Abstract
Electroencephalography (EEG) provides a powerful tool for evaluating the extent of intact cognitive functions in comatose patients at the bedside, mostly by recording responses to sensory stimuli, exemplified by the so-called event-related potentials (ERPs). Different ERP paradigms are informative of various levels of cognitive functions, ranging from basic auditory processing and auditory discrimination (i.e., mismatch negativity (MMN)) to novelty detection and detection of complex sound sequences. Among them, the MMN paradigm has proven especially useful, as the presence of an MMN response has been repeatedly associated with a favorable clinical prognosis. The high predictive power of MMN is basically driven by the absence of such a response in patients who fail to regain consciousness, mainly assessed several weeks or months after coma onset. However, the use of this paradigm in clinical routine has been limited so far, possibly due to the difficulty of assessing the presence of an MMN at the level of single patients. Multivariate EEG decoding methods provide a powerful tool for quantifying the degree of auditory discrimination, at the single-patient level with minimal a priori inclusion criteria, with very promising results in terms of prognostication of awakening. Moreover, recent evidence in patients in acute postanoxic coma treated with therapeutic hypothermia shows that auditory discrimination might still be intact during the first days of coma, irrespective of patients’ outcome. Here, we propose a general framework for assessing the degree of auditory discrimination over time, as a process that degenerates in non-survivors and that improves in patients who eventually awake.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
Patients who survive severe brain damage often fall into a comatose state, during which they may be unresponsive to external stimuli and unable to communicate with their environment. In such conditions, it can be challenging for clinicians to evaluate the extent of preserved neural functions and whether comatose patients are able to consciously perceive their surroundings. Even though the majority of patients surviving a coma recover within a few days, some may remain in a state of reduced consciousness (vegetative or minimally conscious) for prolonged periods (Laureys et al. 2004). Clinical progress in the early resuscitation of these patients, including modern neuroprotective strategies such as therapeutic hypothermia (TH; Bernard et al. 2002), has resulted in an increasing number of patients awakening from coma, especially following cardiac arrest (Oddo et al. 2006). These advances encourage the neuroscientific and clinical community to reassess previous knowledge about preserved functions in the comatose patient and their link to prognosis (Bouwes et al. 2009; Rossetti et al. 2010). In addition, current efforts in translational research aimed at applying state-of-the-art neuroimaging methods to the clinical environment promise to ameliorate our understanding of the physiological basis of coma and ultimately to help improve overall quality of care for comatose patients (Amantini et al. 2011; King et al. 2013; Tzovara et al. 2013) (see also Chaps. 8 and 12). Among these methods, electroencephalography (EEG) provides a powerful and inexpensive tool for measuring neural response to sensory stimuli (event-related potentials, ERPs) in comatose patients at the bedside (Fischer et al. 1999, 2004; Bouwes et al. 2009).
Neuroimaging studies have considerably challenged standard clinical assessment, especially in the chronic phase. One exemplary case is provided by hemodynamic studies showing that patients with disorders of consciousness could be erroneously labeled as vegetative (also called unresponsive wakefulness), though actually able to communicate via “command-following” paradigms (Owen et al. 2006). These paradigms have provided quantitative evidence that patients without overt behavioral response to external stimuli were nevertheless able to modulate brain activity in association with voluntary answers (Owen et al. 2006; Cruse et al. 2011). The vast majority of these studies have, however, exclusively investigated patients with heterogeneous coma etiologies and in a vegetative state, or months after coma onset. The extent of intact cognitive functions during acute coma remains under-investigated, especially since the introduction of TH, which is increasingly used as a neuroprotective strategy during early postanoxic coma (Bernard et al. 2002; Choi et al. 2012). In the following section, we will review the most common applications of event-related potential in coma research, including recent results obtained in postanoxic comatose patients treated with therapeutic hypothermia.
7.2 Overview of Existing ERP Protocols
Measuring ERPs in response to sensory stimuli in patients with disorders of consciousness represents a powerful tool for assessing the extent of intact neural functions at the bedside (Fischer et al. 1999; Luaute et al. 2005; Daltrozzo et al. 2009). The interesting aspect of this research is twofold. On the one hand, a main focus is the understanding of the extent of brain processes that remain unaltered during coma and how this information relates to prognosis. On the other hand, assessing preserved functions during coma opens a window of investigation onto processes that can remain unaltered in the absence of consciousness or in a minimally conscious state (Bekinschtein et al. 2009; Faugeras et al. 2012). This line of research is relevant for addressing unresolved questions in fundamental neuroscience about the relationship between cognitive processes and consciousness in humans (Boly et al. 2013). Access to this kind of investigation in comatose patients may complement similar research performed during sleep and under anesthesia (Chennu and Bekinschtein 2012).
