Abstract
The importance of safe and high-quality food products is doubtless and consumer demand for increased food quality and safety assurances moves down the chain with retailers and service providers asking suppliers and producers to provide verifiable proof that robust food quality and safety control systems have been effectively implemented. Food analysis needs rapid and reliable methods to ensure the quality of products and process control. Food quality control is essential both for consumer protection and also for food industry. Nowadays, the convergence of new technologies, including biotechnology, nanotechnology, and electronic technology, has opened new horizons in development of biosensors. These devices offer advantages as alternatives to conventional methods because they enable real-time detection, portability, and fast laboratory or in-field analysis. This contribution presents a review about the development and application of biosensor technology in foods, and future trends in Brazil and Italy.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Background
In recent years, food-safety emergencies have shaken consumer confidence in the food production chains, focusing attention on how food is produced, processed, and marketed. National Health Agencies, around the world, have recognized food safety and food quality as a top priority. They have established new policies, modernizing legislation into a coherent and transparent set of rules, which can guarantee high level of consumer protection and, thus, human health. An effective food safety policy requires the assessment and monitoring of the risks to consumer health, associated with the presence of contaminants in raw materials, farming practices, and food processing activities.
In the course of the third Spring School organized by the Brazilian State University of Sao Paulo (UNESP) and the Italian University of Padova (Italy) in the Botucatu Campus (Bioscience Institute) in September 18–20th, 2012, these themes were faced and in this chapter recent biosensors developed in the two countries are reviewed.
In fact, the rigorous control of food quality and safety is of growing interest for both consumers and food industries. In the food industry, the quality of a product is evaluated by periodic chemical and microbiological analysis. It has become important to periodically measure a variety of contaminants in food, such as bacteria, viruses, natural toxins, chemical compounds (pesticides, toxic metals, veterinary drugs residues, undesirable fermentation products), and packaging materials. Most of the toxic agents found in foods are natural contaminants from environmental sources, but some of them are chemical compounds deliberately added during food processing (Codex 2009). Consumers are concerned about long-term impacts of mixtures of these chemical additives (pesticides, toxic metals, flavorings, and colors) and about their chronic, as well as, acute effects, especially on children (Jackson 2009).
While the knowledge of phytochemical effects on human health and risks from chemical residues in food has led to a growing interest and attention towards the fast growth of functional and enriched food on the market, only little emphasis has been placed on the analytical aspects (Lavecchia et al. 2013). In this regard, specific, new technologies have recently been developed to examine food components and different analytical procedures were developed to assess food quality and to determine food contaminants. Normally, these procedures are based on various instrumental techniques, such as chromatography, spectrophotometry, electrophoresis, titration etc. These analytical procedures do not easily allow continuous monitoring, mainly because they are based on expensive instrumentations and they need time-consuming multistep sample extraction and pretreatments and well-trained operators, which increase the time and cost of the analysis. Meanwhile, National Health agencies and food industry request affordable methods to determine compounds of interest in foods.
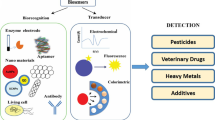
The demand for fast and real-time analyses aimed at the detection of substance related to food quality and safety led to rapid advancements in biosensors technology (Mozaz et al. 2004). These compact analytical devices incorporate a biological sensing element, either closely connected to or integrated within a transducer system able to convert a biological event in an electrical signal (see Fig. 11.1). The recognition mechanism is based on the interaction of biological recognition component with the analyte of interest, by different recognition mechanisms. Information, which is produced in the recognition event, is transformed by means of the transducer into a signal. The Physical Chemistry and Analytical Chemistry Divisions of IUPAC (Thevenot et al. 1999) state a definition applicable to electrochemical biosensors: “A biosensor is a self-contained integrated device that is capable of providing specific quantitative or semi-quantitative analytical information using a biological recognition element (biochemical receptor) which is in direct spatial contact with a transduction element.”
Four major types of transducers can be found: electrochemical, mass, optical, and thermal (Thevenot et al. 1999). They have been used to develop biosensor aimed at the detection of a broad spectrum of analytes present in complex sample matrices. Great promises in different areas, such as clinical diagnostics, food analysis, and environmental monitoring (Table 11.1), have been proposed and the sensitivity of a particular sensor system varies depending on the transducer’s properties and the biological recognizing element.
In general, biosensors consist of three main components as shown in Fig. 11.1: a recognition element, a transducer unit, and a controlling electronic unit, including an input/output interface. The recognition element, which binds to the analyte of interest, is the component producing the primary signal. The transducer represents the biosensor component, which is responsible for the transformation of the primary signal, coming from the recognition element, to a form that can be filtered, amplified, and transferred to the electronic component, which finally processes and displays, and even stores, the analytical result. The choice of the biological element and proper transducer depends on the properties of the sample of interest and the physical magnitude to be measured. The recognition element, that is, the biocomponent, determines the degree of selectivity or specificity of the final biosensor.
