Abstract
The main challenge in the surgical treatment of lumbar degenerative spinal stenosis is to achieve adequate decompression of the neural structures without inducing iatrogenic instability, keeping or restoring a good lordosis and correcting or preventing spinal deformity Sometimes nerve root decompression could be achieved only by restoration of the height of the intervertebral space and by a large opening of the lateral recesses and the foramen. Large bone resection may be indeed required. Decompression surgery for spinal stenosis due to degenerative changes producing claudication is successful in most patients. According to the literature, the rate of further spinal instability is from 5 to 10 % and the risk of postoperative additional forward slip in degenerative spondylolisthesis is assessed between 10 and 18 % of the patients treated without fusion. Even if further horizontal dislocation did not lead to worse clinical results, it is logical for the surgical treatment not only to aim the most efficient decompression of the neurological structures by using adequate bone resection and restoration of the intervertebral height by the distractive interbody fusion, but also the second aim for surgery is to prevent postoperative destabilisation by using the same intervertebral fusion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Historically, lumbar fusion has been described as a treatment of symptomatic spondylolisthesis, degenerative scoliosis and spinal stenosis associated with instability [1–3]. Lumbar fusion is also performed after posterior decompressive procedure when evidence of preoperative lumbar spinal deformity or instability that could worsen after laminectomy alone exists [4].
Burns [5] reported the first case of lumbar interbody fusion in 1933. From an anterior approach (anterior lumbar interbody fusion, i.e. ALIF), he used an autogenous tibial peg to treat an adolescent with spondylolisthesis. The posterior lumbar interbody fusion (PLIF) procedure was first described in 1944 by Briggs and Milligan [6], who used laminectomy bone chips in the disk space as interbody graft. In 1946, Jaslow [7] modified the technique by positioning an excised portion of the spinous process within the intervertebral space. It was not until 1953 when Cloward [1] described his technique, which used impacted blocks of iliac crest autograft that the popularity of PLIF technique increased. The PLIF procedure was found to have substantially increased fusion rates, often in excess of 85 %. Despite controversy about the efficacy of lumbar interbody fusion, because of the introduction of pedicle screw fixation [8], some clinicians have continued to use this procedure as Lin [9], Branch [10] and Takeda [11]. Then, advances in bone physiology, biomechanics, and fusion techniques with synthetic interbody implants have renewed interest in posterior interbody fusion. The BAK cage, which is a perforated stainless steel cylinder and filled with local autologous bone graft, was developed by Bagby and Kulisch [12, 13]. The concept was to use two parallel implants interposed between the vertebral bodies, with distraction, that restored the disk space, and the compression of the implants against the subchondral bone produces immediate stability [14]. More recently, interbody cages have become popular and are now composed of a wide range of materials, such as titanium mesh, carbon fibre and polyether ether ketone (PEEK) [15]. Finally, the addition of pedicle screws increases the stability of the construct and has been reported to significantly increase the fusion rate of this procedure compared with stand-alone grafts [16, 17].
2 Rationale
Damage and degeneration of the lumbar disk can be the result of ageing, activity and trauma. Therefore, the degradation of the disk matrix leads to loss of disk height with or without bulging of the intervertebral disk and distension of the ligaments that create segmental instability. Thus, constraints on facet joints are increased and that may cause deformation, hypertrophy or subluxation of the facet joints like in spondylolisthesis. Moreover, mechanical stress causes hypertrophy and fibrosis of the ligamentum flavum. All these processes, including decreased disk height, facet joint and ligamentum flavum hypertrophy and vertebral endplate osteophytosis, may result in central canal stenosis, and/or lateral recess stenosis and/or foraminal stenosis [18]. Moreover, spinal stenosis may be emphasised by congenital abnormalities, or disorder of postnatal development [19].
Nerve root and cauda equina compression may arise from the combination of prolapsed disk, vertebral bone lesions anteriorly, but also from degenerated facet joint and hypertrophied ligamentum flavum posteriorly. Most often, degenerative spondylolisthesis occurs at the fourth lumbar vertebrae in middle-age women. As a result of a slipping forward of the vertebrae, cauda equina and spinal nerve roots may be tightened between the edge behind the top of lower vertebrae and frontal edge of the lower part of upper lamina, also linked to the subluxation of the facet joints.
