Abstract
Lymphadenopathy is common in children, and frequently presents with palpable lymph nodes amenable to fine needle aspiration. The interpretation of these aspirates can be challenging due to the wide spectrum of benign and malignant causes of lymphadenopathy and the overlapping cytomorphologic features of many of these entities. Ancillary studies are important for resolving the differential diagnosis and excluding lymphoma and non-lymphoid malignancies. This chapter describes the cytomorphologic features of common and uncommon causes of lymphadenopathy in the pediatric age group and the key ancillary studies used to arrive at a definitive diagnosis or to narrow the differential diagnosis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Fine needle aspiration (FNA ) cytology offers a minimally invasive modality for evaluating lymphadenopathy in pediatric patients. In the majority of children and adolescents, lymphadenopathy is due to reactive lymphoid hyperplasia or infection and in this setting, the use of FNA can avoid more invasive and unnecessary core or open biopsies, provide a relatively rapid diagnosis, and by confirming benignity, alleviate the anxiety of patients and/or families [1–5]. Moreover, when coupled with appropriate ancillary studies , FNA allows specific diagnosis of many benign and malignant causes of lymphadenopathy. Overall, the diagnostic accuracy for lymph node FNAs has been reported to be approximately 90 %, with a sensitivity of about 85–95 % and specificity of 98–100 % [4–6].
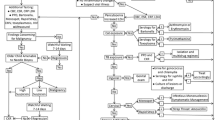
3.2 Approach to the Evaluation of Lymphadenopathy in Children and Adolescents
3.2.1 Gross Examination
At the time of FNA, note the color, consistency and amount of aspirated material, and the presence or absence of fluid indicative of a cystic lesion. If cystic fluid is obtained, note whether it appears purulent, suggesting an infectious process, and in this scenario, material should be reserved for microbial cultures. Mucoid, watery, or bloody aspirates are more suggestive of a non-lymphoid lesion. Non-diagnostic aspirates often yield a dry tap or bloody material, whereas adequate lymphoid aspirates usually are composed of finely granular, opaque material that is easy to smear.
3.2.2 Low Power Microscopic Examination
Dyscohesion is characteristic of benign and malignant lymphoid populations (Fig. 3.1), but does not exclude non-lymphoid entities. Any clustering raises the possibility of metastatic non-lymphoid malignancy, granulomatous inflammation, germinal center fragments, or technical artifacts, such as a thick smear, suboptimal spreading technique, or blood clot [7] (Fig. 3.2).
Reactive lymphadenopathy (Diff-Quik stain, medium power). This case of reactive lymphadenopathy shows a follicle-like organization, in which the lymphoid cells cluster together with tingible body macrophages. The lymphoid population appears polymorphous, which is helpful in excluding a lymphoid malignancy.
Reactive lymphoid hyperplasia with artifact (a–c. Diff-Quik stain, medium power; d. Diff-Quik stain, low power). Some cases of reactive lymphoid hyperplasia have artifactual changes such as air-drying artifact (a, b) and crush artifact (c), that can make the cells appear, paler (a), blown up (b) or spindled (c), and may raise concern for a metastatic neoplasm or lymphoproliferative disorder. In addition, when smears are too thick, the cytological features and lymphoglandular bodies may be difficult to identify (d).
3.2.3 High Power Microscopic Examination
The presence of lymphoglandular bodies is an important diagnostic feature of benign and malignant lymphoid populations (Fig. 3.1). When lymphoglandular bodies are present supporting a lymphoid proliferation, the next step is to assess the homogeneity or heterogeneity of the constituent cells, the size(s) of the cells, and the presence or absence of tingible body macrophages. The size of lymphoid cells is usually described in relation to a histiocyte nucleus or 2–3 red blood cells, with small, intermediate and large lymphoid cells being smaller than, the same size as, and larger than the nucleus of a histiocyte, respectively. The size of the predominant population helps to narrow the differential diagnosis. Features suggestive of malignancy include a homogeneous lymphoid population, predominance of large cells, marked pleomorphism, and/or an absence of tingible body macrophages, and should prompt collection of additional material for flow cytometry, cell block, and/or fluorescence in situ hybridization (FISH). In the setting of clinical findings suggestive of lymphoma, such as extensive lymphadenopathy or an elevated LDH, the presence of numerous mitotic figures and tingible body macrophages should also lead one to consider a high-grade lymphoma, such as Burkitt lymphoma. In contrast, features of reactive lymphoid proliferations include a heterogeneous population of cells with a predominance of small mature lymphocytes, lymphohistiocytic aggregates, and scattered tingible body macrophages (Fig. 3.1). Other features that provide important clues to the differential diagnosis include the presence or absence of other hematolymphoid cells (including eosinophils, neutrophils, plasma cells and histiocytes), granulomas , necrosis, and non-hematolymphoid cells.
Differential Diagnosis
Causes of lymphadenopathy in children and adolescents are summarized in Table 3.1, which categorizes the entities based on whether they are benign or malignant and common or uncommon (Figs. 3.3, 3.4, and 3.5). Differential diagnostic considerations based on eight morphologic patterns are listed in Table 3.2 and those based on the size of the predominant population are listed in Table 3.3. Primary lymphoid malignancies must be distinguished from metastatic small round cell tumors, which are summarized in Table 3.4.
Pearls
-
Avoid examination of areas on a slide with artifactual distortion, such as crush artifact or air-drying artifact, where the cells appear poorly preserved and/or pale (Fig. 3.2).
-
The key features to evaluate in lymph node aspirates from children and adolescents include: presence or absence of cohesion; the type of lymphoid population (heterogeneous versus homogeneous); the size(s) of the constituent cells ; the presence or absence of certain cell types (macrophages and/or granulomas, plasma cells, immunoblasts, eosinophils, neutrophils, and non-hematolymphoid cells ); and the background (clean versus necrotic).
