Abstract
Biorefineries consider lactic acid as one of the most promising platform chemicals which are being extensively used in a wide range of food and nonfood applications. Since lactic acid is produced via biotechnological processes, the microbial strains are in the focus of interest, besides all the other aspects of raw materials, fermentation mode, etc.
Microorganisms, which are able to produce lactic acid and organic lactates, are systematically classified and morphologically and biochemically characterized, and their different metabolic pathways for the formation of various lactic acid enantiomers are described in detail. The genera Lactobacillus and Bifidobacterium are regarded as well as the order of the Bacillales. In addition, the important individual yeasts, moulds and other bacteria were also characterized.
The present review work is summarized on the fermentation systems used for the biotechnological production, the various raw materials and applications of lactic acid and organic lactates. Future developments in this area with respect to the strain selection and modifications, genetic-engineering approaches, carbohydrate sources and their pretreatment, fermentation techniques and the downstream processing options are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The biotechnological production of lactic acid (2-hydroxypropionic acid) as an example of a platform chemical for the subsequent processing (e.g. into PLA) is carried out in technical reactors by using a suitable strain. LA can be produced by several microorganisms classified into bacteria, fungi, yeast, cyanobacteria and algae (Abdel-Rahman et al. 2013; Thongchul 2013). Besides the wide group of Lactobacillus (Antonio et al. 1996; Hofvendahl and Hahn-Hägerdahl 1997; Berry et al. 1999; Fu and Mathews 1999; Kwon et al. 2001), other bacteria like Bacillus (Payot et al. 1999; Danner et al. 2002; Patel et al. 2004), Enterococcus (Walczak et al. 2012), Lactococcus (Ramchandran et al. 2012), Pediococcus (Zhao et al. 2013b), Streptococcus (Tang et al. 2013) and filamentous fungi (Martak et al. 2003), especially Rhizopus oryzae (Yin et al. 1997; Bai et al. 2004), were also used as production strains. These microorganisms convert easily monosaccharides like glucose or fructose into cell mass and LA. LA formation and cell growth are closely coupled in LA fermentation (Zacharof and Lovitt 2013). An overview about the utilization of different renewable resources for LA fermentation, other microorganisms and yields depending on several process parameters was given by Hofvendahl and Hahn-Hägerdal (2000) and Castillo Martinez et al. (2013).
Whereas the fermentation of glucose can be carried out efficiently, the bioconversion of the pentose fraction out of lignocellulosic feedstocks and residues presents a challenge. A lot of attention has therefore been focused on genetically engineering strains that can efficiently utilize both glucose and pentose and convert them to useful compounds. The metabolic engineering objectives so far have focused on higher yields, productivities and expanding the substrate and product spectra (Aristidou and Penttilä 2000; Hua et al. 2006; Singh et al. 2006; Ilmen et al. 2007; Adler et al. 2012).
For the industrial production of l-(+)-LA, it is necessary to provide cheap carbon sources that can be easily metabolized by lactic acid bacteria (LAB) and to obtain the optimal conditions of fermentation with higher yields and production rates (John et al. 2007).
The different microbes have achieved one or more improvements over the others, such as a broader substrate range, improved yield and productivity, reduction of nutritional requirements or improved optical purity of LA (Abdel-Rahman et al. 2013). In view of the above-mentioned several complex substrates, also the use of mixed cultures in fermentation may provide useful combinations of metabolic pathways for the utilization of complex raw materials containing a mixture of carbohydrates (Cui et al. 2011; Trontel et al. 2011; Secchi et al. 2012). Several genetic-engineering approaches have been exploited in order to improve performance, LA yield and optical purity by various microbial producers (Nagamori et al. 2013; Wu et al. 2013; Zhao et al. 2013a). An extensive review by Okano et al. (2010) provides a broad collection of genetically engineered microorganisms for LA production including their characteristics and applicability for fermentation processes, respectively.
2 Lactic Acid-Forming Bacteria
Important LA-forming bacteria include the genera Lactobacillus and Bifidobacteria. Also the genera Bacillus, Lactococcus, Streptococcus, Pediococcus and Enterococcus are able to produce LA. There are also reports about the LA fermentation by some yeast and fungi.
2.1 The Genus Lactobacillus
Lactobacillus (L.) is a very heterogeneous genus, comprising species with a large diversity of phenotypic, biochemical and physiological features. More than 70 species are recognized, and all are able to convert carbohydrates into LA. The most important LA-forming bacteria belong to this genus. These include L. acidophilus, L. brevis, L. casei, L. delbrueckii, L. fermentum, L. helveticus, L. plantarum, L. paracasei and L. rhamnosus.
2.1.1 Short Characteristics of the Genus Lactobacillus
The genus can be divided into three subgroups based on their type of fermentation: obligate homofermentative, obligate heterofermentative and facultative heterofermentative. Important features of this genus are summarized in Table 1.
2.1.2 Carbohydrate Fermentation of Lactobacilli
The metabolism of carbohydrate utilization depends both on the kind of the sugar (e.g. hexoses, pentoses) and from the type of fermentation by the LAB. General, the fermentation types differ in the utilization of hexoses and pentoses.
2.1.2.1 Fermentation of Hexoses
2.1.2.1.1 Obligate Homofermentative LAB
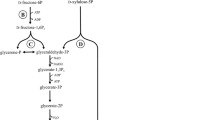
In principle the obligate homofermentative LAB converted hexoses to lactate by the Embden-Meyerhof-Parnas (EMP) glycolytic pathway (Fig. 1) (Wood 1961; Kandler 1983; von Wright and Axelsson 2012).
Glucose is first broken down in glycolysis to pyruvate. Pyruvate is reduced by the enzyme lactate dehydrogenase to lactate, which is present under physiological conditions in dissociated lactate ions and protons. During the glycolysis of glucose or fructose per molecule, two molecules lactate and ATP are formed, so that the sum of the equation homofermentative LA fermentation is
Pentoses and gluconate were not fermented by this pathway because of lack of enzyme phosphoketolase. This type of fermentation includes some species of the genus Lactobacillus. The important LA producers in this genus are L. acidophilus and L. delbrueckii (Hofvendahl and Hahn-Hägerdal 2000; Kwon et al. 2001).
2.1.2.1.2 Obligate Heterofermentative LAB
The pathway of obligate heterofermentative LA fermentation is formed by LAB, which had a lack of enzyme aldolase. This enzyme is required for the glycolysis of fructose-1,6-bisphosphate into the two phosphotriose dihydroxyacetone and glyceraldehyde. This type of fermentation includes organisms of the genera Leuconostoc, Weissella and Oenococcus as well as some species of the genus Lactobacillus (Hammes et al. 1991; Hammes and Vogel 1995). Important obligate heterofermentative LA producers are L. brevis, L. fermentum and L. reuteri. These bacteria can degrade hexoses in the phosphogluconate pathway (Fig. 2) to lactate, ethanol and CO2 or furthermore to acetate.
During the fermentation of glucose per molecule, one molecule lactate and ATP are formed, so that the sum of the equation of heterofermentative LA fermentation is
Other hexoses (such as mannose or fructose) enter the pathway as either glucose-6-phosphate or fructose-6-phosphate. Fructose, however, is reduced not only to lactate and CO2 but also to mannitol and acetate:
For the fermentation of galactose, there are two different pathways, depending on the form it enters in the cells (von Wright and Axelsson 2012). If galactose enters the cells as galactose-6-phosphate, it will ferment also to pyruvate however via the tagatose-6-phosphate pathway (Bisset and Anderson 1974) (Fig. 3a). As a free galactose, imported in the cells by a specific permease, it will ferment via glycolysis to pyruvate by the so-called Leloir pathway (Kandler 1983) (Fig. 3b).
2.1.2.2 Fermentation of Pentoses
Many LAB are able to ferment pentoses. They can only ferment heterofermentatively by entering the phosphogluconate pathway as either ribulose-5 phosphate or xylulose-5 phosphate (Kandler 1983) (Fig. 4). Pentoses (such as arabinose, ribose, xylose) are converted into lactate and acetate; CO2 is not produced. The sum of the equation is
However, there are reports of homofermentative fermentation of pentoses by engineered strains of L. plantarum (Okano et al. 2009a, b).
2.1.2.2.1 Facultative Heterofermentative LAB
LAB, which can ferment hexoses and pentoses, belong to the group of facultative heterofermentative LAB. Hexoses are fermented by the EMP glycolytic pathway to lactate. Under glucose limitation some species can produce also ethanol, acetic acid and formic acid (Hammes and Hertel 2009). Pentoses enter this pathway and are fermented to LA and acetic acid. The important LA producers belong to this fermentation type, e.g. L. casei, L. plantarum and L. paracasei.
2.1.2.3 Fermentation of Other Important Carbohydrates
For the production of LA, the most important carbohydrates are the disaccharides lactose, maltose and sucrose. In principle they are split enzymatically into their monosaccharide, and then they enter the various pathways.
Lactose can enter the cells in two ways, either by means of a specific permease or as lactose-6-phosphate (von Wright and Axelsson 2012). In the first case, lactose is split into glucose and galactose, which can enter the major fermentation pathway (e.g. Streptococcus thermophilus). In the second case, lactose-6-phosphate is cleaved to glucose and galactose-6-phosphate. While glucose is processed by the glycolytic pathway, the galactose-6-phosphate enters the tagatose-6-phosphate pathway (e.g. Lactococcus lactic). In some cases both systems can coexist (Wood and Holzapfel 1995; von Wright and Axelsson 2012).
