Abstract
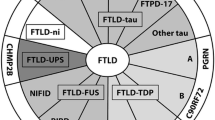
Frontotemporal lobar degeneration (FTLD) is a clinically, genetically, and pathologically heterogeneous group of diseases, including Pick disease, frontotemporal lobar dementia with lacking distinctive histology (FTLD-LDH), and FTLD with motor neuron disease (FTLD-MND). Pick disease is essentially taupathy. FTLD-LDH has inconsistent clinicopathological findings with different immunohistochemical characterization.
FTLD-MND shows characteristic combinations with FTLD and MND. FTLD and FTLD-MND represent distinct clinicopathological entities. The relationship between FTLD-MND and amyotrophic lateral sclerosis is uncertain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Frontotemporal lobar degeneration (FTLD) is one of the most common neurodegenerative disorders among aging societies. FTLD accounts for approximately 20% of neurodegenerative dementias [1, 2]. FTLD is the third most common cause of neurodegenerative dementia syndrome after Alzheimer’s disease and dementia with Lewy bodies. FTLD occurs primarily between the age of 35 and 75 years, and it is rare to have the onset after age of 75. The disease affects both sexes approximately equally. Some cases may carry a diagnosis of Alzheimer’s disease or rapidly dementing illness. Pathologically, it includes a heterogeneous group of sporadic and familial neuropsychiatric diseases.
In the 1980s and 1990s, several research groups recognized that there were many patients with clinical features similar to those of patients with Pick disease who had neither the classical lobar atrophy nor characteristic neuronal changes. These disorders came to be known by several different names, including FTLD-LDH, which consists of frontotemporal lobar degeneration lacking distinct histology, Pick disease, and FTLD with motor neuron disease (FTLD-MND), primary progressive aphasia (PPA), and semantic dementia (SD) [3–10]. As expected, all variants of FTLD have neuronal loss and gliosis affecting the frontal and temporal cortices in keeping with a diagnosis of FTLD. The research criteria have been focused on the need to have subgroups of patients for study during life and accurate pathological diagnosis on autopsy.
Clinical criteria for diagnosing FTLD include the Lund and Manchester Criteria and the more recent consensus criteria [2, 11, 12]. Patients with FTLD present gradual and progressive changes in behavior, or gradual and progressive language dysfunctions. The most common psychiatric symptom of FTLD is an early change in social and personal conduct, characterized by difficulty in modulating behavior to the social demands of a situation. FTLD patients are impaired in the regulation of conduct. This dysfunction is often associated with a lack of inhibition, resembling in impulsive or inappropriate behavior. Progression of the disease may lead to poor financial judgement, and compulsive-like behaviors are common presenting symptoms among FTLD patients [12, 13]. Complex compulsive acts may result from temporal lobe involvement [14]. In some individuals, inappropriate sexual behavior occurs. Perseverative and stereotyped behaviors encompass simple repetitive acts and verbal expression or stereotypies such as lip smacking, habitual hand rubbing or clapping, and humming that may result from frontostriatal circuit dysfunction or involvement of the caudate nuclei. Psychotic symptoms such as delusions and hallucinations are uncommon in FTLD. Nevertheless, there have been patients with an initial schizophrenia-like psychosis or a psychotic affective disorder such as a sign of FTLD [15, 16]. Dietary habits and personal hygiene may also change. Patients become inactive with decreased behavioral motivation and spontaneity, loss of interest in personal hygiene with failure to wash, bathe, groom, and so on. In FTLD, fragments of the Klüver–Bucy syndrome can occur, particularly the hyperorality that manifests as cramming and bingeing, altered food performance especially for sweets or food fads. They may attempt to eat inedible items. FTLD patients can be so hyperoral that require restraint to prevent suffocation or aspiration. There is a loss of concern for one’s personal appearance, and patients may be increasingly unkempt early in the disease. Patients show loss of personal concern for their actions.
