Abstract
Vagus nerve-preserving gastrectomy was first introduced in 1991 for the purpose of alleviating postgastrectomy syndrome. There are three main parts of the vagus nerve that are preserved in vagus nerve-preserving gastrectomy: the hepatic branch, the celiac branch, and the hepatic plexus. Vagus nerve-preserving gastrectomy was first introduced in open surgery, but, as minimally invasive surgery became more pervasive, it has recently also been performed as a minimally invasive procedure, the magnified view of which facilitates identification of the vagus nerve, as well as a precise procedure. At present, there are not enough reports to show conclusive evidence for the feasibility and efficacy of vagus nerve-preserving gastrectomy. However, based on currently available reports, it can be said that vagus nerve-preserving gastrectomy is a technically feasible and oncologically acceptable procedure as long as the proper inclusion criteria are applied, and it brings about positive short-term and long-term outcomes for patients, which improve the patients’ postoperative quality of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
The radical surgical treatment of gastric cancer requires resection of a large portion of the stomach, as well as regional lymphadenectomy. It is well known that gastric resection and reconstruction of the gastrointestinal tract result in a variety of functional and physiological disorders such as dumping syndrome, malabsorption, diarrhea, and so on. These unpleasant alimentary and/or systemic symptoms are collectively referred to as postgastrectomy syndrome [1]. Vagus nerve preservation, which was first introduced in 1991, is one way to alleviate postgastrectomy syndrome [2]. In this chapter, we will describe the surgical anatomy, operational procedure, and postoperative outcomes of vagus-preserving gastrectomy (VPG).
Surgical Anatomy
There are three main parts of the vagus nerve in gastric surgery: (1) the hepatic branch from the anterior vagal trunk, (2) the celiac branch from the posterior vagal trunk, and (3) the hepatic nerve plexus (Fig. 18.1).
Hepatic Branch
The anterior vagal trunk bifurcates into the hepatic branch and the anterior gastric branch at the level of the right cardia. The hepatic branch consists of a few nerves traversing through the compact part of the lesser omentum caudal to the left lobe of the liver, and it joins the hepatic nerve plexus. The anterior gastric branch runs along the lesser curvature, innervating the anterior wall of the stomach from the cardia to the gastric body.
Celiac Branch
The posterior vagal trunk runs behind the abdominal esophagus through the gastropancreatic folds, bifurcating into the celiac branch and the posterior gastric branch. The celiac branch joins the right and left celiac ganglia. The celiac branch and the left gastric artery are often fused after they are joined together.
Hepatic Nerve Plexus
The hepatic nerve plexus arises from the celiac nerve plexus, which consists of the vagus nerve from the celiac ganglia and the sympathetic nerve from the greater splanchnic nerve. The nerve plexus surrounds the common and proper hepatic arteries, especially dorsal to their cranial circular location.
Operational Procedure
VPG was originally introduced in open surgery [2], but recently it has also been performed as a minimally invasive approach, and the magnified view of laparoscopic and robotic surgery facilitates identification of the vagus nerve, as well as a precise procedure. In this section, the laparoscopic procedure will be presented as described by Kojima et al. in their article [3].
The surgical procedure begins with port insertion and lymph node dissection around the greater omentum and infrapyloric area, followed by duodenal resection.
When the lesser omentum is resected, the hepatic branch from the anterior vagal trunk, which runs across the compact part of the lesser omentum near the liver, should be identified and preserved.
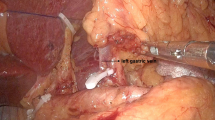
The posterior vagal trunk and celiac branches that run along the posterior wall of the abdominal esophagus and down to the celiac ganglion are identified. With exposure of the diaphragm’s right crus and the anterolaterodorsal side of the abdominal esophagus, the posterior vagal trunk is isolated and retracted toward the right side with a vessel loop (Fig. 18.2).
After exposure of the common hepatic artery and gastropancreatic folds, the No. 8a lymph nodes, located along the common hepatic artery, are dissected. In this step, the hepatic nerve plexus that runs along the common hepatic artery must be preserved. Lymph nodes along the left gastric artery (No. 7) and celiac artery (No. 9) are dissected toward the position where the celiac branch of the posterior vagal trunk joins the left gastric artery (Fig. 18.3). The left gastric artery is divided with double clips after isolation of the celiac branches. The nerves of Latarjet are divided from the celiac branches, along with the perigastric lymph nodes. Retraction of the celiac branches toward the right side facilitates this procedure. The vagus nerve-sparing lymph node dissection is then completed (Fig. 18.4). After this procedure, resection of the stomach and reconstruction are performed.
Short-Term Results
As for the perioperative results, although only one article by Sakuramoto et al. reported that longer operative time and greater intraoperative bleeding were seen in the VPG group [4], two other articles showed comparable perioperative results, such as blood loss, operative time, morbidity, and postoperative hospital stay, between VPG and vagus nerve-resection gastrectomy (VRG) groups [3, 5]. The time to first flatus was also earlier in the VPG group than in the VRG group in some reports [5,6,7].
Two articles investigated the hormonal effects of vagus nerve preservation. Takiguchi et al. found that, on postoperative day 7, although plasma fasting ghrelin, which is a brain-gut peptide with GH-releasing and appetite-inducing properties, decreased significantly in both the VPG and VRG groups by about 50% of the baseline values, the postprandial reduction of ghrelin was maintained only in the VPG group [5]. Kim et al. reported that a lesser increase of peptide YY [8], acting as a satiety signal, was observed in the VPG group compared to the VRG group. They also concluded that less body weight loss was observed 1 month after surgery in the VPG group, which was related to less increase of peptide YY [9].
