Similarly, laser ablation of TH-expressing PGCs was compensated by the integration of new TH-expressing PGCs in the same periglomerular positions where preexisting TH-expressing PGCs were present before ablation (Sawada et al. 2011). The constitution of OB neuronal circuits thus appears to be further maintained by subtype-specific compensatory mechanisms even under extensive turnover (Fig. 6.9c).
Although I have so far explained the role of subtype-specific turnover of OB interneurons in the compensation and maintenance of OB neuronal circuits, neurogenesis at different ages generates overlapping but different populations of interneuron subtypes. The calbindin-expressing PGC subtype is predominantly generated during the embryonic and neonatal period, whereas adult-born PGCs are mostly of the TH- or calretinin-expressing subtype (De Marchis et al. 2007; Ninkovic et al. 2007; Batista-Brito et al. 2008). Calretinin-expressing GCs are predominantly generated after birth, while 5T4-expressing GCs are generated throughout the embryonic and postnatal periods (Batista-Brito et al. 2008). Genetic analysis and transplantation studies of stem cells revealed that the generation of different interneuron subtypes originates from the heterogeneity of stem cells, rather than the putative subtype-specific instruction cues in the OB environment (Merkle et al. 2007; Young et al. 2007).
These observations raise the interesting possibility that the subtype constitution of OB neuronal circuits may gradually change with age, and that neurogenesis at different ages may contribute to odor information processing differently. BrdU labeling of new neurons showed that neonate-born GCs tend to locate in the superficial portion of the GCL while adult-born GCs locate in the deep portion (Lemasson et al. 2005; Imayoshi et al. 2008). Neonate-born GCs tend to extend dendrites into the superficial sublamina of the EPL, whereas adult-born GCs do so into the deep sublamina of the EPL (Kelsch et al. 2007). Given the distribution of mitral cell lateral dendrites in the deep EPL sublamina (Mori 1987), adult-born GCs may make dendrodendritic synaptic contacts preferentially with mitral cells and contribute to the odor information processing conveyed through mitral cell pathways.
Further analysis of the turnover of interneuron subtypes during odor-guided learning and memory formation would give clues to understanding the functional roles of individual interneuron subtypes and their turnover. Different interneuron subtypes respond differently to olfactory sensory experience. Olfactory sensory deprivation remarkably reduces the survival of TH-expressing PGCs compared to calbindin- or calretinin-expressing PGCs (Bastien-Dionne et al. 2010; Sawada et al. 2011). Olfactory sensory deprivation preferentially reduces the survival of newly generated GCs in the deep sublamina of the GCL (Mandairon et al. 2006). Proper control of interneuron subtype turnover may be important both for the maintenance and plastic modulation of OB neuronal circuits.
6.5 Contribution of OB New Neurons in Olfactory Behaviors
6.5.1 Methods for Addressing the Role of Adult Olfactory Neurogenesis
A central question for adult neurogenesis is how it contributes to brain functions. Recent studies in rodents are revealing the roles of olfactory neurogenesis in olfactory behaviors, although contradictory observations have occasionally appeared. The purpose of this section is to provide an overview of how adult neurogenesis has been experimentally addressed and what kinds of functional alteration have been observed in adult neurogenesis-modified animals. For details, please refer also to recent reviews (Lazarini and Lledo 2011; Breton-Provencher and Saghatelyan 2012; Kageyama et al. 2012).
Most studies are based on the loss-of-function approach, whereby olfactory behaviors are addressed in mice with suppressed adult olfactory neurogenesis. Four major techniques to suppress olfactory neurogenesis have been reported to date: antimitotic drug injection, γ-ray irradiation, genetic cell ablation, and utilization of gene-mutant mice. Each method has advantages and disadvantages. Injection of the antimitotic drug Ara-C into the lateral ventricle (LV)/SVZ effectively ablates proliferating cells in the SVZ and reduces the supply of new neurons to the OB. Continual application of Ara-C continues to suppress neurogenesis and drug removal restores it (Enwere et al. 2004). Ara-C application can therefore address the effect of both loss and recovery of olfactory neurogenesis. A major caveat is that application of the drug affects not only olfactory neurogenesis but also hippocampal neurogenesis (Breton-Provencher et al. 2009; Sultan et al. 2010). Some olfactory learning is known to depend on the hippocampus (Sauvage et al. 2008). On the other hand, γ-ray irradiation in restricted brain areas, including the SVZ, enables specific suppression of olfactory neurogenesis by sparing hippocampal neurogenesis. However, both Ara-C treatment and irradiation may have confounding effects on preexisting neuronal circuits, such as direct neuronal toxicity, or indirect effects through the induction of inflammation.
