Abstract
EEG in refractory – and thus often long lasting and eventually subtle – status epilepticus is mostly characterized by generalized or lateralized periodic discharges. Based on EEG recordings alone, it is almost impossible to discern late forms of status epilepticus from various other severe nonepileptic encephalopathies. Periodic EEG patterns are not specific for circumscribed neurological conditions and are not pathognomonic for status epilepticus. Even disappearance of periodic discharges following administration of intravenous anticonvulsant does not prove the epileptic nature of specific EEG patterns. Generalized periodic discharges do not seem to be the cause and are not even a biological marker for poor prognosis. If the electro-clinical course allows making a definite diagnosis of refractory status epilepticus, EEG follow-up may help to tailor further therapeutic management.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
Status epilepticus (SE) represents the second most common neurological emergency following cerebrovascular accidents. Up to one third of patients do not respond to first- and second-line anticonvulsants, defining refractory SE. Especially if associated with severe disturbances of consciousness and/or breathing, this condition generally requires further management in an – at best neurological – intensive care unit. Changes of EEG patterns in the course of SE have been extensively studied. Refractory SE, which is often long lasting and thus subtle, is accompanied by a typical pattern mostly characterized by generalized and sometimes lateralized periodic discharges. The EEG in refractory SE is helpful in guiding further pharmacological management. One of the challenges in clinical practice is that EEGs from critically ill patients are often “cross-sectional,” i.e., detailed information on the electro-clinical evolution of the condition are lacking. This makes critical care EEGs difficult to interpret, as periodic discharge patterns are not pathognomonic for SE, but are seen in severe nonepileptic encephalopathies as well. The main scope of this chapter is to describe the evolution of typical EEG patterns in various SE stages and to discuss the difficulties to discern these patterns from those encountered in nonepileptic conditions.
4.2 Refractory Status Epilepticus: Pathophysiological and Clinical Background
One of the most interesting features of epileptic seizures is the fact that in the vast majority of cases, fits are self-limiting. While pathophysiological processes underlying transition from the interictal to the ictal state, thus the onset of a seizure, are quite well understood, neurobiological mechanisms resulting in seizure cessation are still relatively elusive. One hypothesis is that termination of an epileptic seizure requires energy-dependent processes involving restoration of the Na+–K+ pump. Failure of these processes results in persistence of increased extracellular K+ concentration facilitating continuous seizure activity and subsequently SE. Prolonged seizure activity induces internalization of postsynaptic GABAA receptors into the cell and thus GABAergic impairment (Chen and Wasterlain 2006; Kapur and Macdonald 1997). This concept is termed “receptor trafficking” and well explains both further maintenance of seizure activity – as endogenous GABA is less efficient – and progressive pharmacoresistance of the (exogenous) anticonvulsant drugs, most of which act on the GABAA receptor.
When defining SE clinically, first one has to consider how long self-limiting seizures commonly last. Video-EEG analyses of partial-onset seizures have demonstrated that the median duration of secondary generalized tonic–clonic seizures is 130 s and that of complex partial seizures 80 s (Jenssen et al. 2006). Retrospective data have shown that the likelihood of spontaneous termination of generalized tonic–clonic seizures is tightly related to seizure duration (42 % cessation after 10–29 min vs. 7 % after 30 min and more) (DeLorenzo et al. 1999). Keeping both findings in mind, an operational definition of SE of seizures lasting longer than 5 min has been suggested (Lowenstein et al. 1999); this has been adopted in the current European Federation of Neurological Societies treatment guidelines for all clinical forms of status epilepticus (Meierkord et al. 2010) (Fig. 4.1) and is widely used in clinical practice.