One of the main efforts in the development of neuroimaging techniques applied in coma research is focused on establishing indirect communication channels with patients that may be conscious and potentially able to express willful responses (Owen et al. 2006; Cruse et al. 2011). During acute coma, neuroimaging techniques aim for a different goal, which is to assess the extent of intact neural functions in a condition where any form of conscious access to incoming sensory is extremely unlikely. In this context, the main interest relies in experimental protocols targeting basic sensory processing and cognitive functions which are largely based on implicit and automatic mechanisms (Lew et al. 2006).
Somatosensory evoked potentials (SSEPs; Zandbergen et al. 1998) and brain stem auditory evoked potentials (AEPs) have been widely used in clinical practice for predicting outcome in a variety of coma etiologies (Zandbergen et al. 1998; Gendo et al. 2001; Robinson et al. 2003; Bouwes et al. 2009) (see Chap. 6). The absence of early amplitude modulation in SSEPs provides an indirect measure of damage occurring at the level of the sensory pathways and/or at the level of primary sensory cortices. However, the early cortical response (N20) can be preserved both in patients with poor outcome and in those who later survive (Bouwes et al. 2009). While SSEPs mainly reflect processing at the level of sensory cortices and provide information about poor outcome, other ERPs, mostly reflecting cognitive processes, are informative as regards awakening or regaining of consciousness (Lew et al. 2006).
The auditory modality provides the most straightforward channel for recording cognitive ERPs, as it can be easily targeted in nonresponsive patients, either in coma (Kane et al. 1993; Fischer et al. 1999, 2004; Daltrozzo et al. 2009) or in vegetative/minimally conscious states (Neumann and Kotchoubey 2004; Kotchoubey et al. 2005; Boly et al. 2011). The most notable of these experimental protocols for measuring AEP is the so-called mismatch negativity (MMN) paradigm (Naatanen et al. 1978; Garrido et al. 2009); such a protocol can be based on a simple sequence of stimuli in which a frequent (standard) sound is repeated several times before being interrupted by a rare (deviant) sound with different acoustic properties. Classically, in healthy subjects, the negative difference (i.e., mismatch negativity) between the AEPs in response to deviant and standard sounds is mostly evident over the fronto-central regions at a latency of about 150 ms poststimulus (Garrido et al. 2009) (Fig. 7.1). MMN is considered a pre-attentive and preconscious marker of the brain’s ability to discriminate sounds and can be measured even in conditions where any form of top-down contribution to sensory processing can be excluded, such as during sleep (Ruby et al. 2008; Sculthorpe et al. 2009) and deep anesthesia (Koelsch et al. 2006). The literature on MMN in comatose patients is extensive and has provided robust evidence of the relation between the MMN component and the patient’s chances of awakening (Kane et al. 1993; Fischer et al. 2004; Luaute et al. 2005; Naccache et al. 2005; Wijnen et al. 2007; Tzovara et al. 2013). This has been demonstrated in a wide range of coma etiologies and measured from as early as a few days after coma onset (Kane et al. 1993) to weeks (Fischer et al. 2004; Naccache et al. 2005) or even months (Boly et al. 2011) (see also Chap. 9).
Average AEPs for one exemplar control subject in response to standard and duration-deviant sounds (black and red lines, respectively) at five fronto-central electrodes. Voltage topographies at ~100 ms after stimulus onset exhibit prototypical N100 configurations with central negativity, for both standard and deviant sounds. The occurrence of the first negative difference between deviant and standard sounds and corresponding voltage topographies is observed at a typical MMN latency, between 150 and 200 ms. The white and black dots on the topographies represent the position of the minimum and maximum values across the whole electrode montage, respectively
While MMN is mostly informative with regard to automatic processing of auditory regularities at the level of individual sounds, other more complex paradigms examine auditory processing at the level of groups of sounds (Bekinschtein et al. 2009). In this case, a regularity is established over few seconds, by repeating groups of five sounds and replacing them from time to time with deviant ones. The detection of a rare group of sounds at the neural level is much more demanding than the detection of a single sound, for example, in an MMN protocol, as the former requires subjects to be aware of the regularity, while the latter is thought to be more automatic (Bekinschtein et al. 2009). This more complex paradigm has therefore been linked to conscious processing, as previous studies have shown that the majority of patients who can detect this type of irregularity are either at a minimally conscious level (Bekinschtein et al. 2009) or eventually regain consciousness (Faugeras et al. 2012).
