Abstract
Schistosomiasis is one of the most imposing and widespread helminthic diseases. The immune system strives to be protective vis-à-vis Schistosoma infection, but the inevitable immunopathology may lead to fibrosis and organ dysfunction. Moreover, the skewing of immune response axis to polarized Th2 phenotype can impair resistance to other pathogens and has been associated with neoplasms, further complicating the clinical sequelae of schistosomiasis. Techniques and tools for diagnosing schistosomiasis are either cumbersome or lack sensitivity and specificity. Accordingly, many patients remain undiagnosed and receive no treatment. Currently, the only available drug is praziquantel; the use of which in mass treatment raises concerns about development of drug resistance. Indeed, Schistosoma infection is a fascinating model for gaining insight about the mutual interplay between host and parasite factors, which ultimately determines the overall morbidity. Despite decades of intensive research on schistosomiasis, unresolved issues are still intriguing scientists, one of which is the development of a vaccine. Fortunately, however, significant progress has been achieved towards the elimination of schistosomiasis. Information provided in this review should help opening venues for better diagnosis, treatment, and prevention of schistosomiasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Millions of children suffer anaemia, growth deficiency, abdominal pain, exercise intolerance, poor school performance, cognitive defects, and other sequelae resulting from infection with schistosomes. Millions of adult males and females endure fever, headache, lethargy, and lowered work capacity and quality of life because of severe lesions and damage in the liver, colon, rectum, and/or bladder and lower urinary tract consequent to the infection. At least 200,000 people die annually of haematemesis, liver failure, or cancer of the urinary bladder. Sound information is required for setting the platform for elimination of this serious affliction.
3.2 The Agent
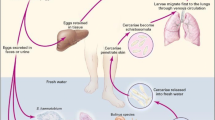
Schistosomes are flatworms (kingdom Animalia, phylum Platyhelminthes, class Trematoda), which are exclusively different from other trematodes in having separate sexes; yet, they form pairs, mimicking the hermaphrodite condition. They are endoparasites (subclass Digenea) of an intermediate host snail where they reproduce asexually and a final vertebrate host where copulation of adult male and female leads to daily production of hundreds of eggs. They belong to the order Strigeidida, characterized by a forktailed cercaria, which infects final hosts using enzymes of penetration glands. The suborder is Strigeata, superfamily Schistosomatoidea, which contains three families: Sanguinocolidae (parasites of fish), Spirorchidae (parasites of turtles), and Schistosomatidae (parasites of crocodilians, birds, and mammals). They demonstrate unsurpassed precision in laying eggs near the conduit for egg passage to the external environment to complete the life cycle. The family Schistosomatidae comprises approximately 100 species, among which Schistosoma mansoni, S. haematobium, and S. japonicum and to a less prevalent extent S. guineensis, S. intercalatum, and S. mekongi cause human schistosomiasis, also named bilharzia, a severe disease of humans in 74 developing countries of the tropics and subtropics (Platt and Brooks 1997).
3.3 Epidemiology of Schistosomiasis
Incidence and prevalence of schistosomiasis reflect the distribution of the freshwater snail the schistosome species use as intermediate host. Thus, the presence of Oncomelania snails (Oncomelania hupensis) in the Philippines (Leonardo et al. 2013) and the marsh and lake regions of southern China (Li et al. 2000; Ross et al. 2001; Zhao et al. 2012a, b) allows the spread of S. japonicum. In the Middle East and sub-Saharan Africa, Oncomelania spp. and schistosomiasis japonicum are not found, while the broad geographical range of susceptible snail species of the genus Biomphalaria and Bulinus coincides with the widespread incidence of schistosomiasis mansoni and schistosomiasis haematobium, respectively (Morgan et al. 2001). In contrast, schistosomiasis is absent from Cape Verde, Comoros, and Seychelles owing to the absence of permissive snail intermediate hosts (Utzinger et al. 2009). In Brazil, the distribution of Biomphalaria spp. (B. glabrata, B. straminea, B. tenagophila) is closely associated with the occurrence of schistosomiasis mansoni (Scholte et al. 2012). Transmission of the parasite to the human population in Zanzibar is related to the distribution of the intermediate snail host, Bulinus globosus (Allan et al. 2013). Abundance of Bulinus truncatus is declining in Egypt and so is urinary schistosomiasis (Barakat 2013). On the other hand, the occurrence of B. truncatus and B. beccari in Saudi Arabia might allow spread of S. haematobium (Mostafa et al. 2009, 2012).
The prevalence of schistosomiasis is also closely related to adequate financial, social, and political conditions that would permit rise in living standards and access to clear water, improved sanitary conditions, and health education to every resident of rural areas (Utzinger et al. 2009, 2011; King 2010). Thus, schistosomiasis transmission was reported to be interrupted in Japan, Iran, Jordan, Morocco, and Tunisia (Utzinger et al. 2009, 2011; World Health Organization 2011). In contrast, more than 90 % of all estimated cases of hepato-intestinal and urinary schistosomiasis reside in sub-Saharan Africa with Nigeria and Tanzania having the highest burden. Of note, the prevalence of infection increased with increase in the population in Tanzania from 19 % in 1977 to 51.5 % in 2012 (Mazigo et al. 2012). On the other hand, the zoonotic behaviour of S. japonicum, which may infect 40 mammalian species, renders eradication of schistosomiasis in China a most difficult task (Ross et al. 2001; Zhou et al. 2012; Hong et al. 2013).
Additionally, epidemiology of schistosomiasis is directly related to completeness and accuracy of information regarding infection prevalence. There is a prominent lack of reliable and updated information on schistosomiasis prevalence in numerous countries (Brooker et al. 2010; Utzinger et al. 2011) as the most complete global resource (Atlas of the Global Distribution of Schistosomiasis) is 26 years old (Brooker et al. 2010; World Health Organization 2010). Thus, several reports indicate that the prevalence rate of schistosomiasis in Egypt has dropped to nearly 1.5 % (EMRO 2007; Curtale et al. 2010), while no national programme for investigation of schistosomiasis prevalence was heard of since the year 2000. Thus, in the Abis area (near Alexandria), which is reportedly a region of “low” S. mansoni prevalence and intensity, 15.2 % of 11-year-old children were found to be infected with S. mansoni with infection intensity of up to 480 egg/g (Allam et al. 2009). Understandably, international travellers (not local residents) were warned that “Egypt is one of the most highly infected countries” (IAMAT 2012).
As importantly, epidemiology is dependent on the level of accuracy of diagnostic methods. Thus, the World Health Organization (WHO) estimate is of 235 million cases of schistosomiasis worldwide, with 732 million people at risk for infection in known transmission areas (WHO 2009). Since standard methods of field testing are admittedly insensitive (Gryseels 1996; Gad et al. 2011), true prevalence and worm loads in endemic communities may be considerably underestimated, and accordingly, the WHO often-quoted statistics can only represent floor estimates for active and potential cases (de Vlas and Gryseels 1992; Enk et al. 2008; Coulibaly et al. 2012). King (2010) argued that probably 40–60 % of patients are likely misdiagnosed and hence suggested that the WHO values should be adjusted to between 391 and 587 million people worldwide. As a consequence, the WHO Preventive Chemotherapy and Transmission Control (PCT) databank no longer provides estimates on population infected or at risk. These have now been replaced by estimates of “population requiring preventive chemotherapy”, which is the population in need of regular treatment with praziquantel, based on WHO recommendations and prevalence thresholds, calculated by “implementation unit”. This indicator is now used in order to avoid the biases inherent in the calculation of number of people infected (by reason of the low sensitivity of the diagnostic tools used) or population at risk (by reason of the geographical focality of the disease) (WHO PCT databank—schistosomiasis. http://www.who.int/neglected_diseases/preventive_chemotherapy/sch/en/index.htm).
Schistosoma mansoni is endemic in a few countries in South America, principally Brazil, where prevalence rate is less than 1 %, and in around 50 countries in the Middle East (prevalence rate in Egypt less than 10 %) and sub-Saharan Africa where prevalence rates exceed 50 % in Nigeria, Ghana, Mozambique, Burkina Faso, Mali, Sierra Leone, Madagascar, and Tanzania (Utzinger et al. 2009, 2011; WHO 2010). In Tanzania, various studies have reported prevalence of up to 100 % in some areas at the Lake Victoria shores (Mazigo et al. 2012). Schistosomiasis haematobium is prevalent only in the Middle East and sub-Saharan African countries (Hürlimann et al. 2011).
Approximately 12.7 million people are infected with schistosomes in the Middle East and North Africa with 7.2 million accounted for in Egypt, 2.9 million in Yemen, 2.3 million in Algeria, and 0.3 million in Libya (Steinmann et al. 2006; Hotez et al. 2012). More than 12,000 schistosomiasis recent prevalence survey locations from 35 Western, Middle, Eastern, and Southern African sub-Saharan countries are now compiled in one database that can be accessed at http://www.gntd.org (Hürlimann et al. 2011). S. haematobium (54.4 %) and S. mansoni (40.8 %) were the most prevalent species. The third schistosome species parasitizing humans in Africa, S. intercalatum (4.8 %), was only reported in surveys carried out in Cameroon and Nigeria, confirming that this species is restricted to some parts of West and Central Africa. The co-occurrence of S. mansoni and S. haematobium was reported in 20 % of the survey locations. Based on the WHO prevalence cut-offs of <10 %, 10–50 %, and >50 % as low-, moderate-, and high-endemicity areas, respectively, S. haematobium is highly prevalent in Western and Southern sub-Saharan African countries, while S. mansoni predominates in East Africa (Hürlimann et al. 2011).
Schistosoma japonicum affects humans and more than 40 mammalian host species, all of which can act as reservoirs of infection, in the Philippines, China, and Indonesia. However, only 7 out of 12 previously endemic provinces in China still report schistosome infections with prevalence rates of 6–7 % (Peng et al. 2010; Zhou et al. 2011a, b, 2013). In the Philippines, there are currently only 560,000 cases of schistosomiasis (Bergquist and Tanner 2010; Gordon et al. 2012). Schistosomiasis transmission is also under control in Indonesia in the two previously endemic areas of Lindu valley and Napu valley, both located in the province of Central Sulawesi, where prevalence rates range between 0 and 13 % (Garjito et al. 2008).
3.4 Immunopathogenesis of Schistosoma Infection
Barsoum et al. (2013) stated that “almost all clinical features of schistosomiasis are caused, directly or indirectly, by the host’s immune response to different stages of the parasite’s life cycle in the body”. Most of our concepts of the immunopathological processes during Schistosoma infection are derived from animal studies especially on S. mansoni and S. japonicum and much less frequently on S. haematobium. Human studies are relatively few and difficult to be controlled and interpreted. Most authorities, however, believe that the immunopathology is rather similar in human and animal hosts.
3.4.1 Acute Schistosomiasis
Cercarial dermatitis is a local IgE-mediated hypersensitivity response directed against penetrating cercariae. It occurs infrequently among endemic populations but is common among visitors and migrants and after primary infections (Gryseels et al. 2006). Upon adherence to the host skin, S. mansoni cercariae exposed to linoleic acid produce prostaglandin E2 (PGE2) and induce mouse and human keratinocytes to produce PGE2 and immunosuppressive interleukin (IL)-10 (Ramaswamy et al. 2000). After invasion of the host skin, cercariae transform into schistosomula and release large amounts of proteins that can activate lymph node cells of irradiated cercariae-vaccinated mice to release copious amounts of interferon (IFN)-γ (Harrop et al. 2000). In vivo, cercariae-derived proteins likely interact with the Langerhans cells and keratinocyte surface membrane toll-like receptor (TLR)2 and TLR4 and/or mannose-binding lectin, leading to the production of nitric oxide, inflammatory cytokines, IL-10, and PGE2 (Ramaswamy et al. 2000; Pivarcsi et al. 2004). Additionally, upon entry in the dermis, skin-stage schistosomula secrete a S. mansoni-derived apoptosis-inducing factor of 23 kDa, which elicits apoptosis in the CD4+ and CD8+ T lymphocytes surrounding the larvae in the skin of naive and irradiated cercariae-vaccinated mice (Chen et al. 2002). Blood cell cultures of humans infected with S. mansoni and/or S. haematobium stimulated with cercarial excretory–secretory products (ESP) also released large amounts of IL-10 (Turner et al. 2013). Fluorescent imaging revealed uptake of cercarial and schistosomular ESP by murine skin neutrophils, dendritic cells, and macrophages, with subsequent production of IL-6, tumour necrosis factor (TNF), IL-12, and IL-10 (Paveley et al. 2009). Indeed, skin schistosomula ESP-mediated immune responses lead to both immune priming and regulation (Mountford and Trottein 2004).
Katayama syndrome is a systemic immune complex-mediated hypersensitivity reaction against migrating schistosomula and early egg deposition. The symptoms of Katayama syndrome manifest 14–84 days after individuals are first exposed to schistosome infection or following heavy reinfection. Acute schistosomiasis due to S. mansoni or S. haematobium infection is common among individuals exposed for the first time such as travellers or migrants but is rare among endemic populations. In contrast, acute disease due to S. japonicum is common in endemic communities and is associated with severe and persistent manifestations that may rapidly progress to hepatosplenomegaly and portal hypertension (Gryseels et al. 2006; Ross et al. 2007; Burke et al. 2009).
3.4.2 Chronic Schistosomiasis
The course of chronic schistosomiasis is variable and is dependent on the anatomical location of adult schistosomes within the vasculature of the mammalian host. In murine models, immune responses to schistosome antigens manifest a striking shift from a moderate T-helper type 1 (Th1) to a robust Th2-dominated response. Fibrosis and much of the pathology are primarily mediated by Th2, while Th1 responses are presumed to be protective (Reiman et al. 2006). However, recent evidence suggests that maintaining a balanced and controlled Th1 or Th2 response is critical in the case of schistosomiasis for protective granuloma formation without excessive pathology (Wilson et al. 2007).
During the first 4–6 weeks of infection in the mouse, a moderate Th1 response is induced against migrating schistosomula and immature adult worms. This response exhibited increased levels of circulating proinflammatory cytokines including TNF-α, IL-1, IL-6, and IFN-γ (Pearce and MacDonald 2002; Wynn et al 2004; Wilson et al 2007). High levels of these cytokines have also been associated with the development of Katayama syndrome in humans (Caldas et al. 2008). The immune response then polarizes to a Th2 response with the start of egg laying, characterized by augmented expression of IL-4, IL-5, IL-10, and IL-13. The Th2 response reaches a peak at approximately 8 weeks postinfection and is then downregulated with progression to chronic infection (Pearce and MacDonald 2002; Wynn et al. 2004; Wilson et al. 2007).
The situation is more complex in humans as the cytokine profile in chronic cases is typically a variable mix of Th1 and Th2 cytokines. Different clinical entities of schistosomiasis are associated with characteristic cytokine patterns (Caldas et al. 2008). For example, one study revealed that whole blood cultures of approximately 340 Egyptian schoolchildren patently infected with S. mansoni produced large amounts of IFN-γ and IL-17 in response to soluble adult worm antigens (SAWA), soluble egg antigens (SEA), and recombinant schistosome antigens, namely, glyceraldehyde 3-phosphate dehydrogenase (rSG3PDH), challenging the dogma that the immune responses to schistosome antigens are dominated by type 2 cytokines, principally IL-4 and IL-5, while Th1 responses are downregulated in human schistosomiasis. Furthermore, the blood cultures of approximately 60 % of these children produced IL-4 or IL-5 to SAWA and SEA (but not to rSG3PDH) only following praziquantel treatment (Barakat et al. [manuscript under preparation]). These findings are in accord with previous reports showing that plasma IL-5, IL-13, and IL-33; serum IgE binding to adult worm antigens; and circulating eosinophil numbers significantly increased only upon treatment in children and adult patients with patent schistosomiasis mansoni (Joseph et al. 2004b; deMorais et al. 2008) or haematobium (Wilson et al. 2013a, b). In other studies of humans with chronic schistosomiasis mansoni, whole blood cultures of adults (Joseph et al. 2004a) and 4–17-year-old children (Wilson et al. 2008) produced significantly (P < 0.05–<0.001) higher levels of IL-4, IL-5, and IL-13 in response to SAWA than to SEA.