Imaging studies are indispensable for diagnostic evaluation and treatment planning in symptomatic patients. There are many morphometric methods for the description of the spinal canal. Such terms as absolute and relative spinal stenosis are defined by purely radiological criteria and lack any clinical correlation. Lumbar MRI is the standard procedure for the demonstration of stenosis and cauda equina compression. As reported in the literature, its sensitivity is 87–96 % and its specificity is 68–75 % [20]. Lumbar CT may be useful for the assessment of bone condition and potential osteoporosis with a view towards the planning of surgery. On the other hand, lumbar myelography with post-myelographic CT should now only be performed in exceptional cases. The main indications for this invasive study are the presence of metal implants in the lumbar spine that would make MRI uninterpretable because of artefacts [21]. In our practice, we perform routinely full spine radiograph in order to analyse sagittal balance and lumbar dynamic radiographs to explore segmental instability. Electrophysiological studies are mainly useful in that they can reveal potential differential diagnoses.
One of the major objectives of spinal fusion is to relieve pain arising from spinal structures by removing potentially pain-generating disk tissue and stabilising one or more motion segments. Various methods of posterior lumbar fusion (PLF) have long been used for this purpose. Interbody fusion procedures became more widely used for their stabilising effect on the spine segment and as the role of the lumbar disk as a pain generator became better appreciated. The primary concept behind lumbar interbody fusion is that by removing all or most of the disk and stabilising the operated segment with bone graft, the primary pain generator is removed. Stabilising the segment should then eliminate mechanical stimulation that may provoke symptoms and may avoid future problems associated with collapse of an unsupported space. In a biomechanical study, interbody fusion was found to be stiffer than posterior lumbar fusion [22]. In addition, the surface area between the host bone and the graft is much greater with interbody fusion than with intertransverse process fusion.
The two primary purposes of interbody fusion are to relieve pain and stabilise the symptomatic spine segment. In cases of disk-related pain, the symptom-related tissue is removed. However, the removal of this tissue may cause the disk space to collapse with a concomitant narrowing of the foramen and related changes of the facet joints, causing nerve root compression. By filling the disk space with bone graft, the disk space height is re-established. This may also increase the height of the foramen. The bone graft grows into the bone of the adjacent vertebra, fusing them into a single unit. This stabilising effect is particularly important in cases of pseudarthrosis, spondylolisthesis, spinal instability and postlaminectomy syndrome.
Evidence supports interbody fusion over posterior fusion alone in the treatment of lumbar disk-related pain. Weatherley [23] reported using discography to identify symptomatic disks at the level of a solid posterior fusion. More recently, successful outcome was reported for such patients with persistent symptoms despite a solid posterior fusion when symptomatic disks within the previously fused segment were treated with ALIF [24]. Results of a biomechanical study found that following simulated posterior fusion with pedicle screw fixation, the intradiscal pressure during spinal flexion was as great as that measured in the intact, nonoperated segment [25]. These studies provide biomechanical and clinical support for the need to use an interbody fusion technique to adequately address pain arising from the disk. ALIF and PLIF have been found to be effective in the treatment of disk-related pain [26–31], particularly that associated with a chemically sensitised disk identified by discography [32]. Fusion not involving an interbody technique has yielded poor results for disk-related pain [32–34]. The potential benefits of using cages in interbody fusion procedures are that they may increase the chances of achieving a successful fusion and they provide some immediate stability to the operated segment whilst the bone graft incorporates [35].
Several cages are designed to be implanted into the disk space using either the anterior or posterior approach. Based on the review of the literature, there is no general preference for the approach to be used. The decision regarding the type of approach should be made based on several factors, such as the sagittal balance, pathology present, spinal anatomy, patient’s history of prior surgery (either approach may be more difficult if there is significant scarring from prior surgeries), vascular anatomy (and conditions that may make an anterior procedure more difficult, such as calcification of vessels) and the surgeon’s individual training and experience.
The main challenge in the surgical treatment of lumbar degenerative spinal stenosis is to achieve adequate decompression of the neural structures without inducing iatrogenic instability, keeping or restoring a good lordosis and correcting or preventing spinal deformity. Sometimes nerve root decompression could be achieved only by restoration of the height of the intervertebral space and by a large opening of the lateral recesses and the foramen. Large bone resection may be indeed required. Decompression surgery for spinal stenosis due to degenerative changes producing claudication is successful in most patients. According to the literature the rate of further spinal instability is from 5 to 10 % and the risk of postoperative additional forward slip in degenerative spondylolisthesis is assessed between 10 and 18 % of the patients treated without fusion [36, 37]. Even if further horizontal dislocation did not lead to worse clinical results, it is logical for the surgical treatment not only to aim the most efficient decompression of the neurological structures by using adequate bone resection and restoration of the intervertebral height by the distractive interbody fusion, but also the second aim for surgery is to prevent postoperative destabilisation by using the same intervertebral fusion.