The genetic method utilizing drug-induced Cre recombinase activation provides a sophisticated way of suppressing adult neurogenesis. For example, induced expression of cytotoxic genes by tamoxifen-mediated activation of CreER recombinase causes cell death in adult-born neurons at desired time points (Imayoshi et al. 2008). The efficacy and specificity of this ablation system are crucially dependent on the availability of tissue- or cell type-specific promoters for expressing exogenous genes. Gene mutant mice showing impairment in olfactory neurogenesis offer a variety of materials to address the role of adult neurogenesis as well as the function of the genes of interest. Possible drawbacks of mutant mice are abnormalities other than olfactory neurogenesis and the recruitment of compensatory mechanisms for the gene mutation from developmental stages.
Many kinds of olfactory behaviors have been examined in these neurogenesis-suppressed mice, including spontaneous approaching behaviors to odors, acquisition of associative olfactory memory, retention of olfactory memory, and odor-guided social and reproductive behaviors. These behaviors differ greatly in motivation, difficulty of tasks, and expected outcomes of the behavioral responses. Although contradictory observations are often seen, the overall tendency of behaviors in olfactory neurogenesis-suppressed mice seems to be that spontaneous odor behaviors are somewhat spared, acquisition and retention of associative olfactory memory are somehow impaired, and social and reproductive olfactory behaviors are remarkably impaired. These effects are explained in the next section.
6.5.2 Possible Roles of Adult Neurogenesis in Olfactory Behaviors
When mice are repeatedly exposed to a given odor without any reward or avoidance cues, the exploratory time devoted to the odor gradually diminishes (habituation). If a different odor is applied after habituation to the first odor, however, the exploratory time increases (dishabituation). This habituation–dishabituation paradigm is frequently used to address a mice’s ability in odor discrimination and the odor-detection threshold. In olfactory neurogenesis-suppressed mice, these functions do not appear to differ from those in intact mice (Imayoshi et al. 2008; Lazarini et al. 2009, but see Breton-Provencher et al. 2009). In contrast, when short-term olfactory memory is assessed by this habituation–dishabituation paradigm, apparent impairment is observed. When the same odor was presented twice at varying time intervals, intact mice showed reduced sniffing to the second presentation even after an interval of 120 min. However, mice injected with Ara-C in the LV did not show reduced sniffing to the second presentation as early as after 30 min, indicating that the Ara-C-injected mice did not retain memory of the odor for longer than 30 min (Breton-Provencher et al. 2009). A different odor presentation paradigm showed that repeated exposure to two different odors improves the ability to discriminate the odors, which is referred to as perceptual olfactory learning (Moreno et al. 2009, 2012). Perceptual olfactory learning is impaired in Ara-C-injected mice (Moreno et al. 2012). These observations indicate that olfactory neurogenesis contributes to olfactory learning even when odors are experienced without any association with reinforcing (attractive or aversive) cues.
In typical experiments of olfactory learning, animals learn to associate odor cues with specific reinforcing outcomes. When one odor is associated with a sugar/water reward while another odor is left unrewarded, mice become selectively attracted to the rewarded odor. In SVZ-irradiated mice and Ara-C-injected mice, this odor–reward associative learning was accomplished normally, but long-term retention of the memory over days was impaired (Lazarini et al. 2009; Sultan et al. 2010). In contrast, odor–sugar reward associative learning was maintained for more than a week in Ara-C-injected mice (Breton-Provencher et al. 2009) and over several months in mice with cell ablation using tamoxifen-dependent Cre-mediated cell toxicity (Imayoshi et al. 2008).