When is a seizure status epilepticus? Definitions of status epilepticus are still heterogeneous. The typical, self-limited epileptic seizure clearly lasts less than 5 min (GTCS generalized tonic–clonic seizure, CPS complex partial seizure) (Jenssen et al. 2006). The longer an epileptic seizure lasts, the less likely it terminates spontaneously, but the higher is the mortality rate (DeLorenzo et al. 1999). These findings resulted in an operational definition of status epilepticus proposing that every epileptic seizure lasting more than 5 min is status epilepticus, requiring instant and appropriate anticonvulsant treatment
Once the SE diagnosis has been made, antiepileptic treatment (or maybe better, anticonvulsant treatment, as the agents used act symptomatically) needs to be initiated instantly. First-line treatment was assessed in some randomized controlled trials, favoring intravenous lorazepam or intramuscular midazolam (Treiman et al. 1998; Alldredge et al. 2001; Silbergleit et al. 2012) over other agents (particularly, phenytoin). In practice, it is reasonable to administer a benzodiazepine, a fast-acting drug class; of note, diazepam redistributes relatively quickly away from the brain and therefore may be less suitable than lorazepam, midazolam, or even clonazepam. Which of the second-line anticonvulsants is most effective is planned to be assessed in the near future in a multicenter randomized controlled trial (ESETT, established status epilepticus treatment trial) (Bleck et al. 2013). Though a unifying definition of refractory SE does not exist, most authors agree that failure of an intravenous benzodiazepine (in most cases lorazepam) and a second-line drug, such as (fos)phenytoin, levetiracetam, or valproic acid, to terminate ongoing seizures defines refractoriness (Holtkamp et al. 2005b; Rossetti et al. 2005; Hocker et al. 2013). As opposed to first-line therapy, treatment of refractory SE is not based on high-evidence studies. Moreover, it seems reasonable to tune the further extent of treatment aggressiveness according to the clinical form. Complex partial SE (i.e., focal SE without severe consciousness impairment) itself does not seem to increase mortality rate or neurological/neuropsychological long-term sequelae. Therefore, pharmacological treatment regimens should try to avoid anesthetic anticonvulsants, at least initially. In contrast, generalized convulsive SE and its late clinical form – subtle SE (also called nonconvulsive SE in coma) – are accompanied by extensive neuronal excitotoxicity and induce potentially severe systemic challenges to the physiological homeostasis; thus long-term neurological and neuropsychological consequences represent a consistent risk (Meierkord and Holtkamp 2007). After failure of first- and second-line anticonvulsants, anesthetics such as barbiturates, midazolam, or propofol are highly recommended (Meierkord et al. 2010). Approximately, every fifth patient does not even respond to anesthetics, and seizure activity recurs after tapering. This condition has been termed malignant (Holtkamp et al. 2005a) or superrefractory (Shorvon 2011) SE.
The outcome of refractory SE is poorer than SE responding to the first-line treatment. In-hospital mortality has been reported to be as high as 30–40 %, either including (Sutter et al. 2013) or excluding (Hocker et al. 2013) hypoxic encephalopathy. Furthermore, duration of refractory SE is associated with poor prognosis (Sutter et al. 2013); survivors develop chronic epilepsy in 85 % of cases (Holtkamp et al. 2005b). However, even after several days of refractory SE, neuropsychological outcome may be favorable at least in some patients (Cooper et al. 2009); therefore, one should not stop treatment, especially in younger individuals, in the absence of clear signs of irreversible and severe brain damage.
4.3 EEG in Critically Ill: Terminology and Criteria for Seizures (See Also Chaps. 3 and 5)
Clinicians reading EEGs are often asked to judge if an EEG recorded from a critically ill patient is SE or not. However, the SE diagnosis, as that of epilepsy in general, is majorly a clinical one, and the EEG is only one piece of the puzzle. Most periodic EEG patterns are unspecific and per se do not allow to make the diagnosis of SE. In this regard, the commonly used term “epileptiform” in descriptions of generalized or lateralized periodic discharges may be misleading and bears the risk of misdiagnosing nonepileptic conditions for SE. Therefore, one can appreciate the American Clinical Neurophysiology Society’s undertaking to standardize EEG terminology in critical care – and the concept behind it (Hirsch et al. 2013). One of the main goals was to eliminate terms with clinical connotations, such indeed as the criticized term “epileptiform.” Following the proposed nomenclature, the former well-known term “periodic lateralized epileptiform discharges (PLED)” is proposed to be replaced by the descriptive term “lateralized periodic discharges” (Fig. 4.2a). Analogously, “generalized periodic epileptiform discharges” (GPEDs) are now better labeled “generalized periodic discharges” (Fig. 4.2b). As stated, these patterns are unspecific; they do not necessarily indicate SE, but depending on the clinical context and course, they may do (Meierkord and Holtkamp 2007) (Fig. 4.3).