3 Indications
The principal indication for lumbar interbody fusion surgery is the stabilisation and fusion of adult spinal instability and/or deformity. Therefore, lumbar fusion has been described as a treatment of symptomatic spondylolisthesis, degenerative scoliosis and spinal stenosis associated with instability [1, 2, 9, 38]. For those with lumbar stenosis but without spondylolisthesis (deformity), the surgical management has traditionally involved posterior decompressive procedures, including laminectomy or laminotomy, and judicious use of partial medial facetectomies and foraminotomies, with or without diskectomy [39, 40]. In patients with evidence of spinal instability, however, in situ posterior lumbar fusion is recommended as a treatment option in addition to decompression in the setting of lumbar stenosis [39]. Due to early surgical failures (the mean rate of poor outcome is 20 % in large series of laminectomies [36, 37]) and late deteriorations due to iatrogenic instability (5–18 %), restenosis (7 %) or disk herniation at adjacent spinal levels (10 %), careful selection of patients for fusion must be carried out by assessing radiological parameters that are associated with the greatest risk of postoperative destabilisation.
Secondary indications include recurrent lumbar disk herniation, where extensive bony removal is necessary for exposure of the disk fragments, lateral or massive disk herniations, failed previous lumbar fusions by other techniques and discogenic low back pain [38]. Because the cause of spinal pain is not completely understood and remains controversial, surgical efforts to treat such conditions also remain controversial [41]. The description of spinal pain is often referred to as “lumbar segmental instability” [42, 43] caused by degenerative disk disease [34] or facet joint syndrome [42, 44] when no signs of increased motion or spondylolisthesis exist [45].
As a consequence, the main parameters for indication of fusion after surgical decompression are (Fig. 31.1) as follows:
-
Sagittal orientation of the facet joints
-
Total facetectomy
-
Lumbar stenosis associated with lumbar previous idiopathic scoliotic deformity
-
Degenerative scoliosis
-
Intracanal synovial cysts alone or associated with listhesis
-
Flat back with loss of lordosis
-
Degenerative spondylolisthesis
-
Recurrent lumbar disk herniation
-
Secondary displacement after failed previous decompressive surgery
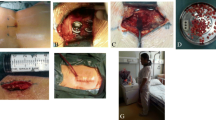
4 Technical Aspects (Fig. 31.2)
Ten main surgical steps for PLIF procedure: (1) Complete exposure of the posterior arches of the two adjacent vertebras, (2) bilateral facetectomy (inferior facets of upper vertebra and superior facets of lower vertebra), (3) insertion of pedicle screws, (4) laminotomy with control of the adjacent nerve roots (i.e. the two upper and the two lower roots), (5) complete diskectomy via bilateral approach, (6) intervertebral distraction through the disk space, (7) cleaning of the end plates using curettes and/or dedicated rasps, (8) insertion of lordotic peek cages filled with autologous bone graft, (9) contouring of the rod, (10) rod placement with compression performed along the rod between the screw heads
To perform PLIF, patients are positioned in the prone on chest and iliac crests rolls in order to lower intra-abdominal pressure and improve venous drainage. Arms are placed on arm boards with abduction limited to 80° as to prevent brachial plexus injury.
A dorsal midline incision is made and subcutaneous tissues are dissected with monopolar until the deep fascia. This fascia is incised adjacent to the spinous processes bilaterally preserving supra-spinous ligament. Then the para-spinous muscles are released from the laminae in a subperiosteal fashion, and the dissection is taken out to the facets bilaterally until the transverse processes are visualised. Lateral radiographs should be obtained to confirm the operative levels prior to arthrodesis. Then, soft tissues should be removed on and around the lamina, pars, facet joint and dorsal transverse processes including the facet capsule and intrafacet synovium. After all, the fixation with pedicle screws is realised prior to the decompression, therefore limiting the risk of neural and dura mater injury during screws insertion and reducing the timing with the canal opened (associated with potential epidural bleeding). In addition slight and gentle distraction between the screw head using appropriate distractor could facilitate the insertion of the interbody implants. For this procedure, a facetectomy is done, keeping the bone that will be morselised for future graft, and then pedicle screws are inserted with lateral radiograph control bilaterally for all interbody fusions. Laminotomy and foraminotomy can be performed as needed for neural decompression of the thecal sac and nerve roots.