In sum, research has indicated that patently infected mice and praziquantel-treated humans produce large amounts of type 2 cytokines and the immunosuppressive IL-10 in response to SEA (Joseph et al. 2004a, b; Wilson et al. 2008), likely leading to the downregulation of granuloma formation and liver fibrosis. On the other hand, persistent production of low levels of IL-10 and IFN-γ and high levels of inflammatory cytokines appears to be associated with severe periportal fibrosis, hepatosplenomegaly, and portal hypertension (Hoffmann et al. 2000; Booth et al. 2004; Wilson et al. 2008; Barsoum 2013).
3.4.2.1 Granuloma Formation
The eggs of Schistosoma deposited in the tissues induce granuloma formation. Upon full maturation, the living embryo, the miracidium, secretes SEA which exit via the microscopic pores of the egg shell (Ashton et al. 2001). For S. mansoni, the egg-derived antigens include Sm-40, cytoskeletal proteins like tubulin, egg-secreted protein 15, proteins of the micro-exon gene 3 (MEG-3) family, as well as the IL-4-inducing factors of S. mansoni eggs, IL-4-inducing principal of S. mansoni eggs (IPSE), and the ribonuclease domain-containing Omega 1 (Fitzsimmons et al. 2005; Jang-Lee et al. 2007; Mathieson and Wilson 2010). Extensive studies of experimental schistosomiasis, mostly on murine S. mansoni, have revealed that granuloma formation is attributable to a vigorous CD4 + Th2-driven response, akin to a form of delayed-type hypersensitivity, that is tightly regulated by various cell populations, cytokines, and chemokines (Wynn et al. 2004).
Although Schistosoma periovular granulomas seem detrimental to the host, it is evident that they serve an indispensible host-protective function, especially during S. mansoni infection. Schistosome eggs and their secreted materials are a continuous antigenic stimulus for the immune system. If these antigens are not sequestered or neutralized effectively, they can harm the surrounding tissues, with hepatocytes being particularly sensitive to toxins secreted by the eggs. Hence, granuloma formation seems to be a compromise, which allows the host to live with the infection for a long time. Hypothetically, the negative aspects associated with granulomas (fibrosis, portal hypertension) represent a better alternative, for host and parasite, than that of the host dying soon after parasite egg production (Pearce and MacDonald 2002; Wilson et al. 2007; Burke et al. 2009).
Histologically, five types of granuloma could be identified during the evolution of Schistosoma-induced granulomatous reaction irrespective of the anatomical site (Fig. 3.1), as indicated by early studies in mice, in rhesus monkeys (Hsu et al. 1972), and, later, in pigs (Hurst et al. 2000): the weakly reactive, exudative, exudative–productive, productive, and involutional stages (Hurst et al. 2000). Initially, there is accumulation of mononuclear cells, neutrophils, and eosinophils around the eggs (weakly reactive), which increases to form a microabscess in the exudative stage. Fibrinoid material is deposited around the eggs (Hoeppli–Splendore reaction) (Lucas 2002). In the exudative–productive stage, histiocytes, epithelioid cells, and foreign body giant cells begin to replace the leucocytic zone. Fibroblasts form a rim around the granuloma. In the productive granuloma, the eggshells are disintegrated and the cellular elements are replaced by fibroblasts with deposition of collagen. Macrophages, lymphocytes, plasma cells, and few eosinophils are still found at the periphery. Finally, the involutional stage is characterized by marked shrinkage of granuloma which is replaced by hyalinized collagen fibres. Eggs may, by then, become calcified (Hsu et al. 1972; Hurst et al. 2000).
(a) Early “exudative” periovular schistosomal granuloma in colonic mucosa. Note the prominent eosinophilic infiltrate (×400). (b) Sections of intravascular adult Schistosoma worms (arrows) in a resected colonic polyp from an Egyptian patient (×200). (c) Squameous cell carcinoma of urinary bladder on top of schistosomiasis in an Egyptian patient. Note Schistosoma eggs (arrows) (×200). (d) Schistosoma eggs in a resected ovarian adenofibroma from an Egyptian female, a common incidental finding in Egyptian patients (×400)
An effective T-cell response is known to be crucial for the development of the granulomatous response and host survival. Nude mice infected with a Chinese strain of S. japonicum supported normal parasite survival and fecundity, although transitory growth retardation was observed during the early stage of infection (Cheng et al. 2008). Moreover, these T-cell-deprived mice developed severe necrosis around the eggs in the liver, a situation similar to the T-cell-deprived mice infected with S. mansoni. Interestingly, B-cell function is required for the development of S. japonicum egg-induced granuloma in early infection (Ji et al. 2008). OBF-1 knockout mice and μMT mice, both with impaired B-cell development, developed significantly smaller hepatic granulomas at 5 weeks postinfection compared to their wild-type counterparts. In contrast, they displayed no significant difference in granuloma pathology at 8 weeks postinfection. This is in agreement with some studies on S. mansoni, also using B-cell-deficient mouse models, which have suggested that B cells are required for Th2 T-cell responses but not for granuloma formation late in infection (Ji et al. 2008).
Elegant studies with IL-13- and IL-4-deficient and IL-13/IL-4 doubly deficient mice have demonstrated that IL-4 launches the development of granulomatous inflammation, whereas IL-13 is the central profibrotic cytokine in the development of schistosome-induced liver fibrosis (Fallon et al 2000). Likewise, there are correlations between severity of hepatic fibrosis and levels of IL-13 expressed by peripheral blood mononuclear cells from individuals with chronic schistosomiasis mansoni (Oliveira et al. 2006). Typically, IL-4 determines the granuloma size, induces the proliferation of Th2 cytokine-producing lymphocytes, and is important for the production of IL-5 and IL-13 by granuloma-associated cells (Cheever et al. 1994). Furthermore, IL-4 is not required for the development of fibrosis but enhances the fibrogenic effects of IL-13. As well as enhancing fibrosis, IL-13 has an additive effect with IL-4 in the development of the Th2-dominant, eosinophil-rich, granulomatous reaction (Fallon et al. 2000). Additionally, IL-5 is required for the recruitment of eosinophils to the granulomatous response as granulomas in mice deficient in IL-5 are virtually devoid of these cells (Cheever et al. 1991). Eosinophils are an important source of Th2 cytokines such as IL-13, and thus, IL-5 indirectly contributes to the polarization of the immune response through the recruitment of these cells (Rumbley et al. 1999; Burke et al. 2009). In contrast, the egg-induced Th2 immune responses and hepatic fibrosis are counter-regulated by IL-10, IL-12, and IFN-γ (Hoffmann et al. 2002).
3.4.2.2 Immunomodulation of the Granulomatous Response
In chronic human infections, two paradoxical situations exist: on the one hand, the older indigenous individuals would harbour fewer worms probably due to the development of concomitant immunity and the fecundity of female worms diminishes. Also, the newly formed granulomas become smaller in size. On the other hand, the granulomas will heal by fibrosis that could, over time, be dense enough to cause morbidity and irreversible sequelae (Butterworth and Thomas 1999).
Strict regulation of the Th1, Th2, and possibly Th17 cytokine responses generated during schistosome infection is essential to prevent excessive pathology. In experimental S. mansoni infection, CD4+ CD25+ Foxp3+ regulatory T-cells (Tregs) appear to regulate schistosome egg-induced immunopathology (Singh et al. 2005; Taylor et al. 2006). Thus, at 8 weeks after infection by S. mansoni, Foxp3 gene expression of splenocytes was similar to that of naive mice, but increased fourfold by 16 weeks. In contrast, granulomatous livers at 8 and 16 weeks showed 10- and 30-fold increase, respectively, in gene expression compared with the normal liver. The percentage of granuloma CD4+ CD25+ T cells rose from 12 % at 8 weeks to 88 % at 16 weeks of the infection. Moreover, retroviral transfer of the Foxp3 gene at the onset of granuloma formation enhanced fourfold Foxp3 expression in the granuloma CD4+ CD25+ T cells and strongly suppressed full granuloma development (Singh et al. 2005).
Several other mechanisms are thought to be involved in the down-modulatory process during Schistosoma infection. Well-designed studies in murine schistosomiasis have revealed that IL-13Rα2 is essential for the downregulation of the granulomatous response and is pivotal in the control of IL-13-mediated fibrosis. IL-13Rα2 acts as a potent decoy receptor, competing with IL-13Rα1 for binding of IL-13 and preventing signalling through the IL-4/IL-13Rα1 receptor complex (Wilson et al. 2007; Burke et al. 2009). A role for apoptosis, particularly apoptosis by neglect of CD4+ T cells, has been suggested to contribute to the down-modulation of the granulomatous response (Rutitzky et al. 2003). Furthermore, B-cell-mediated FcR-dependent signalling has also been implicated in the downregulation of the Th2 response as mice deficient in B lymphocytes or the Fc receptor exhibited marked exacerbation of granulomatous inflammation (Jankovic et al. 1998). Interestingly, some sort of immunomodulation, regarding the future immune and fibrogenic responses, occurs in offsprings born to experimentally Schistosoma-infected hosts in case of postnatal exposure to the parasite (Othman et al. 2010). Perinatal immunological sensitization may occur by transplacental and/or transmammary passage of schistosome antigens, anti-idiotypic antibodies, or immune complexes to the offsprings.
3.4.2.3 Other Immune-Mediated Phenomena in Man
Immune complex glomerulonephritis may occur in all types of Schistosoma infection but most commonly with S. mansoni, and the basic lesion is mesangioproliferative or membranoproliferative glomerulonephritis. Immunofluorescence and electron microscopy reveal the presence of immune complexes containing IgM, IgG, IgA, IgE, complement components, and schistosomal antigens in the mesangium and along the endothelial side of the capillary wall (van Velthuysen and Florquin 2000). Genetic and environmental factors, e.g. chronic salmonellosis, are implicated in the pathogenesis of renal disease. The disease is manifested by proteinuria, hypertension, or nephrotic syndrome. It occurs years after the development of hepatosplenic disease, and it may be attributable, at least in part, to loss of immune complex clearing function of hepatic macrophages due to portosystemic shunting. Amyloid deposition in the kidney occurs infrequently in all types of schistosomiasis and may lead to nephrotic syndrome (van Velthuysen and Florquin 2000; Barsoum 2013). Moreover, Schistosoma-induced arthropathy has probably an underlying allergic immune mechanism (Saidenberg-Kermanac’h et al. 2005).
3.5 Pathogenesis and Clinical Features of Schistosomiasis
3.5.1 Stage of Invasion (Cercarial Dermatitis)
Penetration of human skin by cercariae of human schistosomes causes allergic reaction and dermatitis. There is erythema, accompanied by maculopapular and sometimes vesicular eruption. Scratching and secondary bacterial infection may lead to pustule formation. Exposure to cercariae of avian or bovine schistosomes, even for the first time, may lead to severe dermatitis (swimmer’s itch). Treatment consists mainly of avoidance. Local and systemic antihistamines may be needed, as well as antibiotics (Butterworth and Thomas 1999).
Passage of schistosomula through the lungs and the liver may cause fever, cough, pneumonitis, and abdominal symptoms.
3.5.2 Stage of Maturation (Katayama Fever, Acute Schistosomiasis)
This stage coincides with maturation of adult worms and start of deposition of eggs. The patient may experience fever, rigors, headache, dry cough, muscle and joint pain, hepatosplenomegaly, abdominal pain, lymphadenopathy, skin rash, eosinophilia, and patchy pulmonary infiltrates on chest radiograph. Severe central nervous system involvement may occur. The diagnosis relies on serological tests and finding eggs in the excreta (Butterworth and Thomas 1999; Gryseels et al. 2006; Ross et al. 2007). Treatment consists of schistosomicidal drugs with or without steroids. Artemether treatment given early after exposure may decrease the risk of Katayama fever (Ross et al. 2007; Jauréguiberry et al. 2010).
3.5.3 Stage of Established Infection
The eggs of Schistosoma deposited in the tissues result in granuloma formation. In S. haematobium infection, deposition of eggs in the wall of the urinary bladder results in granulomatous inflammatory reaction and appearance of pseudotubercles. The patient suffers from cystitis and haematuria which is typically terminal but may be total. Haematuria is considered a normal developmental event in adolescent males in some regions of Africa. Symptoms of cystitis include suprapubic discomfort or pain, dysuria, and frequency (Bourée 2005). In S. mansoni and S. japonicum, deposition of eggs in the wall of the large intestine especially in the mucosa and submucosa results in granuloma formation and ulceration. The patient may suffer from chronic or intermittent abdominal pain and discomfort, loss of appetite, diarrhoea, and passage of blood and mucus in stool (schistosomal dysentery). This stage, unfortunately, may be asymptomatic or oligo-symptomatic, thereby the disease may progress unchecked until complications supervene (Butterworth and Thomas 1999; Gryseels et al. 2006).
3.5.4 Stage of Late Infection and Sequelae
The complications are mainly due to healing of schistosomal lesions by fibrosis. The damage produced at this stage is irreversible due to organ damage by fibrosis and vascular changes.
3.5.4.1 Genitourinary Schistosomiasis
3.5.4.1.1 Obstructive Uropathy
Healing of granulomatous lesions in the wall of the urinary bladder and lower ends of the ureters results in fibrosis and urinary obstruction. Various characteristic pathological lesions may appear such as ulceration, sandy patches, cystitis cystica, and calcification. The bladder may become rigid and contracted with reduced capacity. Moreover, urinary stasis predisposes to urinary tract infection and calculus formation which further aggravate the urinary obstruction. The end result is hydroureter and hydronephrosis. The progressive obstructive uropathy could ultimately lead to end-stage renal failure (Butterworth and Thomas 1999; Bourée 2005; Barsoum 2013).
3.5.4.1.2 Bladder Cancer
The mucosa of the urinary bladder undergoes squamous metaplasia due to chronic inflammation. This predisposes to squamous cell carcinoma of the bladder. The typical histopathological lesion reported in many studies over the years is a squamous cell carcinoma in roughly 60 % of cases. Other histological types include transitional cell carcinoma (20 %), adenocarcinoma (10 %), and mixed (10 %). Schistosomal ova were detected in more than 85 % of bladder cancers in an Egyptian series of 1026 cases subjected to surgical cystectomy (Ghoneim et al 1997). The tumour, particularly when of the squamous cell type, remains localized for a long time before spreading to the surrounding pelvic tissues or a distant site, owing to the occlusion of lymphatics by the fibrotic process (Barsoum 2013).
Associated bacterial and viral infections, rather than parasitic products, are suggested to be the main pathogenic factors. Associated infection with human papillomavirus has received considerable recent attention in this respect (el-Mawla et al. 2001), being encountered in about one-fourth of cases. Moreover, specific p53 gene mutations have been observed in one-third of cases (Warren et al. 1995), being attributed to the effect of neutrophil-generated reactive oxygen molecules, the cleavage of conjugated urinary carcinogens, or the production of nitrosamines by bacterial enzymes (Mostafa et al. 1999).