The reason for this discrepancy is not known but it might be attributable to the difference in the task paradigm (Lazarini and Lledo 2011; Mandairon et al. 2011; Breton-Provencher and Saghatelyan 2012). The studies showing impaired long-term memory utilized operant conditioning tasks, in which mice need to learn novel procedures to retrieve a reward, such as poking the nose into odor/water ports or searching in holes made on a platform. On the other hand, the studies showing no impairment used nonoperant (Pavlovian) conditioning tasks, in which mice can retrieve a reward without acquiring a novel procedure, just by digging into their bedding where the cued odor is present. Such differences in learning paradigm may recruit different brain regions to different extents and alter the dependency on OB neurogenesis. Consistent with this notion, the survival of new GCs is substantially potentiated by performing operant tasks compared to nonoperant tasks (Mandairon et al. 2011).
The association of odor cues with noxious stimuli is also affected in neurogenesis-suppressed mice. When an odor was associated with electrical foot shock delivery, mice developed freezing behavior to the odor. In SVZ-irradiated mice, the freezing behavior occurred to the cued odor but the time duration was shorter than in intact mice. In contrast, the irradiated mice showed normal freezing behavior when an auditory cue was used for the association with foot shock (Valley et al. 2009). In mice with genetic and inducible ablation of adult-born neurons, freezing response to TMT, a predator’s odor, was compromised when TMT was force associated with a sugar reward (Sakamoto et al. 2011).
Olfaction mediates a variety of social and reproductive behaviors, and the suppression of olfactory neurogenesis substantially affects these behaviors. Proliferation of progenitors in the SVZ of female mice is enhanced during pregnancy and after partition, which is mediated by the increased secretion of prolactin (Shingo et al. 2003). This prolactin-mediated enhanced neurogenesis is essential for the proper pup-fostering behavior of mothers (Larsen and Grattan 2010), for female mice to show preference to dominant males over subordinate males (Mak et al. 2007), and for male mice to recognize their own offspring (Mak and Weiss 2010). Irradiation of the SVZ in females impaired their recognition of male odors (Feierstein et al. 2010). Genetic and inducible ablation of adult-born neurons induces impairment in male-to-male aggressive behavior, male-to-female mating behavior, and the pup-fostering behavior of mothers (Sakamoto et al. 2011).
In addition to these loss-of-function studies, a recent optogenetic study used a gain-of-function approach. Photoactivation of new OB interneurons during odor discrimination learning facilitated the learning process, in which mice that received photoactivation accomplished the learning with a smaller number of trials (Alonso et al. 2012). Notably, this learning facilitation was evident when mice discriminated very similar odors but not when they discriminated dissimilar odors.
Overall, these studies indicate that adult neurogenesis in the OB contributes to various olfactory behaviors to varying extents. A possible interpretation for the seemingly variable contribution to different olfactory tasks is that adult neurogenesis may be much more involved in “difficult” tasks compared to “easy” tasks. The degree of difficulty in the olfactory tasks may relate to whether the tasks are operant or nonoperant, whether they require the discrimination of very similar odors or dissimilar odors, and whether they require the identification and discrimination of animals of the same species or different species, as well as nonliving odorous objects. Each of the former of these paired cases may represent a more difficult situation for the performance of proper olfactory behaviors. Difficult tasks may require much higher attention and motivation and additional learning/memory processes that must recruit many brain regions and signal systems. The function of adult-born OB interneurons may be fully potentiated by, and required for, situations in which many brain regions and signal systems are required to work in concert.