FTLD patients tend toward decreased verbal output progressively to complete mutism. Early language disturbances of FTLD are empty speech, nonfluent anomia, especially for words connecting action, and semantic anomia where the word loses its meaning. They usually do not have a true amnestic syndrome. In the FTLD, spatial difficulties are seen in patients with mild or moderate impairments. Many of these patients present with troubles in the expression of language, problems using the correct words, including the naming of persons and things, or expressing oneself. Some patients with FTLD have a progressive aphasia several years before other clinical manifestations; in such cases, speech and language changes predominate (PPA and SD) [17].
FTLD patients have relatively preserved visuo-spatial abilities such as spatial localization and orientation in familiar surroundings. FTLD patients lack sympathy, empathy, emotional warmth, or awareness of the needs of others and appear emotionally shallow and indifferent. FTLD patients have deficits of insight, abstraction, planning, and problem solving, in keeping with a frontal “dysexecutive” syndrome. Judgement is abnormal. FTLD patients are often very concrete on proverb interpretation and tests assessing comprehension of similarities and differences. On neuropsychological testing, frontal-executive functions are compromised early in FTLD, and most memory and occipitoparietal functions are compromised early in most Alzheimer patients.
In the early and middle stages, neurological signs are usually absent or confined to the presence of primitive reflexes such as grasp, snout, and sucking reflexes. Dystonia, ideomotor apraxia, dysphagia, or fasciculations and muscle wasting are occasionally observed.
There are no laboratory findings pathognomonic of FTLD. Routine investigations of blood and urine yield unremarkable results. Conventional EEGs tends to remain normal until late in the course, when they show diffuse slowing and occasional focal frontal or temporal slow wave activity. Structural neuroimaging, particularly magnetic resonance imaging (MRI), can help confirmation in the presence of FTLD. Most FTLD patients show frontal (and anterior temporal) atrophy, enlargement of the Sylvius fissures, anterior callosal atrophy, and eventual hippocampal and entorhinal volume loss. Computed tomography (CT)/MRI may show atrophy of the anterior temporal and frontal lobes. Some FTLD patients may have additional MRI evidence of bilateral caudate nuclear atrophy. Functional imaging is more sensitive than structural imaging for the diagnosis of FTLD. Single-photon emission computed tomography/18F-fluorodeoxyglucose-positron emission tomography (SPECT/FDG-PET) typically demonstrates decreased perfusion and metabolism of the frontal and temporal lobes.
The usual clinical duration of FTLD is about 8–11 years. The clinical course of FTLD can be divided into three stages. In the initial stages, there are prominent personality changes, emotional alterations, and impaired insight and judgement. Speech and language changes also may occur. In the second stage, aphasia and other cognitive changes become pronounced, but there is at least partial preservation of memory, visuo-spatial skills, and computational ability. The third and final stage of the disease is often dominated by progressive muteness, and the patients become profoundly demented.
At autopsy, the brains of patients with FTLD show a lobar distribution of atrophy involving the frontal lobes, temporal lobes, or both. Coronal sections reveal deep sulci and may show knife-edged gyri in the atrophic area, especially in Pick disease. The orbitofrontal cortex and the anterior and medial temporal areas show the most severe atrophic changes. The predominant neuropathological abnormalities are frontotemporal neuronal loss and gliosis with ubiquitin-positive, tau-negative inclusions and without detectable amounts of insoluble tau, with MND or without MND. The cortical degeneration involves mainly the grey matter, including the insula and the anterior cingulate gyrus. In the past, clinicians have referred to FTLD patients as having “Pick bodies,” but on neuropathological examination, most FTLD patients lack the pathognomonic Pick bodies. There has been continuing controversy concerning whether identifiable cellular changes of Pick bodies and ballooned neurons are a requirement for diagnosis. Smaller number of FTLD patients have involvement of substantia nigra, striato-pallidum, parietal cortex, thalamus, and other structures [18, 19]. Most FTLD patients do not have senile plaques or neurofibrillary tangles, although some have amyloid beta deposition, particularly late in the course and in patients with an APO-E epsilon 4-allele [20]. In FTLD, about one-third of patients have tau pathology and about 10% have a tau gene mutation [21, 22]. Pathological findings have, to date, not been associated with specific clinical manifestations.