3.5.4.1.3 Other Lesions
Eggs may be deposited in the seminal vesicles, prostate, and spermatic cord in males leading to funiculitis, epididymitis, prostatitis, haemospermia, and rarely infertility. In females the eggs may reach the vagina, uterine cervix, and fallopian tubes, resulting in inflammation and fibrosis that may predispose to infertility or ectopic pregnancy. Schistosomal lesions of the genital organs may increase chances of transmission of sexually transmitted pathogens including HIV. Moreover, lesions in the uterine cervix may be mistaken for carcinoma (Bourée 2005; Barsoum 2013).
3.5.4.2 Intestinal Schistosomiasis
Schistosomiasis mansoni causes patchy fibrosis of the wall of the intestine. Strictures, sinuses, and fistulae may occur. It may lead also to colonic polyposis resulting in bleeding, anaemia, protein loss, and hypoproteinaemia. Polyps range in size from 2 to 20 mm and may be sessile, pedunculated, or showing a cauliflower appearance. They are mainly concentrated in the distal colon, and they range from few to very numerous polyps. The overlying mucosa is usually redder than the surrounding mucosa due to severe congestion and due to focal haemorrhages. Ulceration is common in rectal polyps; the ulcerated areas appear dusky to blackish grey in colour caused by superficial haemorrhage and are frequently secondarily infected (El-Garem 1998; Elbaz and Esmat 2013).
S. japonicum affects similarly the large intestine as well as the stomach. S. mekongi gives manifestations that are similar to those of S. japonicum but usually milder. S. intercalatum infection is usually asymptomatic or causes mild intestinal symptoms. The latter may also infrequently give rise to urinary manifestations (Davis 2009).
There are contradictory reports on the contribution of S. japonicum to the aetiopathogenesis of colorectal or primary liver cancer (Davis 2009). The situation is more ambiguous for S. mansoni. The anecdotal reports of the association of schistosomiasis mansoni and colorectal carcinoma do not differentiate between direct causal relationship and mere accompaniment (Salim et al. 2010). To date, the carcinogenicity of both species is not proved, and the issue awaits further well-designed research for clarification.
3.5.4.3 Hepatic Schistosomiasis
In case of S. mansoni and S. japonicum, more than 50 % of eggs are not passed in the faeces and are retained in the tissues. Eggs swept to the liver via the portal circulation induce granuloma formation and periportal hepatic fibrosis, ending, in some cases, in presinusoidal portal hypertension (Fig. 3.2). Portal hypertension leads to the opening of portosystemic anastomotic venous channels resulting in oesophageal (and gastric) varices, secondary haemorrhoids, and ascites. Bleeding from oesophageal varices may lead to fatal haematemesis and/or melena. Splenomegaly occurs due to passive congestion secondary to portal hypertension and reticuloendothelial and lymphoid hyperplasia. The spleen is markedly enlarged, firm, smooth, and non-tender. A huge spleen may cause discomfort and dyspepsia or pain in the case of perisplenitis or infarction. Hypersplenism may also develop (Butterworth and Thomas 1999; Davis 2009; Elbaz and Esmat 2013).
Radiographic studies indicate amputation of the large portal veins, development of collateral veins, arterioportal venous shunts, and diminished hepatic arterial diameters (Da Silva and Carrilho 1992). When portal fibrosis is established, with its associated distortion of vascular architecture, the incoming eggs can pass through collateral veins around the large portal veins and via the granuloma/fibrosis sequence; this results in progressively expanding tracts of collagen that characterize the clay-pipestem pattern of fibrosis, originally described by Symmers (Lucas 2002). S. japonicum, owing to smaller size of its eggs, produces periportal fibrosis that affects the peripheral and central zones of the liver, whereas S. mansoni affects only the central zones (Burke et al. 2009).
Hepatic function is well preserved in pure schistosomal fibrosis until very late. Decompensated hepatosplenic disease with stigmata of liver cell failure, especially ascites, may supervene. This may occur due to malnutrition, severe collagen deposition in the space of Disse, ischaemic damage from repeated variceal bleeding, and severe distortion of arteriovenous relationships in the portal tracts. Alcohol abuse and viral hepatitis types B, C, D, or E are additional complicating factors (Watt et al. 1991; Davis 2009).
Fibrosis is the ultimate sequel and a major culprit of chronic hepatic schistosomiasis. Apparently, hepatic stellate cells (HSCs) play a major role in the process of fibrous tissue formation in the liver. They are responsible for the synthesis of components of extracellular matrix and several types of collagen as well as fibrogenic cytokines, matrix metalloproteinases, and tissue inhibitors of metalloproteinases that contribute to the remodelling of fibrous tissue (Parola and Robino 2001). HSCs reside in the Disse’s spaces of the liver sinusoids (Fig. 3.3), and they constitute a minor cell type, roughly 5–8 % of the total liver cells (Maubach et al. 2006). Following chronic injury, HSCs differentiate into myofibroblast-like cells, acquiring contractile and fibrogenic properties (Zhang et al. 2006). Remarkably, HSCs affect adversely the hepatic microcirculation. When activated, they transform into myofibroblasts that contract around the hepatic sinusoids, increasing the vascular resistance and contributing to portal hypertension (Friedman 2000). The different stimuli that initiate and perpetuate HSC activation in chronic liver disease are poorly understood, but the role of oxidative stress seems crucial. This explains the multitude of trials of administration of exogenous antioxidants (e.g. coenzyme Q10, melatonin, vitamin E, silymarin, and molecular hydrogen) in an attempt to reverse or limit Schistosoma-induced liver fibrosis (Gharib et al. 2001; El-Sokkary et al. 2002; Othman et al. 2008).
Several immunological and non-immunological determinants govern the progression of schistosomal fibrosis. The role of cytokines, such as IL-4 and IL-13, has been discussed before. Regulatory cytokines such as IL-10, IL-12, and transforming growth factor β also influence the fibrogenic response of the host (Othman et al. 2010). Alternatively activated macrophages (aaMΦ) are hypothesized to contribute to schistosome-induced fibrosis in murine schistosomiasis (Wynn et al. 2004; Wilson et al. 2007; Stavitsky 2004). Alternative activation of macrophages is induced by Th2 responses and promotes collagen synthesis and fibrogenesis via the metabolism of L-arginine to proline and polyamine by arginase-1. Finally, the genetic constitution of the host was found to determine the propensity to fibrosis. In Egypt, patients with human leucocyte antigen (HLA)-A1 and HLA-B5 have been associated with severe hepatosplenic schistosomiasis mansoni, while in the case of S. japonicum infection, HLA-DR and HLA-DQ have been implicated in the differential regulation of immune responses to egg antigens (Butterworth and Thomas 1999).
Research has indicated that over time schistosomal fibrosis becomes established owing to cross-linking of collagen fibres, rather than the difference in collagen isotypes deposited early or late in infection (Ricard-Blum et al. 1992). When the fibrosis is recent in early infection, a process of collagen breakdown occurs with therapy. This breakdown is almost complete, as well as rapid and abrupt. On the other hand, long-standing fibrosis in chronic infection could also undergo regression with therapy, but the process is slow and gradual. Regression of fibrosis entails collagen degradation and vascular remodelling which includes neovascularization (Andrade 2008). The process of regression of schistosomal fibrosis has been demonstrated both in humans and experimental animals. In humans, it can be translated by regression of splenomegaly and oesophageal varices.
Ultrasonography has greatly facilitated the assessment of schistosomal hepatic fibrosis, splenomegaly, portal vein dimensions and the presence of collateral vessels (Lambertucci et al. 2008). It helps to assess the degree of periportal fibrosis by measuring the portal tract thickness: Grade I if thickness is 3–5 mm, Grade II if it is 5–7 mm, and Grade III if it is more than 7 mm. This method reflects the haemodynamic changes and provides a good estimate of the clinical status of patients who have periportal fibrosis (Abdel-Wahab et al. 1992). Notably, in an attempt to harmonize the practices in clinical trials and evaluation of therapeutic measures, WHO elaborated a standard ultrasound scoring protocol (Niamey–Belo Horizonte) which includes a qualitative assessment of liver parenchyma (according to reference patterns A to F) (King et al. 2003). Portal hypertension is suspected when dilatation of one or more of the portal, mesenteric, and splenic veins is detected. For the collateral vessels, the most commonly described are the left and right gastric, the short gastric, the paraumbilical, the splenointercostal, and the splenorenal veins (Lambertucci et al. 2008; Pinto-Silva et al. 2010). Lastly, the hepatic veins in schistosomiasis can be assessed ultrasonographically. They remain patent with normal phasic flow as the disease evolves, which is different from liver cirrhosis (Elbaz and Esmat 2013).
3.5.4.3.1 Interactions with Viral Hepatitis
Co-infection with either hepatitis B virus (HBV) or hepatitis C virus (HCV) is very common since the regions with a high prevalence of schistosomiasis usually have a high endemicity of chronic viral hepatitis as well (Elbaz and Esmat 2013). An important cause of the extraordinary high prevalence of HCV in Egypt was the establishment of a large reservoir of infection as a result of the mass parenteral chemotherapy campaigns against schistosomiasis that ended in the 1980s (Frank et al. 2000). The association between both schistosomiasis and HCV is known to cause earlier deterioration of hepatic functions and more severe morbidity. The liver is the principal site for both HCV replication and egg deposition. The latter downregulates the local immune responses in the liver (Lundy et al. 2001) and results in the suppression of the intrahepatic bystander immune response to HCV. Moreover, this co-infection can produce a unique clinical, virological, and histological pattern manifested by viral persistence with high HCV RNA titres, higher necro-inflammatory and fibrosis scores in liver biopsy specimens as well as poor response to interferon therapy, and rapid progression of liver fibrosis (Kamal et al. 2006).
3.5.4.4 Anaemia
Schistosomiasis-associated anaemia is well documented and the cause is multifactorial. Elevated levels of proinflammatory cytokines, such as TNF-α and IL-6, resulting in anaemia of inflammation, are thought to play a central role. Moreover, for intestinal schistosome species, microcytic hypochromic anaemia may occur due to blood loss in the stool and consumption of blood by adult worms. Colonic polyposis also results in bleeding, protein loss, and anaemia. Hypersplenism, bleeding oesophageal varices, malnutrition, and autoimmune haemolysis are additional factors. In schistosomiasis haematobium, anaemia may develop due to blood loss in urine (Friedman et al. 2005; Davis 2009). Interestingly, S. mansoni SEA has recently been found to trigger erythrocyte cell death in an animal model, thus contributing to the development of anaemia (Kasinathan and Greenberg 2010). Anaemia in turn has been associated with wasting in adults and growth retardation and cognitive impairment in children.
3.5.4.5 Neuroschistosomiasis
Although uncommon in comparison to the total toll of schistosomal disease, neuroschistosomiasis is not uncommon and is probably underrecognized. Spinal cord schistosomiasis, especially due to S. mansoni, is considered as a primary cause of spinal cord parasitic infection in Egypt (Badr et al. 2011).
Central nervous system (CNS) disease may occur in all Schistosoma spp. during the initial stage of maturation (acute schistosomiasis) particularly in case of S. japonicum. The disease tends to be more common and severe in non-endemic exposed individuals. It is probably due to immunologically mediated vasculitis. In the chronic stage, the pathology is due to passage of eggs of Schistosoma to CNS where they induce space-occupying granulomatous reactions (Ferrari et al. 2008; Ferrari and Moreira 2011).
Schistosoma eggs (or occasionally adults) may reach the CNS via two routes: the first is through Batson’s vertebral venous plexus which connects the portal venous system and inferior venae cavae to the spinal cord and cerebral veins. This route permits both anomalous migrations of adult worms in copula to sites close to the CNS followed by in situ deposition and, occasionally, massive embolization of eggs from the portal mesenteric and pelvic venous system towards the CNS. High intra-abdominal pressure, e.g. during defecation and coughing, increases the chance of retrograde flow. The second route is via the arterial system either directly as in S. haematobium or after development of portocaval anastomoses secondary to portal hypertension in S. mansoni (Katchanov and Nawa 2010; Ferrari and Moreira 2011).
In the chronic stage of infection, cerebral disease is more common in S. japonicum than in other species and most commonly presents with seizures. On the other hand, S. mansoni and S. haematobium (to a lesser extent) affect more commonly the spinal cord leading to paresis, radicular symptoms, sphincteric problems, and cauda equina syndrome (Hughes and Biggs 2002). Schistosomal myelopathy has been reported to occur 38 days to 6 years after infection and is acute or subacute with rapidly progressive neurological deficit over the first 24 h. In one review of 26 patients with spinal cord disease, 11.5 % died, 34.6 % remained paraplegic, and 54 % showed moderate to good improvement with therapy (Scrimgeour and Gajdusek 1985).
The diagnosis relies on clinical presentation, demonstration of schistosomal infection by microscopy and serological methods, imaging features, and finally exclusion of other causes of myelopathy (Lambertucci et al. 2008). Cerebrospinal fluid (CSF) examination may be normal but may show increased protein and pleocytosis in the majority, but eosinophilia in only one-quarter of cases. Eggs were never found in CSF (Hughes and Biggs 2002). Histopathology of specimens obtained after surgical removal or biopsy provides definitive diagnosis.
In cerebral disease, CT and MRI usually show a nonspecific tumour-like lesion surrounded by oedema, associated with mass effect and heterogeneous contrast enhancement. The borders are often irregular and poorly defined. Further, MRI is very sensitive in the detection of abnormalities in patients with spinal disease, but the alterations are nonspecific. The most common findings are signal hyperintensity on T2-weighted images, enlargement of the spinal cord (particularly lower cord and conus medullaris), thickening of the spinal roots (especially cauda equina roots), and a heterogeneous pattern of contrast enhancement on T1-weighted images (Silva et al. 2004; Lambertucci et al. 2008).
3.5.4.6 Cardiopulmonary Schistosomiasis
Embolization of eggs into the capillary bed of the lungs may occur via systemic circulation in S. haematobium infection and in intestinal species especially S. mansoni after development of portosystemic venous anastomoses secondary to portal hypertension. In an early study in Upper Egypt, pulmonary schistosomiasis was associated with S. haematobium infection in 58 % of cases, S. mansoni infection in 31 % of cases, and mixed infection in 11 % of cases (Shaw and Ghareeb 1938). Furthermore, the prevalence of pulmonary hypertension in the patients with schistosomal liver fibrosis was found to be 10.7 % in one Brazilian study (Ferreira et al. 2009).
The eggs induce pulmonary granuloma formation and fibrosis, and vascular changes occur in the adjacent vessels, resulting in necrotizing arteritis, thrombi in the affected vessels, medial hypertrophy, and intimal proliferation. Plexiform lesions develop in severe cases where dilated arterioles and venules are detected. The loss of vascular structures can lead eventually to pulmonary hypertension with or without cor pulmonale (Gutierrez 2000). Clinically, the patient may complain of fatigue, exertional dyspnoea, chest pain, cough with occasional haemoptysis, oedema, and later central cyanosis and clubbing of fingers. Electrocardiographic and radiological abnormalities are observed.
3.5.4.7 Schistosoma–Salmonella Interactions
Concurrent Schistosoma–Salmonella infections occur when enteroinvasive Salmonella enter the systemic circulation and attach to the tegument of adult Schistosoma present in the mesenteric vasculature (LoVerde et al. 1980; Melhem and LoVerde 1984). This interaction apparently provides a refuge in which the bacterium can evade systemic antibiotic therapy. Chronic bacteraemic salmonellosis is an individualized clinical entity characterized by prolonged fever with enlargement of the liver and spleen that occurs in Schistosoma-infected individuals who are co-infected with Salmonella. Furthermore, therapy with the anthelmintic praziquantel can lead to a massive release of schistosome-associated Salmonella causing bacteraemia if the appropriate antibiotic is not co-administered to co-infected individuals. Finally, the use of ineffective antibiotics contributes to antibiotic resistance development and the phenomenon of bacterial persistence (Barnhill et al. 2011).