6.5.3 Physiological Studies Addressing the Role of Adult Neurogenesis
In contrast to the many behavioral studies, physiological analyses in adult neurogenesis-suppressed mice are limited and controversial. Patch clamp recordings of mitral cells in the OB slice of Ara-C-injected mice showed a reduced frequency of spontaneous inhibitory postsynaptic potentials, lesser strength of dendrodendritic inhibition, and decreased synchronized activity (Breton-Provencher et al. 2009). However, in vivo recordings under anesthesia in SVZ-irradiated mice showed no alteration in dendrodendritic synaptic inhibition following antidromic stimulation of mitral/tufted cells or in the odor-induced oscillatory LFP in the OB (Valley et al. 2009). Optogenetic activation of new OB interneurons showed that activation reduces spontaneous firing activity of mitral cells in vitro and also in vivo in head-restrained awake mice (Alonso et al. 2012). Interestingly, enhanced firing of mitral cells in response to odor stimuli was suppressed by optogenetic activation of new OB neurons to different extents, with weakly activated mitral cells showing much greater suppression than strongly activated mitral cells, thereby enhancing the contrast of odor responses among mitral cells (Alonso et al. 2012). The combination of behavioral analysis with electrophysiological and imaging analysis would facilitate understanding of how odor information processing by adult-born OB interneurons contributes to the expression of proper olfactory behaviors. Simultaneous analysis of wide brain regions might also be important, given the possible contribution of many brain regions and signal systems to OB interneuron-mediated odor information processing.
6.6 Conclusion
In the OB, odor information is substantially modulated by a large number of local interneurons. GCs and PGCs induce feedback inhibition, lateral inhibition, and synchronization to mitral/tufted cells via dendrodendritic synapses and regulate the firing activity of mitral/tufted cells. Notably, the dendrodendritic synaptic inhibition of mitral/tufted cells is plastically regulated not only by the odor inputs from the external world but also by the activity of the OC and subcortical neuromodulatory centers. In addition, continual generation of OB interneurons during adulthood potentiates the plasticity of OB neuronal circuits. The life-and-death decision of new GCs is dependent on the behavioral state of the animal, namely, the sequence of feeding behavior during waking and subsequent slow-wave sleep, suggesting that utilization of new OB interneurons is also regulated by the behavioral state-dependent activities of higher brain regions. The OB interneurons therefore establish neuronal circuits in which integration of multiple signals from many brain regions leads to the plastic modulation of mitral/tufted cell activities. The importance of this OB interneuron-mediated information processing is supported by the behavioral analysis of adult neurogenesis-suppressed mice. These mice show impaired behaviors, particularly in difficult olfactory tasks, which likely require much higher attention and motivation and additional learning/memory processes and thereby recruit many brain regions and signal systems. Given that OB interneurons consist of various subtypes, revealing the subtype-specific functions and turnover would give further clues to understanding the adaptive roles of OB interneurons in the acquisition and updating of proper odor-guided behaviors in the ever-changing odor circumstances.
References
Abraham NM, Egger V, Shimshek DR et al (2010) Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron 65:399–411
Adrian ED (1950) The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol 2:377–388
Alonso M, Lepousez G, Sebastien W et al (2012) Activation of adult-born neurons facilitates learning and memory. Nat Neurosci 15:897–904
Alvarez-Buylla A, Garcia-Verdugo J, Tramontin AD (2001) A unified hypothesis on the lineage of neuronal stem cells. Nat Rev Neurosci 2:287–293
Andres KH (1965) Der feinbau des bulbus olfactorius der ratte unter beonderer beruksachtigung der synaptischen verbandungen. Z Zellforsch Mikrosk Anat 65:530–561
Araneda RC, Firestein S (2006) Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J Neurosci 26:3292–3298
Balu R, Pressler RT, Strowbridge BW (2007) Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci 27:5621–5632
Bastien-Dionne PO, David LS, Parent A et al (2010) Role of sensory activity on chemospecific populations of interneurons in the adult olfactory bulb. J Comp Neurol 518:1847–1861