In most cases, complete laminectomy is not necessary and only partial laminotomy of the upper vertebra is sufficient to perform the decompression and to permit the insertion of the interbody cages. Epidural veins must be coagulated to avoid bleeding and cut to move apart neural elements without tether and discover disk space. Care should be taken to protect neural structures with nerve root retractor. Another cause of epidural bleeding is the emissary vein of the vertebral body, which can be plugged by haemostatic gauze. Complete diskectomy and endplate preparation are performed, also removing the cartilaginous end plates using rasps. Then, spacers are inserted in order to progressively distract the disk space and determine the adequate gauge implant size (Fig. 31.3). Morselised autogenous bone, obtained from the laminectomy, is packed anteriorly before the implants are placed. According to our experience, the dimensions of the cages have to be high enough (at least 10 mm) and large (25 mm) to obtain a good primary stabilisation and thus a good fusion. Also, wedge-shaped cages (8° lordotic at minimum) are superior to rectangular cages in restoring segmental lordosis and sagittal alignment and avoiding flat back deformity [46]. The cages, filled with autogenous bone (perfectly cleaned with removal of all soft tissues), are inserted into the disk space with the medial aspect on the pedicles bilaterally. Then pedicle screws and rods are compressed to restore segmental lordosis and promote fusion by graft compression. After haemostasis is ensured, the wound is irrigated and closed in layers. A subfascial drain may be left.
Main surgical steps of PLIF procedure with perioperative views. Control of the four adjacent nerve roots, i.e. right and left L4 and L5 roots for L4–L5 level, is crucial to avoid any damage to neurologic structures. Intervertebral distraction on one side can be helpful to complete the decompression on the other side. uf upper facet, ds dural sac
5 Advantages/Limitations
Unlike posterolateral intertransverse fusion, PLIF is a biomechanically optimal fusion because the graft and/or the interbody implant maintains the disk height (i.e. the lateral foraminal opening), protects the nerve roots, restores weight bearing to anterior structures and controls both horizontal and vertical instabilities. The cagelike implants (titanium or polyether ether ketone (PEEK) cages) meet the mechanical requirements for PLIF by serving both a mechanical function and a biologic bone growth function. The cages stretch the intervertebral space to its normal anatomic height and prevent the postoperative collapse of the graft. The implant is packed with cancellous bone graft obtained from the laminectomy [47]. PLIF and anterior lumbar interbody fusion (ALIF) with cages, without a complementary posterior fixation for 360° stabilisation, are associated with pseudo-arthrosis, secondary displacement and subsequent complications. The role of the pedicle screw-based posterior fixation is first to carry out temporary control of AP, lateral or rotational translation before the achievement of the definitive bone fusion, second to enhance osteogenesis and third to allow early mobilisation without the need of a postoperative corset to avoid external contention (except in case of osteoporosis), loss of lordosis and further destabilisation at the adjacent level to the arthrodesis.
PLIF is neither useful nor safer when reoperations are performed and in which the spinal canal was already opened. There exists an increased risk of dural breach and neural injury due to fibrosis and nerve root distortion. ALIF or TLIF may be a good alternative for these patients, thus avoiding the dissection in the region of the epidural fibrosis. Another drawback of this technique is the blood loss that can be excessive, particularly in older patients. Also, in patients with a high pelvic incidence, ALIF may be a better alternative. ALIF facilitates a good fusion and restores an optimal sagittal balance. This parameter is crucial to respect, because the L4–S1 segment represents two-third of the total lumbar lordosis. As a consequence, arthrodesis should be performed with these parameters in mind.
6 Complications (Table 31.1)
Posterior lumbar interbody fusion provides circumferential release of the dural sac and/or nerve roots as well as a biomechanically stable construct with anterior and middle-column load sharing combined with pedicle screw devices. However, PLIF has some risks for surgical complications [48]. Along with risks related to the surgical approach, the use of implants increases the risk for additional complications [49]. Complications are divided here into perioperative complications that occurred during and within 1 month of surgery and late complications after 1 month of surgery.