3.3 Mimics of Lymphadenopathy
Some lesions, particularly superficial masses in the head and neck, mimic a lymph node clinically and/or radiologically due to their well-circumscribed nature and location, but prove to be other structures or processes on pathological examination. It is important to be aware of these entities and recognize their cytological features to ensure appropriate management. Pediatric head and neck lesions that can mimic lymphadenopathy are listed in Table 3.5 and selected lesions are illustrated in Fig. 3.6. Aspirates from ectopic thymic tissue yield a lymphoid population with a predominance of small lymphocytes, which can be particularly challenging to distinguish from true lymphadenopathy. However, thymic aspirates are characterized by a variable number of larger epithelial cells, and if flow cytometry is performed, there is a maturational spectrum from immature to maturing T-cells (Fig. 3.7).
Ectopic thymic tissue (Diff-Quik stain, high power). Thymic tissue can be present in the neck and mimic a lymph node clinically and cytologically. Although there is a predominance of small lymphocytes, larger epithelial cells are also present and flow cytometry or immunohistochemical stains can help to confirm the presence of immature and maturing T-cells.
3.4 Benign Entities
3.4.1 Reactive Lymphoid Hyperplasia
Clinical Features
Reaactive lymphoid hyperplasia (RLH), characterized by follicular hyperplasia, paracortical hyperplasia and/or sinus histiocytosis, is the most common cause of lymphadenopathy in the pediatric population, accounting for approximately 75 % or more of cases. The high incidence of RLH in this population is largely attributable to the repeated antigenic stimulation of naïve immune systems. On physical examination, benign, reactive lymph nodes usually measure less than 3 cm in greatest dimension and most commonly involve the head and neck, axilla, or inguinal region. RLH resolves spontaneously and can be followed clinically. However, lymphadenopathy that persists for more than 3–6 months or has features inconsistent with RLH may prompt an initial or repeat cytologic evaluation.
Cytological Features
RLH is characterized by a heterogeneous lymphoid population spanning the spectrum from immunoblasts to plasma cells, but dominated by small mature lymphocytes with round nuclei and condensed dark chromatin (Fig. 3.1). In addition, scattered tingible body macrophages, lymphohistiocytic aggregates, and follicular dendritic cells are usually seen.
Differential Diagnosis
The main diagnostic considerations include Hodgkin lymphoma, T-cell lymphoma, partial lymph node involvement by a lymphoid or non-lymphoid malignancy, post-transplant lymphoproliferative disorder, and early infection, as well as other possibilities (Tables 3.1, 3.2, and 3.3). Although rare in the pediatric population, some B-cell non-Hodgkin lymphomas, including marginal zone, low grade follicular, and T-cell rich large B-cell lymphomas, have a heterogeneous population of cells dominated by small cells. However, in contrast to RLH, the spectrum of cells in these malignancies is usually limited. Atypical cells should raise the possibility of a malignant process, but may be few in number in T-cell rich large B-cell lymphoma, Hodgkin lymphoma with a paucity of Reed–Sternberg cells, or partial replacement of a lymph node by a primary lymphoid or metastatic non-lymphoid malignancy. In some early infections, particularly mycobacteria and Bartonella, the characteristic granulomatous and/or neutrophilic inflammation may be absent or poorly developed, and therefore, aspirates from these lymph nodes may mimic non-specific RLH. A rare but important cause of reactive-appearing lymphadenopathy in the pediatric population is autoimmune lymphoproliferative syndrome (ALPS) . This inherited disorder is characterized by defects in Fas/CD95/Apo-1 mediated apoptosis, which lead to childhood onset of generalized lymphadenopathy, hypergammaglobulinemia, lymphocytosis, splenomegaly, and autoimmune phenomena. Flow cytometry performed on lymph nodes from ALPS patients shows an increase in CD4, CD8 double negative, T-cell receptor (TCR)-alpha beta T-cells, ranging from 27 to 54 % of mononuclear cells , and representing 51–78 % of alpha beta T-cells [9].
Pearls
-
Collection of additional material for ancillary studies, such as microbial cultures, special stains, flow cytometry, FISH, and immunohistochemical stains, should be considered when the clinical or microscopic features are concerning for infection, a lymphoproliferative disorder or a metastatic malignancy.
-
Use of a needle alone, without an attached syringe or suction (fine needle non-aspiration technique), can help to optimize control and sampling of small, mobile lymph nodes, and may also decrease anxiety for the patient.
3.4.2 Suppurative Lymphadenitis
Clinical Features
Acute suppurative lymphadenitis usually presents as tender, erythematous, superficial lymph nodes, and is most often due to infection with bacteria such as Staphylococcus, Streptococcus, and gram-negative organisms. Cat scratch disease due to Bartonella henselae infection should be considered, particularly if there is a history of a cat scratch or bite or simply the presence of a cat in the patient’s home environment. Although rare, fungal infections can also cause suppurative lymphadenitis. Empiric treatment with antibiotics results in resolution of acute lymphadenitis in many cases and therefore, cytologic evaluation is usually reserved for those cases in which the lymphadenopathy persists despite therapy.
Cytological Features
Aspirates from acute suppurative lymphadenitis yield yellow-tinged, thick, turbid material. Microscopically, numerous neutrophils, as well as variable numbers of lymphocytes and histiocytes, are present in a dirty background of granular and cellular debris (Fig. 3.8). In some cases, intracellular and/or extracellular microorganisms can be identified on routine stains, and are usually more apparent on modified Giemsa than on Papanicolaou-stained smears . The presence of granulomas in addition to suppurative inflammation is suggestive of cat scratch disease (Fig. 3.9) or mycobacterial infection. Special stains, such as Gram, methenamine silver, Steiner, Warthin–Starry, and acid fast, and/or an immunohistochemical stain for Bartonella may be helpful for demonstrating the presence of microorganisms.
Differential Diagnosis
The main differential diagnostic considerations include necrotizing granulomatous lymphadenitis, systemic lupus erythematosus (SLE) , Kikuchi disease, lymph node infarction, and necrotic tumor. Although a dirty background with necrosis is characteristic of these entities, in contrast to acute suppurative lymphadenitis, neutrophils are usually absent, or if present, not a prominent feature. Other distinguishing features include the presence of granulomas in necrotizing granulomatous inflammation, hematoxylin bodies and LE cells in SLE, crescentic histiocytes in Kikuchi disease, and malignant cells in necrotic tumors.
Pearls
-
Material should be obtained for cultures to establish the specific identity of the causative organism, as well as to determine susceptibility and resistance to antimicrobial agents.