Other auditory paradigms that have been well studied in patients with impaired consciousness are based on oddball protocols (Sutton et al. 1965; Friedman et al. 2001). Oddball paradigms consist in repeating one standard event, typically a sound, and intermixing it with unexpected target or salient stimuli. In healthy subjects, the target stimulus elicits the so-called P300 response (see also Chaps. 9 and 11), a positivity on central electrodes occurring about 300 ms after the stimulus onset (Sutton et al. 1965). The difference between P300 and MMN is that the former typically requires the subject’s attention or is associated with a highly salient event or a future action (Friedman et al. 2001). Moreover, the peak of the P300 response appears at later latencies as compared to the MMN (i.e., around 300 ms vs. ~150 ms poststimulus onset). Interestingly, a P300 response has also been reported during sleep (Pratt et al. 1999; Ruby et al. 2008) and even in coma, provided that the target stimuli are sufficiently salient (Signorino et al. 1995). Therefore, most studies have used neutral sounds as the standard stimulus, intermixed with the patient’s own name as the target stimulus, due to its high saliency for the patient (Signorino et al. 1995; Fischer et al. 2008; Holeckova et al. 2008). In clinical conditions, a P300 is assessed at the level of the single electrode as an amplitude modulation over central regions and is typically found in about 40 % of comatose patients, tested on average at 20 days after coma onset (Fischer et al. 2008). The presence of a P300 response in these conditions correlates with the patients’ chances of awakening (around 85 % specificity for awakening (Fischer et al. 2008). However, when patients are in a vegetative or minimally conscious state several months after coma onset, the chances of detecting a P300 response become considerably lower (about 25 %; Fischer et al. 2010). This possibly reflects a long-term degeneration of attention-related processes in those patients who do not manage to recover and fully regain consciousness.
Other cognitive ERP paradigms that have been tested in unconscious patients are based on linguistic material and test neural responses to semantic or phonetic incongruencies (Kutas and Hillyard 1980). Such paradigms, in healthy controls, typically elicit an N400 component, appearing about 400 ms after the incongruency onset (Perrin and Garcia-Larrea 2003), and it can be elicited even in passive listening (Perrin and Garcia-Larrea 2003). Among patients with severe disorders of consciousness, the N400 has been mostly, but not exclusively, observed in patients with some minimal consciousness (Schoenle and Witzke 2004).
To summarize, the presence of ERP components related to novelty detection or semantic processing is likely to indicate a favorable outcome for comatose patients, but none of them seems robust enough to be used as a reliable predictor in a clinical environment. Before introducing them into routine clinical practice, it is imperative that they have practically no false-positive answers and that they have been validated in large cohorts of patients. One of the main potential candidates for implementing a test for prediction of awakening is the MMN.
7.3 Mismatch Negativity in Clinical Practice
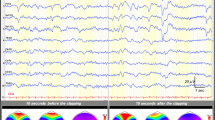
Despite the extensive literature linking the presence of an MMN to the patient’s chance of recovery (Kane et al. 1993; Fischer et al. 2004; Naccache et al. 2005; Wijnen et al. 2007) with a predictive value for awakening that far exceeds other paradigms, the use of MMN protocols in clinical practice has so far been rather limited. We believe that one main reason is the difficulty in assessing its presence at the individual level. Most previous studies assessing MMN in clinical studies rely on the estimation of the presence of an evoked activity at the level of AEP measured at a single-electrode level (Fischer et al. 1999, 2004). This assessment is based on the presence of the so-called N100 component (Hillyard et al. 1973), a negative deflection in the average AEP appearing over fronto-central electrodes, considered an “obligatory” marker of auditory processing at the level of the primary auditory cortices (Naatanen and Picton 1987). The estimation of this component relies on a somewhat subjective threshold regarding how this modulation should differ from an average baseline and has systematically implied the rejection of about 30 % of patients (e.g., in Fischer et al. 1999). Not only does this approach imply the rejection of patients who do not show any sign of intact auditory processing, but also, and more problematically, it implies the exclusion of patients exhibiting AEPs with poor signal-to-noise ratio, a situation occurring quite often in a clinical setting with many sources of electromagnetic noise (see also, e.g., Faugeras et al. 2012) (see also Chap. 2). As a consequence, MMN results based on including those patients who exhibit an N100 response are possibly biased by restricting the analysis to AEPs with a high signal quality and therefore more likely to also exhibit differential activity in response to different kinds of sensory stimuli. In addition, one should consider that AEPs in comatose patients are likely to be different from the stereotypical waveforms that can be measured in healthy subjects and also likely to vary among patients (see the ERPs in response to standard and deviant sounds in a control subject in Fig. 7.1 and a comatose patient in Fig. 7.2). Therefore, the validity of using the N100 component at the typical latency of average AEP measured in the healthy population can be limited, providing a second potential source of biased inclusion of patients exhibiting AEP similar to healthy subjects.