6.1 Perioperative Complications
The incidence of perioperative complications following single-level PLIF has been reported to be 18–37.5 % [48, 50], and the incidence after two-level PLIF has been reported as 46 % [51]. Moreover, Deyo et al. found that patients who underwent lumbar surgery with fusion had a complication rate twice as high as those who underwent surgery without fusion [49]. Amongst several kinds of fusion techniques, PLIF is considered one of the most technically demanding procedures and a definite learning curve exists. One of the most dangerous manipulations in PLIF is excessive retraction of the dural sac with the cauda equine and nerve roots whilst removing disk material and inserting cages and bones. Nerves are often taut and immobile because of severe adhesion due to canal stenosis. Surgeons may unknowingly retract the dural sac beyond a critical pressure and/or period whilst concentrating on the disk space. Neurological deficits have been reported in only 2 % of patients after posterolateral lumbar fusion, in which access to the disk is not required [52]. Hosono et al. found that the surgery duration was the only significant risk factor for neurological complications and therefore suggested that the dural sac or roots should have been retracted for unusually long periods in patients presented with neurological deficits [49]. Also, the rate of neurological complications in procedures with total facetectomy is much lower than procedures with partial preservation of facet joints. It may reduce the intensity and period of retraction of the dural sac and nerve roots and the risks of neurological complications by taking advantages of the large working space provided by total excision of bilateral facet joints.
As a consequence, perioperative complications of PLIF procedures are as follows:
-
Dural laceration, cerebrospinal fluid (CSF) leakage: 4–17 % [53, 54]
-
Neurological complications
-
Hematoma: 1.2 %
-
Pedicle screw misplacement: 4 %
-
Injury to major abdominal vessels [56]
-
Pulmonary embolism: 0.4 %
6.2 Late Complications
The intracorporeal penetration on the cages or subsidence, and thus the loss of the restored intervertebral height, is perhaps the most significant late complication. It mainly occurs in osteoporotic patients, but remains rare – one patient in the authors’ series [55].
Pseudarthrosis is an uncommon complication of PLIF – less than 2 % [54, 55].
Cage retropulsion after PLIF is another complication that has been described. The risk factors are insufficient cage size, multilevel fusion, inadequate seating of the cage anteriorly and surgery at segment L5/S1. Fundamental techniques in performing PLIF must be mastered as follows:
-
The degenerated disk materials must be removed and the end plates cleaned from cartilaginous layers thoroughly.
-
The cage must be inserted without damaging the bony end plates.
-
Undersized cages should not be selected.
-
Adequate compressive force must be applied to the disk space by the pedicle screws.
-
Use of lordotic cages [57].
A prospective randomised study reported that fusion accelerates degenerative changes at the adjacent segment of the fused spine, compared with naturally occurring changes [58]. Spinal fusion alters the biomechanics of spinal motion and increases intradiscal pressure or the load on facet joints of the adjacent motion segment of the fused spine [59]. Within 5 years of lumbar fusion surgery, the clinical incidence of symptomatic adjacent segment disease (ASD) is reportedly 5.2–18.5 % [59] and the incidence of additional surgery for symptomatic ASD is reportedly 3–11 % [60, 61]. Moreover, the deterioration rate for repeat PLIF (44 %) [62] is higher than that for initial PLIF (5.2–18.5 %) [59]. Biomechanical studies have demonstrated greater intradiscal pressure at the adjacent segment in double-level fusion than in single-level fusion [59]. This is one reason why repeat PLIF leads to higher incidence of ASD than the initial PLIF. Deyo et al. [63] reported in their study of 31,543 patients with surgery for lumbar stenosis that prior spinal surgery was the strongest risk factor for repeat surgery and that the hazard ratio for this was 1.58. These results suggest that patients undergoing repeat PLIF for ASD would incur more risk factors for additional surgery than those undergoing single- or double-level PLIF at the initial surgery. Furthermore, age was reported to be a major risk factor for ASD [59, 60].
7 A Comparison of PLIF and TLIF
Interbody fusion techniques have been developed to preserve the load-bearing capacity of the spine, restore local lordosis and facilitate compressive loading onto interbody graft – all of which enhance the potential for fusion acquisition [64]. Lumbar interbody fusion with supplemental posterior pedicle screw fixation (“circumferential” fusion), based on biomechanical evaluation, stabilises all three columns of the spine and has been used routinely for the operative treatment of painful spinal disorders. PLIF, TLIF and ALIF approaches are the most frequently performed options and, when accompanied by posterior pedicle screw fixation, result in circumferential fusion. Each of the former procedures has advantages and drawbacks.
Posterolateral graft and fixation is easily added to the PLIF, further enhancing spinal stability and the induction of fusion.