Pick Disease
Arnold Pick [23–25], in a series of articles based on gross examination of the brain, reported cases of dementia with severe circumscribed atrophy of the frontotemporal regions. The definition of the clinical entity of what became known as Pick disease was presented in a series of articles in the 1920s [26–28]. The initial stage is characterized by prominent personality changes and emotional alterations. Judgment is impaired early, and insight is compromised. Social behavior deteriorates, and language abnormalities are among the earliest intellectual alterations to occur. In the second stage of disease, deterioration of mental status becomes evident and aphasia is more prominent. Cognitive changes are more pronounced, but memory and visuo-spatial skills may remain relatively intact. In the final stage of the disease, progressive extrapyramidal syndrome usually appears and the patient becomes mute and incontinent. Pick disease has a longer illness duration (more than 10 years). CT/MRI may provide supportive evidence for the diagnosis.
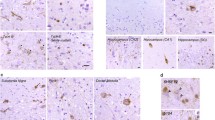
Neuropathology shows severe frontotemporal atrophy, often with “knife-edged” gyri (Fig. 1), and extensive neuronal loss with gliosis. An abrupt transition is sometimes evident between involved and uninvolved cortical regions. There is a tendency for selective sparing of the precentral gyrus and the posterior one-third of the superior temporal gyrus. The concept of Pick disease emphasized the importance of Pick bodies and ballooned neurons in the pathological diagnosis and the clinical distinction of this disorder from Alzheimer disease. The diagnosis of Pick disease occurs when there are Pick bodies with or without Pick cells. Pick bodies are spherical, silver-staining (argyrophilic), ubiquitin- and tau-positive intraneuronal inclusions. Pick bodies are concentrated in frontotemporal neocortical layers II–VI, and the hippocampal formation in the granular layer of the dentate gyrus and sector CA1. Pick bodies do not occur in normal aging. There are pathognomonic microscopic findings showing ballooned neurons or Pick cells and definite positive tau and ubiquitin bodies in neurons of the frontotemporal cortex and granule cells of dentate gyrus of the hippocampus (Pick bodies). Pick disease is essentially taupathy, similar to corticobasal degeneration and progressive supranuclear palsy. The volume of the substantia nigra is decreased, but the concentration of neurons is slightly reduced, and there is no clinical parkinsonism until the terminal stages.
FTLD-LDH
FTLD is a clinical term applied to patients who present with progressive dementia with an insidious onset, prominent behavioral or language dysfunction, or both. We have recognized that this term includes groups of patients whose pathological conditions and genetic mechanisms are heterogeneous. The clinical characteristics of FTLD-LDH are almost similar to those of Pick disease. However, the severity of intellectual and personality deterioration is less than those seen in Pick disease. MRI shows frontotemporal atrophy, and SPECT shows selective hypoperfusion of frontotemporal lobes. The most common pathology of FTLD-LDH is a nonspecific frontotemporal atrophy, and FTLD-LDH is the usual FTLD pathology.
Microscopic study shows mild to slight neuronal loss and astrogliosis with sponginess (minute cavities or microvacuolation) of the outer supragranular (II–III) layers of the frontotemporal cortex with ubiquitin- and TDP-43-positive inclusions (Fig. 2). There is also variable involvement of subcortical and limbic structures. The anterior hippocampal regions are also affected. FTLD-LDH has no Pick bodies, plus depigmentation in the substantia nigra and striato-pallidum.
FTLD-MND
A subset of patients with FTLD develops symptoms suggestive of MND. The mean age at onset and disease duration are 52 years and 2.3 years, respectively. FTLD-MND is a clinicopathological entity characterized by the combination of FTLD and MND. The link between FTLD and MND was suggested by the autopsy in 1984 [29], and the term FTLD-MND was subsequently proposed [30]. The development of MND in patients presenting with a progressive behavioral disorder would strongly support a clinical diagnosis of FTLD-MND. MND is also a clinical term, but it is applied to patients with clinical evidence of corticospinal tract involvement, evidence of brainstem or spinal cord anterior horn cell involvement, or both. FTLD-MND have symptoms of mild forgetfulness and language output impairment, in addition to the more prominent behavioral disorders. These symptoms include weakness and muscle wasting. Symptoms of parkinsonism, such as rigidity, are occasionally seen. Signs of MND may not always be present early. Most of the cases of MND developed approximately 6–24 months after the onset of symptoms of FTLD [29, 31]. Only features of dementia are noted early in the disease course, and both initially carried a diagnosis of FTLD. MRI/SPECT reveals slight to moderate atrophy and selective hypoperfusion in the frontotemporal regions. The patient shows a relatively rapid clinical course, less than 5 years.