Average AEPs in five fronto-central electrodes in response to standard vs. duration-deviant sounds and decoding results for an exemplar patient during the first 24 h of coma and under therapeutic hypothermia after cardiac arrest. The multivariate decoding algorithm provides a data-driven estimation of periods of differential activity across several data shuffles, as shown in the bottom panel. For this exemplar patient, the common periods of differences across all shuffles started around 360 ms and lasted up to ~390 ms poststimulus onset. The voltage topographies shown on the right are the ones observed in response to standard vs. deviant sounds during this period (the white and black dots on the topographies represent the position of the minimum and maximum values across the whole electrode montage, respectively)
Improving the assessment of an evoked activity and of a differential response in auditory paradigms would be extremely worthwhile: in clinical practice, the potential utility of MMN as a marker of good prognosis is enormous (Kane et al. 1993; Fischer et al. 2004; Luaute et al. 2005; Naccache et al. 2005; Wijnen et al. 2007; Tzovara et al. 2013). Its routine application during acute coma would potentially enhance the possibility of informing relatives and caregivers of the patient’s prospects, as early and as accurately as possible; the second aspect to consider is economic since intensive care support is an expensive practice needing to be sustained and motivated by demonstrating its utility for the patients; finally, increasingly accurate information about the patient’s early state could pave the way to optimizing rehabilitation care according to individual situations. Below, we will outline a method and a set of results providing an alternative to the classical estimation of the N100 and MMN response based on ERPs at the level of single electrodes.
7.4 Multivariate Decoding for Assessing Auditory Discrimination
An ideal approach for acute coma patients would be tailored to the individual and provide timely information. Patients can exhibit highly heterogeneous patterns of activity and present various degrees of coma severity and different forms of brain damage. The assessment of a reliable response to auditory and sensory stimuli in general should therefore flexibly adapt to the pattern of response of each individual without specific inclusion criteria based on knowledge gathered in the healthy population. Moreover, there is an increasing need for quantitative evaluation of sensitive markers of intact neural functions in a context where the vast majority of the tests are based on prediction of poor outcome (Bouwes et al. 2009; Rossetti et al. 2010). The implementation of multivariate decoding at the level of single-trial EEG offers a possible solution for an unbiased evaluation of AEPs at the single-patient level (Tzovara et al. 2013).
Multivariate decoding methods are increasingly used in neuroimaging studies (Pereira et al. 2009; Weil and Rees 2010; Blankertz et al. 2011), presenting several benefits over univariate approaches. First, the framework of implementation of these techniques typically offers a clear separation between discovering a discriminative pattern in one set of data and then testing on a separate set of observations (Kriegeskorte et al. 2009). Second, multivariate decoding has turned out to be particularly sensitive in comparison to univariate analysis when the discriminative patterns between experimental conditions range across voxels in functional magnetic resonance imaging (Staeren et al. 2009) or several temporal windows and electrode positions in EEG measurements (Hausfeld et al. 2012). Third, it can typically be optimized by allowing a parameter selection that best adapts to the signal of specific patients without assuming specific a priori hypotheses about the type of response (De Lucia et al. 2011; King et al. 2013). Fourth, decoding algorithms naturally deal with single-trial information and therefore allow the exploration of events occurring at different latencies with respect to stimulus onset; this is in contrast to average ERP, which keeps only time-locked activity to stimulus onset (De Lucia et al. 2012). Many multivariate decoding algorithms rely on the extraction of optimal features in the signal that allow the best discrimination between experimental conditions (Lemm et al. 2011). Sometimes, this feature-extraction stage relies on well-known phenomena characterizing a specific process (De Vos et al. 2012). Many other attempts in the literature for developing optimal strategies of decoding rely on a blind search for features or combinations of the EEG signatures within a set of possible alternatives (Bourdaud et al. 2008; Murphy et al. 2011). The main drawback is obviously the difficulty of interpreting, from a neurophysiological perspective, what insight one gains by finding optimal class separation between experimental conditions. One proposition lies halfway between a blind search for combinations of optimal features and the adoption of some flexibility in the decoding strategy, while still allowing interpretation (Philiastides and Sajda 2006; Simanova et al. 2010). In this chapter, we illustrate a single-trial topographic analysis (Tzovara et al. 2012a, b) and its application in coma research (Tzovara et al. 2013).
This method is based on voltage topographies as measured time point by time point in continuous recordings. The main goal is to extract configurations in the voltage topographies occurring at latencies where the two experimental conditions of interest tend to differ. Discovering such voltage configurations provides information on the underlying distribution of active sources in the brain. Indeed, within each recording and at the level of each patient, occurrence of a different configuration at the topographic level can be interpreted as resulting from a difference at the level of brain regions and by extension of brain regions involved in stimulus processing (Murray et al. 2008). Discriminative voltage configurations can occur at several latencies, even when one could not assume a reliable presence of an evoked activity. In this type of method, we take advantage of possible distributed activity along one or several time windows of different length and appearing at variable latencies. The discovery of the latencies at which two experimental conditions tend to differ is also informative, as typical basic sensory processing occurs early in time (i.e., <150 ms), while processes characterized by increasing complexity and refinement happen at subsequent stages. The co-occurrence of more than one time period of differential activity can also be informative as regards the existence of multiple levels in the process of a specific class of stimuli (De Lucia et al. 2012).