The fermentation of maltose is known; in lactococci the permease system is active (Sjöberg and Hahn-Hägerdahl 1989), while in various strains of L. sanfranciscensis, maltose is converted to glucose-1-phosphate and glucose (von Wright and Axelsson 2012).
In general, sucrose enters the cells also by a specific permease system, and it is split into glucose and fructose. Also it was reported that lactococci can convert lactose into glucose-6-phosphate and fructose (von Wright and Axelsson 2012).
Starch is fermented only by very few homofermentative species such as L. amylophilus (Altaf et al. 2006; Vishnu et al. 2006), L. manihotivorans (Morlon-Guyot et al. 1998; Ohkouchi and Inoue 2006) and L. amylovorus (Zhang and Cheryan 1991; Hammes and Hertel 2009). However, of these species L. amylophilus and L. manihotivorans are important for the production of LA (Altaf et al. 2007; Yen and Kang 2010; Son and Kwon 2013).
It is reported that the strain Lactobacillus plantarum SW14 has a potential for LA production directly from cassava starch under laboratory conditions (Bomrungnok et al. 2012). Also Enterococcus faecium was already described for the direct fermentation of starch containing feedstocks (Shibata et al. 2007; Nolasco-Hipolito et al. 2012).
2.1.3 Enantiomers of LA
An asymmetric C-atom LA exists in two enantiomeric forms l (+) and d (−) and in a racemic form (dl). The stereoisomeric composition of the formed LA by the various species of lactobacilli is enzymatically determined. The configuration of the LA l (+) or d (−) depends on the stereospecificity of the lactate dehydrogenase in the cells. Racemate (dl) formed either when d-(−)- and l-(+)-dehydrogenases are present in the same cells or when an inducible lactate racemate reacts with a constitutive l-(+)-lactate dehydrogenase (Hammes and Hertel 2009).
However Setter and Stetter and Kandler (1973) reported that the d-(−)-lactate formers produce d-(−)-lactate exclusively, whereas all l-(+)-lactate formers always produce a few percent of the other isomer. This is caused by the presence of an NAD-dependent d-lactate dehydrogenase of very low activity.
2.1.4 Characteristics of the Most Important Lactic Acid Producers
For the selection of strains for the LA production, the fermentation type, the fermented carbohydrates and the temperature of growth are very important. These typical facts for the most used LA producers in the genus Lactobacillus are summarized separately in Tables 2, 3 and 4 after the formation of the various LA enantiomer.
2.2 The Genus Bifidobacterium
There are many references of the use of bifidobacteria for LA production. Many investigations have shown that bifidobacteria promote health preferably via their application in the food industry (Shene et al. 2005; Popa and Ustunol 2011). From that perspective the interest has been focused more on the fermentation performance in combination with typical carbohydrates containing foodstuff (Buruleanu et al. 2011; Watson et al. 2012) than an industrial LA production (Li et al. 2008). In the genus Bifidobacterium (B.), B. adolescentis, B. animalis, B. bifidum, B. breve, B. longum and B. thermophilum are important LA producers.
2.2.1 Short Characteristics of the Genus Bifidobacterium
Important features of this genus are summarized in Table 5.
2.2.2 Carbohydrate Fermentation
Bifidobacteria ferment various types of sugars. Lactose, galactose and sucrose are metabolized by a large number of species. The bacteria lack enzyme aldolase (fructose-1,6-bisphosphate-aldolase), like other heterofermentative LAB, but they metabolize sugars via their own complicated pathway so-called bifid shout (De Vries and Stouthamer 1967) (Fig. 5). Hexoses are degraded via phosphoric esters of the hexoses, erythrose, glyceraldehyde and pentoses. At two sites acetyl-phosphate is cleaved and 2-glyceraldehyde-3-phosphate is formed. This is metabolized by the Embden-Meyerhof-Parnas pathway to l-(+)-lactic and acetic acid in the ratio 2:3 (Sgorbati et al. 1995). Gas is not produced. This pathway has a 25 % higher yield of ATP (2.5 moles per mole of glucose) as the homofermentative LA fermentation (2 moles per mole of glucose). The sum of the fermentation of glucose is
Galactose is fermented by the Leloir pathway (Fig. 3) because the enzymes for this are basically available in glucose-grown cells. On this pathway gas is formed (Scardovi 1986).
The fermented carbohydrates from the most important species of the genus Bifidobacterium are summarized in Table 6.
2.3 The Order Bacillales
LA production is traditionally associated with non-spore-forming bacteria. In addition to these organisms, a number of LA-producing spore-forming bacteria have been described. They are allocated to the genera Bacillus and Sporolactobacillus. The most important LA producer in the genus Bacillus is the species Bacillus (B.) coagulans and in the genus Sporolactobacillus (S.) the species S. inulinus and S. laevolacticus.
2.3.1 The Genus Sporolactobacillus
The species S. inulinus and S. laevolacticus belong to the genus Sporolactobacillus, the only genus of the family of Sporolactobacillaceae. Important features of this genus are summarized in Table 7.
The species S. inulinus (Fukushima et al. 2004; Wang et al. 2011; Zheng et al. 2012) and S. laevolacticus (Mimitsuka et al. 2012; Li et al. 2013) are important d-(−)-LA producers particularly because of their ability to metabolize starch and inulin (Table 8).
2.3.2 Bacillus coagulans
A lot of species of the genus Bacillus (B.) are known for producing LA such as B. lentimorbus, B. popilliae, B. smithii, B. stearothermophilus, B. licheniformis and B. subtilis (Thomas et al. 1979; Claus and Berkeley (1986) Fritze and Claus 1995; Abdel-Rahman et al. 2013). One of the most important species forming LA is B. coagulans (Ou et al. 2011; Wang et al. 2012; Tashiro et al. 2013; Ma et al. 2014). Important features of this species are summarized in Table 9.
Glucose is mainly fermented to l-(+)-LA and smaller amounts of 2.3-butanediol, acetoin, acetic acid and ethanol (Fritze and Claus 1995). Some strains of B. coagulans are able to ferment hexoses and pentoses homolactic to l-(+)-LA (Wang et al. 2012; Ou et al. 2011).
2.4 Other LA-Forming Microorganisms
Besides the previously described species of the genera Lactobacillus, Bifidobacteria, Bacillus and Sporolactobacillus, there are a few bacteria of different systematic positions that are significant for forming LA. The most important bacteria are Enterococcus faecium, Lactococcus lactis, Pediococcus acidilactici and Streptococcus thermophilus. Yeasts and fungi are also of increasingly economic importance such as Saccharomyces cerevisiae and Rhizopus oryzae. Since over 10 years there are reports from LA-producing E. coli strains. Most of them are described as metabolically engineered strains for the production of LA (Chang et al. 1999; Dien et al. 2001; Baba et al. 2006; Kim et al. 2013).
2.4.1 Enterococcus faecium
Compared to other LA-producing microorganisms, E. faecium does not play a major role for the industrial application, but it was already described for the direct fermentation of starch containing feedstocks (Shibata et al. 2007; Nolasco-Hipolito et al. 2012). Important features of E. faecium are summarized in Table 10.
2.4.2 Lactococcus lactis
From the family of Streptococcaceae, the species Lactococcus lactis (L. lactis) and Streptococcus thermophilus are particularly very important for LA production. The species of the genus Lactococcus differ from the other LAB by their pH and by their salt and temperature tolerance for growth (Table 9). Among LAB, L. lactis is the most extensively studied regarding its physiology, metabolic pathways and regulatory mechanisms. Its genome was the first LAB genome to be completely sequenced (Oliveira et al. 2005). Important features of L. lactis are summarized in Table 11.
Among the subspecies of L. lactis, L. lactis ssp. lactis (John et al. 2007) and L. lactis ssp. cremoris are most important for the production of LA (Ramchandran et al. 2012; Mukisa et al. 2012). Fermented carbohydrates of the important species are arranged in Table 12.
It is reported in the literature that L. lactis produce LA as the sole metabolic product at high dilution rates during continuous cultivations or at high glucose concentrations during batch growth (Benthin 1994; Melchiorsen et al. 2002). In contrast, growth at low dilution rates in continuous conditions or at low concentrations of glucose in batch conditions results in a mixed-acid fermentation, where formate, ethanol and acetate are produced in a molar ratio of 2:1:1 (Melchiorsen et al. 2001).
2.4.3 Pediococcus acidilactici
There have been not much applications published for P. acidilactici (Hofvendahl and Hahn-Hägerdal (2000), Giurca and Levin (1992, 1993)), but the new strain DQ2 has been recently described, which is characterized by high-temperature tolerance, high lignocellulose-derived inhibitor resistance and high LA production performance in combination with simultaneous saccharification and fermentation at high-solids loading of corn stover (Dao et al. 2013; Zhao et al. 2013b). Important features of P. acidilactici are summarized in Table 13.
2.4.4 Streptococcus thermophilus
The classification and nomenclature of streptococci have undergone significant changes over the years. In recent years, based on biochemical characteristics as well as RNA analysis, members of the genus Streptococcus have been reclassified into Lactococcus, Vagococcus, Enterococcus and Streptococcus. This genus includes both significant human and animal pathogens but also nonpathogenic species, in particular S. thermophilus, for the production of food (especially milk, milk products and cheese) and LA, particularly on lactose-rich substrates (Hofvendahl and Hahn-Hägerdal 2000; Pescuma et al. 2008; Secchi et al. 2012; Tang et al. 2013). Important features of S. thermophilus are summarized in Table 14.
S. thermophilus, like other streptococci that ferment carbohydrates, produce l-(+)-LA as well as minor amounts of acetic and formic acids, ethanol and CO2 (Whiley and Hardie 2009).