Examples of periodic discharges. (a) Lateralized periodic discharges on the right at a frequency of approximately 0.5 Hz. (b) Generalized periodic discharges with sharp morphology at a frequency of approximately 1 Hz. Information on the clinical condition are not available (Hirsch et al. (2013). With permission from Wolters Kluwer Health)
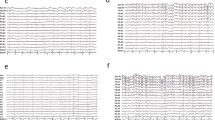
Generalized periodic discharges. Both EEG traces demonstrate generalized periodic discharges punctuated by flat periods. The correct clinical diagnoses can only be made when the clinical course is considered. (a) This EEG is recorded from a 39-year-old woman who presented with new-onset discrete tonic–clonic generalized seizures that increased in frequency, without regain of consciousness. Etiology was assumed to be encephalitis without identification of a specific pathogen. In retrospect, she may had suffered from immune-mediated status epilepticus, but cerebrospinal fluid or serum specimen was not preserved. In the further course, she had continuous generalized motor seizures. She was treated unsuccessfully with benzodiazepines and phenytoin and subsequently with anesthetic anticonvulsants including propofol and thiopental. Even after tapering of the anesthetics, generalized periodic discharges indicated subtle status epilepticus, thus fulfilling the criteria for malignant (or superrefractory) status epilepticus. Clinically, discrete (“subtle”) spontaneous perioral and extremity myoclonus was observed. Eventually, the patient died from electromechanical dissociation after 8 weeks of critical care treatment. (b) This EEG was recorded from a 58-year-old male patient 5 days after cardiopulmonary resuscitation due to ventricular fibrillation. The patient underwent standard treatment with hypothermia and simultaneous intravenous midazolam. Three days after the end of cooling and sedation, the patient was still comatose; he suffered from stimulus-sensitive myoclonus (occurrence during endotracheal suctioning or touching the patient). In this case, generalized periodic discharges indicate severe hypoxic encephalopathy. The patient was transferred to a rehabilitation clinic; the further course is unknown
Electrographic seizures – either discrete or prolonged – are well defined either by repetitive epileptiform discharges at ≥3 Hz or by epileptiform discharges at 1–3 Hz with clear evolution of the seizure pattern by frequency, location, or waveform (Chong and Hirsch 2005). Figure 4.4 illustrates a discrete electrographic seizure against the background of generalized periodic discharges (Foreman et al. 2012).
Electrographic seizure. EEG trace recorded from a woman in her 40s, 3 days after liver transplantation complicated by sepsis and renal failure. She was comatose. (a) Her initial continuous EEG monitoring demonstrated frequent generalized periodic discharges, occasionally with triphasic morphology. (b–d) Three consecutive pages of EEG about 2 h later, when she developed focal status epilepticus with right hemisphere onset, maximal in the right frontal parasagittal region (b, red arrow “onset”) that evolved before ending abruptly (d, red arrow “stop”). There was no clinical correlate on video (Foreman et al. (2012). With permission from Wolters Kluwer Health)
4.4 Temporal Evolution of EEG in Status Epilepticus
The dynamic neurobiological processes underlying SE are well reflected by the evolution of EEG. Treiman was the first to demonstrate that EEG in generalized convulsive SE changes over time following five identifiable and stereotypical patterns (Treiman et al. 1990). At SE onset, (1) discrete seizures were identified that later (2) merge with waxing and waning amplitude and frequency of EEG rhythms. In the further course, the EEG shows (3) continuous ictal activity. With ongoing SE, (4) continuous ictal activity is punctuated by short low-voltage “flat periods,” giving later way to the last pattern, which is characterized by (5) generalized periodic discharges on a “flat background” (Fig. 4.5). These five EEG stages correspond to the extent of generalized motor activity. At onset, (1) single generalized tonic–clonic seizures occur with short intervals that do not allow for full regain of baseline consciousness, defining SE. EEG patterns (2 and 3) correspond to overt generalized convulsive status epilepticus characterized by extensive motor signs. With emergence of EEG flat periods (4 and 5), the intensity of motor activity declines and either disappears completely or presents with only subtle, perioral, or extremity myoclonic twitches.
Temporal evolution of EEG in generalized convulsive status epilepticus. EEG traces show temporal evolution in different stages of generalized convulsive status epilepticus in patients (upper rows) and rodents (lower rows). Stages: (1) discrete seizures, (2) merge with waxing and waning amplitude and frequency of discharges, (3) continuous ictal activity, (4) continuous ictal activity punctuated by short low-voltage “flat periods,” (5) generalized periodic discharges on a “flat background” (Treiman et al. (1990). With permission from Elsevier)
The five stages of EEG in human SE were reproduced in three different animal models, in which seizures were induced by intraperitoneal (IP) injection of kainic acid, by IP injection of homocysteine and prior epidural placement of cobalt, or by IP injection of pilocarpine. Independently of the model used, in all animals the same sequence of EEG stages was recorded (Treiman et al. 1990) (Fig. 4.5). Similarities between the human condition and these three different animal models underline the consistency of EEG findings and thus of the underlying neurobiological processes.
