Abstract
Fatty infiltration (FI) and muscle atrophy are common findings following RC tears. Fatty degeneration varies among rotator cuff muscles. The pathophysiology is complex, and is characterized by increased fibrosis. Age is reported to correlate with degeneration and atrophy. MRI and CT scans are used for exact diagnosis and grading of fatty degeneration according to Goutallier. Rotator cuff atrophy is usually assessed using the occupational ratio (Thomazeau grading) and “tangent sign.” Fatty degeneration is associated with tendon re-tear and postoperative muscle atrophy. Furthermore, fatty changes probably negatively affect the clinical outcome regardless of tendon re-rupture. FI and muscle atrophy are considered as negative prognostic factors for shoulder function and clinical outcomes after repair of RC tears. Tendon repair has a controversial effect on infiltration progression. Most studies demonstrate improvement or reversal of muscle atrophy after cuff repair. However, there are also studies indicating no effect of the repair in degeneration process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Rotator cuff tears (RCTs) are a common cause of shoulder pain and disability. Fatty infiltration (FI) and muscle atrophy can be established and progress following RC tears, and are closely correlated to worse outcomes following rotator cuff repair. These two terms are usually used interchangeably. Fatty degeneration (FD) of the rotator cuff muscles appears due to tendon rupture or less often due to nerve damage. Rotator cuff tears were at the beginning associated with muscle degeneration as reported by Goutallier et al. [1]. FD can involve all muscles with ruptured tendons.
In terms of RC muscles, fatty degeneration takes place in the whole muscle mass of supraspinatus, while it appears in whole infraspinatus only if more than 50% of the tendon is torn [1]. Subscapularis degenerates only in the part of the tendon that is ruptured. Nevertheless, severe fatty infiltration can appear in subscapularis too. There are cases when infraspinatus degenerates without rupture, when the supraspinatus and subscapularis are torn on the antero-superior side. The duration of symptoms seems to cause increase of fatty infiltration, especially of the infraspinatus muscle [1].
Infraspinatus FI is gaining interest recently, because it seems to be the most rapidly affected muscle [2, 3]. It is reported that infraspinatus fatty degeneration is higher when an infraspinatus tear exist, and worsens when multiple tendons are torn [4]. Age, besides the aforementioned duration of symptoms, is found to be a predisposing factor for increased infraspinatus FI [4]. FI of the infraspinatus tendon can appear even without tendon rupture [5].
The pathogenesis and pathophysiology of FI and atrophy are complex and not clearly understood. Tendon rupture has an important effect on muscle physiology, structure, and function. FI is characterized by increased fibrosis and fiber shortening while mechanical unloading and denervation also have been found to contribute to atrophy [6]. Architectural changes take place as a result of tendon release and musculotendinous retraction [7]. Fibrous tissue and fat serve to fill the gap space created among muscle fibers. Furthermore, increased tension on the suprascapular nerve (SSN) may be applied by retracted tears of supraspinatus, causing nerve injury which subsequently leads to muscle atrophy [6, 8].
Although there are limited studies refusing association between age and FI [9], recent data find correlation between age and FI [2, 4, 10]. In a large retrospective study, evaluation of FI with the use of CT arthrography, in intact cuffs, showed a significant influence of aging on FI of supraspinatus, infraspinatus, and subscapularis, with significant acceleration after 40 years [11]. Moreover, the age of 70 seemed to be a particular threshold for FI. Same conclusions were presented by Raz et al. [12], reporting age-related increase of FI for supraspinatus, infraspinatus, and subscapularis, with earlier onset of infiltration in supraspinatus and subscapularis. In late stages, FI and atrophy may even be seen during clinical examination. Patients with massive RCTs and atrophy may appear with diminished external or internal rotation [13, 14].
2 Radiological and Clinical Implications
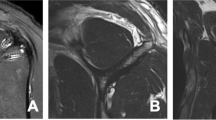
In terms of radiological evaluation, antero-posterior plain radiographs of the shoulder are usually ordered at initial admission, being of minor diagnostic occurrence for FI. However, narrowing of the acromio-humeral interval may gradually cause muscle atrophy, especially in infraspinatus tendon [4]. Computed tomography (CT) was used by Goutallier to establish his grading system (Table 10.1) [1]. Later, Fuchs et al. [15] modified this grading system with the application of magnetic resonance imaging (MRI) (Table 10.2). Their modified grading system includes three grades of fatty infiltration based on Goutallier grading (0 + 1, 2, 3 + 4) (Figs. 10.1 and 10.2). They found that interobserver reproducibility turns from good to excellent by using their modified classification.