Unfortunately, the PLIF is usually limited to use at levels below L3, because of the risk of damage to the conus medullaris and to the cauda equina that may result from bilateral root retraction here. The suggested modification of PLIF presented by Harms and Jeszenszky [65], the TLIF, is equivalent to the PLIF and is simpler and safe, and some believe superior in result. The technical advantages of the TLIF include avoidance of thecal sac and/or nerve root retraction injury, safe performance below L3 and a decrease in epidural bleeding and scarring [65–67]. Harms and Jeszenszky, in their presentation of the original TLIF procedure, as well as many other authors of biomechanical reports, have recommended additional posterior pedicle screw fixation to enhance stability.
The PLIF and TLIF are familiar to most spine surgeons and both require only a single approach. These two procedures have therefore recently become the most popularly used techniques to treat spinal disorders. They are associated with a few differences with regard to the actual surgical technique, however. The TLIF requires a complete unilateral facetectomy and spares the contralateral lamina, facets, and pars interarticularis. The PLIF procedure requires a bilateral laminotomy as well as partial, and at times complete, facetectomy to place an adequate interbody spacer device. The TLIF implants are usually semilunar and only one is implanted, whereas those used for PLIF are cubic or cylindrical in shape and are placed in pairs resulting in a greater surface of bone graft and better distribution of loads. With the PLIF procedure, a portion of the posterior longitudinal ligament (PLL) is cut to position the interbody space devices, whereas the TLIF procedure preserves most of the PLL [68].
On the other hand, the diskectomy and clearing of end plates performed during PLIF procedure via bilateral approach are probably more complete and have better quality compared to TLIF.
From a biomechanical consideration perspective, Sim et al. showed that the PLIF provides a higher immediate stability than the TLIF, especially for the lateral bending motion. The implant position in the disk space, however, is not an important factor for the immediate stability of a single-level TLIF. If the TLIF implant is placed further anteriorly, although there were no statistically significant differences in this study, there is a tendency for this position to be more stable [68].
Another difference between PLIF and TLIF is that performing a TLIF at the L5–S1 segment is quite difficult due to the pelvic position that prevents the good positioning of the cage in the disk space.
8 Tips and Tricks
-
During exposure, a goal should be to avoid dural tear and nerve roots injury that can result from the manipulation of instruments and also to reduce the amount of bleeding coming from the canal (epidural veins).
-
During the procedure when the nerve roots are retracted to prepare the disk space, there is most often significant bleeding that arises from the emissary vein of the vertebral body. This may be difficult to stop. The most effective strategy is to clog the vein with haemostatic gaze and thus to perform an embolisation of the vein.
-
When preparing the disk space, the vertebral bodies must be maximally distracted in order to put the higher cage (10–12 mm of height in most cases). The distractor used should be 1 or 2 mm higher compared to the implant to facilitate the insertion of the cage on the contralateral side. It also facilitates the decompression of the nerve roots in the foramen and confers maximum stability to the spine – thus avoiding the retropulsion of the cage.
-
Morselised and perfectly cleaned bone is compressed into the cage, but the area of fusion is quite reduced. To enhance the chance of fusion, we also put morselised bone into the anterior disk space before inserting the cages.
-
The five key points to restore lumbar lordosis during PLIF procedure are as follows:
-
1.
Patient positioning
-
2.
Use of lordotic cages
-
3.
Optimal size for the cages
-
4.
Optimal AP placement of the cages (placed at the anterior part of the disk space)
-
5.
Posterior inter-pedicular compression after cage insertion
-
1.
9 Clinical Cases
Clinical cases are illustrated in Figs. 31.4, 31.5, 31.6 and 31.7.
A 56-year-old man who underwent an operation for lumbar stenosis via an L3–L4 PLIF procedure that suffered postoperatively from left cruralgia. An emergency CT scan was performed, which demonstrated morselised bone graft located in the left recess (red arrows). The patient underwent immediate reoperation to decompress the nerve root and remove the bone graft that had migrated into the canal
References
Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. Indications, operative technique, after care. J Neurosurg. 1953;10:154–68.
Collis JS. Total disc replacement: a modified posterior lumbar interbody fusion. Report of 750 cases. Clin Orthop Relat Res. 1985;193:64–7.
Cole CD, Mc Call TD, Schmidt MH, et al. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2:118–26.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology. J Neurosurg Spine. 2055;2:637–8.
Burns BH. An operation for spondylolisthesis. Lancet. 1933;1:1233.
Briggs H, Milligan P. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Surg. 1944;26:125–30.
Jaslow I. Intracorporeal bone graft in spinal fusion after disc removal. Surg Gynecol Obstet. 1946;82:215–22.
Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop. 1986;203:7–17.
Lin PM. Posterior lumbar interbody fusion technique. Complications and pitfalls. Clin Orthop. 1985;193:90–102.
