Abstract
Predicting the fate of ICU patients who are in coma is extremely challenging. In this context, somatosensory evoked potential (SSEP) can assist the multimodal neurological evaluation. In this chapter, we discuss the principles, applications and limitations of the SSEP in the ICU, with a focus on prognostication in comatose patients. Registration of the SSEP is a very reliable and reproducible method, if performed and interpreted correctly. During recordings, great care should be taken in improving the signal-to-noise ratio: if the noise level is too high, the peripheral responses are abnormal, or the response is not reproducible in a second set of stimuli; in these cases, interpretation of the SSEP cannot be done reliably. A bilaterally absent cortical response is a reliable predictor for poor neurological outcome in patients with a postanoxic coma, but not in patients with traumatic brain injury or stroke.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic Brain Injury Patient
- Cortical Response
- Poor Neurological Outcome
- Bilateral Absence
- SSEP Amplitude
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Every patient remaining in coma in the intensive care unit (ICU) raises the question regarding outcome prediction (Chap. 1). Both the treating team and family members need information about the chances of recovery of consciousness and long-term functional outcome. Reliable information on this topic is necessary to decide on limitations or even withdrawal of supportive intensive care treatment. Somatosensory evoked potentials (SSEPs) are often used in this situation and can prove very useful depending on the condition underlying the coma.
6.2 SSEP Principles (see also Chap. 2)
The SSEP is a small (<10–50 μV) electrical signal, which can be recorded noninvasively from the skull, after giving a set of electrical stimuli to one of the peripheral nerves, evaluating the complete pathway from the peripheral sensory nervous system to the sensory cortex, running via the dorsal column and lemniscal pathway through the spinal cord, brainstem and thalamus (Cruccu et al. 2008). This pathway consists of four neuronal populations: the cell bodies of the first-order neurons are situated in the dorsal root ganglia, the trigeminal ganglion, the midbrain trigeminal nucleus and the vagal ganglion nodosum. The second-order neuron lies in the rostral part of the dorsal column (cuneate and gracile nuclei). Axons of these second neurons cross the midline and project to the ventroposterior nuclei of the thalamus (third-order neuron). From there, the pathway projects into the network of somatosensory cortex areas (fourth-order neurons), which include the primary and secondary somatosensory cortex, posterior parietal cortex, posterior and mid-insula and mid-cingulate cortex (Fig. 6.1).
SSEPs are usually evoked by bipolar transcutaneous electrical stimulation applied on the skin over the selected nerve and registered with disc electrodes along the tract. For example, in recordings of the median nerve, registration electrodes can be placed at the elbow, Erb’s point (over the plexus, above the clavicle) and cervical, parietal and frontal cortex (Fig. 6.2; see also Chap. 2). Cortical responses can only be interpreted reliably when the peripheral responses are present. In the nomenclature of SSEP waveforms, N or P followed by an integer is used to indicate the polarity (positive, respectively, negative) and the nominal poststimulus latency (in ms) of the recorded wave in a healthy reference population (e.g. P15, N20). The earliest cortical potential is the N20, which is generated in the primary somatosensory cortex, where thalamocortical cells undergo synaptic connections with the superficial and deep pyramidal cell layers (Allison et al. 1991). In comparison to cortical responses of greater latency, the N20 is the most robust, as this is the latest waveform to disappear following increasing levels of encephalopathy or pharmacological sedation; of note, however, the N20 is relatively independent to the level of sedation used in clinical settings (Cruccu et al. 2008). Since the cortical waveforms appearing later (such as P45, N60 and P/N100) are less reliable and more susceptible to changes by sedation, the N20 is widely used in almost all clinical prognostic questions.