2.4.5 Saccharomyces cerevisiae
Recently, there are reports about LA-forming yeasts such as S. cerevisiae (Dequin and Barre 1994; Praphailong and Fleet 1997; Bianchi et al. 2001; Lu et al. 2012) and other Saccharomyces species, Zygosaccharomyces, Candida, Pichia and Kluyveromyces that have been engineered to produce LA (Abdel-Rahman et al. 2013). Important features of S. cerevisiae are summarized in Table 15.
2.4.5.1 Carbohydrate Utilization
Under aerobic conditions, S. cerevisiae respire glucose, producing H2O and CO2. Under anaerobic conditions, cells ferment sugars to ethanol and CO2. Galactose and fructose are shown to be two of the best fermenting carbohydrates. S. cerevisiae do not ferment pentoses such as arabinose, ribose and xylose and also disaccharides, e.g. lactose and cellobiose. Glucose oxidation under aerobic conditions provides more energy than fermentation. Therefore, the mass increases rate, and the cell division rate in oxidative decomposition of sugar is much higher than in fermentation. The ability of yeasts to use different sugars can differ depending on the oxygen conditions. So some strains cannot grow anaerobically on sucrose and trehalose. d-(−)-lactate formation is described by Stewart et al. (2013) (Fig. 6).
d-(−)-Lactate production by S. cerevisiae (mod. Stewart et al. 2013)
Experiments with S. cerevisiae under aerobic conditions demonstrated that during glucose consumption d-(−)-lactate forms as a result of methylglyoxal metabolism. The data suggest that increased glucose uptake by cells grown in a glucose-rich environment results in an increased generation of methylglyoxal with subsequent metabolism to d (−) lactate.
2.4.6 Rhizopus oryzae
It is known that the mould in the genera Rhizopus, Mucor and Monilia form LA (Yadav et al. 2011). Currently, there are increasing reports on the production of LA by various species of Rhizopus, especially R. oryzae but also R. arrhizus (Bai et al. 2008; Taskin et al. 2012; Wu et al. 2011). R. oryzae produce a wide spectrum of metabolites, in the form of enzymes, esters, organic acids, volatile materials, polymers and bioalcohols. Biodiesel can also be produced. The fungus is a rich source of LA but also fumaric acid and to a better extent malic acid (Ghosh and Ray 2011). R. oryzae is a filamentous fungus belonging to the traditional Zygomycota (Hibbett et al. 2007). Its important features are summarized in Table 16.
2.4.6.1 Formation of LA
In R. oryzae, all fermentable carbon sources are metabolized to pyruvate during glycolysis (Fig. 7).
Fermentation routes of glucose in R. oryzae (mod. Meussen et al. 2012)
The pyruvate is subsequently channelled to a number of pathways, including the pathways responsible for the formation of ethanol, lactate and fumarate. The dissolved oxygen in the medium influences the flow of pyruvate. Under anaerobic conditions, the carbon flow is directed towards the formation of ethanol, while under aerobic conditions, with excess of carbon substrate, the flow is directed towards organic acid production (Skory et al. 1998; Meussen et al. 2012). With a mutagenizing strain, they could express almost a tenfold increase in LA production compared to the parental strain. Also the formation of l-(+)-LA is possible by strains of R. oryzae or genetically modified strains (Bai et al. 2004 ; Park et al. 2004). R. oryzae is widely studied as a commercially perspective producer of l-(+)-LA (Miura et al. 2003; Yin et al. 1997; Yamane and Tanaka 2013), because the fungus cells possess better resistance to high concentrations of accumulated LA (Hamamci and Ryu 1994; Schepers et al. 2003). Moreover the cells need lower nutrient requirements compared to the commonly used bacterial producers (Hujanen et al. 2001; Kwon et al. 2000). The use of R. oryzae in immobilized form is one of the most efficient approaches to improve the LA production process for long-term acid production (Tay and Yang 2002; Efremenko et al. 2006; Yamane and Tanaka 2013).
2.4.7 Escherichia coli
There are many challenges for the industrial production of LA, and satisfying all these requirements is very difficult through the traditional use of LAB. Therefore, improving LAB via gene modification and using other microorganisms (e.g. E. coli) and yeast for LA production via gene modification have become an essential and interesting research area (Grabar et al. 2006; Okano et al. 2010). Several research activities are directed towards the recombinant improvement of LA yield and the optical purity since E. coli produces a mixture of organic acids and other metabolites (Zhou et al. 2012; Mazumdar et al. 2013; Zhao et al. 2013a). Important features of E. coli are summarized in Table 17.
2.4.7.1 Lactate Formation
E. coli can grow both under aerobic and anaerobic conditions. Like other species of the family Enterobacteriaceae, E. coli ferments glucose anaerobically via pyruvate to various acids and gas. Pyruvate can be formed either via glycolysis or via the EMP or the Entner-Doudoroff (ED) pathway (Fig. 8). However, the ED pathway is by E. coli of minor importance, because the enzymes of the ED pathway are only induced at the presence of gluconate, glucuronate, galacturonate or idonate (Eisenberg and Dobrogosz 1967). The resulting pyruvate is converted to d-(−)-lactate, acetate, succinate and formate via so-called mixed-acid fermentation. Part of the formic acid is split into equal amounts of CO2 and H2. The sum of the equation is (Schlegel 2007)
Approximately 80 % of the glucose is fermented by these pathways, and the other 20 % are metabolized by the oxidative pentose phosphate pathway (Fuhrer et al. 2005).
3 Organic Lactate-Forming Bacteria
Various organic lactates are formed by LAB, such as 1,4-piperazinium-(l,l)-dilactate (Kamm et al. 1997; Richter et al. 2001), imidazole-(l)-lactate (Kamm et al. 1999), hexamethylenediamine-(l,l)-dilactate (Gutmacher 2008) and lysine-(l)-lactate (Leiss et al. 2010). Piperazinium-(l,l)-dilactate, imidazole-(l)-lactate (Kamm et al. 1999) and hexamethylenediamine-(l,l)-dilactate are applied as intermediates for the production of polylactic acid (Kamm et al. 1999, 2000). These lactates are formed by means of strains of Lactobacillus paracasei ssp. paracasei. Important features of L. paracasei ssp. paracasei are summarized in Table 18.
LA is produced through facultative heterofermentation, which means cells of the strain ferment hexoses and pentoses (see Sect. 2.1). Hexoses are fermented by the EMP glycolytic pathway to l-(+)-lactate. The same strains, which are formerly described as L. pseudoplantarum, produce dl-LA (Hammes and Hertel 2009). Pentoses enter this pathway and are fermented to LA and acetic acid.
4 Platform Chemical Lactic Acid
Currently there are criteria for a platform chemical, such as multiple product applicability, high-volume product and potential industrial scaleup (Bozell and Petersen 2010). LA is widely used in the food, cosmetic, pharmaceutical and chemical application, such as lactic acid derivatives as dyeing assistants, and has received increased attention for use as a monomer for the production of biodegradable PLA (Datta et al. 1995; Kamm et al. 1997, 2000; Nampoothiri et al. 2010; Castillo Martinez et al. 2013). LA is one of the most interesting intermediates for the synthesis of industrial relevant bio-based compounds based on carbohydrates, and it was foreseen as a platform chemical for the production of several downstream chemicals. Lactic acid undergoes ready esterification to give lactate esters, of interest as new bio-based ‘green’ solvents (Kamm et al. 2008). Lactate esters have many potential markets as non-toxic replacements for halogenated and toxic solvents. The use of esters, e.g. ethyl lactate and butyl lactate as solvents for cellulose lacquers or poly(vinyl) compounds, is important, and a great variety of esters have been recommended for use as plasticizers in polymers. Catalytic reduction of lactic acid leads to propylene glycol, which can be further dehydrated to give propylene oxide. Alternatively, lactic acid can be dehydrated to give acrylic acid and esters, but in practice this conversion proceeds in low yield (Walkup 1991).
The global lactic acid demand was estimated to be 714.2 kilo tons in 2013, which is expected to reach 1,960.1 kilo tons by 2020, growing at a compound annual growth rate of 15.5 % from 2014 to 2020 (SpecialChem 2014). Growth in demand for LA and its salts and esters in industrial applications will be driven mainly by LA-based biodegradable polymers and, to a lesser degree, lactate solvents (Abdel-Rahman et al. 2013; Taskila and Ojamo 2013).
5 Intermediate and Speciality Chemicals: Organic Lactates
Aminium lactates, such as piperazinium dilactate, imidazole lactate and hexamethylenediamine dilactate, are applied as intermediates for the production of polylactic acid (Kamm et al. 2000) and for the manufacture of high purity lactic acid (Kamm et al. 1999). Aminium lactates can be applied directly as constituents of pharmaceutical and cosmetic products as well. Piperazine lactate has anthelmintic activity (Chatterjee et al. 1997). Imidazole lactate is useful as a topical antilipolytic (Carreras Ginjaume 1985) and lysine lactate can be applied as component in skin lotions (Parab 1995). The antimicrobial activities of protic ionic liquids with lactate ion were investigated intensively (Pernak et al. 2004).
6 Biotechnological Production of LA
Biotechnological processes and bio-based products are an interesting alternative compared to classical ones of chemistry. The so-called white biotechnology points to an emerging field in biotechnology with immense potentials via utilization of biocatalysts for the manufacture of industrial products. The goal is to develop a fermentation process based on the substitution of expensive nutrient supplements by cheaper materials from renewable resources due to their main proportion of the whole process costs (Akerberg and Zacchi 2000; Okano et al. 2010). Depending on the further processing of the LA (e.g. for bioplastics), the separation of impurities after fermentation is a major process cost too (Fitzpatrick et al. 2003; Ryu et al. 2012). Therefore an optimization is necessary to find a balance between the substitution of expensive nutrients and the limitation of interfering or undesirable components of natural raw materials, respectively.