3.5.4.8 Other Manifestations
Deposition of eggs in the skin causes papular dermatitis. Growth retardation and infantilism may occur in schistosomiasis partly due to decreased levels of somatomedins. Moreover, polyarthritis may occur in S. mansoni infection, while S. haematobium may cause osteomalacia resulting from tubular lesions in association with obstructive uropathy (Butterworth and Thomas 1999).
3.6 Diagnosis
3.6.1 Parasitological Techniques
The detection of eggs in stool or urine remains the “gold standard” for schistosomiasis diagnosis despite its lack of sensitivity because of its unsurpassed level of specificity. The shape, size, and spine of the eggs of the three major schistosome species are useful diagnostic features. The eggs of S. japonicum are round with a reduced lateral spine and are smaller in size (60 × 100 μm) than those of S. mansoni (61 × 140 μm; prominent lateral spine) and S. haematobium (62 × 150 μm, prominent terminal spine), which are both ovoid. The 10 ml urine sedimentation and filtration techniques are reliable field diagnostic methods for S. haematobium (Dazo and Biles 1974). The Kato–Katz technique (Endriss et al. 2005) remains the method of choice for the diagnosis of intestinal schistosomiasis, despite its limited sensitivity in areas of low endemicity and the conspicuous intra- and inter-specimen variation of egg distribution and aggregation in faeces (Krauth et al. 2012). However, Berhe et al. (2004) reported that “examination of five Kato-Katz thick smears from one stool specimen using 41.7 mg template or three Kato-Katz thick smears from one stool specimen, and if these are negative, followed by examination of additional triplet Kato-Katz thick smears from subsequent day stool specimen can adequately assess individuals for infection status with S. mansoni”. Similar findings were recorded for S. japonicum parasitological diagnosis (Lin et al. 2008; Utzinger et al. 2011). The formol–ether concentration technique (Allen and Ridley 1970) is slightly more sensitive than the triplet Kato–Katz slides (Ebrahim et al. 1997) but less sensitive than the FLOTAC method (Cringoli 2006) which is recommended for highly accurate coprological diagnosis and for ascertaining cure following chemotherapy (Glinz et al. 2010; Utzinger et al. 2011). The Percoll separation technique (Eberl et al. 2002) could be used in hospitals and laboratories dedicated to research and clinical investigations.
In light infection, examination of rectal mucosa snips taken with a curette through a proctoscope is more sensitive than stool examination. Material thus obtained is examined as a crush preparation. Viability of eggs is confirmed by movement of the cilia of flame cells or of the miracidia within the eggshell (Davis 2009).
3.6.2 Immunological Techniques
Schistosomes elicit immune responses immediately upon host skin invasion and during development, maturation, copulation, and egg laying in blood vessels. Accordingly, antibody responses to different schistosome adult worm (SAWA)- or egg (SEA)-derived antigens or selected antigens, e.g. species-specific microsomal antigens, cathepsin B, etc., have been used for immunodiagnosis by indirect haemagglutination assay (IHA), enzyme-linked immunosorbent assay (ELISA), and/or dipstick dye immunoassay, all of which showed high sensitivity (Al-Sherbiny et al. 1999; Noya et al. 2002; Xiang et al. 2003; Zhou et al. 2007, 2008, 2011a). IHA is among a few commercial serodiagnostic kits for schistosomiasis and was shown to be ideal for assessing IgM and IgG responses in travellers to, but not residents of, endemic regions (van Gool et al. 2002; Yu et al. 2007; Zhou et al. 2007, 2008; Zhao et al. 2012a, b). Antibody detection methods are limited in their specificity as they do not allow discrimination between current and previous infection or reinfection and are thus not useful for assessing follow-up after chemotherapy (Zhou et al. 2011b).
Immunodiagnostic methods based on antigen detection have overcome some of these limitations. Worm antigens and egg-derived molecules are readily detectable in serum or plasma, faeces, urine, and even saliva, provided the availability of specific capture and detecting antibodies (Lei et al. 2011; Demerdash et al. 2013). Such immunodiagnostic methods are of importance not only for assessment of cure after treatment but even more importantly as the most reliable way for diagnosis of chronically infected patients who fail to excrete eggs in stool or urine (Enk et al. 2008; Zhou et al. 2011b). Research has primarily focused on two glycoproteins, circulating anodic antigen (CAA) and circulating cathodic antigen (CCA), which are released from the gut of viable developing larvae and adult worms and may thus provide information on active infection and intensity (van Dam et al. 1996). The assays require access to CAA- and CCA-specific antibodies (Al-Sherbiny et al. 1999); yet, commercial kits are becoming available and showed exquisitely high sensitivity and specificity for diagnosis of S. mansoni but not S. haematobium (Stothard et al. 2006; Ashton et al. 2011; Shane et al. 2011; Utzinger et al. 2011; Navaratnam et al. 2012; Tchuem Tchuenté et al. 2012; Bergquist 2013; Coulibaly et al. 2013; Erko et al. 2013; Grenfell et al. 2013).
3.6.3 Molecular Techniques
Conventional or real-time polymerase chain reaction (PCR) amplification of repetitive elements specific to each schistosome species has been used for the diagnosis of S. mansoni (Pontes et al. 2002, 2003; Rabello et al. 2002; Abath et al. 2006; Gomes et al. 2006; Allam et al. 2009), S. haematobium (Abbasi et al. 2007; Obeng et al. 2008; Aryeetey et al. 2013), and S. japonicum (Xu et al. 2010; Zhao et al. 2012a) using stool, urine, or serum samples.
3.7 Treatment of Schistosomiasis
3.7.1 Schistosomicidal Agents
3.7.1.1 Praziquantel (PZQ)
Praziquantel is a pyrazino-isoquinoline derivative that is practically insoluble in water, sparingly soluble in ethanol, but very soluble in chloroform and dimethylsulfoxide. PZQ is the mainstay of antischistosomal therapy; it is effective against all five species of human schistosomes in a single oral dose of 40–60 mg/kg body weight, leading to cure rates of 60–90 % in different epidemiological settings (Davis 1993; Kumar and Gryseels 1994). Despite that the initial effects of the drug included a rapid influx of calcium into the worm and calcium-dependent muscle contraction and paralysis, the exact mode of action of PZQ is not known as yet (Greenberg 2005), and the PZQ receptor on schistosomes remains elusive. However, in vivo, PZQ-induced muscle contraction and tegumental lesions produce loss of attachment to the endothelial lining of veins and dislodgment to the liver. Host cells of the defence system attach to the tegumental vacuoles and start to penetrate the interior of the parasite early after treatment (Mehlhorn et al. 1981; Andrews 1985; Day et al. 1992). It has been recently shown that PZQ binds and polymerizes adult schistosome actin, which may account for some of its effects (Tallima and El Ridi 2007). Adverse effects include direct and dose-related effects such as nausea and abdominal pain, headache, and dizziness, as well as indirect effects attributable to the death of worms such as fever, urticaria, pruritus, rashes, arthralgia, myalgia, and eosinophilia (Tracy and Webster 2001). PZQ can be administered to pregnant women at any stage of pregnancy and during lactation, as benefit of treatment outweighs risk. Also, the drug can safely be administered to school-age children (WHO 2006).
Two problems are noteworthy with PZQ: first, the emergence of drug resistance—isolates with decreased sensitivity to praziquantel have been reported from different epidemiological settings, e.g. Egypt, Kenya, and Senegal (Ismail et al. 1996; Cioli et al. 2004; Melman et al. 2009), and may be harbingers of more to come. Second, the drug is not effective against juvenile forms of the parasite. It has little activity against eggs or immature worms (schistosomula) and cannot abort early infection. Therefore, patients treated early in their infection must be retreated with PZQ after the adult worms have matured (usually in 6–12 weeks) (Kappagoda et al. 2011).
3.7.1.2 Other Drugs
3.7.1.2.1 Oxamniquine
When administered orally, it is effective against S. mansoni, male worms being more affected than females, while it has no effect on S. haematobium. A single, two, or three oral doses of 20 mg/kg each are needed for a cure rate of 80–90 %, depending on the geographical region. It is now believed that oxamniquine undergoes esterification by a sulfotransferase uniquely present in sensitive schistosomes. The ester spontaneously dissociates, yielding an electrophilic reactant capable of alkylating schistosome DNA, with subsequent inhibition of DNA and RNA synthesis. The absence of this enzyme in mammals, including humans, explains the low toxicity of oxamniquine (Pica-Mattoccia and Cioli 1985). Oxamniquine is no longer used on a large scale (El Ridi and Tallima 2012).
3.7.1.2.2 Antimalarials
The antimalarial drug artemether, a methoxy derivative of artemisinin, has been shown to be active against S. japonicum, S. mansoni, and S. haematobium in experimentally infected animals (Xiao et al. 2002). Mefloquine, another antimalarial drug, was also found to have significant anti-schistosome activity in vitro, and in vivo as well, since a single dose (200 or 400 mg/ kg), administered orally to mice infected with adult S. mansoni, resulted in worm burden reductions of 72.3–100 % (Keiser et al. 2009). It has been shown that artemether interacts with haemin to exert a toxic effect on schistosomes, while mefloquine is believed to inhibit haemozoin formation. Artemisinin derivatives are particularly effective against the immature stages of S. japonicum, S. mansoni, and possibly S. haematobium. Yet there are objections for use of antimalarials in the treatment of schistosomiasis for fear of development of artemisinin-resistant malaria (Gryseels et al. 2006; Utzinger et al. 2007).
The most successful plant product in the treatment of human schistosomiasis was myrrh, an oleo–gum–resin extracted from the stem of Commiphora molmol (Tonkal and Morsy 2008). The product is believed to affect schistosome musculature, leading to uncoupling of male and female couples and their extravasation to the liver (Badria et al. 2001). The product has been licensed for human use by the Egyptian Ministry of Health and introduced in the Egyptian market. However, conflicting reports on its efficacy shed doubts upon the usefulness of its use as a novel therapy for schistosomiasis (Osman et al. 2010).
3.7.1.3 Novel Therapeutic Approaches
A different approach to therapy of schistosomiasis has relied on plants known for medicinal effects. Extracts and oils of several medicinal plants have been tested for potential therapeutic activity against schistosome infection and have been exhaustively compiled in excellent reviews (e.g. Tagboto and Townson 2001; Yousif et al. 2007). Curcumin, the major constituent in the rhizome of Curcuma longa, has been shown to display potent schistosomicidal activities in vivo and in vitro against adult S. mansoni worms (El-Ansary et al. 2007; El-Banhawey et al. 2007).
An essential fatty acid, a component of our diet and cells, the polyunsaturated fatty acid arachidonic acid has been proposed as a remedy for schistosomiasis, due to its ability to activate the parasite tegument-bound neutral sphingomyelinase, with subsequent hydrolysis of the apical lipid bilayer sphingomyelin molecules, allowing access of specific antibody molecules, and eventual worm attrition. This concept was convincingly supported using larval and adult S. mansoni and S. haematobium worms in in vitro experiments and in vivo studies in inbred mice and outbred hamsters (El Ridi and Tallima 2013a, b).
Of great interest is the class of compounds targeting schistosome histone-modifying enzymes, namely, histone acetyltransferases and histone deacetylases, leading to parasite apoptosis and death in in vitro cultures (Pierce et al. 2011). Further, trioxaquines, which are currently in development for malaria, are hybrid molecules consisting of two pharmacophores, a trioxane and a 4-aminoquinoline moiety (Utzinger et al. 2011). These drugs, used at concentrations of 5–50 μg/ml, rapidly kill 21-day-old juvenile and 49-day-old adult S. mansoni in vitro. Therefore, these evidence-based findings confirm that peroxidic compounds are active against schistosomes and perhaps other trematodes (Keiser and Utzinger 2007; Xiao et al. 2007).
Recently, there has been an interest in multidrug transporters, members of ATP-binding cassette (ABC) superfamily of efflux transporters, such as p-glycoprotein (Pgp, ABCB1), which have been associated with drug resistance in parasites, including helminths such as schistosomes (Greenberg 2013). The role of schistosome ABC transporters in regulating drug susceptibility and in normal schistosome physiology, including reproduction and excretory activity, has been recently explored. For example, S. mansoni expresses higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel (Kasinathan et al. 2010). Greenberg (2013) postulated that schistosome ABC transporters could be useful targets for compounds that enhance the effectiveness of current therapeutics as well as for agents that act as antischistosomals on their own.
3.7.1.3.1 Follow-Up Therapy
Parasitological cure has to be confirmed 6–8 weeks and 4–6 months after therapy. Finding viable eggs at 4–6 months indicates either reinfection, therapeutic failure, or that treatment was given too early after infection. Other parameters of cure include improvement of symptomatology and improvement of radiological, ultrasonographic, and endoscopic findings (Davis 2009). Antibody titres, and eosinophilia if present, decline with therapy. However, there may be transient eosinophilia and rise in antibody titres in the first 2 weeks after therapy (Bourée 2005). Antigen detection, if available, is an excellent test for establishing cure.
3.7.2 Symptomatic Therapy and Management of Complications
For the prevention of bleeding from oesophageal varices due to schistosomal portal hypertension, pharmacotherapy in the form of beta blockers is used together with endoscopic treatment in the form of sclerotherapy or much better band ligation. For the management of acute variceal bleeding, resuscitation is mandatory together with the administration of vasoactive drugs (e.g. terlipressin, somatostatin, or octreotide). Endoscopic treatment particularly band ligation is effective in controlling bleeding. Other procedures for portal hypertension include the minimally invasive transjugular intrahepatic portosystemic shunt (TIPS). Surgery is reserved for those patients who fail to respond to endoscopic therapy or in case of symptomatic hypersplenism and includes oesophagogastric devascularization or shunt procedure together with splenectomy (Bosch et al. 2008; Rajekar et al. 2011). Colonoscopic polypectomy is safe and effective and may be required along with medical therapy to achieve complete symptom relief and prevent complications (Mostafa et al. 1990; Elbaz and Esmat 2013).
Obstructive uropathy is usually managed conservatively. However, a damaged kidney may necessitate nephrectomy or nephrostomy. Corrective surgery may be needed for contracted bladder and ureteric obstruction. Carcinoma of the bladder is treated through the conventional procedures of tumour management. Total or partial cystectomy is usually needed. Schistosomal bladder cancer responds less favourably to radiotherapy (Bourée 2005).
3.8 Prognosis
Schistosomiasis remains a major cause of morbidity in tropical and subtropical countries (Engels et al. 2002). Clinical observations in schistosomiasis endemic areas show that major complications of Schistosoma infection develop in approximately 4–12 % of the population, while the majority of infected people remain asymptomatic or exhibit mild nonspecific symptoms (Tachon and Borojevic 1978). Intensity and duration of the infection are major determinants, but other factors are also involved. These include genetic background of the host, nutritional status, parasite strain differences, and frequency of infection. Maternal infection status—in that offspring of the infected mothers may be primed to mount a modulated type of response at first infection—has been proposed by many authors in order to explain the individual differences (Butterworth and Thomas 1999).
Additionally, schistosomiasis does not cause the annual loss of between 1.7 and 4.5 (Steinmann et al. 2006) but between 24 and 56 (King 2010) and up to 70 (Gray et al. 2010; Siddiqui et al. 2011) million disability-adjusted life years. Unlike malaria, tuberculosis, and HIV/AIDS, schistosomiasis and a host of other helminthic, bacterial, protozoan, and viral diseases remain truly neglected (Utzinger et al. 2011). The close link with poverty and lack of resources, geographical isolation, underestimated global burden, stigmatization, lack of political voice of those affected, and the absence of an established global funding mechanism are some of the factors that explain the general neglect of schistosomiasis (Molyneux 2008; Gray et al. 2010; Utzinger et al. 2011).