Single-trial topographic analysis foresees the existence of prototypical voltage configurations in relation to event-related activity at the level of the single EEG response. If these configurations exist and differ between experimental conditions, then the decoding algorithm should exhibit a significant decoding performance when classifying new independent trials as belonging to one of the two conditions. This method consists of three main steps; in the first, the algorithm learns a discriminative pattern across several possible combinations of the models’ parameters; in the second, the optimized algorithm is tested about providing similar decoding performance on trials independent of those used for estimating and optimizing the models; the third step consists of assessing the significance of the obtained results. This can be carried out at the level of each single patient/recording; this level of analysis entails the estimation of a null distribution for the decoding value in a specific setting (i.e., after relevant parameter selection). The estimation of such a distribution is somewhat standard in classical applications of machine-learning techniques and decoding methods in neuroimaging studies and entails training several models across several label randomizations in the training dataset. The real decoding performance can then be compared by nonparametric tests to the distribution of the values obtained for the randomized version of the decoding algorithm (Pereira et al. 2009).
7.5 Auditory Event-Related Potentials in Early Coma
Clinical tests are usually repeated for several days in comatose patients in order to detect possible improvements over time (Kane et al. 1993). However, the vast majority of the literature on ERP reports the results related to a single measurement for each patient. In addition, these studies include subjects recorded at various delays after coma onset (Fischer et al. 2004; Naccache et al. 2005; Boly et al. 2011). These factors contribute to producing a body of evidence that may lead to an incorrect conclusion, especially regarding the absence of specific responses.
Recently, the importance of carrying out ERP recordings over several days in comatose patients after a cardiac arrest was emphasized by a study conducted in a group of postanoxic patients, assessed using a standard, clinical 10–20 montage with 21 electrodes (Tzovara et al. 2013). These results have shown that the degree of auditory discrimination can change over time, as it can already be detected with a time delay of one day between the two recordings (Figs. 7.3 and 7.4a). In Fig. 7.4, the average decoding performance is grouped with respect to the outcome and timing of the recording (during TH and normothermia –NT respectively), regardless of whether patients exhibited significant results individually. A visual check of these average decoding values provides initial insight into the overall level of auditory discrimination in the different groups and, most importantly, provides qualitative evidence of the relatively higher performance of patients with poor outcomes during TH (Fig. 7.4a). This pattern of results was first discovered in a pilot group of patients and then confirmed in an independent validation group, without any knowledge about patients’ outcome. The higher decoding value at group level was also reflected in the degeneration of decoding performances from TH and NT in all the patients who later died (Fig. 7.4b). At the level of each single patient, the progression of the degree of auditory discrimination over time was highly informative regarding the patients’ chances of survival, as only those who later survived improved their performance from TH to NT (Fig. 7.4b): across thirty patients this test provided a 100 % positive predictive power of survival and awakening within 3 months.
Timeline of EEG recordings in correspondence to the various stages of sedation and TH, with corresponding patient body temperatures, as reported in Tzovara et al. 2013
(a) Decoding performance between standard and deviant sounds in two groups of postanoxic comatose patients (pilot and validation), grouped with respect to outcome and time of the recording (TH and NT); the highest decoding performance was obtained during TH for patients who later died and was followed by a decrease during NT, in both pilot and validation groups of patients. (b) Percentage of difference in the decoding performance between NT and TH, grouped with respect to outcome. An increase in decoding performance was only observed in a subgroup of survivors (100 % predictive power for survival and awakening at 3 months) (Data from Tzovara et al. 2013)
A major advantage of this approach is that the multivariate decoding algorithm did not require any preselection before analyzing individual patients’ data. Provided that these results based on multivariate decoding are confirmed in a larger population, they encourage the implementation of this experimental protocol in clinical practice, where this test could complement existing ones, which so far mostly provide information about poor outcomes (Rossetti et al. 2010) (see Chaps. 5 and 6).
There is still a lot to be done to gain insight into the physiological mechanisms underlying the degeneration of auditory discrimination in patients who later die. Many explanations are possible and need to be tested. The first hypothesis is that auditory progression as measured by the described EEG protocol reflects some generic deterioration of auditory function in relation to neuronal death in poor-outcome subjects. However, this would not account for the relatively higher performance during TH displayed by these patients (Fig. 7.4a). In addition, if this was the only explanation for the results, one would be able to observe the same response pattern with any kind of auditory protocol, which remains to be tested with other types of auditory experiments. A second hypothesis is about the sensitivity of EEG measurement in these different conditions. During TH and sedation (often including neuromuscular blockade), the presence of artifacts due to muscular activity, micro-movements, and eye movements can be considered negligible. This reduction of artifacts in comparison to normal temperature might lead to a higher EEG signal quality and possibly also in higher auditory discrimination performance in comparison to normothermia (Madhok et al. 2012). While this explanation needs further testing, it remains unclear why a higher sensitivity during TH would differentially affect patients who later survive and those who do not (Fig. 7.4a).
