Abstract
Protein-based therapeutic subunit vaccines against cancer have proven efficacy in various preclinical models. The translation of their efficacy into the clinic, however, has been challenging. Although there are many factors impacting the efficacy of vaccines in humans, the most important ones are the prolonged tumor development and progression, altered immune responses due to extensive exposure to environmental pathogens, stage of cancer, standard treatments to control cancer, and effect of such treatments on the patient’s immune system prior to vaccine administration. It is a common consensus that the presence of cancer is an indication of effective immune evasion responses initiated and perpetuated by tumor. Immune evasion involves well-orchestrated cellular and molecular mechanisms that control tumor-specific effector immune responses in favor of tumor progression. Therefore, protein-based subunit vaccine formulations will require immune adjuvants that not only generate the desired effector immune responses, particularly those driven by CD8+ T cells, but also reverse the regulatory immune evasion network in place to combat tumor. Costimulation through tumor necrosis factor receptor (TNFR) superfamily is critical for T-cell activation, expansion, acquisition of effector function, and establishment of long-term memory required for tumor eradication and control of recurrences. As such, agonists of TNFRs have great potential as immune adjuvants. We recently generated a novel form of the 4-1BB ligand, SA-4-1BBL, a member of TNF family, and demonstrated its robust pleiotropic effects on the cells of innate, adaptive, and regulatory immunity. Importantly, as the adjuvant component of tumor-associated antigen (TAA)-based vaccine formulations, SA-4-1BBL demonstrated therapeutic efficacy in various preclinical tumor models in the absence of detectable toxicity. This chapter will discuss SA-4-1BBL as a novel adjuvant with demonstrated desired mechanisms of action for tumor eradication and its prospect for human use as monotherapy or in combination with other immune modulators with synergistic mechanisms of action.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The concept of therapeutic cancer vaccines dates back to 1893 when William B. Coley observed regression of tumor in some cancer patients with acute infections and attempted to use bacteria or bacterial products for the treatment of cancer patients (Coley 1891, 1910). Regression of tumors in some of Coley’s vaccinated patients was believed to be due to infection-induced excessive inflammation. Most importantly, Coley’s studies demonstrated that the immune system of cancer patients can be activated to combat tumor without major adverse effects on nonmalignant cells, a clear indication of the exquisite specificity and efficacy of the immune response. Although these initial observations were extremely exciting and marked the origin of modern cancer immunotherapy, the concept of cancer vaccines faced significant skepticism due to various setbacks in achieving the desired therapeutic efficacy. As such, the role of immune system in fighting tumors was called into question. However, increased tumor incidences in immunodeficient mice and in patients on immunosuppressive regimen, such as transplant recipients, provided undisputable evidence that the immune system plays a critical role in controlling tumor progression. This so-called immunosurveillance theory was further supported by findings that genetically modified mice lacking key immune effector molecules, such as IFNγ, granzyme B, and perforin, develop spontaneous tumors with significantly increased frequencies as compared to immunocompetent mice (Smyth et al. 2000; Kaplan et al. 1998).
The advances in molecular techniques and recombinant DNA technology led to a better understanding of the immune system, tumor development and progression, and most importantly the extensive and complex nature of interactions/regulation between the immune system and the tumor, dictating tumor elimination versus progression. This accumulated knowledge led to a better design of vaccines that yielded consistent and reproducible therapeutic responses in various preclinical models (Lesterhuis et al. 2011). However, the translation of preclinical success of cancer vaccines to the clinic became a far-reaching goal. It is unclear as to why vaccine formulations that work so effectively in rodents have minimal to no clinical benefits in humans. The complex nature of the human immune system, its altered state due to continuous exposure to various environmental antigens, spontaneously arising tumor with protracted progression before diagnosis, coevolution of tumor and immune systems during progression, and most importantly, the stage of tumor in patients and standard treatment history to control the tumor prior to vaccination represent some of the contributing factors. The failure of numerous vaccine concepts in the clinic does not invalidate the potential of this therapy, but it certainly indicates that our understanding of the human immune system and tumor progression has not elevated to the level that will allow for the design of effective vaccine formulations. Irrespectively, the approval of the first ever therapeutic vaccine, DC-based Provenge®, for the treatment of prostate cancer by FDA in 2010 was a major milestone, instilled faith, and renewed interest in cancer vaccines.
In this chapter, we will discuss some of the difficulties in translating the efficacy of vaccines from preclinical settings to the clinic and argue that the use of adjuvants that boost immune effector responses for tumor eradication will be key to the clinical success of vaccines. SA-4-1BBL will be presented as adjuvant with such potential. We will then make a case in favor of combinatorial approaches involving adjuvants and selected immune modulators for the design of cancer vaccines with the potential to overcome various immune setbacks and achieve maximal efficacy in the clinic.
2 Immune System and Cancer: A Love/Hate Relationship
The requisite role of the immune system against infections has been well recognized and confirmed by the development of various prophylactic vaccines that save millions of lives worldwide annually. In marked contrast, the role of the immune system in tumor development, progression, and control has been the subject of significant controversy over the past several decades. The initial report of Coley that acute infections may cause spontaneous tumor remission in patients, presumably because of infection-induced inflammation, provided evidence for the role of the immune system in eradicating cancer (Coley 1891, 1910). The concept that immune system is important in controlling tumors under normal physiological conditions was conceived by Paul Erlich in 1909 and formulized by Lewis Thomas and Macfarlane Burnet under the hypothesis of “tumor immune surveillance” in 1957. However, the lack of direct evidence for this hypothesis resulted in significant controversy. Further fueling this debate were observations that nude mice lacking adaptive immunity and their syngeneic wild-type counterparts develop similar incidences of spontaneously arising, non-virus-driven tumors (Stutman 1974; Rygaard and Povlsen 1974). However, subsequent studies over the years demonstrated that nude mice are not totally immune incompetent as they do generate extrathymically developed T cells and innate immune cells (Ikehara et al. 1984), and irrespective of partial immune competency, such mice have higher incidences of tumor as compared with wild-type mice (Engel et al. 1996).
Technological progresses in biomedical sciences allowed the design of sophisticated studies to delineate mechanistic basis of immune responses, which elevated our understanding of the immune system against cancer. In particular, targeted alteration of the immune system in mice via transgenic technology presented the opportunity for rigorous testing of the immune surveillance hypothesis. Mice lacking selected immune cells, such as T and B cells (Rag−/−); or effector molecules, such as perforin (Smyth et al. 2000); or IFNγ (Shankaran et al. 2001) provided unequivocal evidence that the immune system is critical for the control of tumors. The higher incidences of tumors in immunosuppressed individuals, particularly transplant recipients (Engels et al. 2011), as compared with normal population provided clinical evidence for the role of immune surveillance hypothesis. The accumulated knowledge of the immune system combined with the identification of TAAs resulted in the design of immune therapies, particularly cancer vaccines that showed efficacy in various preclinical models (Lesterhuis et al. 2011; Schlom 2012). The efficacy of various immune therapies in eliminating established tumors not only provided direct evidence for the importance of the immune response in controlling tumor but also set the stage for harnessing the power of the immune system for the eradication of tumor (Lesterhuis et al. 2011; Schlom 2012), renewing faith in the early observations of Coley and generating confidence in the promise of cancer vaccines.
Although the role of immune response in fighting cancer is unquestionable, accumulating evidence in the literature also implicates the immune system in tumor progression. The tumor microenvironment consists of malignant as well as nonmalignant stromal cells, such as immune cells, fibroblasts, endothelial cells, and extracellular matrix. A complex set of molecular and cellular communications within this unique microenvironment determine the fate of tumor progression versus tumor elimination. A robust effector response is associated with tumor elimination, while a chronic immune response may be beneficial for tumor progression as it generates various soluble factors involved in angiogenesis, tumor growth, metastasis, and resistance to standard-of-care treatments (Allavena et al. 2008). Although various effector mechanisms, including humoral and innate immune responses, depending on the cancer type are associated with the control and elimination of altered-self cancer cells, Th1-mediated cellular immunity, particularly CD8+ T-cell cytotoxic response, plays the most determining role. In response, tumor cells have developed direct and indirect evasion mechanisms to counterattack the immune system. Indeed, tumor-modulated regulatory immune responses may serve as one of the most important hurdles affecting the efficacy of therapeutic cancer vaccines. Although these immune evasion mechanisms are complex and yet to be fully elucidated, T-cell anergy or nonresponsiveness (Nind et al. 1973), T regulatory cells (Nishikawa and Sakaguchi 2014), regulatory NK T cells (Terabe et al. 2000), myeloid-derived suppressor cells (Gabrilovich and Nagaraj 2009), various soluble factors (such as TGF-β and IL-10), indoleamine 2,3-dioxygenase, downregulation of costimulatory ligands (such as CD80, 4-1BBL, and MHC molecules), upregulation of co-inhibitory ligands (such as PDL-1), or death-inducing molecules (such as FasL) represent some (Zou 2005). Given this complex cancer/immune system interplay, cancer vaccine design that incorporates adjuvants or adjuvant systems to shift the overall balance from immune evasion that facilitates tumor progression to immune effectors, such as Th1 immune responses, that combat tumor may achieve therapeutic efficacy.
3 Efficacy of Prophylactic Versus Therapeutic Vaccines
Prophylactic vaccines against infections have been extremely effective and are considered the miracle of modern medicine. In marked contrast, the promise of therapeutic vaccines against tumors is yet to be fully realized. There are several factors that may contribute to this discrepancy. Preventive vaccines use foreign strong exogenous antigens for the induction of humoral immune responses in a healthy population with an intact and functional immune system. Therapeutic vaccines, on the other hand, use TAAs for the generation of cellular immune responses required for the eradication of tumors in diseased individuals. The nature of antigens used for immunization and the immune status of the vaccinated individuals may be the key to the observed efficacy differences between prophylactic and therapeutic vaccines. Pathogen-derived proteins serve as strong antigens and as such generate robust immune responses. In marked contrast, TAAs, by their nature of being self-antigens, lack the ability to generate robust T cell-mediated immune responses required for tumor eradication. The status of the immune system in vaccinated individuals is most likely the pivotal factor dictating the efficacy of prophylactic versus therapeutic vaccines. Unlike prophylactic vaccines administered to healthy individuals, therapeutic vaccines are given to cancer patients whose immune system not only has failed to control the tumor but most likely has also been altered by standard-of-care cancer treatments.
Finally, tumors have evolved to combat the immune system by a series of intrinsic and extrinsic mechanisms. Most importantly, some of the evasion mechanisms involve the tumor’s ability to utilize the immune system for its own progression (Zou 2005). In particular, various immunoregulatory mechanisms required for self-tolerance have been exploited by tumors to cheat the immune system (Gabrilovich and Nagaraj 2009; Nishikawa and Sakaguchi 2014; Zou 2005). Therefore, the therapeutic efficacy of the cancer vaccines will depend on their ability to generate robust immune effector responses against tumors as well as overcome various immune evasion mechanisms employed by the progressing tumor. These effects need to be achieved in patients who have undergone standard-of-care cancer treatments, and as a consequence, most likely have compromised immune responses. Therefore, therapeutic vaccines need to be formulated with these considerations in mind and will require novel adjuvants that can drive effective immune responses against tumor. An adjuvant that not only generates a robust Th1 response against tumor but also overcomes the tumor employed regulatory/suppressive mechanisms may have the best chance for achieving efficacy in cancer patients.