The worldwide research is advancing focused on the use of renewable raw materials as carbon substrates as well as nutrient additive resources. In this context, there is a strong interest to reduce costs for raw materials and to use renewable resources.
With respect to the above-mentioned cost aspect of bioprocess feedstock, the utilization of residues and waste materials (Pintado et al. 1999; Huang et al. 2005; Bischoff et al. 2010; Ouyang et al. 2013; Tang et al. 2013) and agricultural by-products (Thomsen 2005; John 2009; Alonso et al. 2011; Li et al. 2012) became the focus of public attention.
LA was produced worldwide at first from glucose or pure starch on fermentative ways (Richter and Berthold 1998). First efforts for developing bioconversion processes for the production of LA directly from agricultural starchy feedstock were published by Shamala and Sreekantiah (1987). During the last years, starchy hydrolysates obtained from agricultural resources like corn or barley (Linko and Javanainen 1996; Oh et al. 2005; Venus and Richter 2006), cassava (Xiaodong et al. 1997; Bomrungnok et al. 2012), wheat (Hetenyi et al. 2010), rye (Otlewska et al. 2012), potatoes (Zhang and Jin 2010; Bilanovic et al. 2011) and sago (Nolasco-Hipolito et al. 2002) were also tested on their suitability as substrates for LA fermentation.
Lignocellulosic biomass represents the most abundant global source of biomass, and for this reason it has been largely utilized in many applications (Taherzadeh and Karimi 2007). Lignocellulosic materials can be used to obtain sugar solutions that may be usefully exploited for the production of LA through the following steps: (a) pretreatment to break down the lignocellulosic structure, (b) enzymatic hydrolysis to depolymerize lignocellulose to fermentative sugars, (c) sugar fermentation to LA by LAB and (d) separation and purification of LA (Moldes et al. 2006, Abdel-Rahman et al. 2011). In recent years, one of the most used processes to obtain LA from lignocellulosic materials is the simultaneous saccharification and fermentation (John et al. 2009; Qi et al. 2011; Zhao et al. 2013b), which is able to prevent enzyme inhibition by the product (Gullon et al. 2007; Castillo Martinez et al. 2013).
LAB need, besides the carbon source, also a source of nitrogen and other nutrients and phosphorus. The latter is available when inorganic phosphate salts are added to the medium. The demand for nitrogen cannot be covered by inorganic salts only. LAB need also a series of nitrogen-containing nutrients (amino acids, peptides, etc.) for growth, and therefore, the medium has to be supplied by complex protein hydrolysates (yeast extract, peptone, etc.). The protein extracts mentioned are very expensive, and their substitution by low-priced nutrient extracts is necessary when a large-scale production is planned. A useful combination of green biomass processing for the production of fodder pellets and the utilization of the pressed juice for the LA fermentation was described by Andersen and Kiel (2000) and Vodnar et al. (2010). The use of date juice together with different nitrogen sources as a substrate for LA production was investigated by Nancib et al. (2001).
7 Future Perspectives
Although LA production by LAB is very efficient, further improvements in the process can help make it more cost competitive with petroleum-based polymers for PLA production. Environmentally friendly, ‘green’ solvents are another potential area for lactic acid derivatives, particularly lactate esters of low-molecular-weight alcohols such as ethyl, propyl and butyl lactate (John et al. 2007; Delgado et al. 2010). From that perspective the lactate esters have also further applications in order to run alternative downstream technology (Kamble et al. 2012) and PLA polymerization process (Marques et al. 2012).
Yield and purity of the LA produced are currently limited by many factors including the production of both l- and d-LA via l-lactate dehydrogenase and d-lactate dehydrogenase, respectively, low yield due to by-product formation, use of nutritionally rich medium, high risk of bacteriophage infection that results in cell lysis and subsequent cessation of LA production (Abdel-Rahman et al. 2013). In addition to the previously used LAB and LA-producing microorganisms, these organisms are interesting for the production of LA that were until now get low attention such as microalgae and cyanobacteria.
Through different approaches, these defects can be partially remedied today. The usage of mixed strains and/or development of phage-resistant strains can prevent bacteriophage infection (Hassan and Frank 2001).
Various studies have investigated methods to overcome some problems in the field of metabolic engineering of the strains, e.g. improvement of optical purity via the deletion of either d- or l-LDH genes (Kyla-Nikkila et al. 2000) and increased LA yields through the reduction of by-product levels by the deletion of genes encoding pyruvate formate lyase (formic acid production), alcohol dehydrogenase (ethanol production) and/or acetate kinase (acetic acid production) (Zhou et al. 2003a).
Moreover, the development of bacterial strains producing LA on chemically defined media (Zhou et al. 2003b) and strains improving blocking steps in the phage life cycle (Allison and Klaenhammer 1998; Forde and Fitzgerald 1999) is advanced.
Another way to more effective LA formation is the search for organisms that can tolerate high pH values. Alkaliphilic LAB strains may be promising producers of LA due to their tolerance to high pH levels that would minimize contamination problems during processing. Calabia et al. (2011) isolated an alkaliphilic Lactobacillus halophilus from a marine environment that produced 65.8 g/L of l-LA at pH 9.0. By comparison, the strategy of NatureWorks as the main global PLA producer is directed on the yeast fermentation at lower pH, thereby significantly reducing the use of calcium hydroxide and sulphuric acid, in turn resulting in significantly lower quantities of gypsum together with less energy demand for the entire process (Vink et al. 2010).
Also not yet used for the formation of LA are organisms such as microalgae and cyanobacteria. These photosynthetic microorganisms offer an alternative LA production approach and would allow carbohydrate feedstock costs to be eliminated. It has long been known that some microalgae have the ability to convert the starch they accumulated under light and aerobic conditions into organic matter, such as LA, ethanol, acetic acid and formic acid under dark and anaerobic conditions (Hirayama and Ueda 2004; Oost et al. 1989). There are also reports about the LA production by the microalgal species Nannochlorum sp. 26A4 from about 26 g/L d-LA production with an optical purity of 99.8 % from their starch (40 % content per dry weight) at yield of 70 % under dark and anaerobic conditions (Hirayama and Ueda 2004).
New strains with new properties alone will not lead to more efficient production of LA, but only in interaction with new raw materials, progress in fermentation technology as well as downstream processing development. Because of the relatively low price of LA, one of the major challenges in its large-scale fermentative production is the cost of the raw material. Lactic acid can be produced from a wide spectrum of carbon sources including starchy materials, many food industry by-products (e.g. molasses, whey), agro-industrial residues and by-products (e.g. lignocellulose hydrolysates, cottonseed hulls, corn cob, corn stalks, wheat bran, brewer’s spent grains) and various other renewable resources. Together with the need of low-cost carbon, there is an additional demand of suitable supplements, which should not cause additional costs and problems in view of impurities. Therefore, the kind of nutrients as well as the optimization of their concentration is essential. It is likely that one of the future trends in lactic acid production will end up in mixtures of different low-cost raw materials in order to avoid the use of expensive complex supplements (Taskila and Ojamo 2013; Koutinas et al. 2014).
Besides the strain optimization and alternative raw materials, the transition from traditional batch including repeated batch and fed-batch fermentation to continuous mode fermentation (Dey and Pal 2013; Gao and Ho 2013) with cell recycle (Venus 2009; Wee and Ryu 2009; Lee et al. 2014) as solutions with free cells and the use of immobilized cells in different reactor types (fixed or fluidized bed) could lead to further performance improvement. The number of downstream processing steps strongly influences the quality and the price of the product. Thus the total costs are determined mainly by the purification rather than by LA production using fermentation (Reimann 2006). Open sources provide only limited data about industrial product recovery processes, but the main technology steps are known for large-scale production of carboxylic acids and ongoing research activities are widely discussed (López-Garzón and Straathof 2014). If the disadvantages of traditional fermentation and recovery process are overcome combined with the huge amount of gypsum as a by-product, significant progress for lactic acid production can be expected in the near future.
Furthermore methods for combining the fermentation of lactic acid and production of chemical sequence products derived from lactic acid are required for the development of intermediates and speciality chemicals. A worthwhile approach could be the direct fermentation on organic lactates, such as substituted aminium lactates.