-
High quality aspirate smears may be superior to a diluted or limited cell block, particularly if there are few organisms.
-
Serology can be used to confirm the presence of Bartonella infection and may be particularly warranted when cat scratch disease is suspected clinically and/or cytologically, but organisms are not identified with Steiner, Warthin–Starry or immunohistochemical stains , and mycobacterial infection has been excluded.
-
When acid fast organisms are seen or when no organisms are seen but the clinical suspicion of mycobacterial infection is high, polymerase chain reaction (PCR)-testing can be performed on material from the cell block to confirm the presence and subtype of mycobacteria.
3.4.3 Granulomatous Lymphadenitis
Clinical Features
Granulomatous lymphadenitis is associated with mycobacterial, fungal, bacterial and parasitic infections, foreign body reactions, drugs, sarcoid, and malignancy, as well as a variety of other causes. Beyond the presence of lymphadenopathy, the clinical presentation depends on and may provide important clues to the underlying etiology. Clinical history, such as exposure to infectious individuals, pets, travel, implanted medical devices or prostheses, innate or acquired immunodeficiency, or malignancy, is also key. Diagnostic evaluation usually includes exclusion of treatable infectious etiologies, and even in the setting of a non-infectious cause of granulomatous lymphadenitis, superimposed infection should be considered.
Cytological Features
The characteristic cytological finding is the presence of nodular clusters of epithelioid histiocytes with syncytial-appearing cytoplasm, oval, reniform or spindled nuclei, fine chromatin and small, distinct nucleoli (Figs. 3.10, 3.11, 3.12, and 3.13). Intermixed small mature lymphocytes are present in the clusters. Multinucleated giant cells may be present in variable numbers or absent. Granulomatous inflammation is classified as necrotizing or non-necrotizing, based on the presence or absence of necrosis, and necrotizing granulomatous inflammation is further divided into suppurative and non-suppurative types, based on the presence or absence of neutrophilic inflammation. These features provide important clues to the differential diagnosis. Special stains, including acid fast, methenamine silver, Steiner, Warthin–Starry, and Gram, and/or an immunohistochemical stain for Bartonella may be helpful for demonstrating the presence of microorganisms. In longstanding granulomatous inflammation, the presence of fibrosis and hyalinization may result in paucicellular, non-diagnostic aspirates. Other cytological features that may be seen in a subset of cases are included in discussion of the differential diagnosis.
Necrotizing granulomatous inflammation (a. Diff-Quik stain, high power; b. H&E stain, high power; c. Acid fast stain, high power). In this a case of lymphadenitis due to atypical mycobacteria, clusters of epithelioid histiocytes with foamy cytoplasm are seen within a background of necrosis. Neutrophils are also present, characteristic of suppurative granulomatous inflammation. The acid fast stain highlights rare acid fast bacilli (c, arrow).
BCGosis (a. Diff-Quik stain, high power; b. H&E stain, high power). Newborns and children vaccinated with the BCG (Bacille de Calmette et Guérin) vaccine may develop ipsilateral lymphadenopathy from the attenuated live Mycobacterium bovis in the vaccine. The lymphadenitis appears similar to Mycobacterium tuberculosis with necrotizing granulomatous inflammation. (Images taken from slides provided by Dr. Pamela Michelow).
Differential Diagnosis
As noted above, the differential diagnostic considerations for granulomatous lymphadenitis include a wide variety of infectious and non-infectious processes. In the pediatric population, the most common causes of suppurative necrotizing granulomatous lymphadenitis are atypical mycobacterial or Bartonella infection, while non-suppurative necrotizing granulomatous lymphadenitis is most often due to infection with M. tuberculosis and fungi. In developing countries where use of the BCG (Bacille de Calmette et Guérin) vaccine is common, necrotizing granulomatous inflammation can be seen in newborns or older children in the lymph nodes draining the site of vaccination (Fig. 3.13). Necrotizing granulomatous inflammation may also be seen in some malignancies, such as Hodgkin and T-cell lymphomas, and if exuberant may obscure the diagnostic malignant cells. Causes of non-necrotizing granulomatous lymphadenitis include toxoplasmosis, early mycobacterial infection, foreign body reaction, drug reaction, and sarcoid, among others. Foreign body reactions are characterized by clusters of histiocytes, foamy histiocytes, and variable numbers of interspersed multinucleated giant cells with engulfed debris (the so-called foreign body giant cells ), and may be due to a variety of causes, such as sclerosing agents for vascular anomalies, talc or other crystalline carriers from intravenous drug abuse, and silicone from breast augmentation. Lipogranulomas can be seen in lymph nodes in response to endogenous or exogenous lipids, and oil droplets from lymphangiography. Sarcoid is characterized by tight, single and confluent, non-necrotizing granulomas, and an absence or paucity of multinucleated giant cells. Asteroid bodies (star-shaped), Hamazaki-Wesenberg inclusions (periodic acid-Schiff positive yellow-brown inclusions), and Schaumann bodies (concentrically laminated spherules composed of calcium and iron) may also be seen in sarcoid, but are not specific for that entity. Occasionally, central necrosis, characterized by granular eosinophilic debris is present in sarcoidal granulomas, but such cases lack the dirty background of necrotizing granulomatous processes. Other causes of lymphadenopathy associated with histiocytic proliferations that may mimic granulomatous inflammation include Langerhans cell histiocytosis (LCH), metabolic and storage disorders, hemophagocytosis and familial hemophagocytic syndrome, and sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease ). In Gaucher disease , the most common lysosomal storage disease, the histiocytes are enlarged with abundant crinkled tissue paper-like cytoplasm (Gaucher cells ) [10]. Gaucher-like cells have also been reported in extramedullary hematopoietic tumors, chronic myeloid leukemia, and crystal storage histiocytosis [11]. Gaucher and Gaucher-like cells may be confused with histiocytes from granulomatous inflammation due to atypical mycobacteria in which negative-images of the mycobacteria impart a striped or wrinkled appearance to the cytoplasm in modified Giemsa-stained preparations. Hemophagocytic disorders, LCH, and sinus histiocytosis with massive lymphadenopathy are discussed below in Sects. 3.4.4, 3.4.5, and 3.4.8.