4 Therapeutic Vaccines
Therapeutic cancer vaccines are designed to generate a productive antitumor immune response that translates into efficacy in cancer patients. Cancer vaccines can be classified into cell-free or cell-based vaccines. Cell-free vaccines include DNA-based vaccines, viral vectored vaccines, oncolytic viral vaccines, and protein-based subunit vaccines. Cell-based vaccines, on the other hand, comprise irradiated or chemically fixed whole tumor cells or dendritic cells (DCs) pulsed with TAAs, such as FDA-approved sipuleucel-T, also known as “Provenge.” This book chapter will focus on subunit, protein-based therapeutic cancer vaccines. Therapeutic cancer vaccines against well-defined TAAs emerged as a promising treatment modality. These subunit vaccines are attractive because of their ease of production, cost-effectiveness, off-the-shelf availability, and ease/practical nature of administration into the patients.
The concept of subunit vaccine formulation is rather simple as it involves the addition of one or more whole TAA proteins or synthetic peptides representing T-cell epitopes of such TAAs along with an adjuvant or adjuvant system that not only drives the desired Th1 antitumor immune responses but is also capable of reversing the unwanted tumor-mediated immunosuppressive mechanisms. Inasmuch as DCs are critical for the generation of adaptive immune responses in general and against cancer in particular, as exemplified by the clinical efficacy of Provenge, vaccine formulations may benefit from incorporating vehicles to deliver TAAs into DC in vivo for optimal antigen presentation and effective T-cell activation, proliferation, acquisition of effector function, and establishment of long-term memory. Several TAAs, such as human epidermal growth factor receptor 2 (HER-2), prostate-specific membrane antigen (PSMA), are expressed on cancer cell surface. An adjuvant system that can optimally prime CD8+ T cell-mediated cytotoxic responses along with B cell-mediated antibody responses may prove to be more effective in these settings. Therefore, therapeutic subunit vaccines need to be formulated based on the cancer type, utilized TAAs, and anticipated effector immune responses necessary for tumor elimination. In this context, careful consideration of adjuvants or adjuvant systems as component of vaccine formulations will be critical to a desired therapeutic outcome.
5 Problems and Prospects for the Design of Subunit TAA-Based Cancer Vaccines
Some of the major challenges in vaccine design are the selection of appropriate TAAs, adjuvant or adjuvant systems that are capable of priming/boosting the anticipated antitumor immune responses, and vehicles/systems to ensure the delivery of TAAs into DCs for accomplishing a robust therapeutic efficacy. Antigenic drift, accumulated mutations in T- and B-cell epitopes due to immune pressure, is a major mechanism of tumor escape from immune attack. Therefore, the selection of a TAA that is not only specifically and/or highly expressed by tumor cells but also is essential for tumor survival, progression, and metastasis is important. Discovery of universal TAAs at least for the same tumor type across a patient population will be a key step for designing a generalized vaccine against a specific type of cancer. Analysis of tumors in humans has shown great TAA heterogeneity among the same cancer type and even within the same tumor tissue. Therefore, the choice of a TAA for the development of cancer vaccines should be dictated by a comprehensive understanding of its expression pattern in the selected cancer type and at various stages of the cancer. Although emerging understanding of cancer immunology provides better opportunities to design more specific vaccines, it also brings greater challenges for vaccine customization for a particular type of cancer. Vaccine formulations may need to be tailored to be best suited for the patient’s cancer profile with respect to TAA expression as well as effector immune responses required for the eradication of tumor. This issue not only presents a challenge for the design of cancer vaccines but also customization of the current standard-of-care treatments for individual patients for a more effective outcome.
The question is if it is feasible to develop a universal vaccine that may have utility for different cancer types. Realistically, vaccine formulations may need to be customized for the patient, but not tumor, for the desired therapeutic efficacy after tumor biopsy followed by genomic and proteomics analyses to determine the precise status of genetic variations and TAA expression profiles. Accumulating evidences indicate that the immune system can adapt to the antigenic changes within a tumor through the process of inter- or intramolecular antigen or epitope spreading (Hardwick and Chain 2011). In response to the tumor, T-cell repertoire expands and recognizes epitopes that are not part of the initial TAA in the vaccine formulation. As tumor cells are damaged and eliminated by the immune system, new TAAs are released within the tumor milieu or systemically and picked up by DCs for cross-presentation to CD4+ and CD8+ T cells for the generation of a broader cellular immune response than has been primed by the vaccine. These findings indicate the feasibility of developing vaccines that may have utility for different tumor types. However, if the initial recognition of TAA within the vaccine formulation is critical to the antigen/epitope spreading, then all the targeted tumor types need to express this TAA for the vaccine to manifest its efficacy. In this context, it may also be feasible to design vaccine formulations containing tumor-related and/or unrelated antigens that serve as universal T-cell epitopes admixed with adjuvants having robust immune stimulatory activities. These vaccines can then be administered to patients in conjunction with tumor-damaging agents, such as standard-of-care chemo and/or localized radiotherapy, to initiate a self-perpetuating immune response against cancer. In this scenario, adjuvants will initiate and boost T-cell responses against the antigen component of the vaccine, while the tumor damage will provide endogenous TAAs initiating the process of epitope spreading. Inasmuch as immune evasion mechanisms are one of the most important hurdles for achieving the efficacy of vaccines in the clinic, vaccine composition must contain adjuvants or adjuvant systems that are not only capable of inducing the anticancer immune responses but also overcome various immune evasion mechanisms employed by the progressing tumor.
The in vivo half-life and bioavailability of the vaccine is another issue worth considering when designing vaccine formulations. The depot effect of alum is still believed to be largely responsible for its superb adjuvant properties for augmenting B cell-mediated antibody responses (Kool et al. 2012). As such, numerous vaccine delivery systems, including liposomal and nano/microparticles-based adjuvant systems, have been developed to enhance T cell-mediated responses (Gregory et al. 2013). The success of DC-based vaccines in preclinical and clinical studies served as impetuous to target these cells for antigen delivery to ensure the optimal vaccine efficacy. DCs have been manipulated ex vivo by various means to present TAAs and achieve clinical responses. However, DC-based cellular vaccines are time and labor intensive, costly, and, most importantly, patient customized, which severely limit their broad clinical application. Therefore, intense efforts have been devoted to target DCs in vivo for the improvement of therapeutic efficacy of TAA-based conventional vaccines (Tacken et al. 2007). Studies in humans demonstrated that DC maturation is obligatory for the generation of effective immunity (de Vries et al. 2003). Therefore, various strategies have been attempted to deliver antigens to DCs in vivo by targeting specific receptors, such as DEC205 (Bonifaz et al. 2002), Clec9A (Sancho et al. 2008), the mannose receptor (He et al. 2007), and Dectin-1 (Carter et al. 2006). These strategies also required adjuvants, such as agonists of Toll-like receptor or CD40, to mature the targeted DCs for the generation of endogenous cytotoxic T-cell responses and effective antitumor immunity. Therefore, adjuvants with dual functions, as antigen delivery vehicle and modulator of DC activation, antigen uptake, and cross-presentation, may significantly improve the therapeutic efficacy of the vaccines. In summary, the efficacy of cancer vaccines, irrespective of their formulation, will depend not only on their ability to prime or boost the existing immune responses but also overcome various immune evasion mechanisms that help tumor progression in cancer patients. In this context, the choice of adjuvants is of paramount importance and those that modulate innate, adaptive, and regulatory immunity for the generation of effective Th1 cellular responses without adverse effects or with tolerable toxicity will deliver the promise of cancer vaccines.
6 Adjuvants for Therapeutic Cancer Vaccines
Adjuvants are molecules, compounds, or macromolecular complexes that traditionally are admixed with antigens to enhance the magnitude, breadth, quality, and longevity of the immune response to the antigens. As such, adjuvants may substantially reduce the amount of antigen and/or number of immunizations required for the generation of an effective immune response. Despite the fact that adjuvants are crucial vaccine components determining their success or failure, there has been great deal of pessimism regarding their use for the development of therapeutic cancer vaccines. This is mainly due to potential toxicity arising from the lack of full understanding of mechanistic insight and precise knowledge of the constituents of many adjuvants (Marrack et al. 2009; Pashine et al. 2005). Some of the tested vaccine formulations, like viral vectors, are designed to express their own adjuvants, while others, like peptide-based vaccines, do not and hence require coadministration of adjuvants for the induction of potent immune response.
The choice of adjuvants available for cancer vaccines has been very limited, mostly or in part due to the toxicity concerns, which raise significant regulatory hurdles. In fact, aluminum-salt-based adjuvants were the only ones used clinically in the United States until 2010 when monophosphoryl lipid A (MPL) in combination with aluminum hydroxide was approved by the FDA (Vacchelli et al. 2013) as adjuvant component of the preventive vaccine, Cervarix, against human papillomavirus (HPV). Recent advances in molecular technologies, in particular genomics and proteomics, led to a better understanding of the immune system and the nature and magnitude of immune responses required for the clearance of infections and to a certain extent, control of tumors. This collective knowledge in turn has enormously contributed to the development of vaccines in general and rationalized the design of adjuvants with known mechanisms of action in particular.
Adjuvants achieve their activity by acting as pathogen-associated molecular patterns (PAMPs) that work on evolutionary conserved innate immune receptors to mimic natural infections. Therefore, almost all clinically approved adjuvants and most under development primarily target innate immunity, particularly antigen-presenting cells that serve as a bridge between innate and adaptive immunity. The receptors targeted by PAMPs are called pattern recognition receptors (PRRs). As oppose to adjuvants whose characterizations are poorly understood, a growing focus has been shifting towards the use of natural ligands or synthetic agonists for well-defined PRRs as adjuvants. Therefore, we will focus on agonists of PRRs because of their well-characterized immune actions, advanced development, and one of the agonists, MPL, being approved for clinical use (Vacchelli et al. 2013).
6.1 PRR Agonists as Vaccine Adjuvants
The innate immune system provides first line of defense to the host against invading organisms, such as viral, microbial, and fungal pathogens. Cells of innate immune system express PRRs to identify PAMPs associated with a wide variety of infectious agents (Table 12.1). PRRs initiate defense mechanisms via several conserved signaling pathways that lead to the production of inflammatory cytokines and type I interferons (IFNs). These inflammatory responses recruit and activate circulating immune cells and are essential for priming adaptive immune responses. There are two main classes of PRRs that have been identified in mammalian cells: membrane-bound receptors, such as Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), and cytoplasmic receptors, such as NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs).
Among all PRRs, TLRs are the largest and most well-characterized family of a diverse set of germ line-encoded receptors that recognize broad classes of conserved molecular structures common to groups of microorganisms (Akira et al. 2006; Janeway and Medzhitov 2002; Sansonetti 2006). Due to the critical role TLR signaling plays for the regulation of innate, adaptive, and regulatory immune responses, TLR agonists have emerged as ideal adjuvants for cancer immunotherapy. These agonists include TLR-3 (poly I:C), TLR-4 (MPL), TLR-5 (flagellin), TLR-7 (Aldara), TLR-7/8 (Resiquimod), and TLR-9 (CpG). Alone or in combination with various other immunomodulators, the TLR agonists have been demonstrated to enhance vaccine efficacy. In preclinical studies, TLR agonists were shown to generate antitumor immunity by enhancing innate immunity through the activation of DCs, NK cells, monocytes, and macrophages and induction of cytokines with both direct and indirect antitumor activities (Kim et al. 2004; Ishii and Akira 2007; Davis et al. 1998; Akira and Takeda 2004). Engagement of TLRs on APCs, such as DCs, results in their maturation and migration to lymph nodes where they initiate adaptive immune responses and generates long-lasting memory against tumors. In case of clinical studies, MPL has already been licensed in the United States as the adjuvant component of a preventive vaccine against HPV (Vacchelli et al. 2013). MPL was also tested as a component of allogeneic tumor cell lysate or defined TAA-based vaccines against melanoma in clinical trials (Marchand et al. 2003; Vantomme et al. 2004). A non-small-cell lung carcinoma (NSCLC) vaccine using MPL as adjuvant is in late-stage clinical trials (Atanackovic et al. 2004; Vansteenkiste et al. 2013). RC-529 (GSK, Dynavax), another synthetic TLR-4 agonist, has been licensed for an HBV vaccine in Europe (Baldwin et al. 2009). A combination of MPL and basic fibroblast growth factor (bFGF) has been shown to enhance IgG titers and IFNγ levels in the serum and antitumor activity in mice (Zhong et al. 2010). A polymeric form of TLR4 agonist, lipopolysaccharide (LPS), known as SP-LPS in combination with paclitaxel showed promising antitumor effects through induction of apoptosis (Roy et al. 2012). CpG oligodeoxynucleotides (ODN) as agonist of TLR9 have also shown great promise as an adjuvant for TAA-based cancer vaccines (Kim et al. 2004). Immunization of mice with hepatitis B virus surface antigen (HBsAg) along with type B CpG-ODN (1826) enhanced HBsAg-specific IgG2a Abs (Davis et al. 1998).