Batista-Brito R, Close J, Machold R et al (2008) The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci 28:3966–3975
Beshel J, Kopell N, Kay LM (2007) Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci 27:8358–8365
Blanes T (1897) Sobre algunos puntos dudosos de la estructura del bulbo olfatorio (On some doubtful points concerning the structure of the olfactory bulb). Rev Trim Micrograf 3:99–127
Boyd AM, Sturgill JF, Poo C et al (2012) Cortical feedback control of olfactory bulb circuits. Neuron 76:1161–1174
Brennan P, Kaba H, Keverne EB (1990) Olfactory recognition: a simple memory system. Science 250:1223–1226
Breton-Provencher V, Saghatelyan A (2012) Newborn neurons in the adult olfactory bulb: unique properties for specific odor behavior. Behav Brain Res 227:480–489
Breton-Provencher V, Lemasson M, Peralta MR 3rd et al (2009) Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci 29:15245–15257
Brown RE, Basheer R, McKenna JT et al (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
Buss RR, Sun W, Oppenheim RW (2006) Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci 29:1–35
Buzsaki G (1989) Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31:551–570
Cajal SR (1890–1891) Origen y terminación de las fibras nerviosas olfatorias (Origin and termination of the olfactory nerve fibers). Gaceta Sanitaria de Barcelona 3:133–139; 174–181; 206–212
Carleton A, Petreanu LT, Lansford R et al (2003) Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 6:507–518
Castillo PE, Carleton A, Vincent JD et al (1999) Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J Neurosci 19:9180–9191
Chen WR, Xiong W, Shepherd GM (2000) Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron 25:625–633
Cooper-Kuhn CM, Winkler J, Kuhn HG (2004) Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res 77:155–165
Corotto F, Henegar J, Maruniak J (1994) Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience 61:739–744
De Marchis S, Bovetti S, Carletti B et al (2007) Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci 27:657–664
de Olmos J, Hardy H, Heimer L (1978) The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol 181:213–244
Debarbieux F, Audinat E, Charpak S (2003) Action potential propagation in dendrites of rat mitral cells in vivo. J Neurosci 23:5553–5560
Devore S, Manella LC, Linster C (2012) Blocking muscarinic receptors in the olfactory bulb impairs performance on an olfactory short-term memory task. Front Behav Neurosci 6:59
Diekelmann S, Born J (2010) The memory function of sleep. Nat Rev Neurosci 11:114–126
Doty RL (1986) Odor-guided behavior in mammals. Experientia (Basel) 42:257–271
Doucette W, Restrepo D (2008) Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol 6:e258
Doucette W, Gire DH, Whitesell J et al (2011) Associative cortex features in the first olfactory brain relay station. Neuron 69:1176–1187
Egger V, Svoboda K, Mainen ZF (2003) Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells. J Neurosci 23:7551–7558
Egger V, Svoboda K, Mainen ZF (2005) Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike. J Neurosci 25:3521–3530
Enwere E, Shingo T, Gregg C et al (2004) Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci 24:8354–8365
Escanilla O, Arrellanos A, Karnow A et al (2010) Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur J Neurosci 32:458–468
Eyre MD, Antal M, Nusser Z (2008) Distinct deep short-axon cell subtypes of the main olfactory bulb provide novel intrabulbar and extrabulbar GABAergic connections. J Neurosci 28:8217–8229
Feierstein CE, Lazarini F, Wagner S et al (2010) Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front Behav Neurosci 4:176
Frey U, Morris RGM (1997) Synaptic tagging and long-term potentiation. Nature (Lond) 385:533–536
Gao Y, Strowbridge W (2009) Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci 12:731–733
Gogos JA, Osborne J, Nemes A et al (2000) Genetic ablation and restoration of the olfactory topographic map. Cell 103:609–620
Golgi C (1875) Sulla fina struttura dei bulbi olfactorii (On the fine structure of the olfactory bulb). Riv Sper Freniatr Med Leg 1:405–425
Gribaudo S, Bovetti S, Garzotto D et al (2009) Expression and localization of the calmodulin-binding protein neurogranin in the adult mouse olfactory bulb. J Comp Neurol 517:683–694
Haberly LB, Price JL (1978) Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol 178:711–740
Halabisky B, Strowbridge BW (2003) Gamma-frequency excitatory input to granule cells facilitates dendrodendritic inhibition in the rat olfactory bulb. J Neurophysiol 90:644–654