At the individual level, antischistosomal therapy given early could lead to resolution of schistosomal lesions and could halt the progression of the disease provided that reexposure to infection is avoided. In case of established fibrosis, antischistosomal therapy is given to prevent further damage, but the current morbidity has to be addressed appropriately (Butterworth and Thomas 1999). However, there is evidence of regression of hepatosplenic disease with therapy (Ohmae et al. 1992; Butterworth and Thomas 1999; Lucas 2002; Andrade 2008).
3.9 Prevention and Control
3.9.1 Preventive Chemotherapy
WHO (2006) recommended PZQ as the basis of preventive chemotherapy in schistosomiasis. PZQ-based preventive therapy is a cost-effective public health tool that aims at morbidity control: periodic treatment of at-risk populations will cure subtle morbidity and prevent infected individuals from developing severe, late-stage morbidity due to schistosomiasis. For the assessment of prevalence of Schistosoma infection in a suspected endemic area, schoolchildren are examined. A few schools are selected close to the water and some a little further away, and 50 students of the upper classes in each school are examined either by stool examination (Kato–Katz method) for intestinal schistosomiasis or by search for haematuria (via a questionnaire or urine examination) or urine analysis for ova for urinary schistosomiasis (WHO 2006, 2013).
According to WHO guidelines, PZQ should be given once yearly to all school-age children and adults at risk in high-risk communities (≥50 % prevalence of infection by parasitological methods), once every 2 years to all school-age children and adults at risk in moderate-risk communities (≥10 % but <50 % prevalence by parasitological methods), and twice during primary schooling age to all school-age children and as a case-directed treatment for adults in low-risk communities (<10 % prevalence by parasitological methods). Safety of PZQ is not established in children below 4 years; therefore, these children should be excluded from mass treatment but can be treated on an individual basis by medical personnel. The dosage of PZQ is determined according to the height of children. Possible indicators for monitoring preventive chemotherapy interventions are as follows: prevalence of infection (by parasitological methods), intensity of infection (proportion of heavy-intensity infections), and prevalence of macrohaematuria, microhaematuria, anaemia, or ultrasound-detectable lesions (urinary tract and liver) (WHO 2006).
3.9.2 Vaccine Development
3.9.2.1 The Immediate Need of a Vaccine
For combating schistosomiasis, chemical and biological molluscicides have proven efficacy, yet may be harmful to the environment (Combes and Cheng 1986). Health and hygiene education and improving sanitary and living conditions are required, yet are not available and are not expected to be available in a near future in most countries where schistosomiasis is endemic (Utzinger et al. 2009, 2011; King 2010). Despite the massive distribution and use of the rather safe, effective, and cost affordable schistosomicide, PZQ, it was given to only 30–40 million patients, while hundreds of millions at large remained without treatment. Chemotherapeutic treatment neither delays nor prevents reinfection, requiring repeated administration, and increases the probability of development of parasite resistance to the drug. Accordingly, a safe, efficacious, and cost-effective vaccine should be available to children in endemic rural areas without any delay, in an aim to interrupt transmission and eliminate schistosomiasis.
3.9.2.2 The Prospects of the Candidate Vaccine Antigens
During the 1990s of the last century, a number of candidate vaccine antigens were found to be the targets of Th1-/Th2-related immune responses in irradiated cercariae-vaccinated mice (reviewed in El Ridi and Tallima 2013a, b) and residents of endemic countries, such as Egypt and Brazil, that are susceptible or resistant to reinfection after praziquantel treatment (de Jesus et al. 2000; Bergquist et al. 2002; Al-Sherbiny et al. 2003). The selected molecules were purified, characterized, and prepared in a recombinant or multiple antigen peptide (MAP) form. The test molecules, likely emulsified in Freund’s or alum adjuvant, failed to induce the benchmark of 50 % protection in mice in independent trials sponsored by the World Health Organization (Bergquist and Colley 1998; Todd and Colley 2002). IrV5 studies were discontinued. Experiments with triose-phosphate isomerase (TPI) MAP-1 and MAP-2 (Reis et al. 2008) showed that no protection was induced in mice following immunization with TPI together with immunogenic epitopes of glutathione S-transferase, paramyosin, calpain, and Sm23, assembled as DNA, recombinant protein, or MAP constructs (Yang et al. 2000). Yet, immunization of BALB/c mice with a codon-optimized S. japonicum TPI construct elicited higher than 50 % reduction in challenge S. japonicum worm burden and worm egg counts (Zhu et al. 2010). Glyceraldehyde 3-phosphate dehydrogenase (SG3PDH) in a recombinant, linear peptide, di- and tetrabranched MAP construct, administered to inbred and outbred mice intramuscularly or subcutaneously without adjuvant or emulsified in Freund’s, alum, Allison’s, or Ribi adjuvant, led to only 10–35 % protection against challenge S. mansoni infection [reviewed in El Ridi (1998) and El Ridi and Tallima (2013a, b)]. Trials with the large chain of calpain Sm-80 in a recombinant or DNA vaccine construct were successful, whereby approximately 50 % reductions in worm burdens and worm egg loads were achieved in inbred mice and outbred baboons (Siddiqui et al. 2011). Regarding paramyosin, a good manufacturing practice-ready, pilot-scale process to produce recombinant full-length S. japonicum paramyosin, rSj97, was established, and efficacy and safety studies are currently conducted in rodents and large-animal models (Jiz et al. 2008, 2009).
Sm23, a member of the tetraspanin family of molecules located at the host–parasite interface, was used in multiple trials in a recombinant (large extracellular hydrophilic domain) construct in conjunction with alum or Th1-type adjuvants, resulting in limited levels of protection of inbred C57BL/6 mice against subsequent challenge infection (Da’Dara et al. 2003). Vaccination with nucleic acid constructs encoding Sm23 or Sj23 effectively induced parasite-specific IFN-γ and Th1-/Th2-related antibody responses yet failed to evoke other critical responses needed for optimal vaccine efficacy (Da’Dara et al. 2002, 2003; Gan et al. 2005; Ganley-Leal et al. 2005). More recently, other members of the tetraspanin family of integral membrane proteins, namely, Sm-TSP-1 and Sm-TSP-2, emulsified in Freund’s adjuvant were found to induce significant (P < 0.001) levels of protection in CBA/CaH mice against schistosomiasis mansoni (Tran et al. 2006). These findings were not reproduced on using S. japonicum tetraspanins (Zhang et al. 2011). However, large amounts of the extracellular domain of Sm-TSP-2 are being now generated at Baylor College of Medicine, ready for phase I clinical studies (Curti 2012), notwithstanding its poor performance (Cai et al. 2008; Zhang et al. 2011; Pearson et al. 2012) and the lack of highly significant protective potential of other integral membranes such as Sm23 (Ganley-Leal et al. 2005), S. mansoni glucose transporter SGTP4 (Mahana 2006), and numerous other molecules residing in the tegument of the parasite (Cardoso et al. 2008; Rofatto et al. 2013; Teixeira de Melo et al. 2013).
Of note, glutathione S-transferase of S. haematobium progressed to clinical trial phase I using alum as adjuvant; safety and tolerability were documented, but no reports on the efficacy against urinary schistosomiasis are available as yet (Riveau et al. 2012). The fatty acid-binding protein Sm14 also progressed to preclinical and clinical studies (Tendler and Simpson 2008); yet, no information concerning efficacy against infection with S. mansoni were reported.
Surprisingly, most if not all schistosomiasis vaccine experiments aimed to achieve preponderance of Th1-related cytokines and antibodies (Da’Dara et al. 2003; Cardoso et al. 2008; Siddiqui et al. 2011; Teixeira de Melo et al. 2013; Wang et al. 2013), while resistance to schistosome reinfection in humans, to whom the vaccine is supposedly dedicated, was previously and recently shown to be dependent on type 2 immune responses (Hagan et al. 1991; Roberts et al. 1993; Fallon et al. 2003; Ganley-Leal et al. 2006; Jiz et al. 2009; Figueiredo et al. 2012; Fitzsimmons et al. 2012).
3.9.2.3 The Breakthrough
3.9.2.3.1 A Plausible Mechanism of Immunity
Schistosomes reside in the bloodstream impervious to immune attacks. If intact parasites were susceptible to killing via antibody-dependent complement activation or cell-mediated cytotoxicity (ADCC), they would not survive a day, least of all a decade, as surface membrane-associated molecules released from dying or dead parasites are readily accessible to the host and are immunogenic (Mahana 2006; Tran et al. 2006). Yet, generated antibodies are not able to access antigens on the apical membrane or the tegument (El Ridi and Tallima 2013a, b and references therein; Migliardo et al. 2014). The immune system effectors may, however, recognize and interact with the “scent” molecules, the ESP of developing and adult worms, resulting into immune cell activation and release of inflammatory and toxic mediators in the vicinity of the parasite. In large vessels, ESP are easily washed away from the parasite. The situation is more difficult for the developing worms in the lung capillaries and liver sinusoids, where the ESP might stagnate, attracting the hunting cells in close proximity to the migrating schistosomula. Accordingly, ESP released by developing schistosomula should be excellent vaccine candidates. Indeed, many of the candidate vaccine molecules are larval ESP (El Ridi and Tallima 2009).
To develop an ESP-based vaccine, it is important to examine the “hunting” team summoned upon parasite invasion (von Lichtenberg et al. 1977). Larval ESP induce predominantly Th1-/Th17-related cytokines and antibody responses in mice and humans during natural infection and in mice following immunization with schistosome antigens and Th1-/Th17-biased adjuvants. Accordingly, circulating monocytes and neutrophils would be readily recruited and activated and certainly contribute to the demise of a proportion of invading larvae. Yet, eosinophils and basophils are not invited to participate in the chase and are actually entirely excluded (von Lichtenberg et al. 1977). To recruit and activate eosinophils and basophils, it is necessary to use an adjuvant that skews the larval-induced Th1/Th17 immune responses towards the type 2 phenotype (El Ridi and Tallima 2012, 2013a, b and references therein).
3.9.2.3.2 Adjuvant Selection
Our efforts for proper adjuvant choice led to selection of the type 2 immune response-inducing thymic stromal lymphopoietin, TSLP (El Ridi and Tallima 2012), and papain. Four consecutive experiments were set up whereby groups of 5–10 outbred CD1 mice were injected subcutaneously with 200 μl Dulbecco’s phosphate-buffered saline (D-PBS), pH 7.1, or 50 μg papain in 200 μl D-PBS, 1 h before whole-body exposure to 125 cercariae of S. mansoni. Worm burden was evaluated 6 weeks after infection. For every experiment, papain injection consistently and reproducibly elicited highly significant (P < 0.0001) reduction in worm burden of 70 % ± 3 (mean ± SD for four independent experiments). The reduction was also highly significant for worm liver (P < 0.0001) and small intestine (P < 0.001) egg counts but only of approximately 50 % (Table 3.1). Papain has been documented to drive ovalbumin immune responses towards the type 2 axis within a few days after injection in mice (Sokol et al. 2008, 2009; Tang et al. 2010), and that was sufficient, without any previous or additional immunization, to lead to highly significant (P < 0.0001) protection against schistosomiasis mansoni in every test mouse as compared to controls. These findings gave a sound proof that a large proportion of invading S. mansoni larvae would succumb if met upon host invasion with type 2 immune responses.
3.9.2.3.3 A Vaccine Formula
A series of 8 successive experiments led us to define a formula for an efficacious vaccine for schistosomiasis, leading to consistent, reproducible, and highly significant (P < 0.0001) reduction in challenge S. mansoni worm burden and worm egg load in the liver and small intestine of 62–75 % in immunized outbred, akin to man, mice as compared to controls (El Ridi and Tallima 2013c). The formula is as follows: larval ESP mix + type 2 cytokine such as TSLP, IL-25, or IL-33. It is important to examine the different ESP for optimal results. In our hands, a mixture of rSG3PDH and a MAP construct based on a peptide of S. mansoni 2-cys peroxiredoxine (TPX MAP Mix) was more protective than a mixture of rSG3PDH and a MAP construct based on a peptide of S. mansoni aldolase (ALD MAP Mix) upon immunization of outbred mice in conjunction with TSLP (200 ng/mouse) or papain (10 μg/mouse) as adjuvant (El Ridi 2012; El Ridi and Tallima 2013a).
3.9.2.3.4 A Proof of Concept Leads to an Adjuvant-Free Vaccine Formulation
Immunization of outbred mice with schistosome antigens, which are both ESP and type 2 immune response inducing, consistently and reproducibly elicited highly significant (P < 0.0001) reduction (60–75 %) of challenge S. mansoni worm burden and worm egg load in the liver and small intestine as compared to unimmunized mice. It is, thus, fortunate that we developed an adjuvant-free Th2 immune response-based vaccine effective in outbred mice, since it is destined to the outbred human population, where adjuvant use remains a considerable challenge and where immunological correlates of resistance to the 3 species of schistosomes are associated with type 2 responses (El Ridi et al. 2014).