Despite promising results, safety profile of TLR agonists has been a major hurdle for clinical development and needs to be addressed for the use of these agonists as a component of vaccine formulations. TLR agonists as vaccine adjuvants caused severe toxicity in selected settings due to nonspecific activation of lymphocytes as well as signaling into nonimmune cells (Akira and Takeda 2004; den Haan et al. 2007; Krieg 2007). The limited efficacy of TLR-signaling in the induction of adaptive immune responses, required for the establishment of long-term immunological memory and prevention of tumor recurrences, has also been one of the major challenges of TLR agonists as adjuvant component of therapeutic cancer vaccines (Gavin et al. 2006; Ishii and Akira 2007; Meyer-Bahlburg et al. 2007). Most importantly, TLR signaling in selected settings is involved in the generation of regulatory immunity, which plays a critical role in immune evasion and allows tumors to counterbalance the antitumor immunity. For example, TLR-4 signaling allows the expansion of CD4+CD25+FoxP3+ T regulatory cells (Treg cells) ex vivo and induces IL-10-producing CD4+ Treg cells in vivo (den Haan et al. 2007). Similarly, CpG, a TLR-9 agonist, was shown to convert CD4+ T effector cells into Treg cells via plasmacytoid DCs (Moseman et al. 2004). This agonist also was found to induce CD19+ DCs to acquire potent T-cell suppressive functions through the production of indoleamine 2,3-dioxygenase (Mellor et al. 2005). Due to undesired outcome of TLR agonists as vaccine adjuvants, there is a dire need for the discovery and development of alternative adjuvants that not only have potent immunomodulatory activities on cells of innate, adaptive, and regulatory immunity with a final outcome measured in the generation of Th1 immune responses critical for cancer eradication and control of recurrences but also demonstrate safety at therapeutic doses.
6.2 Costimulatory Ligands as Alternative Adjuvants
An effective therapeutic cancer vaccine should aim to enhance the activity of DC, T cells, and NK cells for the generation of antitumor immune responses effective in eradicating the existing tumor and promoting immunological memory for control of recurrences. Most importantly, therapeutic cancer vaccines should also ideally prevent the generation and/or function of Treg cells and other immune evasion pathways, which serve as major hurdles for the efficacy of cancer vaccines (Schabowsky et al. 2007). In this context, costimulation plays a critical role in modulating innate, adaptive, and regulatory immune responses. Unlike TLRs, costimulation directly targets adaptive immunity and is critical for the generation of primary as well as memory T- and B-cell responses (Croft 2009). As such, agonistic ligands to costimulatory receptors have the potential to serve as effective immunomodulatory components of therapeutic cancer vaccines. Tumor cells have propensity to downregulate costimulatory signals as a means of immune evasion mechanism. Lack of costimulatory signals limits the magnitude of primary T-cell activation against tumors, leading to T-cell anergy (Cuenca et al. 2003). Therefore, ectopic expression of costimulatory molecules in tumor cells via various means has been a successful strategy for the generation of effective antitumor immune responses with preventive and therapeutic efficacy in various preclinical tumor models (Guckel et al. 2005; Singh et al. 2003).
Costimulatory molecules can be divided into two superfamilies: CD28 and TNFR (Croft 2003). The CD28 family includes molecules with costimulatory, CD28 and ICOS, and co-inhibitory functions, CTLA-4 and PD-1, and those that have both inhibitory and stimulatory functions, such as B7-H3 receptor. The TNFR superfamily includes costimulatory CD30, 4-1BB, OX-40, CD40, CD70, and glucocorticoid-induced TNFR-related protein (GITR) (Table 12.2). In contrast to the members of CD28 superfamily, except CD28 receptor, that are involved in the generation of Th2 responses (ICOS), regulatory immunity (ICOS, PD1), or inhibition of immune responses (PD1, CTLA-4, B7-H3R), the majority of TNFR family members are involved in the generation of Th1 and CD8+ T-cell immune responses critical to the elimination of cancer (Croft 2003). As such, the agonists of TNFR family have drawn considerable attention as potential adjuvants for the development of therapeutic cancer vaccines.
Activation of DCs by PAMPs leads to their activation, enhanced antigen uptake and presentation, expression/upregulation of costimulatory ligands and MHC molecules, and cytokine production critical to the initiation of adaptive immune responses. Naïve T cells that have recognized antigens as peptides in the context of MHC molecules respond to DC-generated cytokines and costimulatory cues by proliferating and acquiring effector functions (Janeway and Medzhitov 2002; Jenkins et al. 2001; Banchereau and Steinman 1998). In principal, the initial costimulatory signals are provided by B7 ligands interaction with the constitutively expressed CD28 receptor on naïve T cells. Once activated, T cells upregulate various members of the TNFR superfamily, such as 4-1BB and OX-40, which in turn interact with their upregulated ligands on DCs to further drive T-cell proliferation, survival, differentiation into effectors, and establishment of long-term memory (Harding et al. 1992; Jenkins et al. 1991; Norton et al. 1992; Watts 2005; Croft 2003). Several studies have demonstrated the utility of agonistic Abs against TNFRs in inducing effective Th1 immune responses with therapeutic efficacy in settings of infection and cancer preclinical models (Melero et al. 1997; Weinberg et al. 2000). Among all the TNFR family members, 4-1BB appears to have the desired attributes for the development of therapeutic cancer vaccines as it is a potent inducer of Th1 responses, critical to long-term CD8+ T-cell memory, and overcomes CD4+CD25+FoxP3+ Treg inhibitory responses by various means (Myers et al. 2006; Sharma et al. 2009). These attributes led us to recently propose 4-1BB costimulatory ligands as adjuvants of choice for the development of therapeutic cancer vaccines (Sharma et al. 2009).
7 4-1BB and 4-1BBL Expression and Signaling in Immune Regulation
4-1BB (also known as CD137) is a member of the TNF receptor superfamily that was first discovered to be overexpressed at mRNA levels in activated T cells (Kwon and Weissman 1989). Subsequent studies confirmed the inducible expression of 4-1BB receptor not only on activated T cells but also various cells of innate immunity, such as NK, NKT cells, monocytes, macrophages, mast cells, and eosinophils (Futagawa et al. 2002; Lee et al. 2005a; Melero et al. 2008; Kim et al. 2008a). Constitutive expression of 4-1BB has been shown for Treg cells, neutrophils, a sub-subpopulation of DCs, and also under selected conditions NK and NKT cells (Futagawa et al. 2002; Lee et al. 2005a, 2009a; Melero et al. 1998, 2008). These cells with constitutive 4-1BB expression further upregulate the expression of the receptor following activation. The duration of 4-1BB receptor expression on activated T cells is variable, lasting hours to days depending on the experimental setting. It has recently been reported that around 10 % of CD8+ T memory cells maintain sustained expression of 4-1BB (Lin et al. 2012, 2013). However, such sustained expression was contextual and limited to CD8+ T memory cells in the liver and bone marrow. Importantly, the sustained expression of 4-1BB on memory CD8+ T cells was regulated by GITR in T cell-intrinsic manner (Lin et al. 2013). The expression of 4-1BB is not restricted to hematopoietic lineage only. Hypoxic endothelial cells in tumor beds, fibroblasts, inflamed blood vessels, and lymphatic epithelial cells in response to cytokines or TLR agonists express 4-1BB, suggesting a role for global homeostatic control of this receptor within and beyond the immune regulation (Teijeira et al. 2012).
4-1BB signaling leads to recruitment of TNFR-associated factor (TRAF) adopter proteins, TRAF-1 and TRAF-2, initiating proinflammatory signaling pathways involving phosphatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) that eventually converge on the activation of NF-κB (Arch and Thompson 1998; Sabbagh et al. 2008; Saoulli et al. 1998). This signaling also promotes the upregulation of antiapoptotic molecules, such as Bcl-2 and bcl-XL, and protects antigen-specific T cells from activation-induced cell death (Sabbagh et al. 2008; Kroon et al. 2007). Although signaling into T cells from 4-1BB receptor is predominantly associated with a positive immune response, CD4+ T cells from 4-1BB−/− mice displayed enhanced proliferation when stimulated with mitogens in vitro and showed improved antigen-specific responses following adoptive transfer into wild-type mice (Kwon et al. 2002; Lee et al. 2005b). This observation indicates that 4-1BB signaling in CD4+ T cells may also have a regulatory role, fine-tuning the pursuing immune responses. Recently, it was shown that agonistic 4-1BB Abs induce a unique CD4+ T-cell subpopulation with robust cytotoxicity against melanomas (Curran et al. 2013). This cell population expresses KLRG1 and the T-box transcription factor eomesodermin and requires 4-1BB signaling in both T cell and APCs and IL-27, IL-15, and IL-10 cytokines for development. Besides the potent proliferative, survival, and functional advantages for effector T cells, 4-1BB ligation on NK and NKT cells is important for their expansion, survival, and secretion of IFNγ, which collectively contribute to the critical role of these cells in immune responses against cancer (Melero et al. 1998).
The ligand of 4-1BB, 4-1BBL (also known as CD137L or TNFSF9), is a member of the TNF superfamily and was discovered to be present mostly on APCs, such as macrophages, B cells, and DCs. The reverse signaling of 4-1BBL in APCs induces the production of cytokines such as IL-6 and IL-12 (Ju et al. 2009; Kim et al. 2009a). Reverse signaling has been shown to involve the direct interaction of 4-1BBL via its extracellular domain with TNFR1 on the plasma membrane of human monocytes (Moh et al. 2013). In as much as DCs and monocytes express 4-1BB upon activation, the engagement of 4-1BB with 4-1BBL on the same cell or two different cells may play a positive feed-forward mechanism for the generation, activation, and survival of DCs for improved immune responses. In addition to a plethora of positive effects of 4-1BB signaling on the effector arms of the immune system, this feature of 4-1BB/4-1BBL system related to APC regulation further provides a strong rationale for the use of agonists as a potential immune adjuvant platform for the development of therapeutic cancer vaccines.