6.3 Pitfalls and Limitations of SSEP Recordings in the ICU
One of the main problems of the SSEP interpretation is the interobserver agreement, which has been extensively described in several studies (Zandbergen et al. 2006a; Pfeifer et al. 2013). Zandbergen et al. investigated 56 consecutive patients with anoxic–ischaemic coma (Zandbergen et al. 2006a); these registrations were interpreted independently by five experienced clinical neurophysiologists. The interobserver agreement for SSEPs in anoxic–ischaemic coma was only moderate (kappa 0.52, 95 % CI 0.20–0.65): the main source of disagreement was related to the underlying electrical noise, implying difficulties in obtaining a reasonable signal-to-noise ratio. For recordings with a noise level of 0.25 μV or more, the mean kappa was as low as 0.34 (fair agreement), while for recordings with noise levels below 0.25 μV, the mean kappa improved to 0.74, which is a substantial agreement. Similar results have been reported by Pfeifer et al., again in subjects admitted after cardiac arrest (Pfeifer et al. 2013). One way of integrating the SSEP information with other neurophysiological variables, in order to limit the aforementioned problems, may be a continuous SSEP registration combined with continuous EEG. This can be used to monitor deterioration in patients with severe brain injury (Amantini et al. 2009); however, such approaches are still rare in clinical practice. Of note, almost all literature regarding the use of SSEP for prognostication uses the absence or presence of short-latency cortical responses (N20). Whether the amplitude of cortical responses can be used is uncertain. During continuous SSEP and EEG registration, an N20 amplitude <1.2 μV has been used as a cut-off to describe an abnormal SSEP (Bosco et al. 2011), but it is important to underscore that this threshold does not reflect any evidence nor current practice for N20 analyses in the prognosis after cardiac arrest.
Efforts should be made to increase the signal-to-noise ratio as much as possible. As an orienting threshold, Zandbergen et al. recommend that the peak-to-peak amplitude of noise of the cortical and cervical leads should be lower than 0.25 μV after averaging, especially in the frequency of the SSEPs themselves (20–500 Hz) (Zandbergen et al. 2006a). Administration of muscle relaxants (together with modest doses of sedation, such as 5 mg of midazolam) often improves the quality of the SSEP registrations in patients with abundant muscle activity. Furthermore, disturbing electrical ICU equipment should be turned off as much as possible. Also, delivering more stimuli (up to 1,000 or more) and increasing the stimulus intensity could contribute to optimize the signal-to-noise ratio. In addition, it has been also suggested that the stimulus rate may influence the results of an SSEP recording (Robinson and Micklesen 2010) (see Chap. 2). Since the interpreting clinician is often not present during the actual SSEP registration itself, the role of the technician is crucial in obtaining reliable results.
6.4 Interpretation Criteria of SSEP
Additional criteria apart from the signal-to-noise ratio should be kept in mind when interpreting SSEP recordings. An N20 peak on one side can only be considered as present if it fulfils all of the following criteria (Zandbergen et al. 2006a):
-
It should have an appropriate latency (i.e. appearing at least 4.5 ms later than the corresponding N13 peak recorded from the posterior cervical region in normal-stature adults).
-
It should be present on the other side, and there should be a clear difference with the recording from the side ipsilateral to the stimulus. Therefore, it is recommended to record not only the contralateral sensory cortex after stimulation but also co-register the ipsilateral side. This prevents misinterpretation of the N18, which has its origin in the brainstem, as an N20 potential.
-
Any potential should be clearly reproducible in a second set of stimuli.
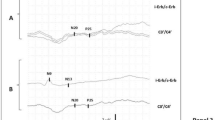
Bilateral absence of N20 peaks requires the presence of normal potentials over Erb’s point and the neck (N13), in order to ensure that the impulses have arrived in the central nervous system through an integer peripheral pathway. Figure 6.3 illustrates an example of an SSEP registration with present and absent N20 cortical responses.