References
Abath FG, Gomes AL, Melo FL, Barbosa CS, Werkhauser RP (2006) Molecular approaches for the detection of Schistosoma mansoni: possible applications in the detection of snail infection, monitoring of transmission sites, and diagnosis of human infection. Mem Inst Oswaldo Cruz 101(Suppl 1):145–148, Review
Abbasi I, King CH, Sturrock RF, Kariuki C, Muchiri E, Hamburger J (2007) Differentiation of Schistosoma haematobium from related schistosomes by PCR amplifying an inter-repeat sequence. Am J Trop Med Hyg 76(5):950–955
Abdel-Wahab MF, Esmat G, Farrag A, El-Boraey YA, Strickland GT (1992) Grading of hepatic schistosomiasis by the use of ultrasonography. Am J Trop Med Hyg 46:403–408
Allam AF, Kader O, Zaki A, Shehab AY, Farag HF (2009) Assessing the marginal error in diagnosis and cure of Schistosoma mansoni in areas of low endemicity using Percoll and PCR techniques. Trop Med Int Health 14(3):316–321
Allan F, Dunn AM, Emery AM, Stothard JR, Johnston DA, Kane RA, Khamis AN, Mohammed K, Rollinson D (2013) Use of sentinel snails for the detection of Schistosoma haematobium transmission on Zanzibar and observations on transmission patterns. Acta Trop 128(2):234–240
Allen AV, Ridley DS (1970) Further observations on the formol-ether concentration technique for faecal parasites. J Clin Pathol 23(6):545–546
Al-Sherbiny MM, Osman AM, Hancock K, Deelder AM, Tsang VC (1999) Application of immunodiagnostic assays: detection of antibodies and circulating antigens in human schistosomiasis and correlation with clinical findings. Am J Trop Med Hyg 60(6):960–966
Al-Sherbiny M, Osman A, Barakat R, El Morshedy H, Bergquist R, Olds R (2003) In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop 88(2):117–130
Alves Oliveira LF, Moreno EC, Gazzinelli G, Martins-Filho OA, Silveira AM, Gazzinelli A, Malaquias LC, LoVerde P, Leite PM, Correa-Oliveira R (2006) Cytokine production associated with periportal fibrosis during chronic schistosomiasis mansoni in humans. Infect Immun 74(2):1215–1221
Andrade ZA (2008) Schistosomiasis and hepatic fibrosis regression. Acta Trop 108:79–82
Andrews P (1985) Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol Ther 29:129–156
Aryeetey YA, Essien-Baidoo S, Larbi IA, Ahmed K, Amoah AS, Obeng BB, van Lieshout L, Yazdanbakhsh M, Boakye DA, Verweij JJ (2013) Molecular diagnosis of Schistosoma infections in urine samples of school children in Ghana. Am J Trop Med Hyg 88(6):1028–1031
Ashton PD, Harrop R, Shah B, Wilson RA (2001) The schistosome egg: development and secretions. Parasitology 122(Pt 3):329–338
Ashton RA, Stewart BT, Petty N, Lado M, Finn T, Brooker S, Kolaczinski JH (2011) Accuracy of circulating cathodic antigen tests for rapid mapping of Schistosoma mansoni and S. haematobium infections in Southern Sudan. Trop Med Int Health 16(9):1099–1103
Badr HI, Shaker AA, Mansour MA, Kasem MA, Zaher AA, Salama HH, Safwat MI (2011) Schistosomal myeloradiculopathy due to Schistosoma mansoni: report on 17 cases from an endemic area. Ann Indian Acad Neurol 14:107–110
Badria F, Abou-Mohamad G, El-Mowafi A, Masoud A, Salama O (2001) Mirazid a new schistosomicidal drug. Pharm Biol 39:127–131
Barakat RMR (2013) Epidemiology of schistosomiasis in Egypt: travel through time. J Adv Res 4(5):425–432
Barnhill AE, Novozhilova E, Day TA, Carlson SA (2011) Schistosoma-associated Salmonella resist antibiotics via specific fimbrial attachments to the flatworm. Parasit Vectors 4:123
Barsoum RS (2013) Urinary schistosomiasis. J Adv Res 4(5):453–459
Barsoum RS, Esmat G, El-Baz T (2013) Human schistosomiasis: clinical perspective. J Adv Res 4(5):433–444
Bergquist R (2013) Good things are worth waiting for. Am J Trop Med Hyg 88(3):409–410
Bergquist NR, Colley DG (1998) Schistosomiasis vaccine: research to development. Parasitol Today 14(3):99–104
Bergquist R, Tanner M (2010) Controlling schistosomiasis in Southeast Asia: a tale of two countries. Adv Parasitol 72:109–144
Bergquist R, Al-Sherbiny M, Barakat R, Olds R (2002) Blueprint for schistosomiasis vaccine development. Acta Trop 82(2):183–192
Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, Moore R, Habte E, Redda A, Gebre-Michael T, Gundersen SG (2004) Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop 92(3):205–212
Booth M, Mwatha JK, Joseph S, Jones FM, Kadzo H, Ireri E, Kazibwe F, Kemijumbi J, Kariuki C, Kimani G, Ouma JH, Kabatereine NB, Vennervald BJ, Dunne DW (2004) Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J Immunol 172(2):1295–1303
Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG (2008) The management of portal hypertension: rational basis, available treatments and future options. J Hepatol 48(Suppl 1):S68–S92
Bourée P (2005) Parasitoses urinaires (urinary parasitosis). Ann Urol 39:232–246
Brooker S, Hotez PJ, Bundy DA (2010) The global atlas of helminth infection: mapping the way forward in neglected tropical disease control. PLoS Negl Trop Dis 4(7):e779
Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP (2009) Immunopathogenesis of human schistosomiasis. Parasite Immunol 31(4):163–176
Butterworth AE, Thomas JEP (1999) Schistosomiasis. In: Weatherall DJ, Ledingham TGG, Warell DA (eds) Oxford textbook of medicine, 3rd ed, vol 1 (Section 7, pp 970–981). Oxford University Press, New York, NY
Cai P, Bu L, Wang J, Wang Z, Zhong X, Wang H (2008) Molecular characterization of Schistosoma japonicum tegument protein tetraspanin-2: sequence variation and possible implications for immune evasion. Biochem Biophys Res Commun 372(1):197–202
Caldas IR, Campi-Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, Gazzinelli G (2008) Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop 108:109–117
Cardoso FC, Macedo GC, Gava E, Kitten GT, Mati VL, de Melo AL, Caliari MV, Almeida GT, Venancio TM, Verjovski-Almeida S, Oliveira SC (2008) Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. PLoS Negl Trop Dis 2(10):e308
Cheever AW, Xu YH, Sher A, Macedonia JG (1991) Analysis of egg granuloma formation in Schistosoma japonicum-infected mice treated with antibodies to interleukin-5 and gamma interferon. Infect Immun 59:4071–4074
Cheever AW, Williams ME, Wynn TA et al (1994) Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol 153:753–759
Chen L, Rao KV, He YX, Ramaswamy K (2002) Skin-stage schistosomula of Schistosoma mansoni produce an apoptosis-inducing factor that can cause apoptosis of T cells. J Biol Chem 277(37):34329–34335
Cheng YL, Song WJ, Liu WQ et al (2008) The effects of T cell deficiency on the development of worms and granuloma formation in mice infected with Schistosoma japonicum. Parasitol Res 102:1129–1134
Cioli D, Botros SS, Wheatcroft-Francklow K, Mbaye A, Southgate V, Tchuenté LA, Pica-Mattoccia L, Troiani AR, El-Din SH, Sabra AN, Albin J, Engels D, Doenhoff MJ (2004) Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int J Parasitol 34:979–987
Combes C, Cheng TC (1986) Control of biomedically important molluscs. Arch Inst Pasteur Alger 55:153–193
Coulibaly JT, Fürst T, Silué KD, Knopp S, Hauri D, Ouattara M, Utzinger J, N’Goran EK (2012) Intestinal parasitic infections in schoolchildren in different settings of Côte d'Ivoire: effect of diagnostic approach and implications for control. Parasit Vectors 5:135
Coulibaly JT, N’gbesso YK, Knopp S, N’guessan NA, Silué KD, van Dam GJ, N’goran EK, Utzinger J (2013) Accuracy of urine circulating cathodic antigen test for the diagnosis of Schistosoma mansoni in preschool-aged children before and after treatment. PLoS Negl Trop Dis 7(3):e2109
Cringoli G (2006) FLOTAC, a novel apparatus for a multivalent faecal egg count technique. Parassitologia 48(3):381–384
Curtale F, Mohamed MY, Youssef ZM (2010) Comprehensive primary health care, a viable strategy for the elimination of schistosomiasis. Trans R Soc Trop Med Hyg 104(1):70–72
Curti E (2012) A robust and reproducible process for production of a Schistosomiasis vaccine. In: 2nd International conference on vaccines and vaccination, 20–22 August 2012, Hilton Chicago/Northbrook, IL
Da Silva LC, Carrilho FJ (1992) Hepatosplenic schistosomiasis. Pathophysiology and treatment. Gastroenterol Clin North Am 21:163–177
Da’dara AA, Skelly PJ, Fatakdawala M, Visovatti S, Eriksson E, Harn DA (2002) Comparative efficacy of the Schistosoma mansoni nucleic acid vaccine, Sm23, following microseeding or gene gun delivery. Parasite Immunol 24(4):179–187
Da’Dara AA, Skelly PJ, Walker CM, Harn DA (2003) A DNA-prime/protein-boost vaccination regimen enhances Th2 immune responses but not protection following Schistosoma mansoni infection. Parasite Immunol 25(8–9):429–437
Davis A (1993) Antischistosomal drugs and clinical practice. In: Jordan P, Webbe G, Sturrock RF (eds) Human schistosomiasis. CAB International, Wallingford, pp 367–404
Davis A (2009) Schistosomiasis. In: Cook GC, Zumla AI (eds) Manson’s tropical diseases. Section 11, Chapter 82, 22nd edn. Saunders Elsevier, pp 1437–1452. International edition. Printed in China
Day TA, Bennett JL, Pax RA (1992) Praziquantel: the enigmatic antiparasitic. Parasitol Today 8:342–344
Dazo BC, Biles JE (1974) Two new field techniques for detection and counting of Schistosoma haematobium eggs in urine samples, with an evaluation of both methods. Bull World Health Organ 51(4):399–408
de Vlas SJ, Gryseels B (1992) Underestimation of Schistosoma mansoni prevalences. Parasitol Today 8(8):274–277
Demerdash Z, Mohamed S, Hendawy M, Rabia I, Attia M, Shaker Z, Diab TM (2013) Monoclonal antibody-based dipstick assay: a reliable field applicable technique for diagnosis of Schistosoma mansoni infection using human serum and urine samples. Korean J Parasitol 51(1):93–98
deMorais CN, Souza JR, Melo WG, Aroucha ML, Miranda P, Domingues AL, Abath FG, Montenegro SM (2008) Cytokine profile associated with chronic and acute human schistosomiasis mansoni. Mem Inst Oswaldo Cruz 103(6):561–568
Eberl M, Al-Sherbiny M, Hagan P, Ljubojevic S, Thomas AW, Wilson RA (2002) A novel and sensitive method to monitor helminth infections by faecal sampling. Acta Trop 83(2):183–187
Ebrahim A, El-Morshedy H, Omer E, El-Daly S, Barakat R (1997) Evaluation of the Kato-Katz thick smear and formol ether sedimentation techniques for quantitative diagnosis of Schistosoma mansoni infection. Am J Trop Med Hyg 57(6):706–708
El Ridi R (1998) More on the search for a schistosomiasis vaccine. Parasitol Today 14(10):436
El Ridi R (2012) The road to a sterilizing vaccine for murine Schistosomiasis mansoni using larval excretory-secretory molecules and papain or type 2 cytokines as adjuvant. In: 2nd International conference of vaccines and vaccination, 20–22 August 2012, Hilton Chicago/Northbrook, IL
El Ridi R, Tallima H (2009) Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine 27(5):666–673
El Ridi R, Tallima H (2012) Adjuvant selection for vaccination against murine schistosomiasis. Scand J Immunol 76(6):552–558
El Ridi RAF, Tallima HA-M (2013a) Novel therapeutic and prevention approaches for schistosomiasis. J Adv Res 4(5):467–478
El Ridi R, Tallima H (2013b) Solving the riddle of the lung-stage schistosomula paved the way to a novel remedy and an efficacious vaccine for schistosomiasis. In: El Ridi R (ed) Parasitic diseases—schistosomiasis. ISBN 978-953-51-0942-6. http://www.intechopen.com/articles/show/title/solving-the-riddle-of-the-lung-stage-schistosomula-paved-the-way-to-a-novel-remedy-and-an-efficaciou
El Ridi R, Tallima H (2013c) Vaccine-induced protection against murine schistosomiasis mansoni with larval excretory-secretory antigens and papain or type-2 cytokines. J Parasitol 99(2):194–202
El Ridi R, Tallima H, Selim S, Donnelly S, Cotton S, Gonzales Santana B, Dalton JP (2014) Cysteine peptidases as schistosomiasis vaccines with inbuilt adjuvanticity. Plos One 9(1):e85401
El-Ansary AK, Ahmed SA, Aly SA (2007) Antischistosomal and liver protective effects of Curcuma longa extract in Schistosoma mansoni infected mice. Indian J Exp Biol 45:791–801
El-Banhawey MA, Ashry MA, El-Ansary AK, Aly SA (2007) Effect of Curcuma longa or parziquantel on Schistosoma mansoni infected mice liver–histological and histochemical study. Indian J Exp Biol 45:877–889
Elbaz T, Esmat G (2013) Hepatic and intestinal schistosomiasis. J Adv Res 4(5):445–452. doi:10.1016/j.jare.2012.12.001
El-Garem AA (1998) Schistosomiasis. Digestion 59(5):589–605
el-Mawla NG, el-Bolkainy MN, Khaled HM (2001) Bladder cancer in Africa: update. Semin Oncol 28(2):174–178
El-Sokkary GH, Omar HM, Hassanein AM, Cuzzocera S, Reiter RJ (2002) Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic Biol Med 32:319–332
EMRO (2007) EMRO Report Schistosomiasis. Inter-Country meeting on strategies to eliminate schistosomiasis from the Eastern Mediterranean Region, Muscat, Oman, 6–8 November. http://www.who.int/schistosomiasis/resources/EM
Endriss Y, Escher E, Rohr B, Rohr H, Weiss N (2005) Kato-Katz technique for helminth eggs, chapter 8. In: Methods in parasitology. Swiss Tropical Institute, Basel. http://www.tropeduweb.ch/Parasitology_Methods_PDF/8_Stool_Kato-Katz.pdf
Engels D, Chitsulo L, Montresor A, Savioli L (2002) The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop 82:139–146
Enk MJ, Lima AC, Drummond SC, Schall VT, Coelho PM (2008) The effect of the number of stool samples on the observed prevalence and the infection intensity with Schistosoma mansoni among a population in an area of low transmission. Acta Trop 108(2–3):222–228
Erko B, Medhin G, Teklehaymanot T, Degarege A, Legesse M (2013) Evaluation of urine-circulating cathodic antigen (Urine-CCA) cassette test for the detection of Schistosoma mansoni infection in areas of moderate prevalence in Ethiopia. Trop Med Int Health 18(8):1029–1035
Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN (2000) Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol 164:2585–2591
Fallon PG, Gibbons J, Vervenne RA, Richardson EJ, Fulford AJ, Kiarie S, Sturrock RF, Coulson PS, Deelder AM, Langermans JA, Thomas AW, Dunne DW (2003) Juvenile rhesus monkeys have lower type 2 cytokine responses than adults after primary infection with Schistosoma mansoni. J Infect Dis 187(6):939–945
Ferrari TC, Moreira PR (2011) Neuroschistosomiasis: clinical symptoms and pathogenesis. Lancet Neurol 10:853–864
Ferrari TC, Gazzinelli G, Correa-Oliveira R (2008) Immune response and pathogenesis of neuroschistosomiasis mansoni. Acta Trop 108:83–88
Ferreira RC, Domingues AL, Bandeira AP, Markman Filho B, Albuqerque Filho ES, Correiade de Araújo AC, Batista LJ, Markman M, Campelo AR (2009) Prevalence of pulmonary hypertension in patients with schistosomal liver fibrosis. Ann Trop Med Parasitol 103(2):129–143
Figueiredo JP, Oliveira RR, Cardoso LS, Barnes KC, Grant AV, Carvalho EM, Araujo MI (2012) Adult worm-specific IgE/IgG4 balance is associated with low infection levels of Schistosoma mansoni in an endemic area. Parasite Immunol 34(12):604–610
Fitzsimmons CM, Schramm G, Jones FM, Chalmers IW, Hoffmann KF, Grevelding CG, Wuhrer M, Hokke CH, Haas H, Doenhoff MJ, Dunne DW (2005) Molecular characterization of omega-1: a hepatotoxic ribonuclease from Schistosoma mansoni eggs. Mol Biochem Parasitol 144(1):123–127
Fitzsimmons CM, Jones FM, Pinot de Moira A, Protasio AV, Khalife J, Dickinson HA, Tukahebwa EM, Dunne DW (2012) Progressive cross-reactivity in IgE responses: an explanation for the slow development of human immunity to schistosomiasis? Infect Immun 80(12):4264–4270
Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS et al (2000) The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet 355:887–891
Friedman SL (2000) Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275:2247–2250
Friedman JF, Kanzaria HK, McGarvey ST (2005) Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol 21:386–392
Gad YZ, Ahmad NA, El-Desoky I, Arafa MM, Farag RE (2011) Colorectal schistosomiasis: is it still endemic in delta Egypt, early in the third millennium? Trop Parasitol 1(2):108–110
Gan Y, Shi YE, Bu LY, Ning CX, Zhu HG (2005) Vaccination of mice with recombinant nucleic acid vaccine encoding the integral membrane protein Sj23 and cytokine IL-12 elicits specific immune responses against Schistosoma japonica. Zhonghua Yi Xue Za Zhi 85(3):193–198
Ganley-Leal LM, Guarner J, Todd CW, Da’Dara AA, Freeman GL Jr, Boyer AE, Harn DA, Secor WE (2005) Comparison of Schistosoma mansoni irradiated cercariae and Sm23 DNA vaccines. Parasite Immunol 27(9):341–349
Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, Colley DG, Secor WE (2006) Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infect Immun 74(4):2169–2176
Garjito TA, Sudomo M, Abdullah, Dahlan M, Nurwidayati A (2008) Schistosomiasis in Indonesia: past and present. Parasitol Int 57(3):277–280
Gharib B, Hanna S, Abdallahi OM, Leidi H, Gardette B, De Reggi M (2001) Antiinflammatory properties of molecular hydrogen: investigation on parasite induced liver inflammation. C R Acad Sci III 324:719–724
Ghoneim MA, El-Mekresh MM, El-Baz MA, el-Attar IA, Ashamallah A (1997) Radical cystectomy for carcinoma of the bladder, critical evaluation of the results in 1026 cases. J Urol 158(2):393–399
Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, Rinaldi L, Cringoli G, N’Goran EK, Utzinger J (2010) Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis 4(7):e754
Gomes AL, Melo FL, Werkhauser RP, Abath FG (2006) Development of a real time polymerase chain reaction for quantitation of Schistosoma mansoni DNA. Mem Inst Oswaldo Cruz 101(Suppl 1):133–136
Gordon CA, Acosta LP, Gray DJ, Olveda RM, Jarilla B et al (2012) High prevalence of Schistosoma japonicum infection in Carabao from Samar Province, the Philippines: implications for transmission and control. PLoS Negl Trop Dis 6(9):e1778
Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG (2010) Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis 10:733–736
Greenberg RM (2005) Ca2+ signalling, voltage-gated Ca2+ channels and praziquantel in flatworm neuromusculature. Parasitology 131(Suppl):S97–S108
Greenberg RM (2013) ABC multidrug transporters in schistosomes and other parasitic flatworms. Parasitol Int 62(6):647–653
Grenfell R, Harn DA, Tundup S, Da’dara A, Siqueira L, Coelho PM (2013) New approaches with different types of circulating cathodic antigen for the diagnosis of patients with low Schistosoma mansoni load. PLoS Negl Trop Dis 7(2):e2054
Gryseels B (1996) Uncertainties in the epidemiology and control of schistosomiasis. Am J Trop Med Hyg 55(5 Suppl):103–108
Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368:1106–1118
Gutierrez Y (2000) Diagnostic pathology of parasitic infections with clinical correlation, 2nd edn. Oxford University Press, Oxford, pp 559–562
Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA (1991) Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349(6306):243–245
Harrop R, Jennings N, Mountford AP, Coulson PS, Wilson RA (2000) Characterization, cloning and immunogenicity of antigens released by transforming cercariae of Schistosoma mansoni. Parasitology 121(Pt 4):385–394
Hoffmann KF, Cheever AW, Wynn TA (2000) IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol 164(12):6406–6416
Hoffmann KF, Wynn TA, Dunne DW (2002) Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv Parasitol 52:265–307
Hong XC, Xu XJ, Chen X, Li YS, Yu CH, Yuan Y, Chen YY, Li RD, Qiu J, Liu ZC, Yi P, Ren GH, He HB (2013) Assessing the effect of an integrated control strategy for schistosomiasis japonica emphasizing bovines in a marshland area of hubei province, china: a cluster randomized trial. PLoS Negl Trop Dis 7(3):e2122
Hotez PJ, Savioli L, Fenwick A (2012) Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis 6(2):e1475
Hsu SY, Hsu HF, Davis JR, Lust GL (1972) Comparative studies on the lesions caused by eggs of Schistosoma japonicum and Schistosoma mansoni in livers of albino mice and rhesus monkeys. Ann Trop Med Parasitol 66:89–97
Hughes AJ, Biggs BA (2002) Parasitic worms of the central nervous system: an Australian perspective. Intern Med J 32:541–553
Hürlimann E, Schur N, Boutsika K, Stensgaard AS, Laserna de Himpsl M, Ziegelbauer K, Laizer N, Camenzind L, Di Pasquale A, Ekpo UF, Simoonga C, Mushinge G, Saarnak CF, Utzinger J, Kristensen TK, Vounatsou P (2011) Toward an open-access global database for mapping, control, and surveillance of neglected tropical diseases. PLoS Negl Trop Dis 5(12):e1404
Hurst MH, Willingham AL 3rd, Lindberg R (2000) Tissue responses in experimental schistosomiasis japonica in the pig: a histopathologic study of different stages of single low- or high-dose infections. Am J Trop Med Hyg 62:45–56
IAMAT (2012) World schistosomiasis. Risk chart 2012 edition. http://www.iamat.org/pdf/World_Schistosomiasis_Risk_Chart.pdf
Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL (1996) Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg 55:214–218
Jang-Lee J, Curwen RS, Ashton PD, Tissot B, Mathieson W, Panico M, Dell A, Wilson RA, Haslam SM (2007) Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol Cell Proteomics 6(9):1485–1499
Jankovic D, Cheever AW, Kullberg MC et al (1998) CD4+ T cell mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J Exp Med 187:619–629
Jauréguiberry S, Paris L, Caumes E (2010) Acute schistosomiasis, a diagnostic and therapeutic challenge. Clin Microbiol Infect 16:225–231
Ji F, Liu Z, Cao J et al (2008) B cell response is required for granuloma formation in the early infection of Schistosoma japonicum. PLoS One 3:e1724
Jiz M, Wu HW, Meng R, Pond-Tor S, Reynolds M, Friedman JF, Olveda R, Acosta L, Kurtis JD (2008) Pilot-scale production and characterization of paramyosin, a vaccine candidate for schistosomiasis japonica. Infect Immun 76(7):3164–3169
Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, Pond-Tor S, Wu HW, Manalo D, Olveda R, Acosta L, Kurtis JD (2009) Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun 77(5):2051–2058
Joseph S, Jones FM, Kimani G, Mwatha JK, Kamau T, Kazibwe F, Kemijumbi J, Kabatereine NB, Booth M, Kariuki HC, Ouma JH, Vennervald BJ, Dunne DW (2004a) Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect Immun 72(2):728–734
Joseph S, Jones FM, Walter K, Fulford AJ, Kimani G, Mwatha JK, Kamau T, Kariuki HC, Kazibwe F, Tukahebwa E, Kabatereine NB, Ouma JH, Vennervald BJ, Dunne DW (2004b) Increases in human T helper 2 cytokine responses to Schistosoma mansoni worm and worm-tegument antigens are induced by treatment with praziquantel. J Infect Dis 190(4):835–842
Kamal SM, Turner B, He Q, Rasenack J, Bianchi L, Al Tawil A et al (2006) Progression of fibrosis in hepatitis C with and without schistosomiasis: correlation with serum markers of fibrosis. Hepatology 43:771–779
Kappagoda S, Singh U, Blackburn BG (2011) Antiparasitic therapy. Mayo Clin Proc 86(6):561–583
Kasinathan RS, Greenberg RM (2010) Schistosoma mansoni soluble egg antigens trigger erythrocyte cell death. Cell Physiol Biochem 26(4–5):767–774
Kasinathan RS, Morgan WM, Greenberg RM (2010) Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel. Mol Biochem Parasitol 173(1):25–31
Katchanov J, Nawa Y (2010) Helminthic invasion of the central nervous system: many roads lead to Rome. Parasitol Int 59:491–496
Keiser J, Utzinger J (2007) Advances in the discovery and development of novel trematocidal drugs. Expert Opin Drug Discov 2(Suppl 1):S9–S23
Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J et al (2009) Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis 3:e350
King CH (2010) Parasites and poverty: the case of schistosomiasis. Acta Trop 113(2):95–104
King CH, Magak P, Salam EA, Ouma JH, Kariuki HC, Blanton RE, World Health Organization (2003) Measuring morbidity in schistosomiasis mansoni: relationship between image pattern, portal vein diameter and portal branch thickness in large-scale surveys using new WHO coding guidelines for ultrasound in schistosomiasis. Trop Med Int Health 8:109–117
Krauth SJ, Coulibaly JT, Knopp S, Traoré M, N’Goran EK, Utzinger J (2012) An in-depth analysis of a piece of shit: distribution of Schistosoma mansoni and hookworm eggs in human stool. PLoS Negl Trop Dis 6(12):e1969
Kumar V, Gryseels B (1994) Use of praziquantel against schistosomiasis: a review of current status. Int J Antimicrob Agents 4:313–320
Lambertucci JR, dos Santos Silva LC, Andrade LM, de Queiroz LC, Carvalho VT, Voieta I, Antunes CM (2008) Imaging techniques in the evaluation of morbidity in schistosomiasis mansoni. Acta Trop 108:209–217
Lei JH, Su BT, Xu H, Shen JL, Guan XH, Feng ZQ, Li YL, Xu MX, Liu WQ (2011) Evaluation of an IgY-based immunomagnetic enzyme-linked immunosorbent assay system for detection of circulating Schistosoma japonicum antigen in serum samples from patients in China. Am J Trop Med Hyg 85(6):1054–1059
Leonardo L, Rivera P, Saniel O, Antonio Solon J, Chigusa Y, Villacorte E, Christoper Chua J, Moendeg K, Manalo D, Crisostomo B, Sunico L, Boldero N, Payne L, Hernandez L, Velayudhan R (2013) New endemic foci of schistosomiasis infections in the Philippines. Acta Trop. doi:10.1016/j.actatropica.2013.03.015, pii: S0001-706X(13)00083-1
Li YS, Sleigh AC, Ross AG, Williams GM, Tanner M, McManus DP (2000) Epidemiology of Schistosoma japonicum in China: morbidity and strategies for control in the Dongting Lake region. Int J Parasitol 30(3):273–281
Lin DD, Liu JX, Liu YM, Hu F, Zhang YY, Xu JM, Li JY, Ji MJ, Bergquist R, Wu GL, Wu HW (2008) Routine Kato-Katz technique underestimates the prevalence of Schistosoma japonicum: a case study in an endemic area of the People’s Republic of China. Parasitol Int 57(3):281–286
LoVerde P, Amento C, Higashi G (1980) Parasite–parasite interaction of Salmonella typhimurium and Schistosoma. J Infect Dis 141:177–185
Lucas SB (2002) Other viral and infectious diseases and HIV-related liver diseases, chapter 8. In: MacSween RM et al (eds) Pathology of the liver, 4th edn. Churchill Livingstone, London, pp 389–392
Lundy SK, Lerman SP, Boros DL (2001) Soluble egg antigen stimulated T-helper lymphocyte apoptosis and evidence for cell death mediated by FasL + T and B cells during murine Schistosoma mansoni infection. Infect Immun 69:271–280
Mahana NA (2006) Human and murine immune responses to the Schistosoma mansoni glucose transporter. Ph.D. Thesis, Faculty of Science, Cairo University. 240 p
Mathieson W, Wilson RA (2010) A comparative proteomic study of the undeveloped and developed Schistosoma mansoni egg and its contents: the miracidium, hatch fluid and secretions. Int J Parasitol 40(5):617–628
Maubach G, Lim MCC, Zhang CY, Zhuo L (2006) GFAP promoter directs lacZ expression specifically in a rat hepatic stellate cell line. World J Gastroenterol 12:723–730
Mazigo HD, Nuwaha F, Kinung’hi SM, Morona D, Pinot de Moira A, Wilson S, Heukelbach J, Dunne DW (2012) Epidemiology and control of human schistosomiasis in Tanzania. Parasit Vectors 5:274
Mehlhorn H, Becker B, Andrews P, Thomas H, Frenkel JK (1981) In vivo and in vitro experiments on the effects of praziquantel on Schistosoma mansoni. A light and electron microscopic study. Arzneimittelforschung 31:544–554
Melhem R, LoVerde P (1984) Mechanism of interaction of Salmonella and Schistosoma species. Infect Immun 44:274–281
Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Barker Wynn N, Mutuku MW, Karanja DMS, Colley DG, Black CL, Secor WE, Mkoji GM, Loker ES (2009) Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis 3:e504
Migliardo F, Tallima H, El Ridi R (2014) Is There a sphingomyelin-based hydrogen bond barrier at the mammalian host-schistosome parasite interface? Cell Biochem Biophys 68:359–367
Molyneux DH (2008) Combating the “other diseases” of MDG 6 changing the paradigm to achieve equity and poverty reduction? Trans R Soc Trop Med Hyg 102:509–519
Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES (2001) Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology 123(Suppl):S211–S228
Mostafa IM, Zakaria S, Khalil A, El-Kaluoby A (1990) The effect of medical treatment and endoscopic polypectomy on clinicopathological, immunological and endoscopic aspects of schistosomal colonic polyposis in Egypt. In: Proceedings of the world congresses of gastroenterology, Sydney, Australia, p 1260
Mostafa MH, Sheweita SA, O’Connor PJ (1999) Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev 12(1):97–111
Mostafa OM, Bin Dajem SM, Abu El Einin HM (2009) Susceptibility of Saudi Bulinus truncatus to infection with Egyptian Schistosoma haematobium with observations on protein electrophoretic pattern of the snails. Vet Parasitol 161(3–4):207–212
Mostafa OM, Bin Dajem SM, Al-Qahtani A, Ibrahim EH, Al-Quraishy SA (2012) Developing species-specific primers to identify Bulinus truncatus and Bulinus beccari, the intermediate hosts of Schistosoma haematobium in Saudi Arabia. Gene 499(2):256–261
Mountford AP, Trottein F (2004) Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol 20(5):221–226
Navaratnam AM, Mutumba-Nakalembe MJ, Stothard JR, Kabatereine NB, Fenwick A, Sousa-Figueiredo JC (2012) Notes on the use of urine-CCA dipsticks for detection of intestinal schistosomiasis in preschool children. Trans R Soc Trop Med Hyg 106(10):619–622
Noya O, Alarcón de Noya B, Losada S, Colmenares C, Guzmán C, Lorenzo MA, Bermúdez H (2002) Laboratory diagnosis of Schistosomiasis in areas of low transmission: a review of a line of research. Mem Inst Oswaldo Cruz 97(Suppl 1):167–169
Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, van Dam GJ, van Lieshout L (2008) Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol 102(7):625–633
Ohmae H, Tanaka M, Hayashi M et al (1992) Improvement of ultrasonographic and serologic changes in Schistosoma japonicum-infected patients after treatment with praziquantel. Am J Trop Med Hyg 46:99–104
Osman MM, El-Taweel HA, Shehab AY, Farag HF (2010) Ineffectiveness of myrrh- derivative Mirazid against schistosomiasis and fascioliasis in humans. East Mediterr Health J 16:932–936
Othman AA, Shoheib ZS, Abdel-Aleem GA, Shareef MM (2008) Experimental schistosomal hepatitis: protective effect of coenzyme-Q10 against the state of oxidative stress. Exp Parasitol 120:147–155
Othman AA, Shoheib ZS, Saied EM, Soliman RH (2010) Congenital exposure to Schistosoma mansoni infection: impact on the future immune response and the disease outcome. Immunobiology 215:101–112
Parola M, Robino G (2001) Oxidative stress-related molecules and liver fibrosis. J Hepatol 35:297–306
Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP (2009) Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl Trop Dis 3(10):e528
Pearce EJ, MacDonald AS (2002) The immunobiology of schistosomiasis. Nat Rev Immunol 2:499–511
Pearson MS, Pickering DA, McSorley HJ, Bethony JM, Tribolet L, Dougall AM, Hotez PJ, Loukas A (2012) Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis 6(3):e1564
