Abstract
The mathematical model of cerebrospinal fluid (CSF) pressure volume compensation, introduced by Anthony Marmarou in 1973 and modified in later studies, provides a theoretical basis for differential diagnosis in hydrocephalus. The Servo-Controlled Constant Pressure Test (Umea, Sweden) and Computerised Infusion Test (Cambridge, UK) are based on this model and are designed to compensate for inadequate accuracy of estimation of both the resistance to CSF outflow and elasticity of CSF pressure volume compensation.
Dr. Marmarou’s further works introduced the pressure volume index (PVI), a parameter used to describe CSF compensation in hydrocephalic children and adults. A similar technique has been also utilized in traumatic brain injury (TBI).
The presence of a vascular component of intracranial pressure (ICP) was a concept proposed in the 1980s. Marmarou demonstrated that only around 30% of cases of elevated ICP in patients with TBI could be explained by changes in CSF circulation. The remaining 70% of cases should be attributable to vascular components, which have been proposed as equivalent to raised brain venous pressure.
Professor Marmarou’s work has had a direct impact in the field of contemporary clinical neurosciences, and many of his ideas are still being investigated actively today.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Models of cerebrospinal fluid (CSF) circulation usually differ from models simulating brain tissue displacement. Anatomical structure and distribution of stress-strain in the tissue are not of interest here compared with the hydrodynamics of CSF flow. Dynamics of intracranial pressure (ICP) may be monitored invasively in clinical practice with a pressure transducer, and dynamics of CSF flow can be measured noninvasively with phase-coded magnetic resonance imaging (MRI). Therefore, models of CSF dynamics have an established clinical application in diagnosis and management of several diseases such as hydrocephalus, idiopathic intracranial hypertension, and syringomyelia.
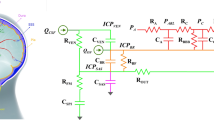
Many theoretical/modeling studies on CSF dynamics were published before the 1970s [3, 6, 8, 10]. However, Professor Anthony Marmarou was one of the first [11, 13] who integrated all components – CSF production, circulation, absorption, and storage – in one elegant theoretical structure expressed as an electrical circuit (see Fig. 1). He analyzed theoretically three basic maneuvers: bolus CSF withdrawal, addition, and constant rate infusion. This model has withstood the test of time and, with only a very few ‘cosmetic’ modifications, it is still used today. Consequently, hydrocephalus and other disorders of CSF circulation are now characterized using parameters from this model such as resistance to CSF outflow, elasticity, and pressure volume index (PVI). These parameters were introduced into clinical practice by Marmarou et al. in 1975 [12]. He also proposed a mathematical explanation of the linear relationship between pulse amplitude and mean ICP [12], which was later elaborated by Avezaat and Eijndhoven [2]. In 1987, he described the “vascular component” of ICP [14]. In patients with traumatic brain injury (TBI), only 30% of cases of elevated ICP can be explained by changes in CSF circulation. Therefore, Marmarou concluded that the remaining 70% of cases of elevated ICP are derived from changes in the intracranial vascular component.
Electrical model of cerebrospinal fluid (CSF) dynamics according to (a) Marmarou. Upper panel: Current source represents formation of CSF, resistor, and diode – unilateral absorption to sagittal sinus (voltage source p ss represents sagittal sinus pressure). Capacitor – nonlinear compliance of CSF space. Lower panel: Extended model showing hydrodynamic consequence of shunting
All three of these milestone achievements in the area of CSF dynamics are used today. The mathematical model of CSF dynamics will be presented briefly in the next section followed by a synopsis of the legacy of Marmarou’s works in contemporary clinical neuroscience.
Marmarou’s Model of CSF Dynamics
The mathematical model of CSF pressure volume compensation, introduced by Marmarou [11, 13] and modified in later studies [2, 16], provides a theoretical basis for differential diagnosis in hydrocephalus.
Under normal conditions, without long-term fluctuations of the cerebral blood volume, production of CSF is balanced by its storage and reabsorption in the sagittal sinus:
Production of CSF is assumed to be constant, although it may not always be the case. Reabsorption is proportional to the gradient between CSF pressure (p) and pressure in the sagittal sinuses (p ss):
p ss is considered to be a constant parameter determined by central venous pressure. However, it is not certain whether an interaction between changes in CSF pressure and p ss exists in all circumstances: in patients with benign intracranial hypertension p ss is frequently elevated due to fixed or variable stenosis of transverse sinuses, and a similar situation can be seen in venous sinus thrombosis.