Halabisky B, Friedman D, Radojicic M et al (2000) Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci 20:5124–5134
Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888
Hirata Y (1964) Some observations on the fine structure of the synapses in the olfactory bulb of the mouse, with particular references to the atypical synaptic configurations. Arch Histol Jpn 24:293–302
Igarashi KM, Ieki N, An M et al (2012) Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci 32:7970–7985
Imamura F, Nagao H, Naritsuka H et al (2006) A leucine-rich repeat membrane protein, 5T4, is expressed by a subtype of granule cells with dendritic arbors in specific strata of the mouse olfactory bulb. J Comp Neurol 495:754–768
Imayoshi I, Sakamoto M, Ohtsuka T et al (2008) Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11:1153–1161
Isaacson JS, Strowbridge BW (1998) Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron 20:749–761
Jahr CE, Nicoll RA (1982) An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol 326:213–234
Kaba H, Nakanishi S (1995) Synaptic mechanisms of olfactory recognition memory. Rev Neurosci 6:125–141
Kageyama R, Imayoshi I, Sakamoto M (2012) The role of neurogenesis in olfaction-dependent behaviors. Behav Brain Res 227:459–463
Kaneko N, Okano H, Sawamoto K (2006) Role of cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 11:1145–1159
Kaplan MS, McNelly NA, Hinds JW (1985) Population dynamics of adult-formed granule neurons of the rat olfactory bulb. J Comp Neurol 239:117–125
Kasa P, Hlavati I, Dobo E et al (1995) Synaptic and non-synaptic cholinergic innervation of the various types of neurons in the main olfactory bulb of adult rat: immunocytochemistry of choline acetyltransferase. Neuroscience 67:667–677
Kashiwadani H, Sasaki YF, Uchida N et al (1999) Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol 82:1786–1792
Katagiri H, Pallotto M, Nissant A et al (2011) Dynamic development of the first synapse impinging on adult-born neurons in the olfactory bulb circuit. Neural Syst Circuits 1:6
Kato HK, Chu MW, Isaacson JS et al (2012) Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron 76:962–975
Kay LM, Laurent G (1999) Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci 2:1003–1009
Kelsch W, Mosley CP, Lin CW et al (2007) Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol 5:2501–2512
Kelsch W, Lin CW, Lois C (2008) Sequential development of synapses in dendritic domains during adult neurogenesis. Proc Natl Acad Sci USA 105:16803–16808
Kelsch W, Sim S, Lois C (2010) Watching synaptogenesis in the adult brain. Annu Rev Neurosci 33:131–149
Kosaka K, Aika Y, Toida K et al (1995) Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. Neurosci Res 23:73–88
Lagace DC, Whitman MC, Nooman MA et al (2007) Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci 27:12623–12629
Lagier S, Carleton A, Lledo PM (2004) Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb. J Neurosci 24:4382–4392
Larsen CM, Grattan DR (2010) Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151:3805–3814
Lazarini F, Lledo PM (2011) Is adult neurogenesis essential for olfaction? Trends Neurosci 34:20–30
Lazarini F, Mouthon MA, Gheusi G et al (2009) Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One 4:e7017
Le Jeune H, Aubert I, Jourdan F et al (1995) Comparative laminar distribution of various autoradiographic cholinergic markers in adult rat main olfactory bulb. J Chem Neuroanat 9:99–112
Lemasson M, Saghatelyan A, Olivo-Marin JC et al (2005) Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci 25:6816–6825
Lledo PM, Alonso M, Grubb MS (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7:179–193
Luskin MB, Price J (1983) The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol 216:264–291
Mak GK, Weiss S (2010) Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci 13:753–758
Mak GK, Enwere EK, Gregg C et al (2007) Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci 10:1003–1011
Manabe H, Kusumoto-Yoshida I, Ota M et al (2011) Olfactory cortex generates synchronized top-down inputs to the olfactory bulb during slow-wave sleep. J Neurosci 31:8123–8133
Mandairon N, Sacquet J, Jourdan F et al (2006) Long-term fate and distribution of newborn cells in the adult mouse olfactory bulb: influences of olfactory deprivation. Neuroscience 141:443–451
Mandairon N, Sultan S, Nouvian M et al (2011) Involvement of newborn neurons in olfactory associative learning? The operant or non-operant component of the task makes all the difference. J Neurosci 31:12455–12460