Pearls
-
Material should be obtained for cultures to establish the specific identity of the causative organism, as well as to determine susceptibility and resistance to antimicrobial agents.
-
High quality aspirate smears may be superior to a diluted or limited cell block, particularly if there are few organisms.
-
Serology can be used to confirm the presence of Bartonella infection and may be particularly warranted when cat scratch disease is suspected clinically and/or cytologically, but organisms are not identified with Steiner, Warthin–Starry, or immunohistochemical stains, and mycobacterial infection has been excluded.
-
When acid fast organisms are seen or when no organisms are seen but the clinical suspicion of mycobacterial infection is high, polymerase chain reaction (PCR)-testing can be performed on material from the cell block to confirm the presence and subtype of mycobacteria.
3.4.4 Hemophagocytic Lymphohistiocytosis and Hemophagocytosis
Clinical Features
Hemophagocytic lymphocytosis (HLH) is a rare, life-threatening condition that can be primary due to inherited defects in NK cells (familial hemophagocytic lymphohistiocytosis), or acquired after strong immunologic activation by infection, particularly herpes viruses, autoimmune disease, or malignancy. HLH is a hyperinflammatory, uncontrolled, ineffective, immune response with clinical features attributable to high levels of cytokines and chemokines. In contrast, hemophagocytosis outside the context of HLH is a non-specific finding that is often associated with viral infections and pursues a benign course. In both HLH and non-specific hemophagocytosis, activated macrophages in various organs phagocytize other hematolymphoid cells, such as erythrocytes and lymphocytes [12].
Cytological Features
The key cytological finding is the presence of macrophages with engulfed erythrocytes or white blood cells, which are typically surrounded by a thin halo (Fig. 3.14). The histiocytes are positive for CD68 and S100, but are negative for CD1a.
Differential Diagnosis
The main differential diagnostic considerations include sinus histiocytosis with massive lymphadenopathy, which is discussed in Sect. 3.4.8, and other histiocytic processes, as summarized in Table 3.2. In addition, an associated lymphoma, particularly T-cell lymphomas, should be excluded.
Pearls
Although hemophagocytosis may be non-specific and pursue a benign course, its presence should prompt consideration of HLH, as well as lymphoma or leukemia.
3.4.5 Langerhans Cell Histiocytosis (Histiocytosis X, Letterer–Siwe Disease, Hand–Schuller–Christian Disease , Eosinophilic Granuloma)
Clinical Features
Langerhans cell histiocytosis (LCH) is a clinically heterogeneous disease, characterized by clonal proliferation of Langerhans cells. Multifocal multisystem LCH (Letterer–Siwe disease) presents in infancy and preferentially involves skin, bone, liver, spleen, and bone marrow, whereas multifocal unisystem LCH (Hand–Schuller–Christian disease ) affects young children and typically involves bone and adjacent soft tissue. Unifocal unisystem LCH (eosinophilic granuloma) usually presents in older children and adults as a lytic bone lesion with or without involvement of the adjacent soft tissue, or less commonly in lymph node, skin, or lung. Males are more commonly affected than females. Whereas unifocal unisystem LCH follows a benign course in virtually all cases, multifocal multisystem disease is associated with a high mortality rate, particularly in patients who fail to respond promptly to therapy [13].
Cytological Features
Approximately 85 % of cases of LCH can be correctly diagnosed by FNA cytology [14]. The key feature is the presence of LCH cells, which are oval, intermediate-sized cells with moderately abundant cytoplasm, and grooved, folded, or indented nuclei with delicate chromatin and inconspicuous nucleoli (Fig. 3.15). In addition to small lymphocytes, the background has variable numbers of eosinophils, histiocytes, multinucleated giant cell of both histiocytic and LCH cell origin, and neutrophils. Eosinophilic microabscesses are present in some cases. Late lesions may be fibrotic and have a paucity of LCH cells. LCH cells are positive for CD68, S100, CD1a, langerin, and fascin [13, 14].
Langerhans cell histiocytosis. (a. Diff-Quik stain, high power; b. H&E stain, high power; c. CD1a stain, high power). The aspirate (a) and biopsy (b) from this cervical lymph node shows LCH cells with irregular, grooved, or folded nuclei. Deep clefts give some nuclei multilobated appearance. Lymphocytes and eosinophils are present in the background. CD1a staining on the cell block (c) is strongly positive in these Langerhans cells.
Differential Diagnosis
The main differential diagnostic considerations include dermatopathic lymphadenitis, sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease), hemophagocytic disorders, lymphoma, and malignant histiocytosis. In dermatopathic lymphadenitis, the accumulation of Langerhans cells that are morphologically indistinguishable from LCH cells and are positive for CD68, S100, CD1a, and langerin, as well as the presence of eosinophils in some cases, can lead to confusion with LCH. However, histiocytes with engulfed pigment are present in dermatopathic lymphadenitis and help to distinguish this entity from LCH [15, 16]. Lymphomas and malignant histiocytosis can be distinguished from LCH by the presence of morphologically malignant cells. Features of sinus histiocytosis with massive lymphadenopathy and hemophagocytic disorders are discussed in Sects. 3.4.8 and 3.4.4, respectively. Additional diagnostic considerations include infections due to parasites or fungus, Kimura disease , Hodgkin lymphoma, and T-cell lymphoma, due to the presence of eosinophils in those entities.
Pearls
-
The distinct nuclear morphology of LCH cells, positivity for CD1a and absence of engulfed cells, help to distinguish LCH from most other histiocytic processes.
-
Dermatopathic lymphadenitis may closely mimic LCH due to the presence of Langerhans cells that are morphologically indistinguishable from LCH cells and positive for CD68, S100, CD1a, and langerin, and in some cases, they have associated eosinophils. Histiocytes with intracellular pigment provide an important clue to the correct diagnosis.
3.4.6 Histiocytic Necrotizing Lymphadenitis (Kikuchi-Fujimoto Disease or Kikuchi Disease)
Clinical Features
This is a benign, self-limited condition of unclear etiology that occurs most often in young Asian women and presents with fever, night sweats, and painless cervical lymphadenopathy [17]. However, in children, there tends to be a male predominance. The condition usually resolves spontaneously within 6 months of the diagnosis.