The coefficient R (symbol R CSF is also used) refers to the resistance to CSF reabsorption or outflow (units: mmHg/(mL/min)).
Storage of CSF is proportional to the cerebrospinal compliance C (units: mL/mmHg) and the rate of change of CSF pressure dp/dt:
The compliance of the cerebrospinal space is inversely proportional to the gradient of CSF pressure p and the reference pressure p 0 (4):
Some authors suggest that relationship (4) is valid only above a certain pressure level called the “optimal pressure” [16]. The coefficient E is termed the cerebral elasticity (or elastance coefficient) (unit: mL−1). Elevated elasticity (> 0.18 mL−1) signifies a poor pressure volume compensatory reserve [2]. This coefficient has recently been confirmed to be useful in predicting a patient’s response to third ventriculostomy [17]. Relationship (4) expresses the most important law of the cerebrospinal dynamic compensation: When the CSF pressure increases, the compliance of the brain decreases.
A combination of (1) with (2) and (4) gives a final equation (5):
where I(t) is the rate of external volume addition and p b is a baseline CSF pressure.
The model described by this equation may be presented in the form of its electric circuit equivalent [11] (Fig. 1).
Equation (5) can be solved for various types of external volume additions I(t). The most common in clinical practice is
-
(a) A constant infusion of CSF (I(t) = 0 for t < 0 and I(t) = I inf for t > 0) – see Fig. 2:
Fig. 2 Methods of identification of the model of cerebrospinal fluid (CSF) circulation during constant rate infusion study. (a) Recording of CSF pressure (ICP) versus time increasing during infusion with interpolated modeling curve (7) Infusion of constant rate of 1.5 mL/min starts from vertical line. (b) Recording of pulse amplitude (AMP) during infusion. Rise in AMP is usually well correlated with rise in ICP. (c) Pressure volume curve. On the x-axis, effective volume increase is plotted (i.e., infusion and production minus reabsorption of CSF). On y-axis, the increase in pressure is measured as a gradient of current pressure minus reference pressure p 0, relative to baseline pressure p b. (d) Linear relationship between pulse amplitude and mean ICP. Intercept of the line with x-axis (ICP) theoretically indicates the reference pressure p 0
$$ P(t)=\frac{\left[{I}_{\mathrm{inf}}+\frac{{p}_{\text{b}}-{p}_{0}}{R}\right] \cdot \left[{p}_{\text{b}}-{p}_{0}\right]}{\frac{{p}_{\text{b}}-{p}_{0}}{R}+{I}_{\mathrm{inf}} \cdot \left[{e}^{-E\left[\frac{{p}_{\text{b}}-{p}_{0}}{R}+{I}_{\mathrm{inf}}\right] \cdot t}\right]}+{p}_{0} $$(6)The analytical curve (6) can be matched to the real recording of the pressure during the test, which results in an estimation of the unknown parameters: R, E, and p 0 (see Fig. 2a).
-
(b) A bolus injection of CSF (volume ΔV):
$$ p(t)=\frac{\left({p}_{\text{b}}-{p}_{0}\right) \cdot \text{e}^{E\left[\Delta V+\frac{{p}_{\text{b}}-{p}_{0}}{R} \cdot t\right]}}{1+{e}^{E\Delta V} \cdot \left[{e}^{E \cdot \frac{{p}_{\text{b}}-{p}_{0}}{t}\cdot t}-1 \right]}+ {p}_{0} $$(7)
The bolus injection can be used for calculation of the PVI, defined as the volume added externally to produce a tenfold increase in the pressure [12]:
p p in the formula (8a) is peak pressure recorded just after addition of the volume ∆V. The PVI is theoretically proportional to the inverse of the brain elastance coefficient E. The pressure volume compensatory reserve is insufficient when PVI <13 mL, and a PVI value above 26 mL signifies an “over-compliant” brain. These norms are valid for the PVI calculated as an inverse of E (according to 8b) using slow infusion. If the bolus test is used, norms for PVI are higher (the threshold equivalent to 13 mL is around 25 mL [15]).