A possible clue for the interpretation of what precedes might come from investigating the properties of the EEG signal at rest (Heine et al. 2012; Lechinger et al. 2013). This line of investigation would provide both insight into the general quality of the measurement during TH, most importantly with regard to the degree of “physiological noise,” and its relation to the evoked activity during the MMN protocol (see Wu et al. (2012) for the impact of hypothermia on SSEP). We expect that patients who later die may exhibit a slower and less rich physiological background activity during TH than the other group. These properties of the ongoing activity could explain a better detection of the discriminative signal between standard and deviant stimuli in the MMN paradigm. Moreover, they could provide insight into whether the obtained results are strongly dependent on the TH treatment or whether they would still be observed during early coma in other patients not treated with TH, as in the case of other coma etiologies (i.e., traumatic).
In previous studies, the sensitivity toward auditory sequence violation in patients who would later die has been mostly overlooked (Fischer et al. 1999, 2004; Daltrozzo et al. 2009). However the significant decoding performance in non-survivors (Tzovara et al. 2013) was obtained in an experimental context that had never been considered before: to the best of our knowledge, no other study focused on AEPs measured during an MMN protocol in patients treated with TH. Moreover, previous literature included patients at variable latencies after coma onset, suggesting that the results obtained so far on ERPs in comatose patients might reflect the tip of the iceberg with regard to a phenomenon which starts very early after coma onset, that is, the degeneration of neural function in patients who will later die (Fischer et al. 1999, 2004; Daltrozzo et al. 2009). In Fig. 7.5, the panel b represents hypothetical EEG responses to standard and deviant sounds at several days after coma onset. This model could explain why at long latencies after coma onset a differential response to auditory stimuli is not observed in non-survivors. The evaluation of such responses over variable latencies of coma can possibly reveal a more complete image of the extent of preserved brain functions. Future ERP studies in early coma will possibly revise many common beliefs about the extent of preserved brain function in comatose patients and help to establish new markers of accurate prognosis.
(a) Early progression of auditory discrimination. (b) Auditory discrimination at variable latencies. Hypothetical evolution of EEG responses to standard and deviant sounds (here schematized as single voltage topographies) over the course of days in survivors and non-survivors (upper and lower panels, respectively). The progression of auditory discrimination, rather than the mere presence of it, allows the prediction of chance of survival in early coma (see also Fig. 7.4 for an overview on 30 patients). Previous literature has focused on variable latencies after coma onset, emphasizing the presence of a discriminative response to standard and deviant sounds in survivors (upper panel b) and the absence in non-survivors (lower panel b)
References
Amantini A, Carrai R, Fossi S, Pinto F, Grippo A (2011) The role of early electroclinical assessment in improving the evaluation of patients with disorders of consciousness. Funct Neurol 26:7–14
Bekinschtein TA, Dehaene S, Rohaut B, Tadel F, Cohen L, Naccache L (2009) Neural signature of the conscious processing of auditory regularities. Proc Natl Acad Sci U S A 106:1672–1677
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563
Blankertz B, Lemm S, Treder M, Haufe S, Muller KR (2011) Single-trial analysis and classification of ERP components – a tutorial. Neuroimage 56:814–825
Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S, Friston K (2011) Preserved feedforward but impaired top-down processes in the vegetative state. Science 332:858–862
Boly M, Seth AK, Wilke M, Ingmundson P, Baars B, Laureys S, Edelman DB, Tsuchiya N (2013) Consciousness in humans and non-human animals: recent advances and future directions. Front Psychol 4:625
Bourdaud N, Chavarriaga R, Galan F, Millan Jdel R (2008) Characterizing the EEG correlates of exploratory behavior. IEEE Trans Neural Syst Rehabil Eng 16:549–556
Bouwes A, Binnekade JM, Zandstra DF, Koelman JH, van Schaik IN, Hijdra A, Horn J (2009) Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology 73:1457–1461
Chennu S, Bekinschtein TA (2012) Arousal modulates auditory attention and awareness: insights from sleep, sedation, and disorders of consciousness. Front Psychol 3:65
Choi HA, Badjatia N, Mayer SA (2012) Hypothermia for acute brain injury – mechanisms and practical aspects. Nat Rev Neurol 8:214–222
Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernandez-Espejo D, Pickard JD, Laureys S, Owen AM (2011) Bedside detection of awareness in the vegetative state: a cohort study. Lancet 378:2088–2094
Daltrozzo J, Wioland N, Mutschler V, Lutun P, Calon B, Meyer A, Pottecher T, Lang S, Jaeger A, Kotchoubey B (2009) Cortical information processing in coma. Cogn Behav Neurol 22:53–62
De Lucia M, Constantinescu I, Sterpenich V, Pourtois G, Seeck M, Schwartz S (2011) Decoding sequence learning from single-trial intracranial EEG in humans. PLoS One 6:e28630
De Lucia M, Tzovara A, Bernasconi F, Spierer L, Murray MM (2012) Auditory perceptual decision-making based on semantic categorization of environmental sounds. Neuroimage 60:1704–1715
De Vos M, Thorne JD, Yovel G, Debener S (2012) Let's face it, from trial to trial: comparing procedures for N170 single-trial estimation. Neuroimage 63:1196–1202
Faugeras F, Rohaut B, Weiss N, Bekinschtein T, Galanaud D, Puybasset L, Bolgert F, Sergent C, Cohen L, Dehaene S, Naccache L (2012) Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia 50:403–418
Fischer C, Morlet D, Bouchet P, Luaute J, Jourdan C, Salord F (1999) Mismatch negativity and late auditory evoked potentials in comatose patients. Clin Neurophysiol 110:1601–1610
Fischer C, Luaute J, Adeleine P, Morlet D (2004) Predictive value of sensory and cognitive evoked potentials for awakening from coma. Neurology 63:669–673
Fischer C, Dailler F, Morlet D (2008) Novelty P3 elicited by the subject’s own name in comatose patients. Clin Neurophysiol 119:2224–2230
Fischer C, Luaute J, Morlet D (2010) Event-related potentials (MMN and novelty P3) in permanent vegetative or minimally conscious states. Clin Neurophysiol 121:1032–1042
Friedman D, Cycowicz YM, Gaeta H (2001) The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci Biobehav Rev 25:355–373
Garrido MI, Kilner JM, Stephan KE, Friston KJ (2009) The mismatch negativity: a review of underlying mechanisms. Clin Neurophysiol 120:453–463
Gendo A, Kramer L, Hafner M, Funk GC, Zauner C, Sterz F, Holzer M, Bauer E, Madl C (2001) Time-dependency of sensory evoked potentials in comatose cardiac arrest survivors. Intensive Care Med 27:1305–1311
Hausfeld L, De Martino F, Bonte M, Formisano E (2012) Pattern analysis of EEG responses to speech and voice: influence of feature grouping. Neuroimage 59:3641–3651
Heine L, Soddu A, Gomez F, Vanhaudenhuyse A, Tshibanda L, Thonnard M, Charland-Verville V, Kirsch M, Laureys S, Demertzi A (2012) Resting state networks and consciousness: alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness States. Front Psychol 3:295
Hillyard SA, Hink RF, Schwent VL, Picton TW (1973) Electrical signs of selective attention in the human brain. Science 182:177–180
Holeckova I, Fischer C, Morlet D, Delpuech C, Costes N, Mauguiere F (2008) Subject’s own name as a novel in a MMN design: a combined ERP and PET study. Brain Res 1189:152–165
Kane NM, Curry SH, Butler SR, Cummins BH (1993) Electrophysiological indicator of awakening from coma. Lancet 341:688
King JR, Faugeras F, Gramfort A, Schurger A, El Karoui I, Sitt JD, Rohaut B, Wacongne C, Labyt E, Bekinschtein T, Cohen L, Naccache L, Dehaene S (2013) Single-trial decoding of auditory novelty responses facilitates the detection of residual consciousness. Neuroimage 83:726–738
Koelsch S, Heinke W, Sammler D, Olthoff D (2006) Auditory processing during deep propofol sedation and recovery from unconsciousness. Clin Neurophysiol 117:1746–1759
Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, Bostanov V, Birbaumer N (2005) Information processing in severe disorders of consciousness: vegetative state and minimally conscious state. Clin Neurophysiol 116:2441–2453
Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI (2009) Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12:535–540
Kutas M, Hillyard SA (1980) Reading senseless sentences: brain potentials reflect semantic incongruity. Science 207:203–205
Laureys S, Owen AM, Schiff ND (2004) Brain function in coma, vegetative state, and related disorders. Lancet Neurol 3:537–546
Lechinger J, Bothe K, Pichler G, Michitsch G, Donis J, Klimesch W, Schabus M (2013) CRS-R score in disorders of consciousness is strongly related to spectral EEG at rest. J Neurol 260:2348–2356
Lemm S, Blankertz B, Dickhaus T, Muller KR (2011) Introduction to machine learning for brain imaging. Neuroimage 56:387–399
Lew HL, Poole JH, Castaneda A, Salerno RM, Gray M (2006) Prognostic value of evoked and event-related potentials in moderate to severe brain injury. J Head Trauma Rehabil 21:350–360
Luaute J, Fischer C, Adeleine P, Morlet D, Tell L, Boisson D (2005) Late auditory and event-related potentials can be useful to predict good functional outcome after coma. Arch Phys Med Rehabil 86:917–923
Madhok J, Wu D, Xiong W, Geocadin RG, Jia X (2012) Hypothermia amplifies somatosensory-evoked potentials in uninjured rats. J Neurosurg Anesthesiol 24:197–202