The pathological features of FTLD-MND are variable. Neuropathology of FTLD-MND shows slight frontotemporal lobe atrophy and more characteristic features of mild neuronal loss with cortical sponginess and subcortical gliosis, affecting the frontal and temporal cortices in keeping with a diagnosis of FTLD. In addition, all cases have pathological evidence of degeneration of lower motor neurons that contain eosinophilic and ubiquitin-positive inclusions. There are also ubiquitin-positive, tau-negative (nonargyrophilic) inclusions in the neurons of the frontotemporal cortex and granule cells in the dentate fascia of the hippocampus, which are characteristic of MND. Ubiquitin-positive and tau-negative inclusions may be present in FTLD with or without MND. Ubiquitin- and TDP-43-positive inclusions are present in the dentate fascia and neocortex in FTLD-MND. Some cases have a mixture of lower motor neuron degeneration and corticospinal tract degeneration (similar to amyotrophic lateral sclerosis, ALS) and the majority have Bunina bodies, which are a histological hallmark of ALS. The severity of motor neuron degeneration is variable, ranging from absent to severe. Some cases have a predominance of corticospinal tract degeneration, but others have no corticospinal tract degeneration. At the present time, there is no way to distinguish between cases on the basis of extramotor ubiquitin-positive pathological features or on the basis of predominant involvement of upper or lower motor neurons. A large sample size would be needed to address possible clinically useful subtypes. FTLD-MND with pathological changes in the hippocampus seems strikingly similar to other FTLD and some evidence of MND, whereas FTLD-MND without hippocampal pathology seems strikingly similar to ALS with some evidence of FTLD.
FTLD-MND should be taught as a spectrum of diseases and include FTLD-MND with hippocampal pathology and FTLD-MND without hippocampal pathology. Detection of signs of clinical MNDs is difficult when motor neuron degeneration is mild. FTLD-MND is diagnosed if there is evidence of brainstem or spinal cord anterior horn cell degeneration with or without ubiquitin-positive inclusions [32–34], degeneration of the corticospinal tract, or both, in addition to histological evidence of FTLD. Histological evidence of motor neuron degeneration includes loss of large anterior horn cells in the spinal cord or hypoglossal neurons plus (1) shrunken residual motor neurons, (2) evidence of neuronophagia, (3) Bunina bodies, or (4) ubiquitin-immunoreactive intraneuronal inclusions. Evidence of FTLD includes the presence of superficial sponginess, neuronal loss, and astrogliosis affecting predominantly layer II of the cortex, with or without the presence of ubiquitin immunoreactive neuronal cytoplasmic inclusions. Neuronal intranuclear inclusions such as Pick bodies are not detected. The ubiquitin-positive inclusions are negative for tau, alpha-synuclein, and neurofilament. Extramotor ubiquitin-positive neuronal inclusions are present in all cases, in the dentate fascia, neocortex, or both regions. Skeletal muscle has evidence of group atrophy with small acutely angulated fibers consistent with neurogenic atrophy.
Neuronal loss and gliosis of the hypoglossal nucleus and spinal anterior horn cells are variable and ranged from absent to severe. The severity of motor neuron involvement tend to correlate with clinical evidence of MND. Corticospinal tract degeneration also ranged from absent to severe. Severity of corticospinal tract degeneration does not correlate with clinical signs of MND or with severity of neuronal loss and gliosis in the hypoglossal nucleus and spinal anterior horn cells. Extramotor ubiquitin-positive inclusions are absent to sparse in all cortical regions.