Margrie TW, Sakmann B, Urban NN (2001) Action potential propagation in mitral cell lateral dendrites is decremental and controls recurrent and lateral inhibition in the mammalian olfactory bulb. Proc Natl Acad Sci USA 98:319–324
Markopoulos F, Rokni D, Gire DH et al (2012) Functional properties of cortical feedback projections to the olfactory bulb. Neuron 76:1175–1188
Martin C, Gervais R, Messaoudi B et al (2006) Learning-induced oscillatory activities correlated to odour recognition: a network activity. Eur J Neurosci 23:1801–1810
McCune SK, Voigt MM, Hill JM (1993) Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience 57:143–151
McLean JH, Shipley MT (1987) Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J Neurosci 7:3016–3028
McLean JH, Shipley MT, Nickell WT et al (1989) Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol 285:39–349
Merkle FT, Mirzadeh Z, Avarez-Buylla A (2007) Mosaic organization of neural stem cells in the adult brain. Science 317:381–384
Mizrahi A, Lu J, Irving R et al (2006) In vivo imaging of juxtaglomerular neuron turnover in the mouse olfactory bulb. Proc Natl Acad Sci USA 103:1912–1917
Moreno MM, Linster C, Escanilla O et al (2009) Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci USA 106:17980–17985
Moreno MM, Bath K, Kuczewski N et al (2012) Action of the noradrenergic system on adult-born cells is required for olfactory learning in mice. J Neurosci 32:3748–3758
Mori K (1987) Membrane and synaptic properties of identified neurons in the olfactory bulb. Prog Neurobiol 29:275–320
Mori K, Kishi K, Ojima H (1983) Distribution of dendrites of mitral, displaced mitral, tufted, and granule cells in the rabbit olfactory bulb. J Comp Neurol 219:339–355
Mori K, Nagao H, Yoshihara Y (1999) The olfactory bulb: coding and processing of odor molecule information. Science 286:711–715
Mouret A, Lepousez G, Gras J et al (2009) Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci 29:12302–12314
Murata K, Imai M, Nakanishi S et al (2011) Compensation of depleted neuronal subsets by new neurons in a local area of the adult olfactory bulb. J Neurosci 31:10540–10557
Murphy GJ, Darcy DP, Isaacson JS (2005) Intraglomerular inhibition: signaling mechanisms of an olfactory microcircuit. Nat Neurosci 8:354–364
Nagayama S, Takahashi YK, Yoshihara Y et al (2004) Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol 91:2532–2540
Nai Q, Dong HW, Hayar A et al (2009) Noradrenergic regulation of GABAergic inhibition of main olfactory bulb mitral cells varies as a function of concentration and receptor subtype. J Neurophysiol 101:2472–2484
Naritsuka H, Sakai K, Hashikawa T et al (2009) Perisomatic-targeting granule cells in the mouse olfactory bulb. J Comp Neurol 515:409–426
Ninkovic J, Mori T, Gotz M (2007) Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci 27:10906–10911
Orona E, Rainer EC, Scott JW (1984) Dendritic and axonal organization of mitral and tufted cells in the rat olfactory bulb. J Comp Neurol 226:346–356
Pager J (1983) Unit responses changing with behavioral outcome in the olfactory bulb of unrestrained rats. Brain Res 289:87–98
Parrish-Aungst S, Shipley MT, Erdelyi F et al (2007) Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol 501:825–836
Petreanu L, Alvarez-Buylla A (2002) Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci 22:6106–6113
Petzold GC, Hagiwara A, Murthy VN (2009) Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci 12:784–791
Pressler RT, Inoue T, Strowbridge BW (2007) Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci 27:10969–10981
Price JL, Powell TP (1970a) The morphology of the granule cells of the olfactory bulb. J Cell Sci 7:91–123
Price JL, Powell TP (1970b) The synaptology of the granule cells of the olfactory bulb. J Cell Sci 7:125–155
Rall W, Shepherd GM (1968) Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol 31:884–915
Rall W, Shepherd GM, Reese TS et al (1966) Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol 14:44–56
Ravel N, Elaagouby A, Gervais R (1994) Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behav Neurosci 108:317–324
Redondo RL, Morris RG (2011) Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci 12:17–30
Rochefort C, Gheusi G, Vincent J et al (2002) Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci 22:2679–2689
Saghatelyan A, Roux P, Migliore M et al (2005) Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46:103–116
Sakamoto M, Imayoshi I, Ohtsuka T et al (2011) Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc Natl Acad Sci USA 108:8479–8484