Branch Jr CL. The case of posterior lumbar interbody fusion. Clin Neurosurg. 1996;43:252–67.
Takeda M. Experience in posterior lumbar interbody fusion. Unicortical versus bicortical autologous grafts. Clin Orthop. 1985;193:120–6.
Butts M, Kuslick S, Bechtold J. Biomechanical analysis of a new method for spinal interbody fusion. Boston: American Society of Mechanical Engineers; 1987.
Bagby G. The Bagby and Kuslich (BAK) method of lumbar interbody fusion. Spine. 1999;24:1857.
Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931–4.
Brantigan JW, Steffee AD, Geiger JM. A carbon fiber implant to aid interbody lumbar fusion. Mecha Test Spine. 1991;16:S277–82.
Steffee AD, Sitkowski DJ. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988;227:99–102.
Brodke DS, Dick JC, Kunz DN, et al. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine. 1997;22:26–31.
Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Rheumatol. 2010;24:253–65.
Ciricillo SF, Weinstein PR. Lumbar spinal stenosis. West J Med. 1993;158(2):171–7.
Wassenaar M, Van Rijn RM, Van Tulder MW, et al. Magnetic resonance imaging for diagnosis lumbar spinal pathology in adult patients with low back pain or sciatica: a diagnostic systematic review. Eur Spine J. 2012;21:220–7.
Kalff R, Ewald C, Waschke A, et al. Degenerative lumbar spinal stenosis in older people: current treatment options. Dtsch Arztebl Int. 2013;110(37):613–24.
Lee CK, Langrana NA. Lumbosacral spinal fusion: a biomechanical study. Spine. 1984;9:574–81.
Weatherley CR, Prickett CF, O’Brein JP. Discogenic pain persisting despite solid posterior fusion. J Bone Joint Surg Br. 1986;68:142–3.
Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25:853–7.
Abe E, Nickel T, Buttermann GR, et al. Lumbar intradiscal pressure after posterolateral fusion and pedicle screw fixation. Tohoku J Exp Med. 1998;186:243–53.
Blumenthal SL, Baker J, Dossett A, et al. The role of anterior lumbar fusion for internal disc disruption. Spine. 1988;13:566–9.
Gill K, Blumenthal SL. Functional results after anterior lumbar fusion at L5–S1 in patients with normal fusion at L5–S1 in patients with normal and abnormal MRI scans. Spine. 1992;17:940–2.
Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20:356–61.
Linson MA, Williams H. Anterior and combined anteroposterior fusion for lumbar disc pain. A preliminary study. Spine. 1991;16:143–5.
Newman MH, Grinstead GL. Anterior lumbar interbody fusion for internal disc disruption. Spine. 1992;17:831–3.
Schechter NA, France MP, Lee CK. Painful internal disc derangements of the lumbosacral spine: discographic diagnosis and treatment by posterior lumbar interbody fusion. Orthopedics. 1991;14:447–51.
Derby R, Howard MW, Grant JM, et al. The ability of pressure-controlled discography to predict surgical and nonsurgical outcomes. Spine. 1999;24:364–71.
Parker LM, Murrell SE, Boden SD, et al. The outcome of posterolateral fusion in highly selected patients with discogenic low back pain. Spine. 1996;21:1909–16.
Wetzel FT, LaRocca SH, Lowery GL, et al. The treatment of lumbar spinal pain syndromes diagnosed by discography. Lumbar arthrodesis. Spine. 1994;19:792–800.
Blumenthal SL, Ohnmeiss DD. Intervertebral cages for degenerative spinal diseases. Spine J. 2003;3:301–9.
Johnsson KE, Redlund-Johnell I, Udén A, et al. Preoperative and postoperative instability in lumbar spinal stenosis. Spine. 1989;14:591–3.
Johnsson KE, Willner S, Johnsson K. Postoperative instability after decompression for lumbar spinal stenosis. Spine. 1986;11:107–10.
Mummaneni PV, Haid RW, Rodts GE. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:24–30.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2:679–85.
Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 10: fusion following decompression in patients with stenosis without spondylolisthesis. J Neurosurg Spine. 2005;2:686–91.
Zdeblick TA. The treatment of degenerative lumbar disorders. A critical review of the literature. Spine. 1995;20:126S–37.
Esses SI, Botsford DJ, Kostuik JP. The role of external spinal skeletal fixation in the assessment of low-back disorders. Spine. 1989;14:594–601.
Stokes IA, Frymoyer JW. Segmental motion and instability. Spine. 1987;12:688–91.