In this chapter, a brief commentary on some aspects of biosensor construction is reported. Current situation, recent development, and applications of biosensors for food technology in Brazil and Italy are reviewed.
2 Biosensor for Small Molecule Determination in Food Analysis
Among proposed biosensors, electrochemical transduction systems were the most used. Among them, amperometric and potentiometric transduction have found the widest applications, even if other transducers are available. The combination of oxidoreductases, as recognition bioelements, and amperometric electrodes, as trasducers, gave good results for food analysis, mainly because enzymatic activity, depending on substrate concentration in food samples, can be easily measured with reasonable sensitivity. This combination constitutes one of the most successful classes of biosensors.
Recently, Ferreira et al. (2004) immobilized two enzymes, namely, β-galactosidase and glucose oxidase, in order to determine lactose in cheese whey. The biosensor was based on the determination of oxygen consumption, which occurs during the enzymatic reaction. Authors studied the influence of temperature on the biosensor signal, observing a nonlinear relationship between the biosensor electric response and lactose concentrations a function of temperature and analyte concentration. This was due to differences in temperature dependencies of enzyme activities. Nonlinear correlations were proposed to automatically compensate the effects of temperature. Mello et al. (2003) developed a biosensor based on horseradish peroxidase (HRP) and immobilized DNA onto silica–titania and applied the novel system to the measurement of polyphenol compounds in vegetables samples. Various analytical parameters influencing the biosensor performances were studied as a function of chlorogenic acid, as reference polyphenol compound. The effect of working potential, type and concentration of the buffer, pH, response time, and interferences was investigated. The biosensor showed a linear response in the range from 1 to 50 μM chlorogenic acid, applying a potential of −50 mV versus Ag/AgCl, with a sensitivity of 181 nA/μM/cm2 and a detection limit of 0.7 μM. The biosensor was tested for polyphenol determination in vegetable extract and the results were compared with the Folin–Ciocalteau traditional method. The biosensor showed suitability to the quantification of the total polyphenol in the tested samples.
Other authors described a highly selective and stable electrochemical biosensor for the determination of glucose in soluble coffee (de Mattos and Areias 2005) The biosensor electrode consisted of a thin film of ferric hexacyanoferrate, electrodeposited on the glassy carbon electrode and glucose oxidase immobilized on top of the electrode surface. Stability of the thin film was evaluated by injecting standard solution of H2O2 and glucose during 4 h in a flow-injection system, with the electrode polarized at −50 mV versus Ag/AgCl. The system was able to handle about 60 samples per hour, with high stability, and was proposed for industrial process control. A linear calibration in the range of 0.15 and 2.50 mM glucose and a detection limit of 0.03 mM were obtained. Another biosensor for glucose determination in food samples was developed by one of the authors (Baratella et al. 2013). The experimental work demonstrated the peculiar electro-catalytic behavior of a new generation of iron oxide nanoparticles (surface-active magnetite nanoparticles, SAMNs), which were used for the development of a cheap carbon paste electrode aimed at hydrogen peroxide detection, containing an ionic liquid, namely, 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM-PF6), and characterized by a sensitivity of 206.51 μA/mM/cm2, a detection limit of 0.8 μM H2O2, and a noise of 1.01 nA. Furthermore, these metal oxide nanoparticles were used to form stable conjugates with rhodamine isothiocyanate, acting as a bridge, permitting the covalent binding of glucose oxidase. The resulting bio-conjugate was used to develop a nanocomposite, carbon paste-BMIM-PF6-based biosensor, characterized by a sensitivity of 48 μA/mM/cm2, in the 0–1.5 mM glucose concentration range, and a detection limit of 0.9 μM glucose. The system was tested on fruit juices as real samples, without any sample preparation procedure and results suggest that BMIM-PF6–(SAMN@RITC–GOx)/CPE biosensor could be a promising, low-cost option for the development of GOx-based biosensors for glucose determination.
Amati et al. (2008) reported on a biosensor for the determination of the total antioxidant capacity and the total polyphenol concentration in extra virgin oil (EVO), as well as the main kinetic parameters of the process during the thermal oxidation of EVO. They also evaluated the increase of radical concentration during the thermal oxidation process, using a superoxide dismutase biosensor. The investigation concerning this important food product was of high interest, as it referred to the state of alteration of the EVO, used for cooking or frying, as a function of temperature.