Sauvage MM, Fortin NJ, Owens CB et al (2008) Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci 11:16–18
Sawada M, Kaneko N, Inada H et al (2011) Sensory input regulates spatial and subtype-specific patterns of neuronal turnover in the adult olfactory bulb. J Neurosci 31:11587–11596
Schneider SP, Macrides F (1978) Laminar distributions of interneurons in the main olfactory bulb of the adult hamster. Brain Res Bull 3:73–82
Schoppa NE (2006) Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron 49:271–283
Schoppa NE, Kinzie JM, Sahara Y et al (1998) Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci 18:6790–6802
Shao Z, Puche AC, Kiyokage E et al (2009) Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 101:1988–2001
Shepherd GM (1963) Neuronal systems controlling mitral cell excitability. J Physiol 168:101–117
Shepherd GM, Chen W, Greer C (2004) Olfactory bulb. In: Shepherd G (ed) The synaptic organization of the brain, 5th edn. Oxford University Press, New York, pp 165–216
Shepherd GM, Chen WR, Willhite D et al (2007) The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev 55:373–382
Shingo T, Gregg C, Enwere E et al (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299:117–120
Shipley MT, Ennis M (1996) Functional organization of olfactory system. J Neurobiol 30:123–176
Sohal VS, Zhang F, Yizhar O et al (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature (Lond) 459:698–702
Sullivan RM, Wilson DA, Leon M (1989) Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci 9:3998–4006
Sultan S, Mandairon N, Kermen F et al (2010) Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J 24:2355–2363
Toida K, Kosaka K, Heizmann CW et al (1998) Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb: III. Structural features of calbindin D28K-immunoreactive neurons. J Comp Neurol 392:179–198
Toida K, Kosaka K, Aika Y et al (2000) Chemically defined neuron groups and their subpopulations in the glomerular layer of the rat main olfactory bulb. IV. Intraglomerular synapses of tyrosine hydroxylase-immunoreactive neurons. Neuroscience 101:11–17
Tononi G, Cirelli C (2006) Sleep function and synaptic homeostasis. Sleep Med Rev 10:49–62
Trombley PQ, Shepherd GM (1992) Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci 12:3985–3991
Tsuno Y, Kashiwadani H, Mori K (2008) Behavioral state regulation of dendrodendritic synaptic inhibition in the olfactory bulb. J Neurosci 28:9227–9238
Valley MT, Mullen TR, Schultz LC et al (2009) Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci 3:51
Veyrac A, Sacquet J, Nguyen V et al (2009) Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology 34:786–795
Vyazovskiy VV, Cirelli C, Pfister-Genskow M et al (2008) Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci 11:200–208
Wellman PJ (2000) Norepinephrine and the control of food intake. Nutrition 16:837–842
Whitman MC, Greer CA (2007) Synaptic integration of adult-generated olfactory bulb granule cells: basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci 27:9951–9961
Winner B, Cooper-Kuhn CM, Aigner R et al (2002) Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci 16:1681–1689
Xiong W, Chen WR (2002) Dynamic gating of spike propagation in the mitral cell lateral dendrites. Neuron 34:115–126
Yamaguchi M, Mori K (2005) Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA 102:9697–9702
Yamaguchi M, Manabe H, Murata K et al (2013) Reorganization of neuronal circuits of the central olfactory system during postprandial sleep. Front Neural Circuits 7:132
Yokoi M, Mori K, Nakanishi S (1995) Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92:3371–3375
Yokoyama TK, Mochimaru D, Murata K et al (2011) Elimination of adult-born neurons in the olfactory bulb is promoted during the postprandial period. Neuron 71:883–897
Young KM, Fogarty M, Kessaris N et al (2007) Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci 27:8286–8296
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from JSPS, and a Grant-in-Aid for Scientific Research on Innovative Areas from MEXT.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Yamaguchi, M. (2014). Interneurons in the Olfactory Bulb: Roles in the Plasticity of Olfactory Information Processing. In: Mori, K. (eds) The Olfactory System. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54376-3_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54376-3_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54375-6
Online ISBN: 978-4-431-54376-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)