Comments
Large clinicopathological series have been published that have clearly demonstrated an overlap between the clinical syndromes subsumed under the term frontotemporal dementia and the progressive supranuclear palsy syndrome, corticobasal degeneration syndrome, and MND. There have also been significant advancements in brain imaging, neuropathology, and molecular genetics that have led to different approaches to the classification.
FTLD and FTLD-MND represents a distinct clinicopathological entity that shows essentially ubiquitin and TDP-43 proteinopathy. There are some cases of FTLD with TDP-43-positive and ubiquitin-negative pathology. TDP-43 proteinopathy has been reported in many neurodegenerative diseases (filament inclusion disease). TDP-43 might be more susceptible to neuronal damage. From these findings, different pathoetiologies could lead to the varied clinicopathological presentations of FTLD.
The pathological features of FTLD-MND are variable. Most cases have a mixture of lower motor neuron degeneration, occasionally associated with corticospinal tract degeneration, similar to progressive spinal muscular atrophy or ALS, and the majority have Bunina bodies, which are a histological hallmark of ALS.
The field is complicated by a barrage of overlapping clinical syndromes, and neuropathological diagnosis that does not always respond to clinical presentations and underlying neuropathology.
It is difficult to distinguish between cases on the basis of extramotor ubiquitin-positive pathological features or on the basis of predominant involvement of upper or lower motor neurons. Hippocampal sclerosis with ubiquitin-positive inclusions in the dentate fascia is a common feature of FTLD with ubiquitin-only immunoreactive changes.
Lower motor neuron involvement including spinal cord anterior horn cell and hypoglossal nucleus degeneration is of the utmost importance for future clinicopathological studies with combined dementia and MND.
It is unclear how neurodegenerative diseases cause dysfunction and death of brain cells or specific neuropsychiatric symptoms. Clinical manifestations of the FTLD cannot accurately predict the nature of the underlying neurodegenerative disease. The neuropathological findings alone cannot establish that a patient had FTLD in the absence of documented clinical information. Although there is no specific treatment for FTLD, many symptomatic therapies can be very helpful. Many of the behavioral psychological symptoms of FTLD may respond to selective serotonin uptake inhibitors (SSRI) [35]. Marked disinhibition, aggressive behavior, or verbal outbursts may respond to small doses of major tranquilizers such as risperidone, olanzapine, or quetiapine. FTLD is very stressful to the caregiver, and support of the family is critically important.
References
Pasquier F, Delacourte A (1998) Non-Alzheimer degenerative dementias. Curr Opin Neurol 11:417–427
The Lund and Manchester Group (1994) Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 57:416–418
Neary D, Snowden JS, Bowen DM et al (1986) Neuropsychological syndromes in presenile dementia due to cerebral atrophy. J Neurol Neurosurg Psychiatry 49:163–174
Neary D, Snowden JS, Northen B et al (1988) Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry 51:353–361
Brun A (1987) Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology. Arch Gerontol Geriatr 6:193–208
Brun A (1993) Frontal lobe degeneration of non-Alzheimer type revised. Dementia 4:126–131
Gustafson L (1987) Frontal lobe degeneration of non-Alzheiemr type II. Clinical picture and differential diagnosis. Arch Gerontol Geriatr 6:209–223
Gustafson L (1993) Clinical picture of frontal lobe degeneration of non-Alzheimer type. Dementia 4:143–148
Snowden JS, Neary D, Mann DM (1996) Frontotemporal lobar degeneration: frontotemporal dementia. Progressive aphasia, semantic dementia. Churchill-Livingston, London
Knopman DS (1993) Overview of dementia lacking distinctive histology: pathological designation of a progressive dementia. Dementia 4:132–136