Cytological Features
The cytological diagnosis can be challenging, with a reported accuracy of about 56 % in one retrospective study [18]. Smears are characterized by heterogeneous lymphoid cells in a granular, necrotic background containing abundant karyorrhectic debris. Typically there are increased histiocytes and an absence of neutrophils. Histiocytes with peripherally situated, crescent-shaped nuclei and engulfed cellular debris (so-called crescentic histiocytes) are also characteristic, in addition to increased numbers of plasmacytoid dendritic cells, which co-express CD68 and CD123 and are negative for fascin, in the background [17, 18]. The background lymphocytes are predominantly CD8-positive T-cells.
Differential Diagnosis
The differential diagnostic considerations include necrotizing lymphadenitis related to autoimmune disease, such as systemic lupus erythematosus (SLE), herpes viruses, or other infections, as well as infarction and malignancy. Features of SLE that are not seen in histiocytic necrotizing lymphadenitis (HNL) are hematoxylin bodies, which are periodic acid Schiff (PAS)-positive amorphous extracellular structures composed of DNA and immunoglobulin, LE cells which are neutrophils with engulfed hematoxylin bodies, and increased plasma cells. The presence of characteristic viral inclusions supports a diagnosis of herpes simplex virus, which can be confirmed by immunohistochemical stains. Infectious mononucleosis is discussed in Sect. 3.4.7. Granulomas, which are a key feature of necrotizing granulomatous lymphadenitis due to mycobacterial and other infections, are absent in HNL.
Pearls
-
Crescentic histiocytes and plasmacytoid dendritic cells are characteristic of HNL, but are non-specific and can be seen in smaller numbers in other disorders.
-
Neutrophils are absent in HNL; however, the abundant karyorrhectic debris may mimic the multilobulated nuclei of neutrophils, leading to misinterpretation as suppurative inflammation.
3.4.7 Infectious Mononucleosis
Clinical Features
Infectious mononucleosis (IM) is an acute illness characterized by marked cervical lymphadenopathy, fatigue, fever, pharyngitis, and hepatomegaly, splenomegaly or both. Epstein–Barr virus (EBV) is the most common cause, but other viruses, particularly cytomegalovirus (CMV) and human immunodeficiency virus (HIV), can produce clinically indistinguishable disease. IM can mimic lymphoma clinically, especially when the lymphadenopathy is pronounced or asymmetrical and associated with constitutional symptoms, and the heterophile antibody test is negative, thereby prompting pathological evaluation. Serological studies for specific causative viruses confirm the diagnosis , but may not be available at the time of presentation for an FNA.
Cytological Features
Aspirates are composed of a heterogeneous population of lymphocytes with increased intermediate-sized to large immunoblasts with nuclear enlargement, prominent nucleoli, and basophilic cytoplasm (Fig. 3.16). In cases due to EBV infection, lymphocytes throughout the spectrum are positive for EBV-encoded RNA (EBER) by in situ hybridization. Large CD30-positive binucleate immunoblasts mimicking Reed–Sternberg (RS) cells are present in some cases; however, in contrast to RS cells, these cells are positive for CD20 and negative for CD15 [19]. Eosinophils and/or necrosis are also present in some cases. Triage in these cases usually includes collection of additional material for flow cytometry to exclude a lymphoproliferative disorder and a cell block for immunohistochemical stains and EBER in situ hybridization.
Infectious mononucleosis. (a. Diff-Quik stain, high power; b. H&E stain, high power; c. EBER in situ hybridization). These aspirates show a shift to intermediate-sized to large immunoblastic cells with immature-appearing chromatin, prominent nucleoli, and basophilic cytoplasm. EBV in situ hybridization (EBER) performed on a cell block is positive (c).
Differential Diagnosis
The main differential diagnostic considerations include drug-related lymphadenopathy, post-vaccine lymphadenopathy, other infections, such as toxoplasmosis, and lymphoproliferative disorders. The heterogeneous lymphoid population in IM helps to distinguish this entity from non-Hodgkin lymphomas, which are composed of a relatively homogenous population of cells. Distinction between classical Hodgkin lymphoma (CHL) and IM can be challenging as both have a heterogeneous population of cells, RS and RS-like cells are morphologically similar, and in some cases, EBER is positive in RS cells. Eosinophils may be present but are not usually a prominent feature of IM and as previously noted, RS-like cells are CD15 negative. In addition, RS-like cells are usually positive for BOB.1 and OCT-2, which helps to distinguish IM from the infrequent cases of classical Hodgkin lymphoma.
Pearls
Ancillary studies, including flow cytometry, immunohistochemical stains, and in situ hybridization for EBER, are important for confirming the diagnosis and excluding malignancies, particularly in cases with atypical clinical presentations.
3.4.8 Sinus Histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease)
Clinical Features
SHML is a rare histiocytic disorder of uncertain etiology that most often presents with bulky, bilateral, painless cervical lymphadenopathy in children, but can occur in any node, in extranodal sites, and at any age. It is a benign, self-limited condition, although the course may be protracted [20].
Cytological Features
The characteristic finding in SHML is the presence of emperipolesis , which is the engulfment of benign lymphocytes and erythrocytes by histiocytes. The cells are markedly enlarged with abundant pale cytoplasm containing intact engulfed cells surrounded by a thin halo. Nuclei are round to oval with smooth nuclear contours, fine chromatin, and small but conspicuous nucleoli. The histiocytes are positive for CD68, S100, and fascin, but are negative for CD1a [20]. The background lymphoid population is heterogeneous. Eosinophils are usually absent.
Differential Diagnosis
The main differential diagnostic considerations are hemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis , which are discussed in Sects. 3.4.4 and 3.4.5, respectively.
Pearls
In emperipolesis , the histiocytes can be distinguished from tingible body macrophages by the presence of intact engulfed lymphocytes and erythrocytes rather than cellular debris.
3.5 Malignant Entities
3.5.1 Hodgkin Lymphoma
Clinical Features
Classical Hodgkin lymphoma (CHL) accounts for 95 % of Hodgkin lymphomas and has a bimodal age distribution, with the earlier peak occurring in adolescence and young adulthood. Patients usually present with cervical or mediastinal lymphadenopathy, and may have B symptoms, including fever, night sweats, and weight loss. Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) accounts for approximately 5 % of Hodgkin lymphomas and primarily affects 30- to 50-year-old males. The majority of patients present with localized peripheral adenopathy [21].
