Abstract
Tamoxifen is a selective estrogen receptor modulator. In this study we investigated whether or not tamoxifen reduces intracerebral hemorrhage (ICH)-induced brain injury in rats. In all experiments, adult male Sprague-Dawley rats received an injection of 100 μL autologous whole blood into the right basal ganglia. In the first set of experiments, rats were treated with tamoxifen (2.5 mg/kg or 5 mg/kg, i.p.) or vehicle 2 and 24 h after ICH and were killed at day 3 for brain edema measurement. In the second set of experiments, rats were treated with tamoxifen (5 mg/kg) or vehicle and magnetic resonance imaging (MRI), and behavior tests were performed at days 1, 7, 14 and 28. Rats were killed at day 28 for brain histology. We found that tamoxifen at 5 but not at 2.5 mg/kg reduced perihematomal brain edema at day 3 (p < 0.05). Brain histology showed that tamoxifen reduced caudate atrophy at day 28 (p < 0.01). Tamoxifen also improved functional outcome (p < 0.05). MRI demonstrated a tendency to smaller T2* lesions in tamoxifen-treated rats. However, two out of five rats treated with tamoxifen developed hydrocephalus. These results suggest that tamoxifen has neuroprotective effects in ICH, but the cause of hydrocephalus development following tamoxifen treatment needs to be examined further.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Gender has an important role in brain injury after ischemic and hemorrhagic stroke. Our previous studies demonstrated that brain edema after intracerebral hemorrhage (ICH) is less in female compared to male rats [1–3], a gender difference that appears to involve activation of estrogen receptors (ER) in the female rats. Several estrogen receptors have been discovered, including estrogen receptor (ER)-α, -β, -X and a membrane estrogen receptor [4]. Estrogen-induced neuroprotection can be ER mediated or ER independent [4].
Tamoxifen is a selective estrogen receptor modulator, which was discovered and named ICI 46,474 in 1967 [5]. Tamoxifen has also been reported to have a neuroprotective in ischemic stroke [6, 7] and Parkinson’s disease [8]. However, it is still not clear whether or not tamoxifen is protective for the brain after ICH. This study examined whether tamoxifen reduces ICH-induced brain edema, brain atrophy and neurological deficits in male rats.
Materials and Methods
Animal Preparation and Intracerebral Infusion
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. Male Sprague-Dawley rats (Charles River Laboratories, Portage, MI), 275–300 g each, were used in this study. Rats were anesthetized by pentobarbital (50 mg/kg, i.p.). Rectal body temperature was maintained at 37.5°C by using a feedback-controlled heating pad. The right femoral artery was catheterized to obtain blood for injection, to monitor blood pressure and to analyze blood pH, PaO2, PaCO2, hematocrit and glucose concentrations. Rats were placed in a stereotactic frame (Kopf Instruments) and a 1-mm cranial burr hole drilled on the right coronal suture 3.5 mm lateral to the midline. A 26-gauge needle was inserted stereotactically into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, 3.5 mm lateral to the bregma). Autologous whole blood (100 μL) was infused at a rate of 10 μL/min using a microinfusion pump. The needle was then removed, the burr hole sealed with bone wax, the skin incision closed with sutures and the animal allowed to recover.
Experimental Groups
There were two sets of experiments in this study. In the first set, ICH rats were treated with tamoxifen (2.5 or 5 mg/kg in 4% DMSO, i.p., 2 and 24 h after ICH, Tocris Bioscience, Ellisville, MO, n = 6) or vehicle (n = 6). Rats were euthanized at day 3 to determine brain water content. In the second set, rats were treated with tamoxifen (5 mg/kg, n = 5) or vehicle (n = 8) at 2 and 24 h after ICH. Rats had both T2-weighted and T2*-weighted gradient-echo MR scans at days 1, 7, 14 and 28 after ICH, and then were euthanized for brain histology. Body weight and neurological deficits were measured on days 1, 3, 7, 14, 21 and 28 after operation.