Murphy B, Poesio M, Bovolo F, Bruzzone L, Dalponte M, Lakany H (2011) EEG decoding of semantic category reveals distributed representations for single concepts. Brain Lang 117:12–22
Murray MM, Brunet D, Michel CM (2008) Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr 20:249–264
Naatanen R, Picton T (1987) The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology 24:375–425
Naatanen R, Gaillard AW, Mantysalo S (1978) Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst) 42:313–329
Naccache L, Puybasset L, Gaillard R, Serve E, Willer JC (2005) Auditory mismatch negativity is a good predictor of awakening in comatose patients: a fast and reliable procedure. Clin Neurophysiol 116:988–989
Neumann N, Kotchoubey B (2004) Assessment of cognitive functions in severely paralysed and severely brain-damaged patients: neuropsychological and electrophysiological methods. Brain Res Brain Res Protoc 14:25–36
Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L (2006) From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med 34:1865–1873
Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD (2006) Detecting awareness in the vegetative state. Science 313:1402
Pereira F, Mitchell T, Botvinick M (2009) Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 45:S199–S209
Perrin F, Garcia-Larrea L (2003) Modulation of the N400 potential during auditory phonological/semantic interaction. Brain Res Cogn Brain Res 17:36–47
Philiastides MG, Sajda P (2006) Temporal characterization of the neural correlates of perceptual decision making in the human brain. Cereb Cortex 16:509–518
Pratt H, Berlad I, Lavie P (1999) ‘Oddball’ event-related potentials and information processing during REM and non-REM sleep. Clin Neurophysiol 110:53–61
Robinson LR, Micklesen PJ, Tirschwell DL, Lew HL (2003) Predictive value of somatosensory evoked potentials for awakening from coma. Crit Care Med 31:960–967
Rossetti AO, Oddo M, Logroscino G, Kaplan PW (2010) Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 67:301–307
Ruby P, Caclin A, Boulet S, Delpuech C, Morlet D (2008) Odd sound processing in the sleeping brain. J Cogn Neurosci 20:296–311
Schoenle PW, Witzke W (2004) How vegetative is the vegetative state? Preserved semantic processing in VS patients–evidence from N 400 event-related potentials. NeuroRehabilitation 19:329–334
Sculthorpe LD, Ouellet DR, Campbell KB (2009) MMN elicitation during natural sleep to violations of an auditory pattern. Brain Res 1290:52–62
Signorino M, D’Acunto S, Angeleri F, Pietropaoli P (1995) Eliciting P300 in comatose patients. Lancet 345:255–256
Simanova I, van Gerven M, Oostenveld R, Hagoort P (2010) Identifying object categories from event-related EEG: toward decoding of conceptual representations. PLoS One 5:e14465
Staeren N, Renvall H, De Martino F, Goebel R, Formisano E (2009) Sound categories are represented as distributed patterns in the human auditory cortex. Curr Biol 19:498–502
Sutton S, Braren M, Zubin J, John ER (1965) Evoked-potential correlates of stimulus uncertainty. Science 150:1187–1188
Tzovara A, Murray MM, Michel CM, De Lucia M (2012a) A tutorial review of electrical neuroimaging from group-average to single-trial event-related potentials. Dev Neuropsychol 37:518–544
Tzovara A, Murray MM, Plomp G, Herzog MH, Michel CM, De Lucia M (2012b) Decoding stimulus-related information from single-trial EEG responses based on voltage topographies. Pattern Recognition 45:2109–2122
Tzovara A, Rossetti AO, Spierer L, Grivel J, Murray MM, Oddo M, De Lucia M (2013) Progression of auditory discrimination based on neural decoding predicts awakening from coma. Brain 136:81–89
Weil RS, Rees G (2010) Decoding the neural correlates of consciousness. Curr Opin Neurol 23:649–655
Wijnen VJ, van Boxtel GJ, Eilander HJ, de Gelder B (2007) Mismatch negativity predicts recovery from the vegetative state. Clin Neurophysiol 118:597–605
Wu D, Xiong W, Jia X, Geocadin RG, Thakor NV (2012) Short- and long-latency somatosensory neuronal responses reveal selective brain injury and effect of hypothermia in global hypoxic ischemia. J Neurophysiol 107:1164–1171
Zandbergen EG, de Haan RJ, Stoutenbeek CP, Koelman JH, Hijdra A (1998) Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet 352:1808–1812
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Wien
About this chapter
Cite this chapter
De Lucia, M., Tzovara, A. (2015). Prognostic Use of Cognitive Event-Related Potentials in Acute Consciousness Impairment. In: Rossetti, A., Laureys, S. (eds) Clinical Neurophysiology in Disorders of Consciousness. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1634-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1634-0_7
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1633-3
Online ISBN: 978-3-7091-1634-0
eBook Packages: MedicineMedicine (R0)