4.5 Subtle Status Epilepticus
Subtle SE is the late manifestation of so far untreated or insufficiently treated overt generalized convulsive SE. The clinical hallmarks of subtle SE comprise a comatose state and the absence of prominent motor features. However, discrete (“subtle”) muscle twitching may be present, and the EEG mostly shows generalized periodic discharges with flat periods (Fig. 4.3a), but lateralized and regional discharges may also occur.
The concept of subtle SE is very useful and has the potential to guide the clinician in cases where the correct diagnosis is immediately relevant for treatment decisions. This approach looses much of its diagnostic power if not used in the strict sense as representing the end point of “overt” SE (the latter denotes in fact generalized convulsive SE (Treiman et al. 1990, 1998)). In the initial descriptions, the concept of subtle SE was used in a wider sense, and also those patients were included in whom the condition was believed to be caused by severe encephalopathy and subtle SE may have been be a possible, unrecognized cause of coma (Treiman et al. 1984). In order to retain the cutting edge of the concept, the diagnosis of subtle SE should only be made in the presence of clearly suggestive EEG changes and if there is evidence of previous overt epileptic seizures or SE (Holtkamp and Meierkord 2011).
Subtle SE should be treated as aggressively as the overt variant. In a landmark randomized controlled trial, the intravenous (IV) administration of lorazepam, diazepam followed by phenytoin, phenobarbital, or phenytoin terminated subtle status epilepticus in 8–24 % of cases only; success rates were not significantly different among the four study groups (Treiman et al. 1998). As a comparison, the response rate in early overt generalized convulsive SE was 44–65 %, and the dramatic loss in efficacy of the predominantly GABAergic substances may be explained by modification of the GABAA receptor due to continuing seizure activity (Kapur and Macdonald 1997). Therefore, the European treatment guidelines recommend a prompt use of anesthetics such as barbiturates, midazolam, or propofol (Meierkord et al. 2010).
4.6 Mimics: Refractory Status Epilepticus Versus Nonepileptic Encephalopathies
As discussed above, periodic EEG patterns are not specific for SE, even if the morphology of discharges is sharp or spiky (Fig. 4.6a). For some clinicians or clinical neurophysiologists, it may be tempting to “treat” these EEG patterns with IV anticonvulsants: all periodic EEG patterns will disappear with administration of anticonvulsant drugs, especially benzodiazepines (Fig. 4.6b). This mere electrographic “treatment success” again bears the risk to misdiagnose nonepileptic conditions as SE and to potentially harm the patient.
EEG “treatment” with anticonvulsants. Both EEG traces are recorded in the same 65-year-old male patient with severe posthypoxic encephalopathy (a) prior to and (b) after intravenous administration of a benzodiazepine. (a) This EEG was recorded 7 days after cardiopulmonary resuscitation presumably due to ventricular fibrillation. The patient was free of sedative substances for more than 72 h and still comatose. The EEG demonstrates generalized periodic discharges with spiky morphology at a frequency of 2 Hz. (b) This EEG was recorded 10 min after an intravenous administration of 10 mg diazepam (indication unclear). Spiky generalized periodic discharges have disappeared; some “abortive” discharges occur at the right end of the trace. The alteration of the EEG pattern did not correlate with clinical improvement; the patient was still comatose
Several severe nonepileptic encephalopathies are relatively frequently accompanied by periodic EEG patterns with discharges of heterogeneous morphology. These include posthypoxic (Fig. 4.6a), septic, metabolic, and even neurodegenerative conditions (Fig. 4.7).