Cytological Features
CHL is characterized by variable numbers of Reed–Sternberg (RS) cells within a polymorphous background containing increased eosinophils (Fig. 3.17). Classical RS cells are large cells with abundant cytoplasm and two nuclei or nuclear lobes. Nuclei are with irregular membranes, pale chromatin, and a single eosinophilic nucleolus surrounded by a clear halo in each nucleus or lobe. Mononuclear variants (Hodgkin cells) and mummified cells are also present. The malignant cells comprise a small minority of the cells, and are positive for CD30 in a Golgi and membranous pattern, CD15, MUM-1, and PAX5 (weak), and negative for OCT-2 and/or BOB.1 [21]. Granulomas may also be seen. NLPHL is characterized by a predominant population of small lymphocytes with admixed histiocytes and scattered malignant cells. Lymphocyte-predominant (LP) or so-called popcorn cells are large with scant cytoplasm and a single convoluted or multilobated nucleus with multiple, basophilic nuclei that are smaller than those in RS cells. Occasional LP cells are morphologically indistinguishable from RS cells. However, in contrast to RS cells, LP cells are positive for CD20, CD79a, BCL6, OCT-2, and BOB.1, and negative for CD30 and CD15 [21].
Classical Hodgkin lymphoma (a. Diff-Quik stain, high power; b. H&E stain, medium power; c. CD30 stain, medium power). The aspirates reveal Reed–Sternberg (RS) cells with binucleation and prominent nucleoli, within a polymorphous background of lymphocytes and eosinophils (a, b). CD30 (c) is positive in the large RS cells.
Differential Diagnosis
The main differential diagnostic considerations are reactive lymphoid proliferations, infectious mononucleosis, and other viral infections that may have large immunoblastic cells that mimic RS cells, anaplastic large T-cell lymphoma (ALCL) and other large cell non-Hodgkin lymphomas, and metastatic malignancies such as seminoma and melanoma.
Pearls
-
Immunohistochemical stains are critical for confirming the diagnosis of Hodgkin lymphoma given the wide variety of different entities in the differential diagnosis, some of which are also CD30-positive.
-
Flow cytometry shows a reactive pattern and therefore is non-contributory.
3.5.2 Lymphoblastic Leukemia/Lymphoma
Clinical Features
Acute lymphoblastic leukemia (ALL) comprises about 75 % of all acute leukemias in the pediatric population, and is also one of the most curable based on modern treatments [22]. These malignancies can be of B (B-ALL) or T (T-ALL) cell origin. B-ALL accounts for 85 % of pediatric ALL and the majority of cases occur in children under 5 years of age [22]. In contrast, T-ALL comprises approximately 15 % of pediatric ALL, is more common in older children and adolescents, and affects males more often than females. Lymph node involvement is common in ALL, whereas primary lymphoblastic lymphoma (LBL) without leukemia occurs less often [22, 23]. B-LBL comprises approximately 10 % of primary lymphoblastic lymphomas and like B-ALL occurs in children. They often present in the head and neck, and patients are usually asymptomatic with limited disease. In contrast, T-LBL comprises 85–90 % of primary lymphoblastic lymphomas, and like T-ALL occurs more frequently in adolescent males. Patients often present with a large mediastinal mass, and may have tracheal compression, superior vena cava syndrome, and/or pleural effusions. B-ALL and B-LBL have favorable prognoses, whereas T-ALL and T-LBL have less favorable outcomes and require more aggressive treatment [22, 23, 24]. Risk stratification is used to determine treatment and is based on clinical, laboratory, and genetic findings.
Cytological Features
B-ALL/LBL and T-ALL/LBL are morphologically indistinguishable. They are characterized by a homogenous population of intermediate-sized lymphoblasts with scant cytoplasm, round, oval or irregular nuclei, dispersed, finely granular chromatin, and prominent nucleoli. Mitotic figures and tingible body macrophages are present and may be numerous, similar to Burkitt lymphoma. The lymphoblasts in both T- and B-ALL/LBL are TdT-positive by flow cytometry or immunohistochemistry. In addition, T-ALL/LBL shows variable expression of CD1a and T-cell markers, as well as clonal rearrangement of T-cell receptor genes [23]. B-ALL/LBL is usually positive for CD19, CD10, and PAX5, is variably positive for CD20 and CD34, and also expresses the myeloid markers CD13 and CD33. Of note, T-cell receptor gene rearrangements may be seen in up to 70 % of B-ALL/LBL and thus are not useful for determination of lineage [22]. Various cytogenetic and molecular abnormalities are present, some of which define specific subtypes of ALL/LBL.
Differential Diagnosis
The main diagnostic considerations: Burkitt lymphoma, diffuse large B-cell lymphoma, thymoma, and reactive immunoblastic proliferations, such as infectious mononucleosis. Burkitt lymphoma and diffuse large B-cell lymphoma are discussed in Sect. 3.5.4.
Pearls
-
When the clinical or cytological features are suspicious for lymphoma, it is essential to collect adequate additional material for flow cytometry, immunohistochemistry, FISH, and molecular studies.
-
Thin, evenly spread, cellular smears may be preferable to sections from a cell block for molecular studies.
-
Aspirates targeting a mediastinal mass may inadvertently sample normal thymic tissue. Normal immature T-cells maturing in the thymus are phenotypically similar to a T-LBL with positivity for TdT, CD3, and CD1a. Flow cytometry of a sample derived from thymic tissue shows a heterogeneous population of T-cells encompassing the maturation spectrum, whereas a clonal population of immature T-cells is present in T-LBL.
3.5.3 Acute Non-Lymphocytic Leukemia
Clinical Features
Acute leukemias of non-lymphoid origin comprise about 20 % of pediatric leukemias, except in newborns where they are more common than lymphoblastic leukemia. In children less than 2 years of age, monocytic leukemias tend to be the most common, whereas in older children, myeloblastic and myelomonocytic leukemias predominate. When non-lymphoid leukemic cells form a mass lesion, it is referred to as a granulocytic/myeloid sarcoma or chloroma; however, this is rare in children and most often these leukemias will present in the peripheral blood or bone marrow, and never undergo FNA evaluation.