Neary D, Snowden JS, Gustafson L et al (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Mendez MF, Perryman KM, Milker BL et al (1997) Comprehensive behaviours as presenting symptoms of frontotemporal dementia. J Geriatr Psychiatry Neurol 10:154–157
Tonkonogy JM, Smith TW, Barreira PJ (1994) Obsessive-compulsive disorders in Pick’s disease. J Neuropsychiatry Clin Neurosci 6:176–180
Rosso SM, Roks G, Stevens M et al (1998) Complex compulsive behaviour in the temporal variant of frontotemporal dementia. Ned Tijdschr Geneeskd 142:1962–1965
Waddington JL, Youssef HA, Farrell MA et al (1995) Initial “schizophrenia-like” psychosis in Pick’s disease: case study with neuroimaging and neuropathology and implications for frontotemporal dysfunction in schizophrenia. Schizophr Res 18:79–82
Gregory CA (1999) Frontal variant of frontotemporal dementia: a cross-sectioned and longitudinal study of neuropsychiatric features. Psychol Med 29:1205–1217
Ostberg P, Bogdanovic N, Fernaeus SE et al (2001) Jargon aphasia in a case of frontotemporal dementia. Brain Lang 79:333–339
Neary D, Snowden JS, Mann DM (1993) Familial progressive aphasia: its relationship of other forms of lobar atrophy. J Neurol Neurosurg Psychiatry 56:1122–1125
Sam M, Gutmann L, Schochet SS et al (1991) Pick’s disease: a case clinically resembling amyotrophic lateral sclerosis. Neurology 41:1831–1833
Gustafson L, Abramason M, Grubb A et al (1997) Apolipoprotein-E genotyping in Alzheimer’s disease and frontotemporal dementia. Dement Geriatr Cogn Disord 8:240–243
Adams E, Chang HT, Stopa EG et al (2001) Tau protein expression in frontotemporal dementias. Neurosci Lett 315:21–24
Poorkaj P, Grossmann M, Steinbart E et al (2001) Frequency of tau gene mutations in familial and sporadic cases of non-Alzheimer dementia. Arch Neurol 58:383–387
Pick A (1882) Über die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Med Wochenschr 17:165–167
Pick A (1901) Senile Hirnatrophie als Grundlage von Herd ersheinungen. Wien Klin Wochenschr 14:403–404
Oick A (1905) Zur Symptomatologie der linksseitigen Schlafen-lappenn Atrophie. Monatsschr Psychiatr Neurol 16:378–388
Gans A (1922) Betrachtungen über Art und Ausbreitung des krankhaften Prozesses in einem Fall von Pickscher Atrophie des Stirnhirns. Z Gesamte Neurol Psychiatr 80:10–28
Onari K, Spatz H (1926) Anatomische Beitrage zur Lehre von Pickschen umschriebenen Grosshirnrinden-Atophie (“Picksche Krankheit”). Z Gesamte Neurol Psychiatr 101:470–511
Schneider C (1927) Über Picksche Krankheit. Monatsschr Psychiatr Neurol 65:230–275
Mitsuyama Y (1984) Presenile dementia with motor neuron disease in Japan: clinico-pathological review of 26 cases. J Neurol Neurosurg Psychiatry 47:53–959
Neary D, Snowden JS, Mann DM et al (1990) Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry 53:23–32
Mitsuyama Y (2000) Dementia with motor neuron disease. Neuropathology 20(Suppl):S79–S81
Mendez MF, Selwood A, Mastri AR et al (1993) Pick’s disease versus Alzheimer’s disease: a comparison of clinical characteristics. Neurology 43:289–292
Schmitt HP, Yang Y, Fortstl H (1995) Frontal lobe degeneration of non-Alzheimer type and Pick’s atrophy: lumping or splitting? Eur Arch Psychiatry Clin Neurosci 245:299–305
Josephs KA, Parisi JE, Knopman DS et al (2006) Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol 63:506–512
Swartz JR, Miller BL, Lesser IM et al (2001) Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry 56:41–45
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer
About this chapter
Cite this chapter
Mitsuyama, Y. (2010). Clinicopathological Characterization of Frontotemporal Lobar Degeneration. In: Miyoshi, K., Morimura, Y., Maeda, K. (eds) Neuropsychiatric Disorders. Springer, Tokyo. https://doi.org/10.1007/978-4-431-53871-4_20
Download citation
DOI: https://doi.org/10.1007/978-4-431-53871-4_20
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-53870-7
Online ISBN: 978-4-431-53871-4
eBook Packages: MedicineMedicine (R0)