Examples of periodic EEG patterns of nonepileptic origin. (a) EEG trace of a 72-year-old woman with septic encephalopathy demonstrating generalized periodic discharges at a frequency of slightly above 1 Hz. (b) EEG trace of a 38-year-old male patient with hepatic encephalopathy due to liver failure, generalized periodic discharges with triphasic morphology. This EEG pattern is typically seen in hepatic and other metabolic encephalopathies. (c) EEG trace of a 75-year-old woman referred to the neurological intensive care unit with the diagnosis of nonconvulsive status epilepticus. EEG demonstrated spiky generalized periodic discharges at a frequency of 2.5 Hz. Discharges disappeared following intravenous administration of 1 mg clonazepam (EEG trace not shown). Later, the diagnosis of sporadic Creutzfeldt–Jakob disease was made (Lapergue et al. (2010). With permission from Wolters Kluwer Health)
There has been a long debate on whether periodic EEG discharges are just the electrophysiological expression of severe brain disease or may reflect an “epileptogenic” potential: even if these discharges are nonepileptic, it was unclear if they have some excitotoxic properties harming the brain in addition to the underlying severe neurological disorder. This question is not just of academic interest, but implies direct treatment consequences. A recent well-designed case–control study has addressed this issue, comparing in-hospital outcome of 200 critically ill patients with generalized periodic discharges and 200 control patients with comparable severe brain injuries but without EEG discharges. It demonstrated that patients with generalized discharges had significantly more often nonconvulsive seizures and status epilepticus (Fig. 4.4). However, multivariate predictors of poor outcome were cardiac arrest, coma, nonconvulsive SE, and sepsis, but not generalized periodic discharges themselves (Foreman et al. 2012).
References
Alldredge BK, Gelb AM, Isaacs SM et al (2001) A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 345:631–637
Bleck T, Cock H, Chamberlain J et al (2013) The established status epilepticus trial. Epilepsia 54(Suppl 6):89–92
Chen JW, Wasterlain CG (2006) Status epilepticus: pathophysiology and management in adults. Lancet Neurol 5:246–256
Chong DJ, Hirsch LJ (2005) Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. Clin Neurophysiol 22:79–91
Cooper AD, Britton JW, Rabinstein AA (2009) Functional and cognitive outcome in prolonged refractory status epilepticus. Arch Neurol 66:1505–1509
DeLorenzo RJ, Garnett LK, Towne AR et al (1999) Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia 40:164–169
Foreman B, Claassen J, Abou Khaled K et al (2012) Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology 79:1951–1960
Hirsch LJ, LaRoche SM, Gaspard N et al (2013) American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 30:1–27
Hocker SE, Britton JW, Mandrekar JN et al (2013) Predictors of outcome in refractory status epilepticus. JAMA Neurol 70:72–77
Holtkamp M, Meierkord H (2011) Nonconvulsive status epilepticus: a diagnostic and therapeutic challenge in the intensive care setting. Ther Adv Neurol Disord 4:169–181
Holtkamp M, Othman J, Buchheim K et al (2005a) A “malignant” variant of status epilepticus. Arch Neurol 62:1428–1431
Holtkamp M, Othman J, Buchheim K et al (2005b) Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry 76:534–539
Jenssen S, Gracely EJ, Sperling MR (2006) How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia 47:1499–1503
Kapur J, Macdonald RL (1997) Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci 17:7532–7540
Lapergue B, Demeret S, Denys VN (2010) Sporadic Creutzfeldt-Jakob disease mimicking nonconvulsive status epilepticus. Neurology 74:1995–1999
Lowenstein DH, Bleck T, Macdonald RL (1999) It’s time to revise the definition of status epilepticus. Epilepsia 40:120–122
Meierkord H, Holtkamp M (2007) Non-convulsive status epilepticus in adults: clinical forms and treatment. Lancet Neurol 6:329–339
Meierkord H, Boon P, Engelsen B et al (2010) EFNS guideline on the management of status epilepticus in adults. Eur J Neurol 17:348–355
Rossetti AO, Logroscino G, Bromfield EB (2005) Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol 62:1698–1702
Shorvon S (2011) Super-refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia 52(Suppl 8):53–56
Silbergleit R, Durkalski V, Lowenstein D et al (2012) Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 366:591–600
Sutter R, Marsch S, Fuhr P et al (2013) Mortality and recovery from refractory status epilepticus in the intensive care unit: a 7-year observational study. Epilepsia 54:502–511
Treiman DM, DeGiorgio CMA, Salisbury SM et al (1984) Subtle generalized convulsive status epilepticus. Epilepsia 25:653
Treiman DM, Walton NY, Kendrick C et al (1990) A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res 5:49–60
Treiman DM, Meyers PD, Walton NY et al (1998) A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 339:792–798
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Wien
About this chapter
Cite this chapter
Holtkamp, M. (2015). EEG in Refractory Status Epilepticus. In: Rossetti, A., Laureys, S. (eds) Clinical Neurophysiology in Disorders of Consciousness. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1634-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1634-0_4
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1633-3
Online ISBN: 978-3-7091-1634-0
eBook Packages: MedicineMedicine (R0)