Currently, the food industry is very receptive to biosensor technology, while a new market will probably be developed in the long run. A method for the rapid detection of common compounds will probably offer the best opportunity for biosensors in this industrial field, but several key issues also need to be resolved before biosensors find widespread applications. Newly, a new biosensor for the direct determination of lactic acid and malic acid in wines was developed (Mazzei et al. 2007). This multi-enzymatic biosensor was realized for the selective determination of three analytes: d(−) and l(+)-lactic acid and l(−)-malic acid. The measurement was based on a multi-enzymatic biosensor employing the catalytic activities of l(+)-lactate oxidase, d(−)-lactate dehydrogenase, and horseradish peroxidase for the determination of total d(−)- and l(+)-lactic acid and a bienzymatic electrode for l(−)-malic acid determination, realized by coupling the l(−)-malic dehydrogenase and horseradish peroxidase. For both electrodes, enzymes were immobilized on an oxygen-selective Clark electrode and the simultaneous determination of the two organic acids was accomplished either in batch or in a flow injection analysis apparatus, using the same biosensors as detectors. The analytical performances were tested in standard aqueous solutions and on real wine samples, showing high repeatability, short response times, and reduced cost of analysis, suggesting that the experimental approach here described could be convenient to monitor the progress of malo-lactic fermentation in wines.
A paper by Centonze and coworkers described a biosensor for simultaneous monitoring of glucose and ethanol content in drinks and alcoholic fermented media (Mentana et. al 2013). The methodology was based on the immobilization of glucose oxidase and alcohol oxidase by co-cross-linking with bovine serum albumin and glutaraldehyde onto a dual gold electrode, modified with a perm selective over oxidized polypyrrole film. The biosensor was integrated in a flow injection system, coupled with an online microdialysis fiber as sampling tool. Flow rates inside and outside the fiber were optimized in terms of linear responses (0.01–1 and 0.01–1.5 M) and sensitivities (27.6 ± 0.4 and 31.0 ± 0.6 μA/mM/cm2) for glucose and ethanol, respectively. Excellent anti-interference characteristics, with total absence of “cross talk,” and good response stability, under the operational conditions, allowed the application of the dual biosensor to the accurate real-time monitoring of alcoholic drinks and white grape musts.
Arecchi et al. (2010) described an amperometric biosensor for the detection of phenolic compounds in food, based on tyrosinase as bioelement. The enzyme was immobilized by drop-coating on a glassy carbon electrode, covered by a polyamidic nanofibrous membrane, prepared by electrospinning. With respect to others examples in the literature, the selectivity of the tyrosinase biosensor resulted in modification by the presence of the nanostructured coating, which seemed to affect the permeability of polyphenols as a function of solution pH, depending on polyphenol dissociation constants. The biosensor exhibited a response time of 16 s, a detection limit of 0.05 μM, and a linearity up to 100 mM. This biosensor was successfully used for real-time monitoring of the release kinetics of phenols, encapsulated in polymeric microcapsules.
Moreover, innovative detection methods for toxic compound detection in foods were proposed by different groups in Brazil and Italy. An example dealt with the development of a biosensor as an analytical device for the detection of beta-lactam residues in milk (Ferrini et al. 2008). This indirect method was based on the measurements of carbon dioxide (CO2), produced by the microbial growth of a reference microorganism, namely, Bacillus stearothermophilus var. calidolactis. The addition of milk samples to the cultivation medium led to microbial growth inhibition, if beta-lactams are present, and, consequently, a decrease of CO2 production rate. The analysis was based on the differences of CO2 production between a milk sample, spiked with beta-lactams, and a twin milk sample, containing beta-lactams plus a broad spectrum beta-lactamases, using an electrochemical device. Moreover, the ability to sense all of the beta-lactams speeds the total time of analysis, when chemical identification and quantification are not required. The analytical method was adequate for milk control for qualitative screening purposes, complying with the requirements stated in Europe by the Decision 2002/657/EC. Campanella et al. (2009) described a new biosensor for rapid determination of nonsteroidal anti-inflammatory drugs (NSAIDs), based on the inhibition of cyclooxygenase by NSAIDs in fresh milk. The results showed the full validity of the method, which was optimized by comparing the inhibition of two enzyme isoforms, COX-1 and COX-2, in the presence of different tested pharmaceutical drugs (diclofenac, naproxen, ibuprofen, tolmetin). Recovery trials were performed in adulterated milk and fresh cheese samples with known concentrations of NSAIDs, always obtaining recovery values >88 %.