Long-Term Results
One article by Kim et al. showed less diarrhea 3 and 12 months after surgery and less appetite loss at 12 months in the VPG group. Yamada et al. reported that, at 1-year follow-up, the incidences of dumping syndrome and gallstone formation were significantly lower and significantly more residual food was seen on endoscopic examination in the VPG group than in the VRG group, although other clinical symptoms, endoscopic findings, and nutritional status were similar between the two groups. Uyama et al. also reported a lower incidence of diarrhea and gallstone formation in the VPG group with a median follow-up period of 23 months [10].
Two articles showed the data of VPG for up to 5 years. In the article by Inokuchi et al., the incidence of clinical symptoms such as gastroesophageal reflux, early dumping syndrome, and chronic diarrhea, endoscopic findings using the RGB score [11], and nutritional status were similar between the VPG and VRG groups, but gallstone formation was significantly less common in the VPG group 5 years after surgery [12]. Kim et al. compared the oncological outcomes between the two groups, concluding that there were no significant differences between the two groups in cancer recurrence and death over 5 years of follow-up [7].
Conclusion
Up to now, although there have been few reports of the feasibility and efficacy of VPG, and the results have varied article to article, based on the currently available evidence, it can be concluded that (1) it is a technically feasible and oncologically acceptable procedure as long as the appropriate inclusion criteria are applied; and (2) several positive postoperative outcomes, such as earlier first flatus after surgery, less diarrhea, less body weight loss, less appetite loss, less incidence of early dumping syndrome, and less gallstone formation, can be achieved by the procedure, which improves the patients’ postoperative quality of life.
In order to further elucidate these issues, more research with larger sample sizes and diverse populations is needed, as well as research examining the molecular biological mechanisms that can explain the efficacy of vagus nerve preservation.
References
Carvajal SH, Mulvihill SJ. Postgastrectomy syndromes: dumping and diarrhea. Gastroenterol Clin N Am. 1994;23(2):261–79. Epub 1994/06/01.
Miwa K, Kinami S, Sato T, Fujimura T, Miyazaki I. Vagus-saving D2 procedure for early gastric carcinoma. Nihon Geka Gakkai Zasshi. 1996;97(4):286–90. Epub 1996/04/01.
Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. Functional evaluation after vagus-nerve-sparing laparoscopically assisted distal gastrectomy. Surg Endosc. 2008;22(9):2003–8. Epub 2008/07/03.
Sakuramoto S, Kikuchi S, Kuroyama S, Futawatari N, Katada N, Kobayashi N, et al. Laparoscopy-assisted distal gastrectomy for early gastric cancer: experience with 111 consecutive patients. Surg Endosc. 2006;20(1):55–60. Epub 2005/11/12.
Takiguchi S, Hiura Y, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, et al. Preservation of the celiac branch of the vagus nerve during laparoscopy-assisted distal gastrectomy: impact on postprandial changes in ghrelin secretion. World J Surg. 2013;37(9):2172–9. Epub 2013/05/07.
Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K. Efficacy of celiac branch preservation in Roux-en-y reconstruction after laparoscopy-assisted distal gastrectomy. Surgery. 2011;149(1):22–8. Epub 2010/04/27.
Kim SM, Cho J, Kang D, Oh SJ, Kim AR, Sohn TS, et al. A randomized controlled trial of vagus nerve-preserving distal gastrectomy versus conventional distal gastrectomy for postoperative quality of life in early stage gastric cancer patients. Ann Surg. 2016; 263(6):1079–84. Epub 2016/01/05.
Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145(6):2585–90. Epub 2004/03/16.
Kim HH, Park MI, Lee SH, Hwang HY, Kim SE, Park SJ, et al. Effects of vagus nerve preservation and vagotomy on peptide YY and body weight after subtotal gastrectomy. World J Gastroenterol. 2012;18(30):4044–50. Epub 2012/08/23.
Uyama I, Sakurai Y, Komori Y, Nakamura Y, Syoji M, Tonomura S, et al. Laparoscopic gastrectomy with preservation of the vagus nerve accompanied by lymph node dissection for early gastric carcinoma. J Am Coll Surg. 2005;200(1):140–5. Epub 2005/01/06.
Nagano H, Ohyama S, Sakamoto Y, Ohta K, Yamaguchi T, Muto T, et al. The endoscopic evaluation of gastritis, gastric remnant residue, and the incidence of secondary cancer after pylorus-preserving and transverse gastrectomies. Gastric Cancer. 2004;7(1):54–9. Epub 2004/03/31.
Inokuchi M, Sugita H, Otsuki S, Sato Y, Nakagawa M, Kojima K. Long-term effectiveness of preserved celiac branch of vagal nerve after Roux-en-Y reconstruction in laparoscopy-assisted distal gastrectomy. Dig Surg. 2014;31(4–5):341–6. Epub 2014/12/17.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Germany, part of Springer Nature
About this chapter
Cite this chapter
Nakagawa, M., Kojima, K. (2019). Vagus-Preserving Gastrectomy. In: Noh, S., Hyung, W. (eds) Surgery for Gastric Cancer. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45583-8_18
Download citation
DOI: https://doi.org/10.1007/978-3-662-45583-8_18
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45582-1
Online ISBN: 978-3-662-45583-8
eBook Packages: MedicineMedicine (R0)