8 Targeting 4-1BB Signaling for Immunomodulation
CD8+ T cytotoxic response is important for the elimination of various intracellular infections caused by bacteria and viruses (Lee et al. 2005a, 2009a; Tan et al. 1999; Lin et al. 2009). CD8+ T cells are a critical component of effector immune responses against tumors, and in selected settings, they are required for the elimination of tumors (Lesterhuis et al. 2011; Sharma et al. 2009; Smyth et al. 2000; Uno et al. 2006). Importantly, these cells are often ignorant or tolerant towards cancer cells (Lesterhuis et al. 2011; Zou 2005). Therefore, agents that promote CD8+ T-cell activation, expansion, and survival and impart strong cytolytic and inflammatory properties are ideal candidates as adjuvants for the development of therapeutic vaccines. The agonists of TNFR family are well suited as strong CD8+ T-cell adjuvants due to their demonstrated roles in the activation, expansion, and survival of these cells and establishment of long-term memory (Croft 2003; Watts 2005; Aggarwal 2003). We primarily focused on 4-1BB/4-1BBL pathway because 4-1BB signaling is (a) the most effective of all the other members of costimulatory pair of the TNFR family in activating T cells (Rabu et al. 2005), (b) critical to the generation and maintenance of CD8+ T-cell responses (Lee et al. 2003; Myers et al. 2006) that play an essential role in the eradication of viral infections and tumors (Feltkamp et al. 1993; Lin et al. 1996), and (c) important in overcoming various immune evasion mechanisms by tumors (Madireddi et al. 2012; Sharma et al. 2009; Wilcox et al. 2004).
The 4-1BB receptor is expressed early after CD8+ T-cell activation and is important to various functions of T cells. Signaling via 4-1BB receptor induces robust amplification of T cell-mediated immune responses, inhibits apoptotic cell death (Laderach et al. 2002; Rogers et al. 2001), and establishes long-term T-cell memory (Bansal-Pakala et al. 2001; Watts 2005). Ligation of 4-1BB on CD8+ T cells can reverse the tumor-induced nonresponsiveness of these cells, leading to regression of established tumors primarily through the activities of CD8+ T and NK cell axis (Sharma et al. 2009, 2010b; Wilcox et al. 2004). Furthermore, the 4-1BB costimulation has recently been demonstrated to induce a distinct KLRG1+Emos+CD4+ T cells with robust cytotoxic function against melanomas (Curran et al. 2013; Qui et al. 2011). Most importantly, 4-1BB ligation renders T effector cells resistant to suppression by Treg cells (Sharma et al. 2009) as well as prevents antigen, TGF-β, and tumor-mediated conversion of T effector cells into Treg cells (Madireddi et al. 2012).
8.1 4-1BB Signaling in Immunity Against Infections
The importance of 4-1BB signaling in immune responses against infections came from initial observations that 4-1BBL−/− mice have reduced CD8+ T-cell recall response against viruses (DeBenedette et al. 1999; Bertram et al. 2002). Ensuing studies have shown that 4-1BB signaling also contributes to the priming phase of CD8+ T-cell response against various viruses, including influenza, herpes simplex virus-1 (HSV-1), and lymphocytic choriomeningitis virus (LCV) (Bertram et al. 2002; Kim et al. 2005; Tan et al. 1999). Unlike CD28−/− mice that exhibited a severe defect in the expansion of influenza virus-specific primary CD8+ T cells, 4-1BBL−/− mice showed a normal response (Bertram et al. 2002). The number of virus-specific CD8+ T cells, however, was significantly reduced late in primary response. Importantly, 4-1BBL−/− mice did not generate a significant recall response against influenza, and as such implicating 4-1BB signaling in the survival and virus-specific responsiveness of CD8+ T cells late in primary and recall responses. Treatment of CD28−/− mice with an agonistic Ab to 4-1BB during priming effectively rescued the secondary CD8+ T-cell responses against influenza (Bertram et al. 2004), while in marked contrast, the same treatment regimen in 4-1BBL−/− mice was ineffective in rescuing secondary recall responses against influenza. The secondary response in 4-1BBL−/− mice, however, was restored by treatment with the agonistic 4-1BB Ab during the virus challenge. Importantly, treatment of mice during challenge with influenza virus was effective in increasing the number of CD8+ T cells responding to a dominant epitope, expanded the CD8+ T-cell repertoire to subdominant epitopes, and rescued a defect in the primary CD8+ T-cell response in CD28−/− mice (Halstead et al. 2002). Taken together, these studies demonstrate a critical role for 4-1BB signaling in the generation of primary late and recall responses against influenza.
The 4-1BB signaling was also shown to be important for the generation of primary and secondary CD8+ T-cell responses to herpes simplex virus 1 (HSV-1). Treatment of mice with an agonistic 4-1BB Ab during HSV-1 challenge resulted in increased numbers of virus-specific primary and memory CD8+ T cells that also expressed CD11c as a distinct marker (Kim et al. 2005). Unlike influenza, the 4-1BB signaling appears to play a dual role in CD8+ T-cell responses to mouse cytomegalovirus (MCMV) infection (Humphreys et al. 2010). Although the 4-1BB−/− mice exhibited exaggerated primary CD8+ T-cell responses to MCMV, the recall responses to the virus were significantly reduced as compared with wild-type mice. The 4-1BB signaling was shown to rescue HIV-specific CD8+ T-cell cytotoxic function from functionally impaired CD8+ T cells that was correlated with the TRAF1-dependent downregulation of the proapoptotic molecule Bim (Wang et al. 2007). Most significantly, it was shown that HIV-specific CD4+ T cells expressing 4-1BB produced more IL-2 than cells lacking 4-1BB (Kassu et al. 2009). The expression of 4-1BB was found to be lower on virus-specific CD4+ T cells producing both IL-2 and IFNγ. Treatment with antiretroviral drugs resulted in increased 4-1BB expression on virus-specific, IL-2 producing CD4+ T cells, and the percentage of HIV-specific CD4+ T cells expressing 4-1BB was inversely correlated with viremia. Similarly, DCs transfected to express 4-1BBL were shown to enhance the proliferation, function, and survival of HIV-specific CD8+ T cells (De et al. 2011). Signaling via 4-1BB resulted in the downregulation of the inhibitory function of Treg cells on CD8+ T-cell proliferation. In a macaque model, an agonistic Ab against 4-1BB was shown to enhance the efficacy of a DNA subunit vaccine against simian immunodeficiency virus (SIV) by increasing IFNγ production (Hirao et al. 2011).
The 4-1BB signaling also plays an important role in immune responses against bacterial infections. 4-1BB stimulation of neutrophils in the early phase of Listeria monocytogenes infection causes rapid production of inflammatory cytokines/chemokines, which leads to subsequent infiltration of neutrophils and monocytes crucial for the elimination of infection (Lee et al. 2005a). Moreover, a recent study demonstrated that 4-1BB/4-1BBL interaction regulates the innate and adaptive immune responses of the host against Mycobacterium tuberculosis (Fernandez Do Porto et al. 2012). Collectively, these studies demonstrate that agonists of 4-1BB signaling can serve as potential adjuvants for the development of vaccines against intracellular infections.
8.2 4-1BB Signaling in Immunity Against Cancer
The impact of 4-1BB signaling on immune responses is extensively studied in settings of cancer vaccines or other cancer immunotherapy modalities. Stimulation via this receptor was shown to have multiple effects on tumor-specific effector immune responses that include (a) DC activation, survival, and enhanced antigen uptake and processing (Sharma et al. 2009; Futagawa et al. 2002; Choi et al. 2009); (b) T-cell activation, expansion, survival, acquisition of Th1 effector function, and establishment of long-term memory (Sharma et al. 2009; Melero et al. 1997; Lee et al. 2003; Futagawa et al. 2002); and (c) activation and improved function of various innate immune cells, including NK cells, NKT cells, γδT cells (Lee et al. 2013), macrophages, neutrophils, eosinophils, and mast cells (Futagawa et al. 2002; Lee et al. 2005a; Melero et al. 2008). Most significant in the context of tumor vaccines is the role of 4-1BB signaling in overcoming various immune evasion mechanisms employed by the progressing tumor. Stimulation with agonistic Abs to 4-1BB was shown to result in reversal of tumor-induced CD8+ T-cell anergy (Sharma et al. 2009; Wilcox et al. 2004). CD4+CD25+FoxP3+ Treg cells constitutively express 4-1BB receptor, and stimulation through this receptor was shown to block their inhibitory function (Choi et al. 2004). Collectively, these immune attributes of 4-1BB qualify this signaling pathway as an important target to be exploited for cancer immunotherapy.
A series of studies using agonistic Abs to 4-1BB have demonstrated the robust efficacy of 4-1BB signaling in eradication of established tumors in various preclinical models (Melero et al. 1997; Shuford et al. 1997). Immunizations with agonistic Abs to 4-1BB as monotherapy or in combination with other immunomodulators generated CD8+ T cell- and NK cell-driven potent antitumor immune responses that translated into tumor eradication in various animal tumor models, including colon carcinoma, P815 mastocytoma, Ag104A sarcoma, and lymphomas (Melero et al. 1997; Shuford et al. 1997). The impressive therapeutic efficacy of agonistic 4-1BB Abs in preclinical cancer models led to the development of these reagents for clinical trials. Indeed, a humanized agonistic Ab has been tested in several Phase I and a Phase II clinical trials for cancer (Li and Liu 2013). Although impressive, one potential drawback to the use of Abs is their reported toxicity both in experimental systems (Mittler et al. 1999; Niu et al. 2007; Schabowsky et al. 2009) and in clinical trials (Pardoll and Drake 2012). The effective exploitation of the 4-1BB signaling for cancer immunotherapy will, therefore, depend on the development of novel agonists that generate robust immune responses in the absence of or tolerable toxicity at therapeutic doses.
9 SA-4-1BBL as an Adjuvant for the Development of Therapeutic Cancer Vaccines
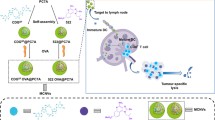
We have recently hypothesized that natural ligand to 4-1BB may serve as a more effective and safe alternative to agonistic Abs for the development of immune therapies (Schabowsky et al. 2009; Sharma et al. 2009, 2010a, b; Srivastava et al. 2012). The 4-1BBL exerts its costimulatory function as trimers expressed on the surface of APCs. The soluble trimers have no costimulatory function (Rabu et al. 2005). Our laboratory has pioneered a novel technology designated as Protex™ that involves the generation of chimeric ligands with a modified form of core streptavidin (SA), modification of the cell membrane with biotin, and engineering of chimeric proteins as an alternative to gene therapy for immunomodulation (Fig. 12.1) (Sharma et al. 2009, 2010c; Singh et al. 2003; Yolcu et al. 2002). In addition, these chimeric proteins have two distinct advantages over native ligands. First, chimeric ligands exist as tetramers and higher-order structures and effectively cross-link their receptors on immune cells in soluble form for effective signal transduction (Sharma et al. 2009, 2010b; Elpek et al. 2007). Second, SA portion of chimeric molecules allows for conjugation to any biotinylated antigen of interest for the development of conjugate vaccines. Inasmuch as a subpopulation of DCs constitutively express 4-1BB receptor, 4-1BBL in the conjugate vaccine may serve as a vehicle to deliver Ags to DCs in vivo for better antigen uptake and cross-presentation to T cells (Fig. 12.2).