Abbreviations
- G + C:
-
Guanine + cytosine
- LA:
-
Lactic acid
- LAB:
-
Lactic acid bacteria
- PLA:
-
Polylactic acid
- ssp.:
-
Subspecies
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2011) Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156:286–301. doi:10.1016/j.jbiotec.2011.06.017
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in LA production by microbial fermentation processes. Biotechnol Adv 31(6):877–902. doi:10.1016/j.biotechadv.2013.04.002
Adler P, Song HS, Kastner K, Ramkrishna D, Kunz B (2012) Prediction of dynamic metabolic behavior of Pediococcus pentosaceus producing lactic acid from lignocellulosic sugars. Biotechnol Prog 28:623–635. doi:10.1002/btpr.1521
Akerberg C, Zacchi G (2000) An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresour Technol 75(2):119–126. doi:10.1016/S0960-8524(00)00057-2
Allison GE, Klaenhammer TK (1998) Phage resistance mechanisms in LA bacteria. Int Dairy J 8(3):207–226. doi:10.1016/S0958-6946(98)00043-0
Alonso JL, Dominguez H, Garrote G, Gonzalez-Munoz MJ, Gullon B, Moure A, Santos V, Vila C, Yanez R (2011) Biorefinery processes for the integral valorization of agroindustrial and forestal wastes. CyTa-J Food 9:282–289. doi:10.1080/19476337.2011.598949
Altaf MD, Naveena BJ, Venkateshwar M, Kumar EV, Reddy G (2006) Single step fermentation of starch to l(+) LA by Lactobacillus amylophilus GV6 in SSF using inexpensive nitrogen sources to replace peptone and yeast extract–optimization by RSM. Process Biochem 41(2):465–472. doi:10.1016/j.procbio.2005.07.011
Altaf MD, Venkateshwar M, Srijana M, Reddy G (2007) An economic approach for L-(+) LA fermentation by Lactobacillus amylophilus GV6 using inexpensive carbon and nitrogen sources. J Appl Microbiol 103(2):372–380. doi:10.1111/j.1365-2672.2006.03254.x
Andersen M, Kiel P (2000) Integrated utilisation of green biomass in the green biorefinery. Ind Crops Prod 11:129–137. doi:10.1016/S0926-6690(99)00055-2
Antonio GV, Pinelli D, Rossi M, Fajner D, Magelli F, Matteuzzi D (1996) Production of L(+) and D(−) LA isomers by Lactobacillus casei subsp. casei DSM 20011 and Lactobacillus coryniformis subsp. torquens DSM 20004 in continuous fermentation. J Ferment Bioeng 81(6):548–552. doi:10.1016/0922-338X(96)81478-4
Aristidou A, Penttilä M (2000) Metabolic engineering applications to renewable resource utilization. Curr Opin Biotechnol 11(2):187–198. doi:10.1016/S0958-1669(00)00085-9
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko A, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia Coli K-12 in-frame, single-gene knockout mutans: the Keio collection. Mol Syst Biol 2:1–11. doi:10.1038/msb4100050
Bai DM, Zhao XM, Li XG, Xu SM (2004) Strain improvement of Rhizopus oryzae for over-production of (+)-LA and metabolic flux analysis of mutants. Biochem Eng J 18:41–48. doi:10.1016/S1369-703X(03)00126-8
Bai DM, Li SZ, Liu ZL, Cui ZF (2008) Enhanced L-(+)-LA production by an adapted strain of Rhizopus oryzae using corncob hydrolysate. Appl Biochem Biotechnol 144:79–85. doi:10.1007/s12010-007-8078-y
Barnett JA, Payne RW, Yarrow D (1990) Yeast: characteristics and identification, 2nd edn. Cambridge University Press, Cambridge, p 1002
Benthin S (1994) Growth energetics of Lactococcus cremoris FD1 during energy-, carbon-, and nitrogen-limitation in steady state and transient cultures. Chem Eng Sci 49(5):589–609. doi:10.1016/0009-2509(94)85006-2
Berry AR, Franco CMM, Zhang W, Middelberg APJ (1999) Growth and LA production in batch culture of Lactobacillus rhamnosus in a defined medium. Biotechnol Lett 21(2):163–167. doi:10.1023/A:1005483609065
Bianchi MM, Brambilla L, Protani F, Liu C, Lievense J, Porro D (2001) Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl Environ Microbiol 67:5621–5625. doi:10.1128/AEM.67.12.5621-5625.2001
Bilanovic D, Chang F-H, Isobaev P, Welle P (2011) Lactic acid and xanthan fermentations on an alternative potato residues media–carbon source costs. Biomass Bioenergy 35:2683–2689. doi:10.1016/j.biombioe.2011.03.001
Bischoff KM, Liu S, Hughes SR, Rich JO (2010) Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol Lett 32:823–828. doi:10.1007/s10529-010-0222-z
Bisset DL, Anderson RL (1974) Lactose and D-galactose metabolism in group N-streptococci: presence of enzymes for both the D-galactose-1-phosphate and d-tagatose-6-phosphate pathways. J Bacteriol 117(1):318–320
Bomrungnok W, Sonomoto K, Pinitglang S, Wongwicharn A (2012) Single step LA production from cassava starch by Lactobacillus plantarum SW14 in conventional continuous and continuous with high cell density. APCBEE Procedia 2:97–103. doi:10.1016/j.apcbee.2012.06.018
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates – the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554. doi:10.1039/B922014C
Buruleanu CL, Leane NC, Manea I, Bratu MG, Avram D (2011) Kinetic study of the LA fermentation of cabbage juice with Bifidobacterium sp. Curr Opin Biotechnol 22(1):S41. doi:10.1016/j.copbio.2011.05.099
Calabia BP, Tokiwa Y, Aiba S (2011) Fermentative production of L-(+)-LA by an alkaliphilic marine microorganism. Biotechnol Lett 33(7):1429–1433. doi:10.1007/s10529-011-0573-0
Carreras Ginjaume E (1985) Process for the preparation of imidazole lactate. ES 540,741(21.02.1985/17.05.1986)
Castillo Martinez FA, Balciunas EM, Salgado JM, Domínguez González JM, Converti A, de Souza P, Oliveira R (2013) LA properties, applications and production: a review. Trends Food Sci Technol 30:70–83. doi:10.1016/j.tifs.2012.11.007
CBS-Knaw (2014) Rhizopus oryzae. http://www.cbs.knaw.nl/Collections. Accessed 5 Jun 2014
Chang DE, Jung HC, Rhee JS, Pan JG (1999) Homofermentative production of D- or L-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microbiol 65(4):1384–1389
Chatterjee P, Basu RK, Shegal JM (1997) Possible anthelmintic use of piperazine acetate and lactate. Indian J Exp Biol 15:568–569
Claus D, Berkeley RCW (1986) The genus Bacillus. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2, 9th edn. William and Wilkins, Baltimore, MD, pp 1105–1138
Cui F, Li Y, Wan C (2011) LA production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour Technol 102(2):1831–1836. doi:10.1016/j.biortech.2010.09.063
Danner H, Madzingaidzo L, Thomasser C, Neureiter M, Braun R (2002) Thermophilic production of lactic acid using integrated membrane bioreactor systems coupled with monopolar electrodialysis. Appl Microbiol Biotechnol 59(2–3):160–169. doi:10.1007/s00253-002-0998-4
Dao TH, Zhang J, Bao J (2013) Characterization of inulin hydrolyzing enzyme(s) in commercial glucoamylases and its application in LA production from Jerusalem artichoke tubers (Jat). Bioresour Technol 148:157–162. doi:10.1016/j.biortech.2013.08.123
Datta R, Tsai SP, Bonsignore P, Moon SH, Frank JR (1995) Technological and economic potential of poly(lactic acid) and lactic acid derivatives. FEMS Microbiol Rev 16:221–231. doi:10.1111/j.1574-6976.1995.tb00168.x
De Hoog GS, Guarro J, Gené J, Figueras MJ (2000) Atlas of clinical fungi, 2nd edn. Centraalbureau voor Schimmelcultures, Utrecht
De Vries W, Stouthamer AH (1967) Pathway of glucose fermentation in relation to the taxonomy of bifidobacteria. J Bacteriol 93(2):574–576
De Vries W, Stouthamer AH (1969) Factors determining the degree anaerobiosis of Bifidobacterium strains. Arch Microbiol 65:275–287. doi:10.1007/BF00407109
Delgado P, Sanz MT, Beltrán S, Núñez LA (2010) Ethyl lactate production via esterification of lactic acid with ethanol combined with pervaporation. Chem Eng J 165:693–700. doi:10.1016/j.cej.2010.10.009
Dequin S, Barre P (1994) Mixed LA-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei L-(+)-LDH. J Biotechnol 12:173–177. doi:10.1038/nbt0294-173
Devriese LA, Pot B (1995) The genus Enterococcus. In: Wood BJB, Holzapfel WH (eds) The LA bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London, pp 327–367
Dey P, Pal P (2013) Modelling and simulation of continuous L (+) lactic acid production from sugarcane juice in membrane integrated hybrid-reactor system. Biochem Eng J 79:15–24. doi:10.1016/j.bej.2013.06.014
Dien BS, Nichols NN, Bothast RJ (2001) Recombinant Escherichia coli engineered for production of L-LA from hexose and pentose sugars. J Ind Microbiol Biotechnol 27(4):259–264
Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000, on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC)
Efremenko E, Spiricheva O, Varfolomeyev S, Lozinsky V (2006) Rhizopus oryzae fungus cells producing L(+)-LA: kinetic and metabolic parameters of free and PVA- cryogel-entrapped mycelium. Appl Microbiol Biotechnol 72:480–485. doi:10.1007/s00253-005-0297-y