To date control strategies in detecting anabolic agents for promoting growth of food producing animals are mainly related to screening techniques based on immunochemical and physiochemical methods, whose major limit is represented by relative low analytical sensitivity. As a consequence, consumers are currently exposed to molecules with potential carcinogenic effects, such as 17β-estradiol, the most powerful substance with estrogenic effect. Therefore, high analytical sensitivity screening and confirmatory methods are required, coupling easiness of use and efficiency. Ricciardi et al. (2013a, b) reported on the immunodetection of 17β-estradiol in serum by antibody-immobilized microcantilever resonators, an innovative biosensing platform able to quantify an adsorbed target mass (such as cells, nucleic acids, biomolecules, etc.) thanks to a shift in resonance frequency. The analytical tool showed to be capable of discriminating treated and untreated animals, showing the ability of detecting traces of 17β-estradiol in serum at concentrations lower than the present accepted physiological serum concentration threshold value (40 ng/kg) and commercial ELISA tests (25 ng/kg). The method exhibits a limit of detection of 20 ng/kg and a limited cross-reactivity with high concentrations (10 μg/kg) of similar molecules (testosterone).
3 Biosensors for Bacteria and Bacteria Toxins Detection
The presence and diffusion of pathogenic bacteria in foodstuff represent the main concern for food safety, and innovative determination methods for microorganism and biological toxin detection, based on biosensor technology, were proposed. Recently, Rejeb et al. (2009) reported on a biosensor based on acetylcholinesterase (AChE) inhibition by mycotoxins, namely, aflatoxin B-1 (AFB-1). AChE was present in solution and an amperometric choline oxidase biosensor was used for monitoring AChE residual activity by determining choline produced from acetylcholine hydrolysis. To create the biosensor, choline oxidase was immobilized by cross-linking onto a screen-printed electrode modified with Prussian Blue and this was used to detect the H2O2 produced by choline oxidation at low applied potential (−0.05 V versus a screen-printed internal silver pseudo-reference electrode). For the development of the AFB-1 assay, several parameters, such as AChE and substrate concentrations, the effect of methanol, and pH, were evaluated and optimized. Authors found a linear working range of 10–60 ppb for AFB-1, and concentrations as low as 2 ppb, corresponding to the legal limit of AFB-1 in food for humans, were detected, after a pre-concentration step. The suitability of the method was evaluated using commercial olive oil samples.
Reis group’s reported a new methodology based on the colorimetric response induced by pathogenic bacteria (Staphylococcus aureus and Escherichia coli) (Pires et al. 2011). The addition of bacterial supernatants caused a colorimetric modification in the presence of 10,12-pentacosadyinoic acid (PCDA) and N-[(2-tetradecanamide)-ethyl]-ribonamide (TDER) vesicles, even in diluted concentrations, indicating that chemical interactions occur between the vesicles and bacteria. It was observed that bacterial substrates released from S. aureus induced a more intense color change, compared to the optical response induced by E. coli. S. aureus metabolites also induced a more pronounced color change when TDER/PCDA vesicles were incorporated into cellulose strips. Authors analyzed the colorimetric response in the presence of interferent molecules, using apple juice as food matrix. Both apple juice samples, sterile and inoculated with bacteria, induced a TDER/PCDA color change; however, the S. aureus supernatants induced a slightly greater color response both in the suspensions and in the cellulose strips. TDER/PCDA vesicles showed a great potential for the development of biosensors to detect food pathogens in intelligent food packaging.
A simple and fast response biosensor for screening nisin, directly identifying nisinogenic bacteria, by bioluminescence detection of Lactococcus lactis was proposed (Virolainen et al. 2012). The method is based on the nisin-controlled gene expression system which facilitates efficient overexpression of heterologous genes. The overlay of putative nisinogenic colonies with the biosensor strain gives identification results within 1 h. Functionality and specificity of the method were verified by screening for the presence of nisin producing bacteria among 144 raw milk colonies and a panel of 91 lactococcal strains. Studies performed on strains and colonies, which did not induce bioluminescence but inhibited Lactococcus lactis NZ9800lux growth, demonstrated that only nisinogenic bacteria can cause bioluminescence induction. Bacteria known to produce bacteriocins, other than nisin, failed to induce bioluminescence, further confirming the specificity of the assay. Moreover, authors discovered a new non-inducing, but inhibitory, lactococcal strain harboring a modified nisin Z gene and demonstrated that the source of the inhibitory action is not a non-inducing variant of nisin, but a bacteriocin of lower molecular weight. The concentration of nisin producing bacteria in a raw milk sample was 1.3 × 102 CFU/mL. A total of seven nisin Z producing colonies of L lactis subsp. lactis, which were shown to belong to three different groups by genetic fingerprinting, were also identified in raw milk samples. The presented biosensor is robust, cost-effective, and simple to use, avoiding the pitfalls of traditional screening methods by directly specifying the identity of the toxic substance.