ProtexTM technology for cell surface engineering of exogenous proteins of interest for immunomodulation. Cells, tissues, or organs can be modified with biotin followed by engineering with SA-chimeric proteins in a rapid, efficient, and cost-effective manner as an alternative to gene therapy for immunomodulation. SA-P1, SA-chimeric protein 1. Modified from Shirwan et al., Cancer Vaccines Methods and Protocols, series Methods in Molecular Biology, Vol. 1139, Lawman, Michael J.P., Lawman, Patricia D. (Eds.), Springer 2013
SA-4-1BBL as a vehicle for targeted delivery of conjugated TAAs into 4-1BB expressing DCs. TAAs can be biotinylated and mixed with SA-4-1BBL for the generation of a conjugate vaccine. In vivo, 4-1BBL portion of the vaccine facilitates the delivery of TAAs to 4-1BB expressing DCs for activation and enhanced antigen uptake and presentation to T cells. SA-4-1BBL at a subsequent stage interacts with the upregulated 4-1BB receptor on newly activated T cells and drives their expansion, survival, acquisition of effector function, and establishment of long-term memory
We thus generated a novel form of 4-1BBL by fusing the extracellular domains of mouse or human 4-1BBL molecules C-terminus to a modified core streptavidin. This chimeric 4-1BBL (SA-4-1BBL) molecule has various desired features relevant for the development of cancer vaccines. First, SA-4-1BBL exists as stable tetramers and higher-order structures owing to the structural features of SA (Sharma et al. 2010a), which endows this molecule with robust costimulatory activity in soluble form for both CD4+ and CD8+ T cells (Schabowsky et al. 2009). This is plausibly due to the ability of SA-4-1BBL to effectively cross-link 4-1BB receptors on immune cells for potent signal transduction. Second, SA-4-1BBL effectively activates DCs in vivo for antigen uptake and cross-presentation to CD8+ T cells (Sharma et al. 2009, 2010a). Third, SA-4-1BBL endowed T effector cells resistant to suppression by CD4+CD25+FoxP3+ Treg cells and blocked antigen, TGF-β, and tumor-induced conversion of T effector into Treg cells (Madireddi et al. 2012; Sharma et al. 2009). Fourth and most importantly, treatment of mice with SA-4-1BBL alone or in combination with various antigens did not result in measurable acute toxicity, immune abnormalities, or autoimmunity (Schabowsky et al. 2009; Srivastava et al. 2012) as had been reported for agonistic Abs (Niu et al. 2007).
As the adjuvant component of various TAA-based vaccine formulations, SA-4-1BBL demonstrated robust therapeutic activity in various tumor models. A single vaccination with SA-4-1BBL and a peptide representing the dominant CD8+ T-cell epitope for human papillomavirus (HPV) E7 oncogene (E749-57) resulted in the eradication of E7 TAA-expressing TC-1 tumor cells (Sharma et al. 2009). SA-4-1BBL in this setting performed better than three other TLR agonists, LPS, MPL, and CPG (Sharma et al. 2009). SA-4-1BBL as the adjuvant component of complete E7 protein vaccine formulation also proved effective in eradicating the TC-1 tumor (Sharma et al. 2010b). This therapeutic efficacy was not restricted to E7 as a xenoantigens as a vaccine formulation containing SA-4-1BBL and survivin as a bona fide self-TAA also demonstrated robust therapeutic efficacy in survivin overexpressing 3LL lung carcinoma model (Sharma et al. 2009; Srivastava et al. 2012). Importantly, therapeutic efficacy of SA-4-1BBL-based vaccines in both models was enhanced to 100 % by multiple vaccinations (Srivastava et al. 2012; Sharma et al. 2013). The vaccine showed no efficacy against tumors grown in 4-1BB mutant mice, demonstrating the requisite role of signaling through 4-1BB receptor (Sharma et al. 2010b).
The therapeutic efficacy of the vaccines correlated with augmented CD8+ T-cell effector/memory responses, IFNγ production, reversal of CD8+ T cell anergy, and increased intratumoral ratio of CD8+ T/Treg cells (Sharma et al. 2009, 2010a). CD8+ T cells played a requisite role for the vaccine therapeutic efficacy, while NK cells played a non-requisite, secondary role (Fig. 12.3) (Sharma et al. 2010b; Srivastava et al. 2012). Importantly, CD4+ T cells did not appear to play a critical role in vaccine therapeutic efficacy at the effector phase, but were required at the priming phase (Sharma et al. 2013). The depletion of CD4+ T cells 1 day before vaccination had no effect on the therapeutic efficacy of SA-4-1BBL/E7 and SA-4-1BBL/survivin-based vaccines in both TC-1 and 3LL established tumor models. However, the depletion of CD4+ T cells was associated with lack of long-term immune memory in the 3LL, but not TC-1 model, suggesting that priming with a weak self-antigen requires CD4+ T-cell help for the establishment of long-term memory as demonstrated for various infection models (Sharma et al. 2013). Importantly, there was a significant decrease in the efficacy of these vaccines when CD4+ T cell was depleted 1 day before tumor challenge. This finding suggests that challenge with the tumor is sufficient to prime CD4+ T cells, which plausibly set in motion a tumor-specific CD8+ T cell response targeted for augmentation by vaccination with SA-4-1BBL/Ags. These observations are not only important specifically in the context of SA-4-1BBL-based vaccines but also in general for all cancer vaccines as they demonstrate the requisite role of CD4+ T cells for effective immune responses against cancer. The therapeutic efficacy of the vaccines was achieved in the absence of detectable acute toxicity or autoimmunity. Potent pleiotropic immune activities (Fig. 12.3) combined with the lack of toxicity highlight the potential SA-4-1BBL holds as an adjuvant platform for the development of therapeutic cancer vaccines (Schabowsky et al. 2009; Sharma et al. 2009, 2010b).
Pleiotropic effects of SA-4-1BBL as the adjuvant component of subunit protein vaccines on tumor-specific immune responses. SA-4-1BBL works on various cells of innate, adaptive, and regulatory immunity to generate antitumor effector immune response that eradicate primary tumors. SA-4-1BBL also exploits 4-1BB signaling to establish and retain long-term T-cell responses for the eradication of micrometastasis and control of tumor recurrences locally and systemically
10 Qualitative and Quantitative Differences Between SA-4-1BBL and Agonistic 4-1BB Antibody
SA-4-1BBL in its signaling outcome shows quantitative and qualitative differences with an agonistic 4-1BB Ab (3H3) widely used in various preclinical settings (Melero et al. 1997; Uno et al. 2006). These differences are summarized as follows: (a) SA-4-1BBL generates better primary CD4+ and CD8+ T-cell responses compared with the agonistic 4-1BB Ab (Schabowsky et al. 2009); (b) the agonistic 4-1BB Ab preferentially activates the proliferation of CD8+ T cells, while at equimolar levels, SA-4-1BBL activates proliferation of both CD4+ and CD8+ T cells (Mittler et al. 1999; Niu et al. 2007; Schabowsky et al. 2009); (3) SA-4-1BBL lacks toxicity and immune perturbation reported for the agonistic 4-1BB Ab (Schabowsky et al. 2009); (4) the agonistic 4-1BB Ab acts as superagonist and activates naive T cells for proliferation and type I cytokine release, whereas SA-4-1BBL lacks such effects and it appears to expand antigen-activated T cells (Schabowsky et al. 2009); (5) a single vaccination with SA-4-1BBL with E749-57 peptide has better efficacy than the agonistic Ab for the eradication of established E7-expressing TC-1 tumor (Sharma et al. 2009); (6) SA-4-1BBL induces greater infiltration of CD8+ T cells into tumors than the agonistic 4-1BB Ab (Sharma unpublished observation), which may suggest a differential chemokine effect; (7) agonistic Ab induces in vivo expansion of CD4+CD25+ Treg cells and increased infiltration into tumor, while SA-4-1BBL has minimal favorable effect on Treg cells (Sharma unpublished observation); (8) vaccination with the agonistic Ab leads to reduction in NK cell numbers as well as diminished B cell-mediated antibody responses (Lee et al. 2009b; Mittler et al. 1999), while SA-4-1BBL vaccination enhances the NK cell-mediated killing responses (Sharma et al. 2010b; Srivastava et al. 2012); (9) the agonistic Ab appears to directly affect the suppressive function of Treg cells (Choi et al. 2004), while SA-4-1BBL makes CD4+ T effector cells resistant to suppression by Treg cells (Sharma et al. 2009); and (10) SA-4-1BBL blocks in vitro and in vivo conversion of CD4+ T effector cells into Treg cells (Madireddi et al. 2012), while the effect of agonistic 4-1BB Abs is yet to be assessed. The mechanistic basis of these key differences between the agonistic 4-1BB Abs and SA-4-1BBL is yet to be fully elucidated. These divergent efficacy/safety features of these agents will require further studies to delineate their mechanistic basis of efficacy/toxicity, which will further guide their development for safe and effective use in cancer immunotherapy.
11 Prospect of 4-1BB Signaling in Combination with Standard-of-Care and Other Cancer Immunotherapies
Given that cancer has evolved to overcome immune surveillance by various mechanisms and TAAs are weak antigens, therapeutic subunit vaccines may benefit from formulations that incorporate more than one adjuvant or immune potentiator to recruit multiple immune effector arms for efficacy. Agonists of 4-1BB receptor may also improve the efficacy of standard-of-care chemo and/or localized radiotherapy by capitalizing on tumor cell death induced by such treatments and the ensuing TAA cross-priming and immune responses.
11.1 Combinatorial Use of 4-1BB Agonists with Other Positive and Negative Costimulatory Molecules
The presence of numerous costimulatory pathways involved in T-cell responses may reflect the fact that individual pathways program unique facets of T-cell functions. For example, although OX40 and 4-1BB are both expressed on activated CD4+ and CD8+ T cells, OX40 costimulation preferentially impacts the CD4+ T-cell effector function (Dawicki et al. 2004; Gramaglia et al. 1998, 2000; Kopf et al. 1999), while 4-1BB more significantly drives CD8+ T-cell responses (Dawicki et al. 2004; Sharma et al. 2009; Pollok et al. 1993). Moreover, while costimulation through OX-40 directly affects the suppressive function of CD4+CD25+FoxP3+ Treg cells (Voo et al. 2013), 4-1BB costimulation makes T effector cells resistant to suppression by Treg cells (Sharma et al. 2009). The combination of agonists of 4-1BB and OX40 may program CD4+ T cells to differentiate into cytotoxic Th1 effector cells. These cytotoxic CD4+ T cells may not only produce IFNγ but also possess the ability to kill target cells presenting cognate MHC class II-restricted peptides (Qui et al. 2011), which might be useful in targeting tumors that have propensity to downregulate their MHC class I (Ferrone and Marincola 1995). Consistent with this notion, we have shown that combination of SA-4-1BBL and SA-OX40L was effective in inducing potent proliferative responses in both CD4+ OT-II and CD8+ OT-I cells in vivo and improved therapeutic efficacy, as compared with single agents, in the established TC-1 tumor model (Srivastava et al. unpublished data).
The combinatorial use of an agonistic 4-1BB Ab and a blocking CTLA-4 Ab resulted in significant improvement in the therapeutic efficacy of a whole tumor cell-based vaccine, whereas individual agents had no effect (Curran et al. 2011). Therapeutic efficacy was associated with 4-1BB-driven intratumoral increase in both KLRG1+CD4+ and CD8+ T effector cells, increase in inflammatory cytokines, and decrease in Treg cells. A triple therapy with Abs to 4-1BB, CD40, and DR5 (apoptosis-inducing receptor for TNF-related apoptosis-inducing ligand, TRAIL) resulted in robust therapeutic efficacy in primary fibrosarcomas, initiated with the carcinogen 3-methylcholanthrene, multiorgan metastases, and primary tumor resistant to DR5 Ab treatment (Uno et al. 2006). Importantly, therapeutic efficacy of this triple Ab treatment was neither associated with detectable toxicity nor autoimmunity and required CD8+ T cells and IFNγ production.