Cytological Features
The morphologic features of the blasts vary, depending on the degree of maturation and type of cell. Myeloid blasts tend to have round, ovoid, or irregular nuclei similar to those seen in ALL/LBL, whereas myelomonocytic and monocytic leukemias tend to have either round or more convoluted nuclei. The blasts are intermediate to large in size with scant-to-abundant cytoplasm with or without vacuoles, granules, or Auer rods, depending on the type of cells. Nuclei have pale, delicate, or lacey chromatin with one or more nucleoli. Flow cytometry and immunohistochemical stains are important for determining maturation and cell lineage markers.
Differential Diagnosis
The main differential diagnostic considerations include lymphoblastic or other non-Hodgkin lymphoma, as well as other malignancies such as germ cell tumors, melanoma, and sarcomas with epithelioid morphology.
Pearls
-
Leukemias are rarely seen in FNA specimens, except for myeloid/granulocytic sarcomas that present with a mass lesion. Although rare, these tumors should be considered when a sample consists of a monomorphous population of intermediate to large, dyscohesive epithelioid cells.
-
According the 2008 World Health Organization (WHO) classification, the term myeloid sarcoma should be used, opposed to previously used terms like chloroma and granulocytic sarcoma.
3.5.4 Other Non-Hodgkin Lymphomas
Clinical Features
In addition to lymphoblastic lymphomas (described above), the most common non-Hodgkin lymphomas (NHLs) in the pediatric population include Burkitt lymphoma, large B-cell lymphomas including diffuse (DLBCL) and primary mediastinal (thymic) PMBL, and anaplastic large cell lymphoma (ALCL) [24]. Of these, Burkitt lymphoma is the most common, comprising 50–60 % of NHL children and adolescents, may be endemic related to EBV infection or sporadic, and usually presents in head and neck or abdomen [25]. Large B-cell lymphomas account for approximately 10–15 % of pediatric NHLs, occur more frequently in adolescents than younger children, and include DLBCL and PMBL subtypes [26, 27]. DLBCL can present with nodal and/or extranodal disease. PBML is likely of thymic origin and by definition arises in the anterior mediastinum without evidence of lymphoma elsewhere. ALCL comprises 10–20 % of childhood NHLs, has a peak incidence in the second decade of life, and often presents with nodal and extranodal disease and B symptoms [28]. Small B-cell lymphomas, which are common in adults, are rare in children.
Cytological Features
These NHLs are characterized by homogeneous populations of intermediate to large malignant cells and, with the exception of PMBL, typically yield highly cellular cytologic specimens.
In Burkitt lymphoma , the cells are intermediate in size, have scant, deeply basophilic cytoplasm with “punched-out” vacuoles, and round or slightly irregular nuclei with clumped, dispersed chromatin, and multiple nucleoli. Numerous mitoses, apoptotic bodies, and tingible body macrophages are present (Fig. 3.18). The cells have a mature B-cell phenotype and are positive for CD10, CD19, CD20, CD79a, Bcl-6, and surface immunoglobulin, and negative for TdT and MUM1. Ki-67 (MIB1) is positive in virtually 100 % of cells. In situ hybridization for EBER is positive in all endemic and up to 30 % of sporadic cases. Translocations involving the MYC gene on chromosome 8 and an IG gene, usually IGH on chromosome 14, are present in almost 100 % of cases [24, 25].
Burkitt lymphoma (a. Diff-Quik stain, high power; b. Papanicolaou stain, high power; c. FISH with the CMYC breakapart probe). This aspirate from a 14-year-old girl with Burkitt lymphoma presenting as a neck mass shows a uniform intermediate-sized lymphoid population with mitoses and tingible body macrophages (a, b), which had a high proliferation index on Ki-67. FISH studies confirmed a CMYC gene rearrangement (c).
DLBCLs are morphologically diverse tumor with three main variants recognized. Centroblastic , the most common variant, is composed of large cells with scant cytoplasm and round, irregular, or lobated vesicular nuclei with fine chromatin and multiple nucleoli. Immunoblastic DLBCL is composed of large cells with moderate to abundant cytoplasm, and round, oval, or irregular nuclei with a single, prominent central nucleolus. Anaplastic DLBCL is composed of pleomorphic, large, round, oval, or polygonal cells with bizarre nuclei and/or multinucleation, and may resemble Hodgkin lymphoma and/or ALCL. Mitotic figures, apoptoses, and variable numbers of tingible body macrophages are present. DLBCLs have a mature B-cell phenotype and are positive for CD19, CD20, CD79a, variably positive for CD10, Bcl-6, and surface immunoglobulin, usually negative for MUM1 and negative for TdT. CD30 is often positive, particularly in the anaplastic variant. Many DLBCL have translocations involving IGH and various partner genes, including BCL6 (up to 30 %), BCL2 (20–30 %), and MYC (6 %) [24, 26]. In addition, some tumors have morphologic features intermediate between Burkitt lymphoma (BL) and DLBCL, and for these, immunophenotyping and genetic analysis are essential for diagnosis and treatment, as these tumors may represent BL, DLBCL, or B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL [29].
PMBLs typically yield paucicellular specimens with poorly preserved large cells with a spectrum of morphologies similar to those seen in DLBCL. On corresponding biopsies distinctive compartmentalizing thin fibrous bands are present throughout (Fig. 3.19). The cells are positive for CD19, CD20, CD79a, and MUM1, variably positive for Bcl-6 and CD30, usually negative for CD10, and negative for surface immunoglobulin and TdT [27].
Mediastinal thymic large B-cell lymphoma (a. Diff-Quik stain, high power; b. H&E stain, high power; c. CD30 stain, high power). This lymphoma typically has intermediate-to-large lymphoid cells with artifactual distortion and limited aspirates due to the interlacing fibrosis. The core biopsy highlights the compartmentalizing fibrosis and the CD30 shows the weak but diffuse staining that is usually seen in these tumors.