Eisenberg RC, Dobrogosz WJ (1967) Gluconate metabolism in Escherichia coli. J Bacteriol 93(3):941–949
Fitzpatrick JJ, Murphy C, Mota FM, Pauli T (2003) Impurity and cost considerations for nutrient supplementation of whey permeate fermentations to produce lactic acid for biodegradable plastics. Int Dairy J 13(7):575–580. doi:10.1016/S0958-6946(03)00072-4
Forde A, Fitzgerald GF (1999) Bacteriophage defense systems in LA bacteria. Anton Leeuw Int J G 76(1–4):89–113. doi:10.1023/A:1002027321171
Fritze D, Claus D (1995) Spore-forming, LA producing bacteria of the genera Bacillus and Sporolactobacillus. In: Wood BJB, Holzapfel WH (eds) The LA bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London, pp 368–391
Fu W, Mathews AP (1999) LA production from lactose by Lactobacillus plantarum: kinetic model and effects of pH, substrate, and oxygen. Biochem Eng J 3(3):163–170. doi:10.1016/S1369-703X(99)00014-5
Fuhrer T, Fischer E, Sauer U (2005) Experimental identification and quantification of glucose metabolism in seven bacterial species. J Bacteriol 187:581–1590. doi:10.1128/JB.187.5.1581-1590.2005
Fukushima K, Sogo K, Miura S, Kimura Y (2004) Production of D-LA by bacterial fermentation of rice starch. Macromol Biosci 4(11):1021–1027. doi:10.1002/mabi.200400080
Gao T, Ho K-P (2013) L-Lactic acid production by Bacillus subtilis MUR1 in continuous culture. J Biotechnol 168:646–651. doi:10.1016/j.jbiotec.2013.09.023
Garvie EI (1986) Genus Pediococcus. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2, 9th edn. William and Wilkins, Baltimore, MD, pp 1075–1079
Ghosh B, Ray RR (2011) Current commercial perspective of Rhizopus oryzae: a review. J Appl Sci 11(14):2470–2486. doi:10.3923/jas.2011.2470.2486
Giraud E, Champailler A, Raimbault M (1994) Degradation of raw starch by a wild amylolytic strain of Lactobacillus plantarum. Appl Environ Microbiol 60(12):4319–4323
Giurca R, Levin RE (1992) Optimization of the lactic acid fermentation of hydrolyzed cod gurry with molasses. J Food Biochem 16:83–97
Giurca R, Levin RE (1993) Optimization of the lactic acid fermentation of hydrolyzed cod (Gadus morhua) gurry with corn syrup as carbohydrate source. J Food Biochem 16:277–289
Grabar TB, Zhou S, Shanmugam KT, Yomano LP, Ingram LO (2006) Methylglyoxal bypass identified as source of chiral contamination in L(+) and D(−)-lactate fermentations by recombinant Escherichia coli. Biotechnol Lett 28(19):1527–1535. doi:10.1007/s10529-006-9122-7
Gullon B, Garrote G, Alonso JL, Parajo JC (2007) Production of L-lactic acid and oligomeric compounds from apple pomace by simultaneous saccharification and fermentation: a response surface methodology assessment. J Agric Food Chem 55:5580–5587. doi:10.1021/jf070442v
Gutmacher F (2008) Fermentationsuntersuchungen zur Herstellung von Aminiumlactat. Diploma Thesis, Beuth-University of Applied Science, Berlin
Hamamci H, Ryu DDY (1994) Production of L (+)-LA using immobilized Rhizopus oryzae reactor performance based on kinetic model and simulation. Appl Biochem Biotechnol 44:125–133. doi:10.1007/BF02921650
Hammes WP, Hertel C (2009) Genus I. Lactobacillus Beijerinck 1901. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY, pp 465–511
Hammes WP, Vogel RF (1995) The genus Lactobacillus. In: Wood BJB, Holzapfel WH (eds) The LA bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London, pp 19–54
Hammes W, Weiss N, Holzapfel W (1991) The genera Lactobacillus and Carnobacterium. In: The prokaryotes, vol II, 2nd edn. Springer, Berlin, pp 1535–1594
Hardie JM (1986) Other Streptococci. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2, 9th edn. William and Wilkins, Baltimore, MD, pp 1068–1071
Hardie JM, Whiley RA (1995) The genus Streptococcus. In: Wood BJB, Holzapfel WH (eds) The LA bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London, pp 55–124
Hassan A, Frank J (2001) Starter cultures and their use. In: Marth EH, Steele JL (eds) Applied dairy microbiology. Marcel Dekker, New York, NY, pp 151–206
Hetenyi K, Nemeth A, Sevella B (2010) First steps in the development of a wheat flour based lactic acid fermentation technology. Culture medium optimization. Chem Biochem Eng Q 24(2):195–201
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, Mclaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüßler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N (2007) A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547. doi:10.1016/j.mycres.2007.03.004
Hirayama S, Ueda R (2004) Production of optically pure D-LA by Nannochlorum sp. 26A4. Appl Biochem Biotechnol 119(1):71–78
Hofvendahl K, Hahn-Hägerdahl B (1997) L-LA production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci. Enzyme Microb Technol 20(4):301–307. doi:10.1016/S0141-0229(97)83489-8
Hofvendahl K, Hahn-Hägerdal B (2000) Factors affecting the fermentative LA production from renewable resources. Enzyme Microb Technol 26:87–107. doi:10.1016/S0141-0229(99)00155-6
Holzapfel WH, Franz CMAP, Ludwig W, Dicks LMT (2009) Genus III. Pediococcus. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY, pp 513–532
Hua Q, Joyce R, Fong SS, Palsson BØ (2006) Metabolic analysis of adaptive evolution for in silico-designed lactate-producing strains. Biotechnol Bioeng 95:992–1002. doi:10.1002/bit.21073
Huang LP, Jin B, Lant P, Zhou J (2005) Simultaneous saccharification and fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Biochem Eng J 23(3):265–276. doi:10.1016/j.bej.2005.01.009
Hujanen M, Linko S, Linko YY, Leisola M (2001) Optimisation of media and cultivation conditions for L(+)(S)-LA production by Lactobacillus casei NRRL B-441. Appl Microbiol Biotechnol 56:126–130. doi:10.1007/s002530000501
Ilmen M, Koivuranta K, Ruohonen L, Suominen P, Penttilä M (2007) Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl Environ Microbiol 73:117–123. doi:10.1128/AEM.01311-06
John RP (2009) Biotechnological potentials of cassava bagasse. In: Singh nee’ Nigam P, Pandey A (eds) Biotechnology for agro-industrial residues utilisation. Springer Science + Business Media B.V., pp 225–237. doi:10.1007/978-1-4020-9942-7_11
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of LA from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74(3):524–534. doi:10.1007/s00253-006-0779-6
John RP, Anisha GS, Nampoothiri KM, Pandey A (2009) Direct lactic acid fermentation: focus on simultaneous saccharification and lactic acid production. Biotechnol Adv 27:145–152. doi:10.1016/j.biotechadv.2008.10.004
Kamble SP, Barve PP, Joshi JB, Rahman I, Kulkarni BD (2012) Purification of lactic acid via esterification of lactic acid using a packed column, followed by hydrolysis of methyl lactate using three continuously stirred tank reactors (CSTRs) in series: a continuous pilot plant study. Ind Eng Chem Res 51:1506–1514. doi:10.1021/ie200642j
Kamm B, Kamm M, Richter K (1997) Formation of aminium lactates in lactic acid fermentation. Preparation and characterization of 1.4-Piperazinium-(L, L)-dilactate obtained from L(+)-lactic acid (Part I). Acta Biotechnol 17(1):3–18. doi:10.1002/abio.370170102
Kamm B, Kamm M, Richter K (1999) New method for preparing poly(lactic acid) from renewable raw material. In: 6th symposium on renewable resources and 4th European symposium on industrial crops & products, Bonn, 23–25 March, Schriftenreihe Nachwachsende Rohstoffe Bd 14, Landwirtschaftsverlag GmbH Münster, pp 685–692. ISBN 3-7843-3019-3
Kamm B, Kamm M, Richter K, Reimann W, Siebert A (2000) Formation of aminium lactates in lactic acid fermentation. Fermentative production of 1.4-Piperazinium-(L, L)-Dilactate and its use as a starting material for the synthesis of dilactide (Part 2). Acta Biotechnol 20(3–4):289–304. doi:10.1002/abio.370200310
Kamm B, Kamm M, Schönicke P, Bohnen F (2008) Technology for production of carbonic acid ester. WO 2009/083551, 23.12.2008/09.07.2009
Kandler O (1983) Carbohydrate metabolism in LA bacteria. Anton Leeuw Int J G 49(3):209–224. doi:10.1007/BF00399499
Kandler O, Weiss N (1986) Sektion 14 regular, nonsporing gram-positive rods. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2, 9th edn. William and Wilkins, Baltimore, MD, pp 1208–1234
Kim HJ, Hou BK, Lee SG, Kim JS, Lee DW, Lee SJ (2013) Genome-wide analysis of redox reactions reveals metabolic engineering targets for d-lactate overproduction in Escherichia coli. Metab Eng 18:44–52. doi:10.1016/j.ymben.2013.03.004
Koutinas AA, Vlysidis A, Pleissner D, Kopsahelis N, Lopez Garcia I, Kookos IK, Papanikolaou S, Kwan TH, Lin CSK (2014) Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem Soc Rev 43:2587–2627. doi:10.1039/c3cs60293a