Significant in the context of this book chapter are the results of two recent studies comparing the efficacy of Abs to various costimulatory, CD137 and CD40, and co-inhibitory, CTLA-4, PD-1, TIM-3, and LAG-3, molecules as single agents or in various combinations in the stringent ID8 mouse ovarian cancer model (Wei et al. 2013; Guo et al. 2013). In a 3-day established tumor model, treatment with Ab to 4-1BB resulted in a better therapeutic efficacy as compared with treatments with Abs to PD-1 or CTLA-4 as monotherapy. However, treatment with single agents in a 10-day established ID8 model had no therapeutic benefit, whereas combinations of Abs to 4-1BB and PD-1 or CTLA-4 or all three Abs significantly prolonged survival as compared with any other double or multiple combinations without 4-1BB Ab. Importantly, the combination of Abs to 4-1BB and PD-1 had the most antitumor effect as compared with any other combinations. Therapeutic efficacy of anti-4-1BB and PD-1 Abs was associated with increased number of splenic CD8+ T effector cells, IFNγ production, and decreased numbers of myeloid-derived suppressor cells (MDSCs) and Treg cells (Wei et al. 2013; Dai et al. 2013). Therefore, the combination of positive costimulatory agonists with co-inhibitory agents, i.e., immune checkpoint blockers, may work in synergy to influence the overall functional outcome of T effector cell responses and manifest better therapeutic efficacy against cancer.
11.2 Combinatorial Use of 4-1BB Agonists with Other Immune Potentiators
Several studies have demonstrated enhanced antitumor responses culminating into therapeutic efficacy when agonistic 4-1BB Ab is used in combination with other immunostimulatory agents. Treatment with an agonistic 4-1BB Ab in combination with IL-12 gene transfer resulted in robust therapeutic efficacy in the poorly immunogenic pulmonary metastatic B16.F10 melanoma model, where the individual agents alone had no measurable efficacy (Huang et al. 2010; Xu et al. 2004). Both NK and CD8+ T cells were critical to the observed therapeutic efficacy with CD4+ T cells having no measurable contribution. Similarly, intratumoral injection with an oncolytic adenovirus vector expressing either 4-1BBL or IL-12 resulted in significant immune responses and therapeutic efficacy in the B16-F10 subcutaneous tumor model (Huang et al. 2010; Xu et al. 2004). However, the combination of both agents significantly improved immune and therapeutic efficacy. An agonistic 4-1BB Ab when used in combination with irradiated tumors engineered to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) as vaccine resulted in increased infiltration of CD8+ T cells into the tumor, their expansion, and long-term memory, which collectively translated into therapeutic efficacy in the established B16 melanoma model (Li et al. 2007). Taken together, these observations justify the combinatorial use of 4-1BB agonists with other immune potentiators for the treatment of cancer.
11.3 Combinatorial Use of 4-1BB Agonists with Standard-of-Care Cancer Treatments
The 4-1BB costimulation was exploited in combination with standard-of-care treatment agents in various preclinical tumor models. Treatment with an agonistic 4-1BB Ab (BMS-469492) along with single-dose irradiation therapy at 5, 10, or 15 Gy resulted in measurable therapeutic efficacy at all radiation doses for a breast and only at higher radiation dose for a lung carcinoma model (Shi and Siemann 2006). Treatment with the 4-1BB and PD-1 Abs worked in synergy with cisplatin to achieve an impressive therapeutic efficacy in the aforementioned 10-day-established ID8 tumor model (Wei et al. 2013). Similarly, agonistic 4-1BB Abs worked in synergy with cisplatin resulting in robust therapeutic efficacy in the CT-26 colon carcinoma model (Kim et al. 2008b). The therapeutic efficacy of agonistic Ab was associated with a rapid recovery of T and B cells from cisplatin-induced lymphopenia and generation/expansion of CD11c+CD8+ T cells expressing IFNγ. Importantly, cisplatin treatment induced the expression of 4-1BB on kidney tubular epithelium and enhanced 4-1BB expression on antigen-primed T cells. Agonistic Ab treatment blocked cisplatin-induced apoptosis of both T cells and kidney epithelium by increasing the expression of antiapoptotic molecules.
In a separate study, combinatorial treatment with a 4-1BB agonistic Ab and 5-fluorouracil (5-FU) resulted in the eradication of radiotherapy- and chemotherapy-resistant renal cell carcinomas in more than 70 % of the mice, where individual agents had no significant effect (Ju et al. 2008). Combination therapy was associated with enhanced proportion of apoptotic cells and higher number of lymphocytes in the spleen and tumor-draining lymph nodes of treated mice. Importantly, mice that had eradicated primary tumors were completely resistant to rechallenge with the original tumor, demonstrating establishment of long-term immune memory. In the B16 melanoma study, combinatorial treatment with 4-1BB Ab and cyclophosphamide resulted in significant antitumor immune responses and tumor eradication, which was associated with increased numbers of IFNγ-producing effector CD11c+CD8+ T cells (Kim et al. 2009b). Agonistic 4-1BB Ab treatment facilitated rapid recovery of T and B cells from drug-induced lymphopenia and protected naïve T cells from drug-induced toxicity. Importantly, treatment with cyclophosphamide increased the expression of 4-1BB on both CD4+ and CD8+ T cells and suppressed peripheral Treg cells. Taken together, these studies provide compelling rationale for exploiting 4-1BB signaling using agonistic Abs or 4-1BBL in combination with standard-of-care agents for cancer treatment.
11.4 Combinatorial Use of 4-1BB and TLR Agonists
Critical to the activation and maintenance of an immune response are the signals transduced by TLR and costimulatory receptor pathways (Croft 2009; Kawai and Akira 2010). As such, agonistic ligands to receptors of these two pathways have significant potential as adjuvants for therapeutic vaccines. Consistent with this notion is the approval of TLR-4 agonist MPL by FDA to be used as the adjuvant component of the preventive vaccine against HPV infection (Romanowski et al. 2009). However, the efficacy of MPL as the adjuvant component of therapeutic vaccines against cancer remains to be fully assessed. MPL primarily targets innate immunity, leading to the recruitment, activation, and maturation of APCs, such as DCs, that facilitate the generation of adaptive immune responses (Didierlaurent et al. 2009). MPL primarily targets APCs for the initiation of adaptive immunity (Didierlaurent et al. 2009) and 4-1BBL targets CD8+ T cells for activation, acquisition of effector function, survival, and long-term memory (Watts 2005; Bukczynski et al. 2004; Cannons et al. 2001). Given the critical role of CD8+ T cells for tumor eradication, we recently hypothesized that an adjuvant system composed of both of these molecules may have potent therapeutic efficacy as the component of TAA-based vaccine formulations against cancer. In preliminary studies, we demonstrated that a single vaccination with a formulation containing both adjuvants and E7 as TAA resulted in the effective eradication of E7-expressing TC-1 tumor in all mice (Srivastava et al. unpublished data). This effect was extendable to the 3LL pulmonary lung carcinoma model where survivin was used as a bona fide self-TAA. Importantly, the therapeutic efficacy of the vaccines required CD8+ T cells and associated with high intratumoral CD8+ T effector/Treg cell ratios (Srivastava et al. unpublished data).
A series of recent studies demonstrated synergy between 4-1BB and TLR4 signaling. Stimulation of macrophages with a TLR agonist was recently shown to upregulate 4-1BBL expression on macrophages (Kang et al. 2007). Importantly, 4-1BBL expression on macrophages was critical to sustained expression of proinflammatory cytokines, particularly TNFα, and achieved this effect by physically interacting with TLR4. The effect of 4-1BBL was 4-1BB receptor independent and required TLR4-induced activation of transcription factors CREB and C/EBP to sustain the late TNFα response. This new and direct interplay between 4-1BB and TLRs is more likely not unique to TLR4 and provides another means of regulation between innate immunity and adaptive immunity. Furthermore, reverse signaling through 4-1BBL in human monocytes converts them into mature DCs with potent antigen-presenting function and production of cytokines such as IL-6 and IL-12 (Ju et al. 2009). Such DCs inhibit the development of Treg cells and induce the expression of perforin, IFNγ, IL-13, and IL-17 in T effector cells (Kwajah and Schwarz 2010). The 4-1BB signaling is also critical for the activation and survival of DCs and trafficking to the T-cell zone in lymph nodes (Choi et al. 2009). Given the demonstrated role and importance of TLRs in activation and effector function of APCs, the synergy between 4-1BB and TLRs provides an important opportunity for the combinatorial use of their agonists as adjuvant systems for the development of therapeutic vaccines against chronic infection and cancer.
12 Conclusions
Cancer immunotherapy field without question is at the brink of exciting developments. The FDA approval of DC-based Provenge therapeutic cancer vaccine against hormone refractory prostate cancer in 2010 followed by anti-CTLA-4 Ab for melanoma (Hodi et al. 2010) marked the beginning of many immunotherapies to follow. The impressive clinical results with anti-PD-1 and PDL-1 Abs are good indication of their approval in a near future as another class of therapeutics (Brahmer et al. 2012; Topalian et al. 2012). These clinical developments combined with our better understanding of the immune system in general and immune response against cancer in particular will further accelerate the development of cancer immunotherapies. In particular, immunomodulation to boost effector immune responses and control immune escape mechanism by progressing tumors combined with the positive impact of some standard-of-care agents on tumor immune responses provides a plethora of possibilities for rationale development of combinatorial therapies. The efficacy of such therapies will require careful consideration of various parameters, which include the nature of tumor, stage/burden of cancer, characteristic of required antitumor immune responses for efficacy, prior standard-of-care treatment history of the patient, and potential treatment to be applied during or post vaccination.
Cancer vaccines stand a good chance of changing the course of cancer treatment. However, their efficacy will require careful consideration of not only TAAs but most importantly also adjuvants for vaccine formulations. Adjuvants having pleiotropic effects on various cells of the immune system with a net outcome of generating tumor destructive immune responses without significant toxicity harbor great potential. In this context, SA-4-1BBL stands a good chance of serving an adjuvant of choice because of several desired features of 4-1BB expression and signaling. In the context of T cells, 4-1BB is expressed in activated T effector cells and plays a critical role for their proliferation, survival, and acquisition of Th1 function, which is critical for the eradication of many tumors. Most importantly, 4-1BB expression marks tumor-specific T cells, and signaling via this molecule has been exploited for effective expansion of these cells ex vivo for cancer adoptive immunotherapy (Lin et al. 2010). The 4-1BB signaling is also involved in DC activation, survival, and enhanced antigen uptake and presentation (Choi et al. 2009; Sharma et al. 2009, 2010a). Most importantly, 4-1BB regulates the suppressive function of Treg cells directly or indirectly for the benefit of generating potent effector immune responses (Sharma et al. 2009; Choi et al. 2004). SA-4-1BBL or agnostic 4-1BB Abs as a single agent has shown impressive therapeutic efficacy in various tumor models (Sharma et al. 2009, 2010b; Srivastava et al. 2012). In combination with other immune potentiators or standard-of-care treatments, agonists of 4-1BB have shown even improved therapeutic efficacies (Ju et al. 2008; Kim et al. 2008b, 2009b; Li et al. 2007; Wei et al. 2013). Importantly, 4-1BB is expressed by nonmalignant cells, such as endothelial or epithelial, within tumor microenvironment in response to tumor-induced hypoxia, and signaling via 4-1BB was shown to generate various cytokines and chemokines that feed on 4-1BB signaling in T effector cells for a more pronounced therapeutic efficacy against tumors (Melero et al. 2008). Signaling via 4-1BB on tubular epithelium cells also protects against standard chemotherapy-induced toxicities (Kim et al. 2008b), further making this pathway an attractive target for cancer combinatorial immunotherapy/chemotherapy/radiotherapy.