Zamolo et al. (2012) developed an ultrasensitive electrochemiluminescence-based sensor for the detection of palitoxin (PlTX), one of the most potent marine toxins, frequently detected in seafood, taking advantage of the specificity provided by anti-PlTX antibodies, the good conductive properties of carbon nanotubes, and the excellent sensitivity achieved by a luminescence-based transducer. The sensor was able to produce a concentration-dependent light signal, allowing PlTX quantification in mussels, with a limit of detection of 2.2 μg/kg of mussel meat), more than two orders of magnitude more sensitive than that of the commonly used detection techniques, such as LC-MS/MS.
An antibody-immobilized microcantilever resonator system was proposed for the detection of mycotoxins, such as aflatoxins and ochratoxin A, which are considered as the most important chronic dietary risk factor, more than food additives or pesticide residues (Ricciardi et al. 2013a, b). The feasibility of using microcantilever resonator arrays to effectively identify total aflatoxins and ochratoxin A, at low concentrations (3 ng/mL and less than 6 ng/mL, respectively), with relatively low uncertainty (about 10 %) and good reproducibility for the same target concentration, was shown. Furthermore, the developed immunosensing method shows a limited cross-reactivity to different mycotoxins, paving the way to a highly specific technique, able to identify different mycotoxins in the sample.
Surface plasmon resonance (SPR)-based DNA biosensors were shown to be rapid, label-free, and selective tools for the detection of PCR products (Pascale et al. 2013). An SPR sensor based on DNA hybridization for the detection of Fusarium culmorum, a fungal pathogen of wheat, was described. A 0.57 kb DNA fragment of F. culmorum was amplified by specific primers, and a 25-mer oligonucleotide probe was selected within the sequence of the PCR amplified. The biotin-labeled probe was immobilized on a streptavidin sensor chip and tested for biospecific interaction with PCR products of F. culmorum. The SPR biosensor was applied to the detection of F. culmorum in fungal cultures and in naturally infected wheat samples.
Another example from Italy dealt with an electrochemical immunoassay, developed using magnetic beads as solid phase and carbon screen-printed arrays as transducers, was developed for the detection of sulfonamides in food matrices (Centi et al. 2010). Magnetic beads, coated with protein A, were modified by immobilization of specific antibodies and a competition between the target analyte and the corresponding labeled analyte was carried out. Analyte labeling was performed with alkaline phosphatase. After the immunosensing step, beads were captured by a magnet onto the working electrode surface of a screen-printed eight-electrode array for a multiple electrochemical detection. Screen-printed eight-electrode arrays were chosen as transducers due to the possibility to repeat multiple analysis and to test simultaneously different samples. The determination was performed by differential pulse voltammetry, as fast electrochemical technique. Calibration curves demonstrated that the developed electrochemical immunoassay was able to detect concentrations as low as ng/mL. The short incubation times and the fast electrochemical measurement make this system a possible alternative to classic ELISA tests.
Nucleic acid aptamers have been presented as a new way to detect pathogenic compound. These macromolecules have attracted intense interest and found wide applications in a range of areas (Palchetti and Mascini 2012; Tombelli and Mascini 2009). Aptamers exhibit many advantages as recognition elements in biosensing when compared to traditional antibodies. They are small in size, chemically stable, and cost effective. More importantly, aptamers offer remarkable flexibility and convenience in the design of their structures, which has led to novel biosensors that have exhibited high sensitivity and selectivity. Recently, Castillo et al. (2012) development a biosensor based on DNA aptamers for detection of ochratoxin A (OTA). The thiolated DNA aptamers specific to OTA of different configurations have been immobilized by chemisorption to the surface of a gold electrode. Electrochemical impedance spectroscopy in the presence of a redox probe, such as [Fe(CN)6]−3/−4, has been used for the determination of the charge transfer resistance, following the addition of OTA containing samples. Charge transfer resistance increased with increasing OTA concentration in the range 100–0.1 nM. The limit of detection (0.12 nM) depended on aptamer configuration. The sensor was renewable and validated on food samples with satisfactory recovery.
Silva et al. (2012) described a new DNA biosensor for the detection of toxigenic Penicillium sclerotigenum in pure culture or infected yams. The P. sclerotigenum detection takes place on a self-assembled monolayer of a (magnetite)/(poly(allylamine hydrochloride) (Fe3O4–PAH) composite that serves as an anchoring layer for the DNA hybridization interaction. Electrical impedance spectroscopy (EIS) was used to evaluate and quantify the hybridization degree. The Fe3O4–PAH composite represents a good platform for the immobilization of biomolecules, due to the presence of many possible binding sites for nucleotides and to its large surface-to-volume ratio, and good biocompatibility. The biosensor was capable of not only qualitatively detecting the presence of the fungus genome at low concentrations, but also showed a good quantitative impedimetric response. A Fe3O4–PAH-probe biosensor would require only small volumes and low concentrations of the analyte when used, for instance, in detecting P. sclerotigenum contamination of food, besides presenting many competitive advantages, such as selectivity, specificity, and reproducibility, relative to alternative techniques.