Kwon S, Lee PC, Lee EG, Chang YK, Chang N (2000) Production of LA by Lactobacillus rhamnosus with vitamin supplemented soybean hydrolysate. Enzyme Microb Technol 26:209–215. doi:10.1016/S0141-0229(99)00134-9
Kwon S, Yoo IK, Lee WG, Chang HN, Chang YK (2001) High-rate continuous production of LA by Lactobacillus rhamnosus in a two-stage membrane cell-recycle bioreactor. Biotechnol Bioeng 73(1):25–34. doi:10.1002/1097-0290(20010405)
Kyla-Nikkila K, Hujanen M, Leisola M, Palva A (2000) Metabolic engineering of Lactobacillus helveticus CNRZ32 for production of pure L-(+)-LA. Appl Environ Microbiol 66(9):3835–3841. doi:10.1128/AEM.66.9.3835-3841.2000
Lee RK, Ryu HW, Oh H, Kim M, Wee YJ (2014) Cell-recycle continuous fermentation of Enterococcus faecalis RKY1 for economical production of lactic acid by reduction of yeast extract supplementation. J Microbiol Biotechnol 24:661–666. doi:10.4014/jmb.1402.02017
Leiss S, Venus J, Kamm B (2010) Fermentative production of L-Lysine-L-lactate with fractionated press juices from the green biorefinery. Chem Eng Technol 33(12):2102–2105. doi:10.1002/ceat.201000314
Li Y, Shahbazi A, Williams K, Wan C (2008) Separate and concentrate LA using combination of nanofiltration and reverse osmosis membranes. Appl Biochem Biotechnol 147(1–3):1–9. doi:10.1007/s12010-007-8047-5
Li Z, Lu J, Yang Z, Han L, Tan T (2012) Utilization of white rice bran for production of l-lactic acid. Biomass Bioenergy 39:53–58. doi:10.1016/j.biombioe.2011.12.039
Li Y, Wang L, Ju J, Yu B, Ma Y (2013) Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM442 with agricultural waste cottonseed as the sole nitrogen source. Bioresour Technol 142:186–191. doi:10.1016/j.biortech.2013.04.124
Linko YY, Javanainen P (1996) Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb Technol 19:118–123. doi:10.1016/0141-0229(95)00189-1
Logan NA, de Vos P (2009) Genus I. Bacillus Cohn 1872. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY, pp 21–128
López-Garzón CS, Straathof AJJ (2014) Recovery of carboxylic acids produced by fermentation. Biotechnol Adv 32(5):873–904. doi:10.1016/j.biotechadv.2014.04.002
Lu Z, Wei M, Yu L (2012) Enhancement of pilot scale production of L(+)-lactic acid by fermentation coupled with separation using membrane bioreactor. Process Biochem 47:410–415. doi:10.1016/j.procbio.2011.11.022
Ludwig W, Schleifer K-H, Whitman WB (2009) Genus I. Sporolactobacillus. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY
Ma K, Maeda T, You H, Shirai Y (2014) Open fermentative production of L-LA with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresour Technol 151:28–35. doi:10.1016/j.biortech.2013.10.022
Martak J, Schlosser S, Sabolova E, Kristofikova L, Rosenberg M (2003) Fermentation of lactic acid with Rhizopus arrhizus in a stirred tank reactor with a periodical bleed and feed operation. Process Biochem 38(11):1573–1583. doi:10.1016/S0032-9592(03)00059-1
Marques DS, Gil MH, Baptista CMSG (2012) Bulk polytransesterification of L-lactic acid esters: an alternative route to synthesize poly(lactic acid). J Appl Polym Sci 125:E283–E289. doi:10.1002/app.36825
Mazumdar S, Blankschien M, Clomburg J, Gonzalez R (2013) Efficient synthesis of L-LA from glycerol by metabolically engineered Escherichia coli. Microb Cell Fact 12(7):1–11
Melchiorsen CR, Jensen NBS, Christensen B, Jokumsen KV, Villadsen J (2001) Dynamics of pyruvate metabolism in Lactococcus lactis. Biotechnol Bioeng 74(4):271–279. doi:10.1002/bit.1117
Melchiorsen CR, Jokumsen KV, Villadsen J, Israelsen H, Arnau J (2002) The level of pyruvate-formate lyase controls the shift from homolactic to mixed-acid product formation in Lactococcus lactis. Appl Microbiol Biotechnol 58(3):338–344. doi:10.1007/s00253-001-0892-5
Meussen BJ, de Graaff LH, Sanders JPM, Weusthuis RA (2012) Metabolic engineering of Rhizopus oryzae for the production of platform chemicals. Appl Microbiol Biotechnol 94:875–886. doi:10.1007/s00253-012-4033-0
Mimitsuka T, Kyungsu NK, Moriata K, Sawai H, Minegishi S, Henmi M, Yamada K, Shimizu S, Yonehara T (2012) A membrane-integrated fermentation reactor system: its effects in reducing the amount of sub-raw materials for D-LA continuous fermentation by Sporolactobacillus laevolacticus. Biosci Biotechnol Biochem 76(1):67–72. doi:10.1271/bbb.110499
Miura S, Arimura T, Hoshino M, Kojima M, Dwiarti L, Okabe M (2003) Optimization and scale-up of L-LA fermentation by mutant strain Rhizopus sp. MK-96-1196 in airlift bioreactors. J Biosci Bioeng 96:65–69. doi:10.1016/S1389-1723(03)90098-3
Moldes AB, Torrado A, Converti A, Dominguez JM (2006) Complete bioconversion of hemicellulosic sugars from agricultural residues into lactic acid by Lactobacillus pentosus. Appl Biochem Biotechnol 135:219–227. doi:10.1385/ABAB:135:3:219
Morlon-Guyot J, Guyot JP, Pot B, Jacobe de Haut I, Raimbault M (1998) Lactobacillus manihotivorans sp. nov., a new starch-hydrolysing LA bacterium isolated during cassava sour starch fermentation. Int J Syst Bacteriol 48:1101–1109. doi:10.1099/00207713-48-4-1101
Mukisa IM, Byaruhanga YB, Muyanja CMBK, Aijuka M, Schuller RB, Sahlstrom S, Langsrud T, Narvhus JA (2012) Influence of cofermentation by amylolytic Lactobacillus plantarum and Lactococcus lactis strains on the fermentation process and rheology of sorghum porridge. Appl Environ Microbiol 78(15):5220–5228. doi:10.1128/AEM.00857-12
Nagamori E, Shimizu K, Fujita H, Tokuhiro K, Ishida N, Takahashi H (2013) Metabolic flux analysis of genetically engineered Saccharomyces cerevisiae that produces lactate under micro-aerobic conditions. Bioprocess Biosyst Eng 36(9):1261–1265. doi:10.1007/s00449-012-0870-6
Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101(22):8493–8501. doi:10.1016/j.biortech.2010.05.092
Nancib N, Nancib A, Boudjelal A, Benslimane C, Blanchard F, Boudrant J (2001) The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. rhamnosus. Bioresour Technol 78:149–153. doi:10.1016/S0960-8524(01)00009-8
Nolasco-Hipolito C, Matsunaka T, Kobayashi G, Sonomoto K, Ishizaki A (2002) Synchronised fresh cell bioreactor system for continuous L(+) LA production using Lactococcus lactis IO-1 in hydrolysed sago starch. J Biosci Bioeng 93:281–287. doi:10.1263/jbb.93.281
Nolasco-Hipolito C, Zarrabal O, Kamaldin R, Teck-Yee L, Lihan S, Bujang K, Nitta Y (2012) LA production by Enterococcus faecium in liquefied sago starch. AMB Express 2:53. doi:10.1186/2191-0855-2-53
Oh H, Wee YJ, Yun JS, Han SH, Jung S, Ryu HW (2005) Lactic acid production from agricultural resources as cheap raw materials. Bioresour Technol 96(13):1492–1498. doi:10.1016/j.biortech.2004.11.020
Ohkouchi Y, Inoue Y (2006) Direct production of l(+)-LA from starch and food wastes using Lactobacillus manihotivorans LMG18011. Bioresour Technol 97(13):1554–1562. doi:10.1016/j.biortech.2005.06.004
Okano K, Yoshida S, Tanaka T, Fukuda H, Kondo A (2009a) Homo D-LA fermentation from arabinose by redirection of phosphoketolase pathway to pentose phosphate pathway in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:5175–5178. doi:10.1128/AEM.00573-09
Okano K, Yoshida S, Yamda R, Tanaka T, Ogino C, Fukuda H, Kondo A (2009b) Improved production of homo-D-LA via xylose fermentation by introduction of xylose assimilation genes and redirection of the phosphoketolase pathway to pentose phosphate path- way in L-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:7858–7861. doi:10.1128/AEM.01692-09
Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure LA from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85(3):413–423. doi:10.1007/s00253-009-2280-5
Oliveira AP, Nielsen J, Förster J (2005) Modeling Lactococcus lactis using a genome-scale flux model. BMC Microbiol 5:39, http://www.biomedcentral.com/1471-2180/5/39.doi: 10.1186/1471-2180-5-39
Oost JVD, Bulthuis BA, Feitz S, Krab K, Kraayenhof R (1989) Fermentation metabolism of the unicellular cyanobacterium Cyanothece PCC 7822. Arch Microbiol 152(5):415–419. doi:10.1007/BF00446921
Otlewska A, Walczak P, Kalinowska H, Dybka K, Oltuszak-Walczak E, Czyzowska A, Rygala A (2012) l-Lactic acid production from rye and oat grains. New Biotechnol 29(Suppl):S174. doi:10.1016/j.nbt.2012.08.485
Ou MS, Ingram LO, Shanmugam KT (2011) L(+)-LA production from non-food carbohydrates by thermotolerant Bacillus coagulans. J Ind Microbiol Biotechnol 38:599–605. doi:10.1007/s10295-010-0796-4
Ouyang J, Ma R, Zheng Z, Cai C, Zhang M, Jiang T (2013) Open fermentative production of l-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour Technol 135:475–480. doi:10.1016/j.biortech.2012.09.096
Parab P (1995) Topical preparations having α-hydroxy carboxylic acids for treatment of skin disorders. US 5.420,106. 22.03.1994/30.05.1995