SA-4-1BBL may also serve as a platform to develop adjuvant systems using agents with different mechanisms of action and as such expected potential therapeutic synergy. Moreover, the next-generation vaccines may benefit from the targeted delivery of TAA/tumor-specific antigens (TSAs) into DCs given the importance of these cells in the initiation of potent adaptive immune responses against tumors. In this context, our published studies demonstrating that SA-4-1BBL conjugated with TAAs effectively delivers the antigens to DCs constitutively expressing 4-1BB for activation, enhanced antigen uptake, and cross-presentation to T cells are significant (Sharma et al. 2010a). The ability of SA-4-1BBL to endow T effector cells refractory to suppression by CD4+CD25+FoxP3+ Treg cells (Sharma et al. 2009), block the conversion of T effector cells in the Treg cells (Madireddi et al. 2012), and reverse tumor-induced CD8+ T cell anergy (Sharma et al. 2009) are important added advantages of this molecule as an immune adjuvant (Fig. 12.3). Although SA-4-1BBL did not show detectable toxicity and autoimmunity in rodent tumor models at therapeutic doses, it remains to be demonstrated if this safety profile translates to humans. Inasmuch as mouse SA-4-1BBL does not cross-react with human 4-1BB, it remains to be seen if the human version of SA-4-1BBL will have the same immune and therapeutic attributes of the mouse version of the molecule. Most importantly, it is critical to evaluate if the therapeutic doses of the mouse version will be applicable to the clinic, or much higher dose will be required, and if the pharmacokinetics and pharmacodynamics of these two molecules are different. Testing this novel form of SA-4-1BBL in clinical trials will be important for its development, and if proven efficacious in therapeutic clinical settings, this vaccine concept may have broad application to all types of cancers with well-defined TAAs as well as chronic infections since the antigenic component of the vaccine can easily be tailored to formulate into conjugate or non-conjugate vaccines for the targeted indications.
Abbreviations
- Abs:
-
Antibodies
- Ags:
-
Antigens
- APCs:
-
Antigen-presenting cells
- bFGF:
-
Basic fibroblast growth factor
- CD:
-
Cluster of differentiation
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen 4
- CTLs:
-
Cytotoxic T lymphocytes
- DC:
-
Dendritic cells
- DNA:
-
Deoxyribonucleic acid
- FasL:
-
Fas ligand
- FDA:
-
Food and Drug Administration
- GITR:
-
Glucocorticoid-induced tumor necrosis factor receptor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- HBsAg:
-
Hepatitis B virus surface antigen
- HER-2:
-
Human epidermal growth factor receptor 2
- HIV:
-
Human immunodeficiency virus
- HPV:
-
Human papillomavirus
- HSV:
-
Herpes simplex virus
- HVB:
-
Hepatitis B virus
- ICOS:
-
Inducible T-cell costimulator
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFNγ:
-
Interferon γ
- IgG:
-
Immunoglobulin G
- IL-10:
-
Interleukin-10
- LCV:
-
Lymphocytic choriomeningitis virus
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MCMV:
-
Mouse cytomegalovirus
- MDSCs:
-
Myeloid-derived suppressor cells
- MHC:
-
Major histocompatibility complex
- MPL:
-
Monophosphoryl lipid
- NK cells:
-
Natural killer cells
- NKT cells:
-
Natural killer T cells
- NLRs:
-
NOD-like receptors
- NSCLC:
-
Non-small-cell lung carcinoma
- ODN:
-
Oligodeoxynucleotides
- PAMPs:
-
Pathogen-associated molecular patterns
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death protein ligand 1
- PI3K:
-
Phosphatidylinositol-3-kinase
- PRRs:
-
Pattern recognition receptors
- PSMA:
-
Prostate-specific membrane antigen
- Rag2−/− mice:
-
Recombination-activating 2-deficient mice
- RLRs:
-
RIG-I-like receptors
- SA:
-
Streptavidin
- SIV:
-
Simian immunodeficiency viruses
- TAA:
-
Tumor-associated antigen
- TGF-β:
-
Transforming growth factor beta
- Th1:
-
T helper 1
- TLRs:
-
Toll-like receptors
- TNFR:
-
Tumor necrosis factor receptor
- TRAFs:
-
TNFR-associated factors
- Treg cells:
-
T regulatory cells
- 5-FU:
-
5-fluorouracil
References
Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3:745–756
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A (2008) Pathways connecting inflammation and cancer. Curr Opin Genet Dev 18:3–10
Arch RH, Thompson CB (1998) 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol 18:558–565
Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA, Matsuo M, Selvakumar A, Dupont B, Chen YT, Hoffman EW, Ritter G, Old LJ, Gnjatic S (2004) Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol 172:3289–3296
Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, Mompoint F, Vedvick TS, Bertholet S, Coler RN, Reed SG (2009) Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine 27:5956–5963
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Bansal-Pakala P, Jember AG, Croft M (2001) Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med 7:907–912
Bertram EM, Lau P, Watts TH (2002) Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol 168:3777–3785
Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH (2004) A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol 172:981–988
Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196:1627–1638
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465.
Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH (2004) Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc Natl Acad Sci U S A 101:1291–1296
Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, Watts TH (2001) 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol 167:1313–1324
Carter RW, Thompson C, Reid DM, Wong SY, Tough DF (2006) Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol 177:2276–2284
Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS (2004) 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol 75:785–791
Choi BK, Kim YH, Kwon PM, Lee SC, Kang SW, Kim MS, Lee MJ, Kwon BS (2009) 4-1BB functions as a survival factor in dendritic cells. J Immunol 182:4107–4115
Coley WB (1891) II. Contribution to the Knowledge of Sarcoma. Ann Surg 14:199–220
Coley WB (1910) The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med 3:1–48
Croft M (2003) Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol 3:609–620
Croft M (2009) The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol 9:271–285
Cuenca A, Cheng F, Wang H, Brayer J, Horna P, Gu L, Bien H, Borrello IM, Levitsky HI, Sotomayor EM (2003) Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res 63:9007–9015
Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP (2011) Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 6:e19499
Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP (2013) Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med 210:743–755
Dai M, Wei H, Yip YY, Feng Q, He K, Popov V, Hellstrom I, Hellstrom KE (2013) Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation. J Immunother 36:248–257
Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM (1998) CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol 160:870–876
Dawicki W, Bertram EM, Sharpe AH, Watts TH (2004) 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol 173:5944–5951
de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ (2003) Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 9:5091–5100
De KB, Heirman C, Corthals J, Empsen C, van Grunsven LA, Allard SD, Pen J, Lacor P, Thielemans K, Aerts JL (2011) The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J Leukoc Biol 89:989–999
DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, Peschon JJ, Watts TH (1999) Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol 163:4833–4841
den Haan JM, Kraal G, Bevan MJ (2007) Cutting edge: lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol 178:5429–5433
Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van MM, Garcon N (2009) AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 183:6186–6197
Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H (2007) Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol 179:7295–7304
Engel AM, Svane IM, Mouritsen S, Rygaard J, Clausen J, Werdelin O (1996) Methylcholanthrene-induced sarcomas in nude mice have short induction times and relatively low levels of surface MHC class I expression. APMIS 104:629–639
Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M (2011) Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306:1891–1901
Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM (1993) Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 23:2242–2249
Fernandez Do Porto DA, Jurado JO, Pasquinelli V, Alvarez IB, Aspera RH, Musella RM, Garcia VE (2012) CD137 differentially regulates innate and adaptive immunity against Mycobacterium tuberculosis. Immunol Cell Biol 90:449–456
Ferrone S, Marincola FM (1995) Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today 16:487–494
Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K (2002) Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol 14:275–286
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D (2006) Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314:1936–1938
Gramaglia I, Weinberg AD, Lemon M, Croft M (1998) Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol 161:6510–6517
Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M (2000) The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol 165:3043–3050
Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 3:13
Guckel B, Stumm S, Rentzsch C, Marme A, Mannhardt G, Wallwiener D (2005) A CD80-transfected human breast cancer cell variant induces HER-2/neu-specific T cells in HLA-A*02-matched situations in vitro as well as in vivo. Cancer Immunol Immunother 54:129–140
Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, Zhang S (2013) Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med 11:215
Halstead ES, Mueller YM, Altman JD, Katsikis PD (2002) In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol 3:536–541
Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP (1992) CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 356:607–609
Hardwick N, Chain B (2011) Epitope spreading contributes to effective immunotherapy in metastatic melanoma patients. Immunotherapy 3:731–733
He LZ, Crocker A, Lee J, Mendoza-Ramirez J, Wang XT, Vitale LA, O’Neill T, Petromilli C, Zhang HF, Lopez J, Rohrer D, Keler T, Clynes R (2007) Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol 178:6259–6267
Hirao LA, Hokey DA, Morrow MP, Jure-Kunkel MN, Weiner DB (2011) Immune modulation through 4-1BB enhances SIV vaccine protection in non-human primates against SIVmac251 challenge. PLoS One 6:e24250
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723.