Mooney V, Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976;115:149–56.
Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–7.
Gödde S, Fritsch E, Dienst M, et al. Influence of cage geometry on sagittal alignment in instrumented posterior lumbar interbody fusion. Spine. 2003;28(15):1693–9.
Perrin G. Surgical treatment of severe lateral and foraminal spine degenerative stenosis. In: Robert G, Marek S, editors. Lumbar spinal stenosis. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 313–20.
Hosono N, Nakameta M, Makino T, et al. Perioperative complications of posterior lumbar interbody fusion for nonisthmic spondylolisthesis: analysis of risk factors. J Neurosurg Spine. 2008;9:403–7. Clinical article.
Deyo RA, Ciol MA, Cherkin DC, et al. Lumbar spinal fusion. A cohort study of complications, reoperations and resources use in the medicare population. Spine. 1993;18:1463–70.
Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4:304–9.
Makino T, Hosono N, Mukai Y, et al. Perioperative complications of patients undergoing two-level posterior lumbar interbody fusion for degenerative lumbar diseases. Rinsho Shinkei Geka. 2008;43:459–63.
Carreon LY, Puno RM, Dimar II JR, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2000;85:2089–92.
Sakaura H, Yamashita T, Miwa T, et al. Outcomes of 2-level posterior lumbar interbody fusion for 2-level degenerative lumbar spondylolisthesis. J Neurosurg Spine. 2013;19:90–4. Clinical article.
Mehta VA, McGirt MJ, Garcés Ambrossi GL, et al. Trans-foraminal versus posterior lumbar interbody fusion: comparison of surgical morbidity. Neurol Res. 2011;33(1):38–42.
Perrin G, Barrey C. Chapter 22. When should PLIF (intervertebral fusion) be done in lumbar canal decompression for degenerative stenosis? In: Advanced concepts for lumbar degenerative diseases. Rio de Janeiro: Dilivros; 2010. p. 259–72.
Postacchini R, Cinotti G, Postacchini F. Injury to major abdominal vessels during posterior lumbar interbody fusion. A case report and review of the literature. Spine J. 2013;13:7–13.
Kimura H, Shikata J, Odate S, et al. Risk factors for cage retropulsion after posterior lumbar interbody fusion. Analysis of 1070 cases. Spine. 2012;37(13):1164–9.
Ekman P, Moller H, Shalabi A, et al. A prospective randomized study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine. 2009;18:1175–86.
Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–44.
Cho KS, Kang SG, Yoo DS, et al. Risk factors and surgical treatment for symptomatic adjacent segment degeneration after lumbar spinal fusion. J Kor Neurosurg Soc. 2009;46:425–30.
Sears WR, Sergides IG, Kazemi N, et al. Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J. 2011;11:11–20.
Miwa T, Sakura H, Yamashita T, et al. Surgical outcomes of additional posterior lumbar interbody fusion for adjacent segment disease after single-level lumbar interbody fusion. Eur Spine J. 2013;22(12):2864–8.
Deyo RA, Martin BI, Kreuter W, et al. Revision surgery following operation for lumbar stenosis. J Bone Joint Surg Am. 2011;93:1979–86.
McLaughlin MR, Haid Jr RW, Rodts GE, et al. Posterior lumbar interbody fusion: indications, techniques and results. Clin Neurosurg. 2000;47:514–27.
Harms JG, Jeszenszky D. The unilateral, transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol. 1998;6:88–99.
Hee HT, Castro Jr FP, Madj ME, et al. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14:533–40.
Humphreys SC, Hodges SD, Patxardhan AG, et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine. 2001;26:567–71.
Sim HB, Murovic JA, Cho BY, et al. Biomechanical comparison of single level posterior versus transforaminal lumbar interbody fusion with bilateral pedicle screw fixation: segmental stability and the effect on adjacent motion segments. J Neurosurg Spine. 2010;12:700–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Launay, O., Perrin, G., Barrey, C. (2016). Instrumented PLIF in Lumbar Degenerative Spine: Principles, Indications, Technical Aspects, Results, Complications and Pitfalls. In: Pinheiro-Franco, J., Vaccaro, A., Benzel, E., Mayer, H. (eds) Advanced Concepts in Lumbar Degenerative Disk Disease. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-47756-4_31
Download citation
DOI: https://doi.org/10.1007/978-3-662-47756-4_31
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-47755-7
Online ISBN: 978-3-662-47756-4
eBook Packages: MedicineMedicine (R0)