The formula (7) for time t = 0 describes the shape of the relationship between the effective volume increase ΔV and the CSF pressure, called the pressure volume curve (Fig. 2c):
Finally, Eq. (7) can be helpful in the theoretical evaluation of the relationship between the pulse wave amplitude of ICP and the mean CSF pressure. If we presume that the rise in blood volume after a heart contraction is equivalent to a rapid bolus addition of CSF fluid at the baseline pressure p b, the pulse amplitude (AMP) can be expressed as:
In almost all cases, when CSF pressure is being increased by the addition of an external volume, the pulse amplitude rises [2, 12] – see Fig. 2b, d. The gradient of the regression line between AMP and p is proportional to the elasticity. The intercept, theoretically, marks the reference pressure p 0.
Synopsis of Clinical Applications of the Model
-
The Servo-Controlled Constant Pressure Infusion Test [7] is used for assessment of CSF disorders. Its aim is to evaluate the resistance to CSF outflow in a repetitive and reliable way.
-
Full identification of the model, including elasticity, can be made using a computerized constant rate infusion test [4] supported by the dedicated software ICM+ (http://www.neurosurg.cam.ac.uk/icmplus/ ).
-
Use of the constant rate infusion test in many centers contributed to a definition of profiles of ICP and its pulse amplitude in different possible clinical scenarios, including normal pressure hydrocephalus, brain atrophy, acute hydrocephalus, and in nondisturbed CSF circulation (see Fig. 3).
Fig. 3 Examples of a constant rate infusion test. ICP mean ICP (10-s average), AMP pulse amplitude of ICP. The blue section is the duration of infusion. (a) Normal pressure hydrocephalus (NPH): Although the base line pressure is normal, the resistance to cerebrospinal fluid (CSF) outflow increased, there are lots of strong vasogenic waves, and changes in pulse amplitude are fairly well correlated with changes in mean ICP. (b) Acute hydrocephalus post-subarachnoid hemorrhage (SAH): The normal baseline pressure was measured, but the resistance to CSF outflow is high. Good response of shunt surgery was expected. (c) Cerebral brain atrophy: the base line pressure is low, but the resistance to CSF outflow is low. No vasogenic waves were recorded, and pulse amplitude does not respond. (d) Normal: the base line pressure, the resistance to CSF outflow, and other parameters are normal, and thus the result demonstrates normal CSF circulation
-
Analysis of the constant rate infusion test in shunted patients can be helpful in shunt assessment in vivo. The electrical circuit model proposed by Professor Marmarou, supplemented by a branch-defining nonlinear pressure flow performance curve, is presented in Fig. 1. Direct knowledge of the curve, as assessed in shunt evaluation laboratories [1, 5] allows in vivo identification of the model and sensitive prediction whether the shunt is working properly, underdraining, or overdraining.
-
The proportional increase of the pulse waveform of ICP with mean ICP, has been explained by Marmarou [12] as a consequence of the exponential pressure volume curve. Although further works demonstrated that in a system with a good pressure volume compensatory reserve, the pressure volume curve is linear [2, 16], at higher pressures, the curve becomes exponential [2] This led to analysis of a moving correlation coefficient (20-s to 2-min period) between mean ICP and AMP. The resulting RAP coefficient indicates the state of compensatory reserve. RAP = 0 suggests good compensatory reserve; RAP = 1, poor compensatory reserve [9].
-
The idea of a vasogenic component of ICP led to modification of Davson’s equation: ICP = R CSF * CSFformation + p ss+ ‘Arterial vasogenic component’
The “arterial vasogenic component” is a component of ICP which is derived by detection of pulsatile blood flow in nonlinear components of cerebrospinal space (intracranial and arterial bed compliance, resistance of collapsible bridging veins, and autoregulation-controlled main cerebrovascular resistance).
Conclusion
When Professor Marmarou was terminally ill and was asked by his coworkers what they should do in future years, he simply said “Continue” (Dr. G. Aygok, personal communication). There are certainly a lot of directions to continue in and many questions initiated by Anthony Marmarou in the field of clinical neurosciences that remain unanswered.