Huang JH, Zhang SN, Choi KJ, Choi IK, Kim JH, Lee MG, Kim H, Yun CO (2010) Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther 18:264–274
Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, Benedict CA, Ware CF, Croft M (2010) Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol 40:2762–2768
Ikehara S, Pahwa RN, Fernandes G, Hansen CT, Good RA (1984) Functional T cells in athymic nude mice. Proc Natl Acad Sci U S A 81:886–888
Ishii KJ, Akira S (2007) Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol 27:363–371
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216
Jenkins MK, Taylor PS, Norton SD, Urdahl KB (1991) CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol 147:2461–2466
Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA (2001) In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 19:23–45
Ju SA, Cheon SH, Park SM, Tam NQ, Kim YM, An WG, Kim BS (2008) Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. Int J Cancer 122:2784–2790
Ju S, Ju S, Ge Y, Qiu H, Lu B, Qiu Y, Fu J, Liu G, Wang Q, Hu Y, Shu Y, Zhang X (2009) A novel approach to induce human DCs from monocytes by triggering 4-1BBL reverse signaling. Int Immunol 21:1135–1144
Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, Watts TH, Han J (2007) Cell surface 4-1BBL mediates sequential signaling pathways ‘downstream’ of TLR and is required for sustained TNF production in macrophages. Nat Immunol 8:601–609
Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD (1998) Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 95:7556–7561
Kassu A, D’Souza M, O’Connor BP, Kelly-McKnight E, Akkina R, Fontenot AP, Palmer BE (2009) Decreased 4-1BB expression on HIV-specific CD4+ T cells is associated with sustained viral replication and reduced IL-2 production. Clin Immunol 132:234–245
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Kim TG, Kim CH, Won EH, Bae SM, Ahn WS, Park JB, Sin JI (2004) CpG-ODN-stimulated dendritic cells act as a potent adjuvant for E7 protein delivery to induce antigen-specific antitumour immunity in a HPV 16 E7-associated animal tumour model. Immunology 112:117–125
Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS (2005) 4-1BB costimulation enhances HSV-1-specific CD8+ T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol 238:76–86
Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS, Kang CY (2008a) 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol 180:2062–2068
Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS (2008b) Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res 68:7264–7269
Kim DK, Lee SC, Lee HW (2009a) CD137 ligand-mediated reverse signals increase cell viability and cytokine expression in murine myeloid cells: involvement of mTOR/p70S6 kinase and Akt. Eur J Immunol 39:2617–2628
Kim YH, Choi BK, Oh HS, Kang WJ, Mittler RS, Kwon BS (2009b) Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther 8:469–478
Kool M, Fierens K, Lambrecht BN (2012) Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol 61:927–934
Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF (1999) OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity 11:699–708
Krieg AM (2007) Toll-free vaccines? Nat Biotechnol 25:303–305
Kroon HM, Li Q, Teitz-Tennenbaum S, Whitfield JR, Noone AM, Chang AE (2007) 4-1BB costimulation of effector T cells for adoptive immunotherapy of cancer: involvement of Bcl gene family members. J Immunother 30:406–416
Kwajah MMS, Schwarz H (2010) CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur J Immunol 40:1938–1949
Kwon BS, Weissman SM (1989) cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A 86:1963–1967
Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, Koller BH, Wolisi G, Broxmeyer HE, Vinay DS (2002) Immune responses in 4-1BB (CD137)-deficient mice. J Immunol 168:5483–5490
Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A (2002) 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int Immunol 14:1155–1167
Lee HW, Nam KO, Park SJ, Kwon BS (2003) 4-1BB enhances CD8+ T cell expansion by regulating cell cycle progression through changes in expression of cyclins D and E and cyclin-dependent kinase inhibitor p27kip1. Eur J Immunol 33:2133–2141
Lee SC, Ju SA, Pack HN, Heo SK, Suh JH, Park SM, Choi BK, Kwon BS, Kim BS (2005a) 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect Immun 73:5144–5151
Lee SW, Vella AT, Kwon BS, Croft M (2005b) Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol 174:6803–6808
Lee SC, Ju SA, Sung BH, Heo SK, Cho HR, Lee EA, Kim JD, Lee IH, Park SM, Nguyen QT, Suh JH, Kim BS (2009a) Stimulation of the molecule 4-1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect Immun 77:2168–2176
Lee SW, Salek-Ardakani S, Mittler RS, Croft M (2009b) Hypercostimulation through 4-1BB distorts homeostasis of immune cells. J Immunol 182:6753–6762
Lee SJ, Kim YH, Hwang SH, Kim YI, Han IS, Vinay DS, Kwon BS (2013) 4-1BB signal stimulates the activation, expansion, and effector functions of gammadelta T cells in mice and humans. Eur J Immunol 43:1839–1848
Lesterhuis WJ, Haanen JB, Punt CJ (2011) Cancer immunotherapy–revisited. Nat Rev Drug Discov 10:591–600
Li SY, Liu Y (2013) Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin Pharmacol 5:47–53
Li B, Lin J, Vanroey M, Jure-Kunkel M, Jooss K (2007) Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol 125:76–87
Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC (1996) Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 56:21–26
Lin GH, Sedgmen BJ, Moraes TJ, Snell LM, Topham DJ, Watts TH (2009) Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol 182:934–947
Lin GH, Liu Y, Ambagala T, Kwon BS, Ohashi PS, Watts TH (2010) Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS One 5:e11003
Lin GH, Edele F, Mbanwi AN, Wortzman ME, Snell LM, Vidric M, Roth K, Hauser AE, Watts TH (2012) Contribution of 4-1BBL on radioresistant cells in providing survival signals through 4-1BB expressed on CD8(+) memory T cells in the bone marrow. Eur J Immunol 42:2861–2874
Lin GH, Snell LM, Wortzman ME, Clouthier DL, Watts TH (2013) GITR-dependent regulation of 4-1BB expression: implications for T cell memory and anti-4-1BB-induced pathology. J Immunol 190:4627–4639
Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H (2012) SA-4-1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-gamma. PLoS One 7:e42459
Marchand M, Punt CJ, Aamdal S, Escudier B, Kruit WH, Keilholz U, Hakansson L, van Baren N, Humblet Y, Mulders P, Avril MF, Eggermont AM, Scheibenbogen C, Uiters J, Wanders J, Delire M, Boon T, Stoter G (2003) Immunisation of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer 39:70–77
Marrack P, McKee AS, Munks MW (2009) Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 9:287–293
Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L (1997) Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 3:682–685
Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L (1998) NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol 190:167–172
Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL (2008) Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci 29:383–390
Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH (2005) Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol 175:5601–5605
Meyer-Bahlburg A, Khim S, Rawlings DJ (2007) B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med 204:3095–3101
Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK (1999) Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med 190:1535–1540
Moh MC, Lorenzini PA, Gullo C, Schwarz H (2013) Tumor necrosis factor receptor 1 associates with CD137 ligand and mediates its reverse signaling. FASEB J 27:2957–2966
Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W (2004) Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol 173:4433–4442
Myers L, Lee SW, Rossi RJ, Lefrancois L, Kwon BS, Mittler RS, Croft M, Vella AT (2006) Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. Int Immunol 18:325–333
Nind AP, Nairn RC, Rolland JM, Guli EP, Hughes ES (1973) Lymphocyte anergy in patients with carcinoma. Br J Cancer 28:108–117
Nishikawa H, Sakaguchi S (2014) Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 27C:1–7
Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L, Vella AT, Mittler RS (2007) Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol 178:4194–4213
Norton SD, Zuckerman L, Urdahl KB, Shefner R, Miller J, Jenkins MK (1992) The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol 149:1556–1561
Pardoll D, Drake C (2012) Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med 209:201–209
Pashine A, Valiante NM, Ulmer JB (2005) Targeting the innate immune response with improved vaccine adjuvants. Nat Med 11:S63–S68
Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS (1993) Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol 150:771–781
Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, Mittler RS, Gordon SM, Reiner SL, Vella AT, Adler AJ (2011) CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol 187:3555–3564
Rabu C, Quemener A, Jacques Y, Echasserieau K, Vusio P, Lang F (2005) Production of recombinant human trimeric CD137L (4-1BBL). Cross-linking is essential to its T cell co-stimulation activity. J Biol Chem 280:41472–41481
Rogers PR, Song J, Gramaglia I, Killeen N, Croft M (2001) OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 15:445–455
Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, Somani R, Barbier S, Blatter MM, Chambers C, Ferris D, Gall SA, Guerra FA, Harper DM, Hedrick JA, Henry DC, Korn AP, Kroll R, Moscicki AB, Rosenfeld WD, Sullivan BJ, Thoming CS, Tyring SK, Wheeler CM, Dubin G, Schuind A, Zahaf T, Greenacre M, Sgriobhadair A (2009) Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 374:1975–1985
Roy A, Chandra S, Mamilapally S, Upadhyay P, Bhaskar S (2012) Anticancer and immunostimulatory activity by conjugate of paclitaxel and non-toxic derivative of LPS for combined chemo-immunotherapy. Pharm Res 29:2294–2309
Rygaard J, Povlsen CO (1974) Is immunological surveillance not a cell-mediated immune function? Transplantation 17:135–136
Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH (2008) ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol 180:8093–8101
Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C (2008) Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 118:2098–2110
Sansonetti PJ (2006) The innate signaling of dangers and the dangers of innate signaling. Nat Immunol 7:1237–1242
Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, Watts TH (1998) CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med 187:1849–1862
Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H (2007) Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Investig Drugs 8:1002–1008
Schabowsky RH, Elpek KG, Madireddi S, Sharma RK, Yolcu ES, Bandura-Morgan L, Miller R, MacLeod KJ, Mittler RS, Shirwan H (2009) A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4-1BB Ab without Ab-associated severe toxicity. Vaccine 28:512–522
Schlom J (2012) Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 104:599–613
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410:1107–1111
Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, Shirwan H (2009) Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res 69:4319–4326
Sharma RK, Schabowsky RH, Srivastava AK, Elpek KG, Madireddi S, Zhao H, Zhong Z, Miller RW, MacLeod KJ, Yolcu ES, Shirwan H (2010a) 4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res 70:3945–3954
Sharma RK, Srivastava AK, Yolcu ES, MacLeod KJ, Schabowsky RH, Madireddi S, Shirwan H (2010b) SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model. Vaccine 28:5794–5802
Sharma RK, Yolcu ES, Elpek KG, Shirwan H (2010c) Tumor cells engineered to codisplay on their surface 4-1BBL and LIGHT costimulatory proteins as a novel vaccine approach for cancer immunotherapy. Cancer Gene Ther 17:730–741
Sharma RK, Yolcu ES, Srivastava AK, Shirwan H (2013) CD4+ T cells play a critical role in the generation of primary and memory antitumor immune responses elicited by SA-4-1BBL and TAA-based vaccines in mouse tumor models. PLoS One 8:e73145
Shi W, Siemann DW (2006) Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res 26:3445–3453
Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS (1997) 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 186:47–55
Singh NP, Yolcu ES, Taylor DD, Gercel-Taylor C, Metzinger DS, Dreisbach SK, Shirwan H (2003) A novel approach to cancer immunotherapy: tumor cells decorated with CD80 generate effective antitumor immunity. Cancer Res 63:4067–4073
Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA (2000) Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med 192:755–760
Srivastava AK, Sharma RK, Yolcu ES, Ulker V, MacLeod K, Dinc G, Shirwan H (2012) Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner. PLoS One 7:e48463
Stutman O (1974) Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science 183:534–536
Tacken PJ, de Vries IJ, Torensma R, Figdor CG (2007) Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol 7:790–802
Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP (1999) 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol 163:4859–4868
Teijeira A, Palazon A, Garasa S, Marre D, Auba C, Rogel A, Murillo O, Martinez-Forero I, Lang F, Melero I, Rouzaut A (2012) CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J 26:3380–3392
Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA (2000) NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol 1:515–520
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454.
Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ (2006) Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 12:693–698
Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L (2013) Trial watch: toll-like receptor agonists for cancer therapy. Oncoimmunology 2:e25238
Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, Lopez-Brea M, Vanakesa T, Jassem J, Kalofonos H, Perdeus J, Bonnet R, Basko J, Janilionis R, Passlick B, Treasure T, Gillet M, Lehmann FF, Brichard VG (2013) Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 31:2396–2403
Vantomme V, Dantinne C, Amrani N, Permanne P, Gheysen D, Bruck C, Stoter G, Britten CM, Keilholz U, Lamers CH, Marchand M, Delire M, Gueguen M (2004) Immunologic analysis of a phase I/II study of vaccination with MAGE-3 protein combined with the AS02B adjuvant in patients with MAGE-3-positive tumors. J Immunother 27:124–135
Voo KS, Bover L, Harline ML, Vien LT, Facchinetti V, Arima K, Kwak LW, Liu YJ (2013) Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J Immunol 191:3641–3650
Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH (2007) 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J Immunol 179:8252–8263
Watts TH (2005) TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 23:23–68
Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, Wang H, Dai M, Hellstrom I, Hellstrom KE, Guo Y (2013) Combinatorial PD-1 Blockade and CD137 Activation Has Therapeutic Efficacy in Murine Cancer Models and Synergizes with Cisplatin. PLoS One 8:e84927
Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J (2000) Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol 164:2160–2169
Wilcox RA, Tamada K, Flies DB, Zhu G, Chapoval AI, Blazar BR, Kast WM, Chen L (2004) Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood 103:177–184
Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH (2004) NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer 109:499–506
Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H (2002) Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity 17:795–808
Zhong Z, Wei X, Qi B, Xiao W, Yang L, Wei Y, Chen L (2010) A novel liposomal vaccine improves humoral immunity and prevents tumor pulmonary metastasis in mice. Int J Pharm 399:156–162
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5:263–274
Acknowledgments
Supported in parts by the NIH (R43 CA 109866, R43 AI071618, R41 CA121665), 5T32 HL076138-07 Training Fellowship (HZ), Kentucky Science and Technology Corporation, Kentucky Lung Cancer Research Program, W.M. Keck Foundation, and the Commonwealth of Kentucky Research Challenge Trust Fund.
Conflict of Interest
The Protex™ technology and SA-4-1BBL described herein are patented by the University of Louisville Research Foundation. Haval Shirwan and Esma S. Yolcu are inventor on these patents. All the other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Sharma, R.K., Srivastava, A.K., Zhao, H., Yolcu, E.S., Shirwan, H. (2014). SA-4-1BBL: A Novel Form of the 4-1BB Costimulatory Ligand as an Adjuvant Platform for the Development of Subunit Cancer Vaccines. In: Lukashevich, I., Shirwan, H. (eds) Novel Technologies for Vaccine Development. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1818-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1818-4_12
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1817-7
Online ISBN: 978-3-7091-1818-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)