6.5 Confounding Factors and Sedation
Cortical responses are generally not influenced by moderate pharmacological sedation or metabolic disturbances, factors that often hamper the clinical neurological examination in the ICU. However, massive intoxications, very severe biochemical or metabolic disturbances and anatomical (e.g. a high cervical) lesions should be actively ruled out. The cortical N20 responses may remain still visible even at sedation levels sufficient to induce an isoelectric EEG (Cruccu et al. 2008; Rothstein 2004); nevertheless, care should be taken when high-dosed barbiturates are administered: high-dosed sodium thiopental induces an increase in latencies and decrease in amplitudes for median nerve SSEP and brainstem auditory evoked responses (Drummond et al. 1985). It is uncertain whether the amplitudes decrease to a level that the cortical response can no longer be identified. Patients with absent cortical responses during thiopental (or pentobarbital) coma prescribed to treat increased intracranial pressures who made a good recovery in the end have been reported in the literature (Robe et al. 2003). This suggests that very high-dosed barbiturates can depress SSEP cortical responses. Propofol produces minimal suppression of the SSEP amplitude, which at most may be quantified to a loss of less than 10 % (Langeron et al. 1999). Also, midazolam and opioids have only moderate effects on SSEP amplitudes and latencies (Langeron et al. 1999; Asouhidou et al. 2010; Laureau et al. 1999; Taniguchi et al. 1992). Remifentanil can lower the cortical components by 20–80 % when given at a high dose (0.8 mcg/kg/min) as used during neuromonitoring in the operating room (Asouhidou et al. 2010). On the other hand, as stated above, in some cases it may be even advisable to administer low-dose sedation to improve the quality of the SSEP recordings. This is especially the case in patients with generalized periodic discharges on the EEG (see Chaps. 3, 4 and 5), which in some situations can be suppressed after the administration of propofol. These periodic discharges often have larger amplitudes in comparison to the evoked potentials and can disturb the detection of cortical response.
6.6 Prognostication in Postanoxic Coma
Bilateral absence of short-latency (N20) SSEP responses has been identified as the most powerful prognosticator of poor outcome in patients who remain unconscious after a circulatory arrest (Rossetti et al. 2010; Bouwes et al. 2012). In patients not treated with hypothermia, bilateral absence of cortical N20 responses 24 h or more after the event represents a reliable predictor for a poor neurological outcome (which is understood as no recovery of awareness) (Zandbergen et al. 2006b). A recent systematic review of all SSEP registrations reported in patients admitted to the ICU after resuscitation from a cardiac arrest and treated with hypothermia showed a false-positive rate (FPR) as low as 0.007, with a 95 % confidence interval of 0.001–0.047 (Kamps et al. 2013). These registrations were performed after return of normothermia. Even registration during therapeutic hypothermia might already have a solid good prognostic value, but the confidence interval is wider (Tiainen et al. 2005; Bouwes et al. 2009).
Unfortunately, a bilateral preservation of the N20 does not imply a favourable outcome in patients after cardiac arrest. In fact, only a small proportion of patients with a poor outcome after resuscitation have negative SSEP responses resulting in a low sensitivity (Kamps et al. 2013; Cloostermans et al. 2012). This low sensitivity of the SSEP is also reflected in the large variability of EEG patterns that can be observed in patients with a preserved N20, including status epilepticus, or even extremely low-voltage EEG. As pyramidal cell synaptic function is mainly reflected by the EEG, while SSEP mainly evaluates the thalamocortical synaptic function, a possible explanation for this peculiar constellation is selective hypoxic damage to the cortical pyramidal cells’ synaptic function, with preserved thalamocortical synapses (van Putten 2012)
The prognostic value of late cortical SSEP responses reflects the function of associative cortical areas beyond the primary sensory cortex; although promising from a scientific point of view, it seems still not to be reliable enough to be used in daily clinical practice for treatment decisions in the clinical setting (Pfeifer et al. 2013; Zandbergen et al. 2006c).
6.7 Prognostication in Traumatic Brain Injury
In patients with severe traumatic brain injury (TBI), the results available on the reliability of SSEP to predict outcome have been contradictive. Sleigh et al. suggested, in a prospective and blinded cohort study including 105 patients, that median nerve SSEPs are reliable predictors for poor neurological outcome, with a 43 % sensitivity and no false positives (Taniguchi et al. 1992). In contrast, in several other studies, TBI patients have been described initially showing bilaterally absent N20 responses, who nevertheless later regained consciousness, with only minor disabilities (Cruccu et al. 2008). These results show that the absence of cortical SSEP responses does not represent a reliable predictor in this clinical context. The most likely explanation is that in head trauma, a transient N20 disappearance may be consecutive to focal midbrain dysfunction due to oedema (Cruccu et al. 2008) or focal cortical lesions. Therefore, SSEP should always be integrated with other neurophysiologic tools and clinical examination to improve the predictive value (Cruccu et al. 2008; Taniguchi et al. 1992). Moreover, in TBI patients, it is especially important to rule out traumatic lesions of the peripheral nerves, nerve roots or spinal cord, when using clinical neurophysiologic tests. To complicate issues, clinical examination of the peripheral nerves at times can prove difficult in patients with diminished consciousness.