Like DLBCL, ALCL is morphologically heterogeneous, with common, lymphohistiocytic, small cell, Hodgkin-like, and composite patterns. Common to all patterns is the presence of variable numbers of the so-called hallmark cells, which have eccentric , reniform, or U-shaped nuclei. ALCLs are positive for CD30 and ALK, usually positive for EMA, variably positive for T-cell antigens, and negative for CD15 and B-cell markers. Of note approximately 75 % of ALCLs are CD3 negative. Some ALCLs have an apparent null cell phenotype due to loss of multiple pan T-cell antigens, but are positive for cytotoxic markers such as perforin, granzyme B, and TIA-1. ALK rearrangements are present in over 90 % of cases [28].
Differential Diagnosis
The differential diagnosis varies depending on the type of non-lymphoblastic NHL, but in general includes Hodgkin lymphoma, lymphoblastic lymphoma, and metastatic melanoma, sarcoma, germ cell tumors, carcinoma, and pediatric round blue cell tumors. Ancillary studies are required to distinguish between these entities.
Pearls
-
Collection of additional material for flow cytometry, immunohistochemical studies, and FISH is essential.
-
Cellular, evenly spread unstained smears, may be preferable to sections from a cell block for FISH.
-
CD30 positivity is present in many tumors in the differential diagnosis, and therefore must be interpreted in the context of other immunophenotypic and molecular findings.
3.5.5 Other Malignancies
In the pediatric population, the more common non-lymphoid malignancies metastatic to lymph nodes include small round cell tumors, germ cell tumors (Fig. 3.20), malignant melanoma, and several carcinomas, including papillary thyroid carcinoma, medullary carcinoma, and nasopharyngeal carcinoma. Non-lymphoid small round cell malignancies are summarized in Table 3.4.
Metastatic seminoma (a. Diff-Quik stain, high power; b. Papanicolaou stain, high power; c. H&E stain, high power). Seminomas show a discohesive pattern of cells with prominent nucleoli and a tigroid background on modified Giemsa-stained slides (a) due to the glycogen in the cells. There is also a lymphohistiocytic background, often with granulomas (a–c). The tigroid background is not typically seen in alcohol fixed material (b, c).
References
Cardillo MR. Fine-needle aspiration cytology of superficial lymph nodes. Diagn Cytopathol. 1989;5:166–73.
Rimm DL, Stastny JF, Rimm EB, Ayer S, Frable WJ. Comparison of the costs of fine needle aspiration and open surgical biopsy as methods for obtaining a pathologic diagnosis. Cancer. 1997;81:51–6.
Steel BL, Schwartz MR, Ramzy I. Fine needle aspiration biopsy in the diagnosis of lymphadenopathy in 1103 patients: role, limitations, and analysis of diagnostic pitfalls. Acta Cytol. 1995;39:76–81.
Lioe TF, Elliott H, Allen DC, Spence RAJ. The role of fine needle aspiration cytology (FNAC) in the investigation of superficial lymphadenopathy: uses and limitations of the technique. Cytopathology. 1999;10:291–7.
Prasad RR, Narasimhan R, Sankaran V, Veliath AJ. Fine-needle aspiration cytology in the diagnosis of superficial lymphadenopathy: an analysis of 2,418 cases. Diagn Cytopathol. 1996;15:382–6.
Thomas JO, Adeyi D, Amanguno H. Fine-needle aspiration in the management of peripheral lymphadenopathy in a developing country. Diagn Cytopathol. 1999;21:159–62.
O’Dowd GJ, Frable WJ, Behm FG. Fine needle aspiration cytology of benign lymph node hyperplasia: diagnostic significance of lymphohistiocytic aggregates. Acta Cytol. 1985;29:554–8.
Monaco SE, Teot LA. Cytopathology of pediatric malignancies: where are we today with fine-needle aspiration biopsies in pediatric oncology? Cancer Cytopathol. 2014;122:322–36.
Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, Sneller MC, Puck JM, Lenardo MJ, Elenitoba-Johnson KSJ, Lin AY, Raffeld M, Jaffe ES. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153:1541–50.
Chen M, Wang J. Gaucher disease: review of the literature. Arch Pathol Lab Med. 2008;132:851–3.
Mokhtari M, Kumar PV, Talei AR. Gaucher-like cells in retroperitoneal extramedullary hematopoietic tumor diagnosed by fine needle aspiration: a case report. Acta Cytol. 2010;54:903–6.
Janka GE, Lehmberg K. Hemophagocytic syndromes—an update. Blood Rev. 2014;28:135–42.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 358–60.
Kakkar S, Kapila K, Verma K. Langerhans cell histiocytosis in lymph nodes: cytomorphologic diagnosis and pitfalls. Acta Cytol. 2001;45:327–32.
Sudilovsky D, Cha I. Fine needle aspiration cytology of dermatopathic lymphadenitis. Acta Cytol. 1998;42:1341–7.
Iyer VK, Kapila K, Verma K. Fine-needle aspiration cytology of dermatopathic lymphadenitis. Acta Cytol. 1998;42:1347–51.
Tong TR, Chan OW, Lee KC. Diagnosing Kikuchi disease on fine needle aspiration biopsy: a retrospective study of 44 cases diagnosed by cytology and 8 by histopathology. Acta Cytol. 2001;45:953–7.
Deaver D, Horna P, Cualing H, Sokol L. Pathogenesis, diagnosis and management of Kikuchi-Fujimoto disease. Cancer Control. 2014;21:313–21.
Louissnat A, Ferry JA, Soupir CP, et al. Infectious mononucleosis mimicking lymphoma: distinguishing morphological and immunophenotypic features. Mod Pathol. 2012;25:1149–59.
Shi Y, Griffin AC, Zhang PJL, et al. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): a case report and review of 49 cases with fine needle aspiration cytology. CytoJournal. 2011;8:3.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 323–30.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 168–70.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 176–8.
Minard-Colin V, Brugières L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33:2963–74.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 262–4.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 233–7.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 250–1.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 312–6.
Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of hematopoietic and lymphoid tissue. 4th ed. Lyons: IARC Press; 2008. p. 267–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Monaco, S.E. (2017). Lymph Nodes. In: Monaco, S., Teot, L. (eds) Pediatric Cytopathology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-53441-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-662-53441-0_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-53439-7
Online ISBN: 978-3-662-53441-0
eBook Packages: MedicineMedicine (R0)