Brain Water Content
Animals were euthanized by decapitation under deep pentobarbital anesthesia (100 mg/kg, i.p.) to measure brain water content. The rat brains were removed immediately, and a 3-mm-thick coronal brain slice 4 mm from the frontal pole was cut with a sharp blade. The slice was divided into four samples, ipsi- and contralateral basal ganglion and ipsi- and contralateral cortex. The cerebellum was also obtained as a control. Each of these five brain tissue samples was weighed on an electronic analytical balance to the nearest 0.1 mg to obtain the wet weight (WW). Samples were then dried in a gravity oven at 100°C for 24 h to obtain the dry weight (DW). Brain water content (%) was calculated as ((WW – DW)/WW)*100%.
Behavioral Tests
To assess neurological deficits, forelimb placing, forelimb use asymmetry and corner turn tests were used. These have been described in detail previously [9]. All animal behavioral tests were evaluated at 1, 3, 7, 14, 21 and 28 days by an investigator who was blinded to the treatment.
Magnetic Resonance Imaging
Head MRI scans were performed to get T2 and T2* images at 1, 7, 14 and 28 days after ICH as described in our previous studies [10, 11]. All measurements were repeated three times and mean values used.
Brain Atrophy Measurement
Brain atrophy was measured as previously described [10]. Coronal sections from 1 mm posterior to the blood injection site were stained with hematoxylin and eosin (H&E) and scanned. The caudate in each hemisphere was outlined on a computer and caudate size measured using Image. Brain tissue loss was calculated by the formula as: (contralateral basal ganglia – ipsilateral basal ganglia)/contralateral basal ganglia x 100%.
Statistical Analysis
All data in this study are presented as mean ± SD. Data were analyzed with Student’s t test or one-way analysis of variance (ANOVA). Differences were set significant at p <0.05.
Results
During the long-term experiments, body weight was monitored. It decreased on the first and third day after ICH, but then gradually increased. There was no significant difference in body weight between tamoxifen-treated and vehicle-treated groups (p > 0.05).
ICH causes brain edema in rats. Tamoxifen treatment at the dose 5 mg/kg significantly reduced brain water content (82.2 ± 0.7 vs. 83.4 ± 1.1% in vehicle treated group, p < 0.05) in the ipsilateral basal ganglia 3 days after ICH. However, tamoxifen at a dose of 2.5 mg failed to reduce ICH-induced brain edema at day 3 (p > 0.05).
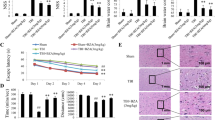
Behavioral tests, including forelimb placing, corner turn and forelimb use asymmetry tests, were performed at days 1, 3, 7, 14, 21 and 28 after ICH. Tamoxifen treatment improved the forelimb placing score (e.g., day 14: 40 ± 10 vs. 24 ± 15% in the vehicle-treated group, p < 0.05), forelimb using asymmetry score (e.g. day 28: 3 ± 6 vs. 22 ± 8% in the vehicle-treated group, p < 0.05) and corner turn score (p < 0.05). Brain tissue loss in the ipsilateral caudate at day 28 was significantly less in tamoxifen-treated rats (21.9 ± 4.3 vs. 30.3 ± 1.8% in vehicle-treated group, p < 0.05, Fig. 1).
On T2* images, there was no significant difference between the two groups (p > 0.05) in T2* lesion size from day 1 to 28 (data not shown), although at day 28 there was a tendency for smaller T2* lesions in tamoxifen-treated rats (28.5 ± 4.7 vs. 34.3 ± 6.1 mm3 in vehicle-treated group, p = 0.14, Fig. 2). On T2 images, we found that two of five tamoxifen-treated rats developed obvious dilation of ventricles starting 7 days after treatment (Fig. 3), but no vehicle-treated rats showed such dilation.
Discussion
In this study, we found that tamoxifen treatment (5 mg/kg) reduced brain edema, brain atrophy and neurological deficits after ICH. However, surprisingly, we found that with this dosage, hydrocephalus developed in two out of five rats.
The half-life of tamoxifen is 9–12 h after an initial dose, and tamoxifen is still detectable after 28 days after 7 days of chronic use [12]. We therefore chose 2 and 24 h for injection time points. We tried two different doses and found that only the higher dose (5 mg/kg) reduced acute brain edema after ICH, and this higher dose was chosen for our long-term study.