Two most popular systems for grading of RC atrophy are the occupational ratio and the so-called “tangent sign.” Occupational ratio, described by Thomazeau et al. [16], is defined (Table 10.3) as the surface area of the supraspinatus muscle/surface area of the entire supraspinatus fossa (Fig. 10.3). “Tangent sign” is seen on an oblique-sagittal MRI image (“Y” view) and is negative if the supraspinatus crosses a line between the superior aspect of the coracoids process and the superior border of the scapular spine (Fig. 10.4) [17]. Tangent sign is a useful MR sign for atrophy of the supraspinatus muscle. It is worth mentioning that interobserver agreement for evaluating FI in MRI is remarkably low according to some studies [18]. Ultrasonography has been also demonstrated to be a reliable means of diagnosing FI. Its advantages, such as low cost, fast and easy to perform, and dynamic evaluation of the RC, make it useful and preferable for the patient [6]. Nevertheless, the use of ultrasonography by an experienced physician is of major importance for proper diagnosis. Ultrasonography can also predict shoulder function after arthroscopic repair by evaluating morphological changes of the supraspinatus muscle [19].
Fatty degeneration is associated with tendon re-tear and postoperative muscle atrophy as well. As FI and muscle atrophy progress, the outcomes worsen. Fatty changes may negatively affect the clinical outcome regardless of tendon re-rupture. Nevertheless, both FI and muscle atrophy are considered as negative prognostic factors for shoulder function and clinical outcomes after repair of RC tears [20,21,22,23]. Goutallier et al. [24] proposed a cut-off of stage 2 according to their staging system for better results after repair. This finding was enhanced by Ohzono et al. [25] who reported that fatty degeneration of Goutallier stage 2 or more before surgery is a predisposing factor for unsatisfactory outcome. In another study, by Gladstone et al. [23], functional outcome and strength of the RC were compromised by FI [23]. The remarkable clue of their analysis was that FI and atrophy of the infraspinatus were found to be the only preoperative factors which deteriorate functional scores. Patients with stage 4 FI seem to experience much lower functional scores in comparison with patients with stage 1 FI after open repair [26]. Godeneche et al. [20] found that Constant scores were mainly influenced by preoperative FI of supraspinatus and not infraspinatus. Additionally Constant scores were significantly lower in patients with stage 1 or 2 FI compared to those with stage 0. Valencia et al. [27] reported FI as a strong indicator of muscle weakness and subsequent loss of supraspinatus strength and function in an experimental study. Muscles with atrophy and FI are much weaker than muscles with atrophy alone.
Literature is controversial regarding the outcome of tendon repair on infiltration progression, regardless of the success or recurrent tears. Probably there is a level of damage in the muscles which cannot be regenerated. Therefore surgery should probably be performed before a high grade of fatty degeneration [4, 28]. Some authors have reported that infiltration and atrophy remain irreversible or progress even in successful repairs [23, 29]. They believe that atrophy remains irreversible, or is slowed by successful repair and advances after failure [30, 31]. Fuchs et al. [30] reported no significant change of muscle atrophy after RC repair, and statistically significant FI increase, despite repair. Nevertheless, RC atrophy was much greater in patients who experienced re-tear of RC after repair [30].
On the other hand, there are studies demonstrating reversal or improvement of atrophy after RC repair. Muscles seem to show an individual behavior regarding infiltration and atrophy after repair. In a study by Gerber et al. [32] with patients operated for massive RCTs, supraspinatus atrophy was slightly improved, while infraspinatus atrophy deteriorated even in successful repairs. In an experimental study in sheep, muscle atrophy was found to be partially reversible at 35 weeks following repair [33]. Butt et al. [34] demonstrated that atrophy is reversible following repair, since the mean cross-sectional area of muscle fibers of supraspinatus increases after repair. In a retrospective study, Fabbri et al. [35] compared two groups of patients with medium to large RCTs and found that surgery can stop muscular degenerative changes. They reported no progression of the muscle atrophy in the operative treatment group, while significant worsening was observed in the nonoperative group [35].
Improvement of muscle atrophy and FI can appear at 2 years after surgery, more than initially after operation according to the results of a study by Hamano et al. [36]. MRI findings confirmed the regeneration of muscle fibers at 2 years, while patients with improved FI experienced a better range of motion in flexion and abduction.