The development of a novel electrochemical immunosensor for the sensitive detection of staphylococcal enterotoxin A (SEA) based on self-assembly monolayer (SAM) and protein A immobilization on gold electrode was reported (Pimenta-Martins et al. 2012). Three different methods of protein A immobilization were tested: physical adsorption, cross-linking using glutaraldehyde, and covalent binding after activation with N-hydroxysuccinimide/N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride on cysteamine-modified gold electrode. The EDC/NHS method for protein A immobilization was selected to lead development of the biosensor. The coating steps of the surface modification were characterized by cyclic voltammetry and the biosensor response by chronoamperometry. The advantages of the immunosensor were exposed in its high sensitivity and specificity. The proposed amperometric immunosensor was successfully used for determination of SEA in contaminated and non-contaminated cheese samples.
4 Pesticide Biosensors
Pesticides represent another class of compounds that have been studied in food analysis by different research groups in Brazil and Italy. Pesticides have been associated with many health hazards and rapid and reliable monitoring of these contaminants is mandatory.
Traditional chromatographic methods, such as high-performance liquid chromatography and capillary electrophoresis, equipped with mass spectrometry detectors, are effective for the analysis of pesticides in the environment, but present previously cited limitations. Thus, the use of biosensors to replace classical analytical methods by simplifying or eliminating sample preparation and making field-testing easier and faster with significant decrease in cost per analysis is attractive (Kantiani et al. 2010; McGrath et al. 2012).
A sensitive electrochemical acetylcholinesterase (AChE) biosensor was successfully developed with polyaniline (PANI) and multi-walled carbon nanotubes (MWCNTs) core–shell modified glassy carbon electrode (GC) and used to detect carbamate pesticides in fruit and vegetables (apple, broccoli, and cabbage). The pesticide biosensors were applied in the detection of carbaryl and methomyl pesticides in food samples using chronoamperometry. The GC/MWCNT/PANI/AChE biosensor exhibited detection limits of 1.4 and 0.95 μM, respectively, for carbaryl and methomyl. These detection limits were below the allowable concentrations set by Brazilian regulation standards for the samples in which these pesticides were analyzed. Reproducibility and repeatability values of 2.6 % and 3.2 %, respectively, were obtained with the procedure. The proposed biosensor was successfully applied for the determination of carbamate pesticides in cabbage, broccoli, and apple samples, without any spiking procedure. The obtained results were in full agreement with those from HPLC analysis (Cesarino et al. 2012).
Pedrosa et al. (2007) reported on an acetylcholinesterase (AchE)-based amperometric biosensor developed by immobilization onto a self-assembled modified (SAM) gold electrode. Cyclic voltammetric experiments performed with the SAM-AchE biosensor in phosphate buffer solutions, containing acetylthiocholine, confirmed the formation of thiocholine and its electrochemical oxidation at +0.28 V vs Ag/AgCl. An indirect methodology involving the inhibition effect of parathion and carbaryl on the enzymatic reaction was developed and employed to measure pesticides in food samples without pretreatment or pre-concentration steps. Values higher than 91–98.0 % recovery indicated the feasibility of the proposed electroanalytical methodology to determine pesticides in food samples. The results obtained by the biosensor were compared with HPLC measurements, confirming the amperometrically measured values. The same research group reported on the detection of carbamates (a common class of pesticides in Brazil) in different vegetables (Cesarino et al. 2012). An electrochemical acetylcholinesterase biosensor was successfully developed on polyaniline and multi-walled carbon nanotubes core–shell modified glassy carbon electrode. The pesticide biosensor was applied to the detection of carbaryl and methomyl pesticides in food samples, by chronoamperometry. The biosensor exhibited detection limits of 1.4 and 0.95 μM for carbaryl and methomyl, respectively, which were below the allowable concentrations set by Brazilian regulation standards. Reproducibility and repeatability values of 2.6 % and 3.2 %, respectively, were obtained, and the proposed biosensor was successfully applied on cabbage, broccoli, and apple samples, without any spiking procedure. The obtained results were in full agreement with those obtained by HPLC.