In animal experiments, high-dose tamoxifen has also been used in studies of cerebral ischemia [6, 7] and Parkinson’s disease [8, 13]. Most of the experiments conclude that doses higher, but not lower, than 5 mg/kg/day, have beneficial effects and that the high doses are generally well tolerated. Tamoxifen-induced neuroprotection may be estrogen receptor mediated, although some reports indicate that protection with high dose tamoxifen is not through estrogen receptors but through anti-oxidative actions [14]. It is well known that oxidative stress has a major role in brain injury following ICH [3].
Hydrocephalus developed in two of five tamoxifen-treated rats in our study, suggesting that tamoxifen may affect the process of production, circulation or absorption of cerebrospinal fluid. There are only a few experiments focusing on long-term changes and side effects in animals after tamoxifen treatment [15]. So far, in clinical trials with MRI follow-up, no tamoxifen-induced hydrocephalus cases have been reported [16, 17]. Whether or not tamoxifen only induces hydrocephalus after intracerebral hemorrhage should be studied further.
References
Hurn PD, Macrae IM (2000) Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 20:631–652
Nakamura T, Hua Y, Keep RF, Park JW, Xi G, Hoff JT (2005) Estrogen therapy for experimental intracerebral hemorrhage in rats. J Neurosurg 103:97–103
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5:53–63
Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM (2003) Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci 23:11420–11426
Harper MJ, Walpole AL (1967) A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil 13:101–119
Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW Jr, Tranmer BI (2000) Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. NeuroReport 11:2675–2679
Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK (2005) Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol 196:41–46
Obata T, Kubota S (2001) Protective effect of tamoxifen on 1-methyl-4-phenylpyridine-induced hydroxyl radical generation in the rat striatum. Neurosci Lett 308:87–90
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G (2002) Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33:2478–2484
Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G (2010) Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke 41:375–382
Wu G, Xi G, Hua Y, Sagher O (2010) T2* magnetic resonance imaging sequences reflect brain tissue iron deposition following intracerebral hemorrhage. Transl Stroke Res 1:31–34
Fabian C, Sternson L, El-Serafi M, Cain L, Hearne E (1981) Clinical pharmacology of tamoxifen in patients with breast cancer: correlation with clinical data. Cancer 48:876–882
Smith CP, Oh JD, Bibbiani F, Collins MA, Avila I, Chase TN (2007) Tamoxifen effect on L-DOPA induced response complications in parkinsonian rats and primates. Neuropharmacology 52:515–526
Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK (2007) Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurol 204:819–827
Atakisi E, Kart A, Atakisi O, Topcu B (2009) Acute tamoxifen treatment increases nitric oxide level but not total antioxidant capacity and adenosine deaminase activity in the plasma of rabbits. Eur Rev Med Pharmacol Sci 13:239–243
Puchner MJ, Giese A, Lohmann F, Cristante L (2004) High-dose tamoxifen treatment increases the incidence of multifocal tumor recurrences in glioblastoma patients. Anticancer Res 24:4195–4203
Sankar T, Caramanos Z, Assina R, Villemure JG, Leblanc R, Langleben A, Arnold DL, Preul MC (2008) Prospective serial proton MR spectroscopic assessment of response to tamoxifen for recurrent malignant glioma. J Neurooncol 90:63–76
Acknowledgment
This study was supported by grants NS-017760, NS-039866 and NS-057539 from the National Institutes of Health (NIH) and 0755717Z, 0840016N from the American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA. Dr. Xie was supported by NSFC-30901549 from the China National Natural Science Foundation.
Conflict of interest statement We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag/Wien
About this chapter
Cite this chapter
Xie, Q., Guan, J., Wu, G., Xi, G., Keep, R.F., Hua, Y. (2011). Tamoxifen Treatment for Intracerebral Hemorrhage. In: Zhang, J., Colohan, A. (eds) Intracerebral Hemorrhage Research. Acta Neurochirurgica Supplementum, vol 111. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0693-8_45
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0693-8_45
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0692-1
Online ISBN: 978-3-7091-0693-8
eBook Packages: MedicineMedicine (R0)