References
Aschoff A, Kremer P (1998) Determining the best cerebrospinal fluid shunt valve design: the pediatric valve design trial. Neurosurgery 42(4):949–951
Avezaat CJJ, Eijndhoven JHM (1984) Cerebrospinal fluid pulse pressure and craniospinal dynamics. Ph.D. thesis, The Jongbloed an Zoon Publishers, The Hague
Benabid AL (1970) Contribution a l’etude de l’hypertension intracranienne modele mathematique. M.D. thesis, Grenoble University
Czosnyka M, Whitehouse H, Smielewski P et al (1996)Testing of cerebrospinal compensatory reserve in shunted and non-shunted patients: a guide to interpretation based on an observational study. J Neurol Neurosurg Psychiatry 60:549–558
Czosnyka Z, Czosnyka M, Richards HK et al (1998) Posture-related overdrainage: comparison of the performance of 10 hydrocephalus shunts in vitro. Neurosurgery 42(2):327–333
Davson H, Hollingsworth JR, Segal MD (1970) The mechanism of drainage of the cerebrospinal fluid. Brain 93:665–678
Eklund A, Lundkvist B, Koskinen LO, Malm J (2004) Infusion technique can be used to distinguish between dysfunction of a hydrocephalus shunt system and a progressive dementia. Med Biol Eng Comput 42(5):644–649
Guinane JE (1972) An equivalent circuit analysis of cerebrospinal fluid hydrodynamics. Am J Physiol 223:425–430
Kim DJ, Czosnyka Z, Keong N et al (2009) Index of cerebrospinal compensatory reserve in hydrocephalus. Neurosurgery 64(3):494–501
Lofgren J, Zwetnow NN (1973) The pressure-volume curve of the cerebrospinal fluid space in dogs. Acta Neurol Scand 49:557–574
Marmarou A (1973) A theoretical and experimental of cerebrospinal fluid system. Ph.D. thesis, Drexel University
Marmarou A, Schulman K, LaMorgese J (1975) Compartmental analysis of compliance and outflow resistance of cerebrospinal fluid system. J Neurosurg 43:523–534
Marmarou A, Shulman K, Rosende RM (1978) A non-linear analysis of CSF system and intracranial pressure dynamics. J Neurosurg 48:332–344
Marmarou A, Maset AL, Ward JD, Choi S, Brooks D, Lutz HA, Moulton RJ, Muizelaar JP, DeSalles A, Young HF (1987) Contribution of CSF and vascular factors to elevation of ICP in severely head-injured patients. J Neurosurg 66(6):883–890
Marmarou A, Foda MA, Bandoh K et al (1996) Posttraumatic ventriculomegaly: hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J Neurosurg 85(6):1026–1035
Sliwka S (1980) A clinical system for the evaluation of selected dynamic properties of the intracranial system. Ph.D. thesis, Polish Academy of Sciences, Warsaw (in Polish)
Tisell M, Edsbagge M, Stephensen H et al (2002) Elastance correlates with outcome after endoscopic third ventriculostomy in adults with hydrocephalus caused by primary aqueductal stenosis. Neurosurgery 50:70–76
Acknowledgements
This work was supported by the National Institute of Health Research, Biomedical Research Centre, Cambridge University Hospital Foundation Trust – Neurosciences Theme, and a Senior Investigator Award (to J. D. P.).
Disclosure
ICM+ is a software for brain monitoring in clinical/experimental neurosciences (http//www.neurosurg.cam.ac.uk/icmplus/). It is licensed by the University of Cambridge (Cambridge Enterprise Ltd). M.C. has a share in a fraction of the licensing fee.
Conflicts of interest statement what is in Disclosure may be in Conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag/Wien
About this paper
Cite this paper
Czosnyka, M., Czosnyka, Z., Agarwal-Harding, K.J., Pickard, J.D. (2012). Modeling of CSF Dynamics: Legacy of Professor Anthony Marmarou. In: Aygok, G., Rekate, H. (eds) Hydrocephalus. Acta Neurochirurgica Supplementum, vol 113. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0923-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0923-6_2
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0922-9
Online ISBN: 978-3-7091-0923-6
eBook Packages: MedicineMedicine (R0)