6.8 Prognostication in Stroke
The use of median nerve SSEP registration in patients with severe ischaemic or haemorrhagic stroke was investigated by Su et al. (2010) and Zhang et al. (2011). The absence of cortical N20 response, at least on one side, or an abnormal bilateral N20–P25 amplitude ratio was reported to be statistically significantly correlated with a poor outcome (Su et al. 2010). Zhang reported that especially the absence of the N20 or the N60 (a late potential) contralateral to the lesion could help to predict a poor outcome in patients with severe stroke (Zhang et al. 2011). Besides the scientific interest of these studies, a sequential clinical assessment integrated with brain imaging studies appears more reliable and robust than the use of SSEP in this clinical setting.
In patients with subarachnoid haemorrhage, neither median nor tibial SSEP nor central conduction time of the median nerve SSEP can be reliably used as a valid outcome predictor. The patient’s initial clinical grading still provides the only satisfying predictor (Wachter et al. 2011).
6.9 Prognostication in Sepsis
In patients with severe sepsis and septic shock, prolonged cortical SSEP peak latencies occur in 84 % of the patients. These latencies can be used to diagnose septic encephalopathy and its severity (Zauner et al. 2002). In these patients, however, SSEPs have not been reported to be helpful in determining the clinical prognosis.
6.10 Conclusions
SSEP can be used for prognostication in ICU patients with coma, as it represents a simple technique available at bedside, which can therefore be implemented relatively easily. Especially in patients with coma after cardiac arrest, this technique can give valuable additional information. However, physicians who base their treatment decisions on these techniques should be well aware of the inherent limitations and pitfalls. Technicians who perform the recordings should be instructed on how to optimize the signal-to-noise ratio, in order to increase the reliability of this test.
References
Allison T, McCarthy G, Wood CC, Jones SJ (1991) Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114(Pt 6):2465–2503
Amantini A, Fossi S, Grippo A, Innocenti P, Amadori A, Bucciardini L, Cossu C, Nardini C, Scarpelli S, Roma V, Pinto F (2009) Continuous EEG-SEP monitoring in severe brain injury. Neurophysiol Clin 39:85–93
Asouhidou I, Katsaridis V, Vaidis G, Ioannou P, Givissis P, Christodoulou A, Georgiadis G (2010) Somatosensory Evoked Potentials suppression due to remifentanil during spinal operations; a prospective clinical study. Scoliosis 5:8
Bosco E, Marton E, Feletti A, Scarpa B, Longatti P, Zanatta P, Giorgi E, Sorbara C (2011) Dynamic monitors of brain function: a new target in neurointensive care unit. Crit Care 15:R170
Bouwes A, Binnekade JM, Zandstra DF, Koelman JH, van Schaik IN, Hijdra A, Horn J (2009) Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology 73:1457–1461
Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, Biemond HS, Kors BM, Koelman JH, Verbeek MM, Weinstein HC, Hijdra A, Horn J (2012) Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol 71:206–212
Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ (2012) Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med 40:2867–2875
Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, Garcia-Larrea L (2008) Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol 119:1705–1719
Drummond JC, Todd MM, U HS (1985) The effect of high dose sodium thiopental on brain stem auditory and median nerve somatosensory evoked responses in humans. Anesthesiology 63:249–254
Kamps MJ, Horn J, Oddo M, Fugate JE, Storm C, Cronberg T, Wijman CA, Wu O, Binnekade JM, Hoedemaekers CW (2013) Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive Care Med 39:1671–1682