Peng WX, Tao B, Clements A, Jiang QL, Zhang ZJ, Zhou YB, Jiang QW (2010) Identifying high-risk areas of schistosomiasis and associated risk factors in the Poyang Lake region, China. Parasitology 137(7):1099–1107
Pica-Mattoccia L, Cioli D (1985) Studies on the mode of action of oxamniquine and related schistosomicidal drugs. Am J Trop Med Hyg 34:112–118
Pierce RJ, Dubois-Abdesselem F, Caby S, Trolet J, Lancelot J, Oger F et al (2011) Chromatin regulation in schistosomes and histone modifying enzymes as drug targets. Mem Inst Oswaldo Cruz 106:794–801
Pinto-Silva RA, Queiroz LC, Azeredo LM, Silva LC, Lambertucci JR (2010) Ultrasound in schistosomiasis mansoni. Mem Inst Oswaldo Cruz 105:479–484
Pivarcsi A, Kemény L, Dobozy A (2004) Innate immune functions of the keratinocytes. A review. Acta Microbiol Immunol Hung 51(3):303–310
Platt TR, Brooks DR (1997) Evolution of the schistosomes (Digenea: Schistosomatoidea): the origin of dioecy and colonization of the venous system. J Parasitol 83(6):1035–1044
Pontes LA, Dias-Neto E, Rabello A (2002) Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg 66(2):157–162
Pontes LA, Oliveira MC, Katz N, Dias-Neto E, Rabello A (2003) Comparison of a polymerase chain reaction and the Kato-Katz technique for diagnosing infection with Schistosoma mansoni. Am J Trop Med Hyg 68(6):652–656
Rabello A, Pontes LA, Dias-Neto E (2002) Recent advances in the diagnosis of Schistosoma infection: the detection of parasite DNA. Mem Inst Oswaldo Cruz 97(Suppl 1):171–172
Rajekar H, Vasishta RK, Chawla YK, Dhiman RK (2011) Noncirrhotic portal hypertension. J Clin Exp Hepatol 1:94–108
Ramaswamy K, Kumar P, He YX (2000) A role for parasite-induced PGE2 in IL-10-mediated host immunoregulation by skin stage schistosomula of Schistosoma mansoni. J Immunol 165(8):4567–4574
Reiman RM, Thompson RW, Feng CG, Hari D, Knight R, Cheever AW, Rosenberg HF, Wynn TA (2006) Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun 74:1471–1479
Reis EA, Mauadi Carmo TA, Athanazio R, Reis MG, Harn DA Jr (2008) Schistosoma mansoni triose phosphate isomerase peptide MAP4 is able to trigger naïve donor immune response towards a type-1 cytokine profile. Scand J Immunol 68(2):169–176
Ribeiro de Jesus A, Araújo I, Bacellar O, Magalhães A, Pearce E, Harn D, Strand M, Carvalho EM (2000) Human immune responses to Schistosoma mansoni vaccine candidate antigens. Infect Immun 68(5):2797–2803
Ricard-Blum S, Ville G, Grimaud JA (1992) Pyridinoline, a mature collagen cross-link, in fibrotic livers from Schistosoma mansoni-infected mice. Am J Trop Med Hyg 47:816–820
Riveau G, Deplanque D, Remoué F, Schacht AM, Vodougnon H, Capron M, Thiry M, Martial J, Libersa C, Capron A (2012) Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis 6(7):e1704
Roberts M, Butterworth AE, Kimani G, Kamau T, Fulford AJ, Dunne DW, Ouma JH, Sturrock RF (1993) Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun 61(12):4984–4993
Rofatto HK, Araujo-Montoya BO, Miyasato PA, Levano-Garcia J, Rodriguez D, Nakano E, Verjovski-Almeida S, Farias LP, Leite LC (2013) Immunization with tegument nucleotidases associated with a subcurative praziquantel treatment reduces worm burden following Schistosoma mansoni challenge. PeerJ 1:e58
Ross AG, Sleigh AC, Li Y, Davis GM, Williams GM, Jiang Z, Feng Z, McManus DP (2001) Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev 14(2):270–295
Ross AG, Vickers D, Olds GR, Shah SM, McManus DP (2007) Katayama syndrome. Lancet Infect Dis 7:218–224
Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM (1999) Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol 162:1003–1009
Rutitzky LI, Mirkin GA, Stadecker MJ (2003) Apoptosis by neglect of CD4+ Th cells in granulomas: a novel effector mechanism involved in the control of egg-induced immunopathology in murine schistosomiasis. J Immunol 171:1859–1867
Saidenberg-Kermanac’h N, Boissier M-C, Bouchaud O (2005) Manifestations articulaires des parasitoses. EMC-Maladies infectieuses 2:146–156
Salim OE, Hamid HK, Mekki SO, Suleiman SH, Ibrahim SZ (2010) Colorectal carcinoma associated with schistosomiasis: a possible causal relationship. World J Surg Oncol 8:68
Scholte RG, Carvalho OS, Malone JB, Utzinger J, Vounatsou P (2012) Spatial distribution of Biomphalaria spp., the intermediate host snails of Schistosoma mansoni, in Brazil. Geospat Health 6(3):S95–S101
Scrimgeour EM, Gajdusek DC (1985) Involvement of the central nervous system in Schistosoma mansoni and S. haematobium infection: a review. Brain 108:1023–1038
Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PN, Butler SE, Karanja DM, Secor WE (2011) Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Negl Trop Dis 5(1):e951
Shaw AFB, Ghareeb AA (1938) The pathogenesis of pulmonary schistosomiasis in Egypt with special reference to Ayerza’s disease. J Pathol Bacteriol 46:401–429
Siddiqui AA, Siddiqui BA, Ganley-Leal L (2011) Schistosomiasis vaccines. Hum Vaccin 7(11):1192–1197
Silva LCS, Maciel PE, Ribas JG, Souza-Pereira SR, Antunes CM, Lambertucci JR (2004) Treatment of schistosomal myeloradiculopathy with praziquantel and corticosteroids and evaluation by magnetic resonance imaging: a longitudinal study. Clin Infect Dis 39:1619–1624
Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL (2005) Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology 114:410–417
Sokol CL, Barton GM, Farr AG, Medzhitov R (2008) A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 9(3):310–318
Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R (2009) Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 10(7):713–720
Stavitsky AB (2004) Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun 72:1–12
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6(7):411–425
Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, Webster JP, Fenwick A (2006) Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop 97(2):219–228
Tachon P, Borojevic R (1978) Mother–child relation in human schistosomiasis mansoni: skin test and cord blood reactivity to schistosomal antigens. Trans R Soc Trop Med Hyg 72:605–609
Tagboto S, Townson S (2001) Antiparasitic properties of medicinal plants and other naturally-occurring products. Adv Parasitol 50:199–295
Tallima H, El Ridi R (2007) Praziquantel binds Schistosoma mansoni adult worm actin. Int J Antimicrob Agents 29:570–575
Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B (2010) The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol 11(7):608–617
Taylor JJ, Mohrs M, Pearce ED (2006) Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol 176:5839–5847
Tchuem Tchuenté LA, Kueté Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, Gipwe NF, Nana ED, Stothard JR, Rollinson D (2012) Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl Trop Dis 6(7):e1758
Teixeira de Melo T, Araujo JM, Campos de Sena I, Carvalho Alves C, Araujo N, Toscano Fonseca C (2013) Evaluation of the protective immune response induced in mice by immunization with Schistosoma mansoni schistosomula tegument (Smteg) in association with CpG-ODN. Microbes Infect 15(1):28–36
Tendler M, Simpson AJ (2008) The biotechnology-value chain: development of Sm14 as a schistosomiasis vaccine. Acta Trop 2–3:263–266
Todd CW, Colley DG (2002) Practical and ethical issues in the development of a vaccine against schistosomiasis mansoni. Am J Trop Med Hyg 66(4):348–358
Tonkal AM, Morsy TA (2008) An update review on Commiphora molmol and related species. J Egypt Soc Parasitol 38:763–796
Tracy JW, Webster LT (2001) Drugs used in the chemotherapy of helminthiasis. In: Hardman JG, Limbind LE, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics, 10th edn. McGraw-Hill, New York, pp 1134–1136
Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, Don TA, McManus DP, Correa-Oliveira R, Loukas A (2006) Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med 12(7):835–840
Turner JD, Meurs L, Dool P, Bourke CD, Mbow M, Dièye TN, Mboup S, Polman K, Mountford AP (2013) Schistosome infection is associated with enhanced whole blood IL-10 secretion in response to cercarial excretory/secretory products. Parasite Immunol 35(5–6):147–156
Utzinger J, Xiao SH, Tanner M, Keiser J (2007) Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs 8:105–116
Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N, Singer BH, N’goran EK (2009) Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136(13):1859–1874
Utzinger J, N’goran EK, Caffrey CR, Keiser J (2011) From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop 120(Suppl 1):S121–S137
Van Dam GJ, Bogitsh BJ, van Zeyl RJ, Rotmans JP, Deelder AM (1996) Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J Parasitol 82(4):557–564
Van Gool T, Vetter H, Vervoort T, Doenhoff MJ, Wetsteyn J, Overbosch D (2002) Serodiagnosis of imported schistosomiasis by a combination of a commercial indirect hemagglutination test with Schistosoma mansoni adult worm antigens and an enzyme-linked immunosorbent assay with S. mansoni egg antigens. J Clin Microbiol 40(9):3432–3437
van Velthuysen ML, Florquin S (2000) Glomerulopathy associated with parasitic infections. Clin Microbiol Rev 13(1):55–66
von Lichtenberg F, Sher A, McIntyre S (1977) A lung model of schistosome immunity in mice. Am J Pathol 87(1):105–124
Wang X, Dong L, Ni H, Zhou S, Xu Z, Hoellwarth JS, Chen X, Zhang R, Chen Q, Liu F, Wang J, Su C (2013) Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine. PLoS Negl Trop Dis 7(4):e2164
Warren W, Biggs PJ, El-Baz M, Ghoneim MA, Stratton MR, Venitt S (1995) Mutations in p53 gene in schistosomal bladder cancer. Carcinogenesis 16:1181–1189
Watt G, Padre LP, Tuazon M, Wotherspoon A, Adapon B (1991) Hepatic parenchymal dysfunction in Schistosoma japonicum infection. J Infect Dis 164(6):1186–1192
WHO (2006) Preventive Chemotherapy in Human Helminthiasis. Coordinated use of anthelmintic drugs in control interventions: a manual for health professionals and programme managers. World Health Organization, Geneva
WHO (2013) Strategic plans 2012–2020. WHO, Geneva, 2013
Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA (2007) Immunopathology of schistosomiasis. Immunol Cell Biol 85:148–154
Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW (2008) Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasis and malaria coinfection. Infect Immun 76(5):2212–2218
Wilson S, Jones FM, Fofana HK, Doucouré A, Landouré A, Kimani G, Mwatha JK, Sacko M, Vennervald BJ, Dunne DW (2013a) Rapidly boosted plasma IL-5 induced by treatment of human Schistosomiasis haematobium is dependent on antigen dose, IgE and eosinophils. PLoS Negl Trop Dis 7(3):e2149
Wilson S, Jones FM, Fofana HK, Landouré A, Kimani G, Mwatha JK, Sacko M, Vennervald BJ, Dunne DW (2013b) A late IL-33 response after exposure to Schistosoma haematobium antigen is associated with an up-regulation of IL-13 in human eosinophils. Parasite Immunol 35(7–8):224–228
World Health Organization (2009) Preventive chemotherapy databank. http://www.who.int/neglected_diseases/preventive_chemotherapy/databank/en/index.html
World Health Organization (2010) Atlas of the global distribution of schistosomiasis. Available at http://www.who.int/wormcontrol/documents/maps/en. Accessed 30 June 2010
World Health Organization (2011) Elimination of schistosomiasis. http://apps.who.int/gb/ebwha/pdf_files/EB130/B130_20-en.pdf
Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM (2004) Immunopathogenesis of schistosomiasis. Immunol Rev 201:156–167
Xiang X, Tianping W, Zhigang T (2003) Development of a rapid, sensitive, dye immunoassay for schistosomiasis diagnosis: a colloidal dye immunofiltration assay. J Immunol Methods 280(1–2):49–57
Xiao S, Tanner M, N’Goran EK, Utzinger J, Chollet J, Bergquist R et al (2002) Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop 82:175–181
Xiao SH, Keiser J, Chollet J, Utzinger J, Dong Y, Endriss Y, Vennerstrom JL, Tanner M (2007) In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob Agents Chemother 51:1440–1445
Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM (2010) Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int J Parasitol 40(3):327–331
Yang W, Jackson DC, Zeng Q, McManus DP (2000) Multi-epitope schistosome vaccine candidates tested for protective immunogenicity in mice. Vaccine 19(1):103–113
Yousif F, Hifnawy MS, Soliman G, Boulos L, Labib T, Mahmoud S et al (2007) Large-scale in vitro screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm Biol 45:501–510
Yu JM, de Vlas SJ, Jiang QW, Gryseels B (2007) Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int 56(1):45–49
Zhang LJ, Zheng WD, Shi MN, Wan XZ (2006) Effects of interleukin-10 on activation and apoptosis of hepatic stellate cells in fibrotic rat liver. World J Gastroenterol 12:1918–1923
Zhang W, Li J, Duke M, Jones MK, Kuang L, Zhang J, Blair D, Li Y, McManus DP (2011) Inconsistent protective efficacy and marked polymorphism limits the value of Schistosoma japonicum tetraspanin-2 as a vaccine target. PLoS Negl Trop Dis 5(5):e1166
Zhao GH, Li J, Blair D, Li XY, Elsheikha HM, Lin RQ, Zou FC, Zhu XQ (2012a) Biotechnological advances in the diagnosis, species differentiation and phylogenetic analysis of Schistosoma spp. Biotechnol Adv 30(6):1381–1389
Zhao QP, Jiang MS, Dong HF, Nie P (2012b) Diversification of Schistosoma japonicum in Mainland China revealed by mitochondrial DNA. PLoS Negl Trop Dis 6(2):e1503
Zhou YB, Yang MX, Wang QZ, Zhao GM, Wei JG, Peng WX, Jiang QW (2007) Field comparison of immunodiagnostic and parasitological techniques for the detection of schistosomiasis japonica in the People’s Republic of China. Am J Trop Med Hyg 76(6):1138–1143
Zhou YB, Yang MX, Tao P, Jiang QL, Zhao GM, Wei JG, Jiang QW (2008) A longitudinal study of comparison of the Kato-Katz technique and indirect hemagglutination assay (IHA) for the detection of schistosomiasis japonica in China, 2001-2006. Acta Trop 107(3):251–254
Zhou YB, Liang S, Chen GX, Rea C, He ZG, Zhang ZJ, Wei JG, Zhao GM, Jiang QW (2011a) An integrated strategy for transmission control of Schistosoma japonicum in a marshland area of China: findings from a five-year longitudinal survey and mathematical modeling. Am J Trop Med Hyg 85(1):83–88
Zhou YB, Zheng HM, Jiang QW (2011b) A diagnostic challenge for schistosomiasis japonica in China: consequences on praziquantel-based morbidity control. Parasit Vectors 4:194
Zhou YB, Liang S, Jiang QW (2012) Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors 5:275
Zhou YB, Liang S, Chen GX, Rea C, Han SM, He ZG, Li YP, Wei JG, Zhao GM, Jiang QW (2013) Spatial-temporal variations of Schistosoma japonicum distribution after an integrated national control strategy: a cohort in a marshland area of China. BMC Public Health 13:297
Zhu Y, Lu F, Dai Y, Wang X, Tang J, Zhao S, Zhang C, Zhang H, Lu S, Wang S (2010) Synergistic enhancement of immunogenicity and protection in mice against Schistosoma japonicum with codon optimization and electroporation delivery of SjTPI DNA vaccines. Vaccine 28(32):5347–5355
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Othman, A., El Ridi, R. (2014). Schistosomiasis. In: Bruschi, F. (eds) Helminth Infections and their Impact on Global Public Health. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1782-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1782-8_3
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1781-1
Online ISBN: 978-3-7091-1782-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)