Nonoperative treatment is also proposed by some authors for RC tears. Success of nonoperative treatment seems to depend on FI of RC muscles. Jain et al. [37] reported that the absence of FI along with shorter duration of symptoms have more favorable outcomes in nonoperative treatment of RC tears.
3 Summary
In conclusion, fatty infiltration seems to be a progressive, aging process of the muscles. Trauma and/or rupture initiate, accelerate, or worsen this process. Rotator cuff repair after rotator cuff tear probably stops this degeneration process, improving or even sometimes reversing the established muscle atrophy.
References
Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78.
Barry JJ, Lansdown DA, Cheung S, Feeley BT, Ma CB. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J Shoulder Elbow Surg. 2013;22:18. https://doi.org/10.1016/j.jse.2011.12.014.
Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39:2099. https://doi.org/10.1177/0363546511415659.
Melis B, Wall B, Walch G. Natural history of infraspinatus fatty infiltration in rotator cuff tears. J Shoulder Elbow Surg. 2010;19:757. https://doi.org/10.1016/j.jse.2009.12.002.
Cheung S, Dillon E, Tham SC, Feeley BT, Link TM, Steinbach L, et al. The presence of fatty infiltration in the infraspinatus: its relation with the condition of the supraspinatus tendon. Arthroscopy. 2011;27:463. https://doi.org/10.1016/j.arthro.2010.09.014.
Kuzel BR, Grindel S, Papandrea R, Ziegler D. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg. 2013;21:613. https://doi.org/10.5435/JAAOS-21-10-613.
Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22:1004. https://doi.org/10.1016/j.orthres.2004.02.009.
Albritton MJ, Graham RD, Richards RS, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg. 2003;12:497. https://doi.org/10.1016/S1058-2746(03)00182-4.
Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550. https://doi.org/10.1016/S1058-2746(03)00211-8.
Kany J, Flurin PH, Richardi G, Hardy P. Rotator cuff tear imaging in patients over 70years: specific MRI findings? Orthop Traumatol Surg Res. 2013;99:S385. https://doi.org/10.1016/j.otsr.2013.10.003.
Gueniche J, Bierry G. Rotator cuff muscles fatty infiltration increases with age: retrospective review of 210 patients with intact cuff on computed tomography arthrography. J Shoulder Elbow Surg. 2019;28:617. https://doi.org/10.1016/j.jse.2018.09.020.
Raz Y, Henseler JF, Kolk A, Riaz M, van der Zwaal P, Nagels J, et al. Patterns of age-associated degeneration differ in shoulder muscles. Front Aging Neurosci. 2015;7:236. https://doi.org/10.3389/fnagi.2015.00236.
Walch G, Boulahia A, Calderone S, Robinson AHN. The “dropping” and “hornblower’s” signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br. 1998;80:624. https://doi.org/10.1302/0301-620x.80b4.8651.
Gerber C, Hersche O, Farron A. Isolated rupture of the subscapularis tendon. J Bone Joint Surg Am. 1996;78:1015.
Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;80:599. https://doi.org/10.1016/S1058-2746(99)90097-6.
Thomazeau H, Boukobza E, Morcet N, Chaperon J, Langlais F. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res. 1997;(344):275.
Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Invest Radiol. 1998;33:163. https://doi.org/10.1097/00004424-199803000-00006.
Spencer EE, Dunn WR, Wright RW, Wolf BR, Spindler KP, McCarty E, et al. Interobserver agreement in the classification of rotator cuff tears using magnetic resonance imaging. Am J Sports Med. 2008;36:99. https://doi.org/10.1177/0363546507307504.
Kim YK, Choi ES, Kim KT, Yoon JR, Chae SH. Quantitative measurement of muscle atrophy and fat infiltration of the supraspinatus muscle using ultrasonography after arthroscopic rotator cuff repair. Ann Rehabil Med. 2018;42:260. https://doi.org/10.5535/arm.2018.42.2.260.
Godenèche A, Elia F, Kempf JF, Nich C, Berhouet J, Saffarini M, et al. Fatty infiltration of stage 1 or higher significantly compromises long-term healing of supraspinatus repairs. J Shoulder Elbow Surg. 2017;26:1818. https://doi.org/10.1016/j.jse.2017.03.024.
Barth J, Andrieu K, Fotiadis E, Hannink G, Barthelemy R, Saffarini M. Critical period and risk factors for retear following arthroscopic repair of the rotator cuff. Knee Surg Sport Traumatol Arthrosc. 2017;25:2196. https://doi.org/10.1007/s00167-016-4276-x.
Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16:691. https://doi.org/10.1016/j.jse.2007.02.122.
Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719. https://doi.org/10.1177/0363546506297539.
Goutallier D, Postel JM, Radier C, Bernageau J, Zilber S. Long-term functional and structural outcome in patients with intact repairs 1 year after open transosseous rotator cuff repair. J Shoulder Elbow Surg. 2009;18:521. https://doi.org/10.1016/j.jse.2008.11.006.
Ohzono H, Gotoh M, Nakamura H, Honda H, Mitsui Y, Kakuma T, et al. Effect of preoperative fatty degeneration of the rotator cuff muscles on the clinical outcome of patients with intact tendons after arthroscopic rotator cuff repair of large/massive cuff tears. Am J Sports Med. 2017;45:2975. https://doi.org/10.1177/0363546517724432.
Warner JJP, Higgins L, Parsons IM IV, Dowdy P. Diagnosis and treatment of anterosuperior rotator cuff tears. J Shoulder Elbow Surg. 2001;10:37. https://doi.org/10.1067/mse.2001.112022.
Valencia AP, Lai JK, Iyer SR, Mistretta KL, Spangenburg EE, Davis DL, et al. Fatty infiltration is a prognostic marker of muscle function after rotator cuff tear. Am J Sports Med. 2018;46:2161. https://doi.org/10.1177/0363546518769267.
Yamaguchi H, Suenaga N, Oizumi N, Hosokawa Y, Kanaya F. Will preoperative atrophy and fatty degeneration of the shoulder muscles improve after rotator cuff repair in patients with massive rotator cuff tears? Adv Orthop. 2012;2012:195876. https://doi.org/10.1155/2012/195876.
Kuenzler MB, Nuss K, Karol A, Schär MO, Hottiger M, Raniga S, et al. Neer Award 2016: reduced muscle degeneration and decreased fatty infiltration after rotator cuff tear in a poly(ADP-ribose) polymerase 1 (PARP-1) knock-out mouse model. J Shoulder Elbow Surg. 2017;26:733. https://doi.org/10.1016/j.jse.2016.11.009.
Fuchs B, Gilbart MK, Hodler J, Gerber C. Clinical and structural results of open repair of an isolated one-tendon tear of the rotator cuff. J Bone Joint Surg Am. 2006;88:309. https://doi.org/10.2106/JBJS.E.00117.
Schaefer O, Winterer J, Lohrmann C, Laubenberger J, Reichelt A, Langer M. Magnetic resonance imaging for supraspinatus muscle atrophy after cuff repair. Clin Orthop Relat Res. 2002;(403):93. https://doi.org/10.1097/00003086-200210000-00015.
Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505. https://doi.org/10.2106/00004623-200004000-00006.
Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, Von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;89:1973. https://doi.org/10.2106/00004623-200409000-00016.
Butt U, Rashid MS, Temperley D, Crank S, Birch A, Freemont AJ, et al. Muscle regeneration following repair of the rotator cuff. Bone Joint J. 2016;98-B:1389. https://doi.org/10.1302/0301-620X.98B10.37231.
Fabbri M, Ciompi A, Lanzetti RM, Vadalà A, Lupariello D, Iorio C, et al. Muscle atrophy and fatty infiltration in rotator cuff tears: can surgery stop muscular degenerative changes? J Orthop Sci. 2016;21:614. https://doi.org/10.1016/j.jos.2016.06.001.
Hamano N, Yamamoto A, Shitara H, Ichinose T, Shimoyama D, Sasaki T, et al. Does successful rotator cuff repair improve muscle atrophy and fatty infiltration of the rotator cuff? A retrospective magnetic resonance imaging study performed shortly after surgery as a reference. J Shoulder Elbow Surg. 2017;26:967. https://doi.org/10.1016/j.jse.2016.10.016.
Jain NB, Ayers GD, Fan R, Kuhn JE, Baumgarten K, Matzkin E, et al. Predictors of pain and functional outcomes after the nonoperative treatment of rotator cuff tears. Orthop J Sports Med. 2018;6:2325967118788531. https://doi.org/10.1177/2325967118788531.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 ESSKA
About this chapter
Cite this chapter
Hantes, M., Komnos, G. (2020). Fatty Infiltration and Muscle Atrophy. What It Means and What Happens After Repair?. In: Sampaio Gomes, N., Kovačič, L., Martetschläger, F., Milano, G. (eds) Massive and Irreparable Rotator Cuff Tears. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-61162-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-662-61162-3_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-61161-6
Online ISBN: 978-3-662-61162-3
eBook Packages: MedicineMedicine (R0)