Other paper describes the development of methodology for carbaryl determination in tomatoes (Caetano and Machado 2008). The measurements were carried out using an amperometric biosensor based on the inhibition of acetylcholinesterase activity due to carbaryl adsorption. The analytical curve obtained in buffered solutions showed excellent linearity in the 5.0 × 10−5 to 75 × 10−5 M range, with a limit of detection of 0.4 × 10−3 g/L. The application of the developed methodology on tomato samples involved a simple sample solubilization, followed by carbaryl spiking at different concentrations. Recovery values were in the 92.4–99.0 % range. For comparison, HPLC experiments were also carried out under similar conditions. However, with this analytical procedure, tomato samples have to be manipulated by an extraction procedure, which yielded much lower recovery values (78.3–84.8 %). Finally, the biosensor was employed to analyze carbaryl directly inside the tomato fruit, without any previous manipulation. In this case, the biosensor was immersed in the tomato pulp, which was previously spiked with the pesticide for 8 min, and removed and inserted in the electrochemical cell. A recovery of 83.4 % was obtained, showing a very low interferent effect of matrix constituents.
5 Conclusions and Perspectives
Advances were recently made in biosensor applications for the determination of food composition and contaminants in Brazil and in Italy. Innovative devices were developed, aimed at the determination of food quality and at the presence of toxic substances, microorganisms, and residues. The proposed biosensors, using electrochemical, optical, and piezoelectric transducers, have the potential to achieve the low limits of detection imposed by legislation, and, at the moment, some of them could be transferred for further industrialization.
It is clear from the reviewed literature that common needs are more often being addressed. Nevertheless, it appears that further research into biosensor technologies and sample preparation techniques must be performed to create new systems that should be truly portable from laboratory to field. Anyway, biosensor technologies allow to provide simple to use, inexpensive, and portable systems that can be used to ensure the health and safety of consumers around the world.
References
Amati L, Campanella L, Dragone R, Nuccilli A, Tomassetti M, Vecchio S (2008) New investigation of the isothermal oxidation of extra virgin olive oil: determination of free radicals, total polyphenols, total antioxidant capacity, and kinetic data. J Agric Food Chem 24:8287–8295
Arecchi A, Scampicchio M, Drusch S, Mannino S (2010) Nanofibrous membrane based tyrosinase-biosensor for the detection of phenolic compounds. Anal Chim Acta 659:133–136
Baratella D, Magro M, Sinigaglia G, Zboril R, Salviulo G, Vianello F (2013) A glucose biosensor based on surface active maghemite nanoparticles. Biosens Bioelectron 45:13–18
Caetano J, Machado SAS (2008) Determination of carbaryl in tomato “in natura” using an amperometric biosensor based on the inhibition of acetylcholinesterase activity. Sens Actuators B Chem 129:40–46
Campanella L, di Persio G, Pintore M, Tonnina D, Caretto N, Martini E, Lelo D (2009) Determination of nonsteroidal anti-inflammatory drugs (NSAIDs) in milk and fresh cheese based on the inhibition of cyclooxygenase. J Food Technol Biotechnol 47:172–177
Castillo G, Lamberti I, Mosiello L, Hianik T (2012) Impedimetric DNA aptasensor for sensitive detection of ochratoxin a in food. Electroanalysis 24:512–520
Centi S, Stoica AI, Laschi S, Mascini M (2010) Development of an electrochemical immunoassay based on the use of an eight-electrodes screen-printed array coupled with magnetic beads for the detection of antimicrobial sulfonamides in honey. Electroanalysis 22:1881–1888
Cesarino I, Moraes FC, Lanza MRV, Machado SAS (2012) Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline–carbon nanotubes. Food Chem 135:873–879
Codex Alimentarius Commission (2009) Codex General Standard for contaminants and toxins in food and feed. Codex Stan 193-1995
De Mattos IL, Areias MCD (2005) Automated determination of glucose in soluble coffee using Prussian blue-glucose oxidase-Nafion (R) modified electrode. Talanta 66:1281–1286
Ferreira LS, Trierweiler JO, De Souza Jr MB, Folly ROM (2004) A lactose fia-biosensor system for monitoring and process control. Braz J Chem Eng 21:307–315
Ferrini AM, Mannoni V, Carpico G, Pellegrini GE (2008) Detection and identification of beta-lactam residues in milk using a hybrid biosensor. J Agric Food Chem 13:784–788