Langeron O, Vivien B, Paqueron X, Saillant G, Riou B, Coriat P, Lille F (1999) Effects of propofol, propofol-nitrous oxide and midazolam on cortical somatosensory evoked potentials during sufentanil anaesthesia for major spinal surgery. Br J Anaesth 82:340–345
Laureau E, Marciniak B, Hebrard A, Herbaux B, Guieu JD (1999) Comparative study of propofol and midazolam effects on somatosensory evoked potentials during surgical treatment of scoliosis. Neurosurgery 45:69–74
Pfeifer R, Weitzel S, Gunther A, Berrouschot J, Fischer M, Isenmann S, Figulla HR (2013) Investigation of the inter-observer variability effect on the prognostic value of somatosensory evoked potentials of the median nerve (SSEP) in cardiac arrest survivors using an SSEP classification. Resuscitation 84:1375–1381
Robe PA, Dubuisson A, Bartsch S, Damas P, Laureys S (2003) Favourable outcome of a brain trauma patient despite bilateral loss of cortical somatosensory evoked potential during thiopental sedation. J Neurol Neurosurg Psychiatry 74:1157–1158
Robinson LR, Micklesen PJ (2010) Does stimulus rate matter when performing somatosensory evoked potentials for coma patients? Neurocrit Care 12:69–73
Rossetti AO, Oddo M, Logroscino G, Kaplan PW (2010) Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 67:301–307
Rothstein TL (2004) Recovery from near death following cerebral anoxia: a case report demonstrating superiority of median somatosensory evoked potentials over EEG in predicting a favorable outcome after cardiopulmonary resuscitation. Resuscitation 60:335–341
Su YY, Xiao SY, Haupt WF, Zhang Y, Zhao H, Pang Y, Wang L, Ding JP, Zhao JW (2010) Parameters and grading of evoked potentials: prediction of unfavorable outcome in patients with severe stroke. J Clin Neurophysiol 27:25–29
Taniguchi M, Nadstawek J, Pechstein U, Schramm J (1992) Total intravenous anesthesia for improvement of intraoperative monitoring of somatosensory evoked potentials during aneurysm surgery. Neurosurgery 31:891–897
Tiainen M, Kovala TT, Takkunen OS, Roine RO (2005) Somatosensory and brainstem auditory evoked potentials in cardiac arrest patients treated with hypothermia. Crit Care Med 33:1736–1740
van Putten MJ (2012) The N20 in post-anoxic coma: are you listening? Clin Neurophysiol 123:1460–1464
Wachter D, Christophis P, Stein M, Oertel MF (2011) Use of multimodal electrophysiological monitoring to predict outcome after subarachnoid hemorrhage? A prospective series. J Neurosurg Sci 55:179–187
Zandbergen EG, Hijdra A, de Haan RJ, van Dijk JG, de Visser Ongerboer BW, Spaans F, Tavy DL, Koelman JH (2006a) Interobserver variation in the interpretation of SSEPs in anoxic-ischaemic coma. Clin Neurophysiol 117:1529–1535
Zandbergen EGJ, Hijdra A, Koelman JHTM, Hart AAM, Vos PE, Verbeek MM, de Haan RJ, PROPAC Study Group (2006b) Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 66:62–68
Zandbergen EG, Koelman JH, de Haan RJ, Hijdra A (2006c) SSEPs and prognosis in postanoxic coma: only short or also long latency responses? Neurology 67:583–586
Zauner C, Gendo A, Kramer L, Funk GC, Bauer E, Schenk P, Ratheiser K, Madl C (2002) Impaired subcortical and cortical sensory evoked potential pathways in septic patients. Crit Care Med 30:1136–1139
Zhang Y, Su YY, Ye H, Xiao SY, Chen WB, Zhao JW (2011) Predicting comatose patients with acute stroke outcome using middle-latency somatosensory evoked potentials. Clin Neurophysiol 122:1645–1649
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Wien
About this chapter
Cite this chapter
Tjepkema-Cloostermans, M.C., van Putten, M.J.A.M., Horn, J. (2015). Prognostic Use of Somatosensory Evoked Potentials in Acute Consciousness Impairment. In: Rossetti, A., Laureys, S. (eds) Clinical Neurophysiology in Disorders of Consciousness. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1634-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1634-0_6
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1633-3
Online ISBN: 978-3-7091-1634-0
eBook Packages: MedicineMedicine (R0)