Jackson LS (2009) Chemical food safety issues in the United States: past, present, and future. J Agric Food Chem 57:8161–8170
Kantiani L, Llorca M, Sanchís J, Farré M, Barceló D (2010) Emerging food contaminants: a review. Anal Bioanal Chem 398:2413–2427
Lavecchia T, Rea G, Antonacci A, Giardi MT (2013) Healthy and adverse effects of plant-derived functional metabolites: the need of revealing their content and bioactivity in a complex food matrix. Crit Rev Food Sci Nutr 53:198–213
Mazzei F, Botrè F, Favero G (2007) Peroxidase based biosensors for the selective determination of D, L-lactic acid and L-malic acid in wines. Microchem J 87:81–86
McGrath TF, Elliott CT, Fodey TL (2012) Biosensors for the analysis of microbiological and chemical contaminants in food. Anal Bioanal Chem 403:75–92
Mello LD, Sotomayor MDPT, Kubota LT (2003) HRP-based amperometric biosensor for the polyphenols determination in vegetables extract. Sens Actuators B 96:636–645
Mentana A, Palermo C, Nardiello D, Quinto M, Centonze D (2013) Simultaneous and accurate real-time monitoring of glucose and ethanol in alcoholic drinks, must, and biomass by a dual-amperometric biosensor. J Agric Food Chem 61(1):61–68
Mozaz SR, Marco MP, Lopez de Alda MJ, Barceló D (2004) Biosensors for environmental applications: future development trends. Pure Appl Chem 2004(76):723–752
Palchetti I, Mascini M (2012) Electrochemical nanomaterial-based nucleic acid aptasensors. Anal Bioanal Chem 402:3103–3114
Pascale M, Zezza F, Perrone G (2013) Surface plasmon resonance genosensor for the detection of Fusarium culmorum. Methods Mol Biol 968:155–165
Pedrosa VA, Caetano J, Machado SAS, Freire RS, Bertotti M (2007) Acetylcholinesterase immobilization on 3-mercaptopropionic acid self assembled monolayer for determination of pesticides. Electroanalysis 19:1415–1420
Pimenta-Martins MGR, Furtado RF, Heneine LGD, Dias RS, Borges MF, Alves CR (2012) Development of an amperometric immunosensor for detection of staphylococcal enterotoxin type A in cheese. J Microbiol Methods 91:138–143
Pires ACD, Soares NDF, da Silva LHM, da Silva MDH, De Almeida MV, Le Hyaric M, de Andrade NJ, Soares RF, Mageste AB, Reis SG (2011) A colorimetric biosensor for the detection of foodborne bactéria. Sens Actuators B 153:17–23
Rejeb IB, Arduini F, Arvinte A, Amine A, Gargouri M, Micheli L, Bala C, Moscone D, Palleschi G (2009) Development of a bio-electrochemical assay for AFB(1) detection in olive oil. Biosens Bioelectron 24:1962–1968
Ricciardi C, Castagna R, Ferrante I, Frascella F, Marasso SL, Ricci A, Canavese G, Lorè A, Prelle A, Gullino ML, Spadaro D (2013a) Development of a microcantilever-based immunosensing method for mycotoxin detection. Biosens Bioelectron 40:233–239
Ricciardi C, Ferrante I, Castagna R, Frascella F, Marasso SL, Santoro K, Gili M, Pitardi D, Pezzolato M, Bozzetta E (2013b) Immunodetection of 17b-estradiol in serum at ppt level by microcantilever resonators. Biosens Bioelectron 40:407–411
Silva GJL, Andrade CAS, Oliveira IS, de Melo CP, Oliveira MDL (2012) Impedimetric sensor for toxigenic Penicilliumsclerotigenum detection in yam based on magnetite-poly(allylamine hydrochloride) composite. J Colloid Interface Sci 396:258–263
Thevenot DR, Tóth K, Durst RA, Wilson GS (1999) Electrochemical biosensors: recommended definitions and classification. Pure Appl Chem 71:2333–2348
Tombelli S, Mascini M (2009) Aptamers as molecular tools for bioanalytical methods. Curr Opin Mol Ther 11:179–188
Virolainen N, Guglielmetti S, Arioli S, Karp M (2012) Bioluminescence-based identification of nisin producers – a rapid and simple screening method for nisinogenic bacteria in food samples. Int J Food Microbiol 158:126–132
Zamolo VA, Valenti G, Venturelli E, Chaloin O, Marcaccio M, Boscolo S, Castagnola V, Sosa S, Berti F, Fontanive G, Poli M, Tubaro A, Bianco A, Paolucci F, Prato M (2012) Highly sensitive electrochemiluminescent nanobiosensor for the detection of palytoxin. ACS Nano 6:7989–7997
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Wien
About this paper
Cite this paper
Pedrosa, V.A., Fleuri, L.F., Lima, G.P.P., Magro, M., Vianello, F. (2013). Recent Biosensors for Food Analysis in Brazil and Italy. In: Lima, G., Vianello, F. (eds) Food Quality, Safety and Technology. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1640-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1640-1_11
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1639-5
Online ISBN: 978-3-7091-1640-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)