Park EY, Anh PN, Okuda N (2004) Bioconversion of waste office paper to L (+)-LA by the filamentous fungus Rhizopus oryzae. Bioresour Technol 93:77–83. doi:10.1016/j.biortech.2003.08.017
Patel M, Ou M, Ingram LO, Shanmugam KT (2004) Fermentation of sugar cane bagasse hemicellulose hydrolysate to L(+)-lactic acid by a thermotolerant acidophilic Bacillus sp.**. Biotechnol Lett 26(11):865–868. doi:10.1023/B:bile.0000025893.27700.5c
Payot T, Chemaly Z, Fick M (1999) LA production by Bacillus coagulans – kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzyme Microb Technol 24(3–4):191–199. doi:10.1016/S0141-0229(98)00098-2
Pernak J, Goc I, Mirska I (2004) Green Chem 6:323–329. doi:10.1039/b404625k
Pescuma M, Hérbert EM, Mozzi F, de Valdez DF (2008) Whey fermentation by thermophilic LA bacteria: evolution of carbohydrates and protein content. Food Microbiol 25:442–451. doi:10.1016/j.fm.2008.01.007
Pintado J, Guyot JP, Raimbault M (1999) Lactic acid production from mussel processing wastes with an amylolytic bacterial strain. Enzyme Microb Technol 24(8–9):590–598. doi:10.1016/S0141-0229(98)00168-9
Popa D, Ustunol Z (2011) Influence of sucrose, high fructose corn syrup and honey from different floral sources on growth and acid production by LA bacteria and bifidobacteria. Int J Dairy Technol 64:247–253. doi:10.1111/j.1471-0307.2011.00666.x
Praphailong W, Fleet GH (1997) The effect of pH, sodium chloride, sucrose, sorbate and benzoate on the growth of food spoilage yeasts. Food Microbiol 14(2):459–468. doi:10.1006/fmic.1997.0106
Qi X, Tang Y, Jian H, Li X, Jiang J (2011) Production of lactic acid by simultaneous saccharification and fermentation using steam pretreated lespedeza stalks as inexpensive raw materials. Adv Mater Res 152–153:1404–1411. doi:10.4028/www.scientific.net/AMR.152-153.1404
Ramchandran L, Sanciolo P, Vasiljevic T, Broome M, Powell I, Duke M (2012) Improving cell yield and LA production of Lactococcus lactis ssp. cremoris by a novel submerged membrane fermentation process. J Memb Sci 403:179–187. doi:10.1016/j.memsci.2012.02.042
Reimann W (2006) Downstreaming of lactic acid from hydrolysate of rye after fermentation. Agric Eng Int: the CIGR Ejournal. Manuscript FP 06 003. Vol. VIII. April 2006. http://hdl.handle.net/1813/10538
Richter K, Berthold C (1998) Biotechnological conversion of sugar and starchy crops into lactic acid. J Agric Eng Res 71(2):181–191. doi:10.1006/jaer.1998.0314
Richter K, Kose F, Kamm B, Kamm M (2001) Fermentative production of piperazinium dilactate. Acta Biotechnol 21(1):37–47. doi:10.1002/1521-3846(200102)21
Ryu HW, Kim YM, Wee YJ (2012) Influence of operating parameters on concentration and purification of L-lactic Acid using electrodialysis. Biotechnol Bioprocess Eng 17(6):1261–1269. doi:10.1007/s12257-012-0316-7
Scardovi V (1986) Genus Bifidobacterium. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2, 9th edn. William and Wilkins, Baltimore, MD, pp 1418–1434
Schepers AW, Thibault J, Lacroix C (2003) Lactobacillus helveticus growth and LA production during pH-controlled batch cultures in whey permeate/yeast extract medium. Part I. Multiple factor kinetic analysis. Enzyme Microb Technol 30(2):176–186. doi:10.1016/S0141-0229(01)00465-3
Scheutz F, Strockbine NA (2009) Genus I Escherichia. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, Part B, vol 2, 2nd edn. Springer, New York, NY, pp 607–624
Schlegel HG (2007) Allgemeine mikrobiologie, 8th edn. Thieme, Stuttgart, p 364ff
Schmidt HL (2002) Eine Mykothek. Zum Schimmelpilzvorkommen in Futtermitteln und Nahrungsgrundstoffen. LUFA Speyer, Speyer, pp 153–154
Secchi N, Giunta D, Pretti L, García MR, Roggio T, Mannazzu I, Catzeddu P (2012) Bioconversion of ovine scotta into LA with pure and mixed cultures of LA bacteria. J Ind Microbiol Biotechnol 39(1):175–181
Sgorbati B, Biavati B, Palenzona D (1995) The genus Bifidobacterium. In: Wood BJB, Holzapfel WH (eds) The LA Bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London, pp 279–303
Shamala TR, Sreekantiah KR (1987) Degradation of starchy substrates by a crude enzyme preparation and utilization of the hydrolysates for lactic fermentation. Enzyme Microb Technol 9(12):726–729. doi:10.1016/0141-0229(87)90032-9
Shene C, Mardones M, Zamora P, Bravo S (2005) Kinetics of Bifidobacterium longum ATCC 15707 fermentations: effect of the dilution rate and carbon source. Appl Microbiol Biotechnol 67(5):23–630. doi:10.1007/s00253-004-1792-2
Shibata K, Flores DM, Kobayashi G, Sonomoto K (2007) Direct l-LA fermentation with sago starch by a novel amylolytic LA bacterium, Enterococcus faecium. Enzyme Microb Technol 41(1–2):149–155. doi:10.1016/j.enzmictec.2006.12.020
Simpson WJ, Taguchi H (1995) The genus Pediococcus, with notes on the genera Tetratogenococcus and Aerococcus. In: Wood BJB, Holzapfel WH (eds) The LA bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London
Singh SK, Ahmed SU, Pandey A (2006) Metabolic engineering approaches for lactic acid production. Process Biochem 41:991–1000. doi:10.1016/j.procbio.2005.12.004
Sjöberg A, Hahn-Hägerdahl B (1989) β glucose-phosphate, a possible mediator for polysaccharide formation in maltose-assimilation Lactococcus lactis. Appl Environ Microbiol 55(6):1549–1554
Skory CD, Freer SN, Bothas RJ (1998) Production of L-LA by Rhizopus oryzae under oxygen limiting conditions. Biotechnol Lett 20:191–194. doi:10.1023/A:1005397028700
Son MS, Kwon YJ (2013) Direct fermentation of starch to L(+)-LA by fed-batch culture of Lactobacillus manihotivorans. Food Sci Biotechnol 22(1):289–293. doi:10.1007/s10068-013-0040-x
SpecialChem (2014) http://www.specialchem4bio.com/news/2014/05/23/global-lactic-acid-market-to-grow-at-a-cagr-of-15-5-from-2014-20-grand-view-research#sthash.McKc8RdH.dpuf. Accessed 18 Jun 2014
Stackebrandt E, Rainey FA, Warde-Rainey NL (1997) Proposal for a new hierarchic classification system, Actinobacteria classis nov. Int J Syst Bacteriol 47(2):479–491. doi:10.1099/00207713-47-2-479
Stetter KO, Kandler O (1973) Untersuchungen zur Entstehung von DL-Milchsäure bei Lactobacillen und Charakterisierung einer Milchsäureracemase bei einigen Arten der Untergattung Streptobacterium. Arch Mikr 94(3):221–247. doi:10.1007/BF00417453
Stewart BJ, Navid A, Kulp KS, Knaack JLS, Bench G (2013) D-Lactate production as a function of glucose metabolism in Saccharomyces cerevisiae. Yeast 30:81–91. doi:10.1002/yea.2942
Suh SO, Blackwell M, Kurtzman CP, Lachance MA (2006) Phylogenetics of Saccharomycetales, the ascomycete yeasts. Mycologia 98(6):1006–1017. doi:10.3852/mycologia.98.6.1006
Svec P, Devriese LA (2009) Genus I. Enterococcus. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY, pp 594–607
Taherzadeh MJ, Karimi K (2007) Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: a review. [Elektronisk resurs]. Bioresources 2(4):707–738. http://hdl.handle.net/2320/2960. Accessed 18 Jun 2014
Tang Y, Bu L, He J, Jiang J (2013) L(+)-LA production from furfural residues and corn kernels with treated yeast as nutrients. Eur Food Res Technol 236:365–371. doi:10.1007/s00217-012-1865-x
Tashiro Y, Matsumoto H, Miyamoto H, Okugawa Y, Pramod P, Miyamoto H, Sakai K (2013) A novel production process for optically pure l-LA from kitchen refuse using a bacterial consortium at high temperatures. Bioresour Technol 146:672–681. doi:10.1016/j.biortech.2013.07.102
Taskila S, Ojamo H (2013) The current status and future expectations in industrial production of lactic acid by lactic acid bacteria. In: Kongo M (ed) Lactic acid bacteria - R & D for food, health and livestock purposes. InTech, Rijeka, pp 615–632. doi:10.5772/51282
Taskin M, Esim N, Ortucu S (2012) Efficient production of L-LA from chicken feather protein hydrolysate and sugar beet molasses by the newly isolated Rhizopus oryzae TS-61. Food Bioprod Process 90:773–779. doi:10.1016/j.fbp.2012.05.003
Tay A, Yang ST (2002) Production of L(+)-LA from glucose and starch by immobilised cell of Rhizopus oryzae in a rotating fibrous bed bioreactor. Biotechnol Bioeng 80:1–12. doi:10.1002/bit.10340
Teuber M (1995) The genus Lactococcus. In: Wood BJB, Holzapfel WH (eds) The LA bacteria, vol 2, The genera of LA bacteria. Blackie Academic and Professional, London, pp 173–234
Teuber M, Geis A (2006) The genus Lactococcus. In: Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, Dworkin M (eds) The prokaryotes. Springer, Berlin, pp 205–228. doi:10.1007/0-387-30744-3_7
Teuber M (2009) Genus II. Lactococcus. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY, pp 711–722
Thomas TD, Ellwood DC, Longyear VM (1979) Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol 138(1):109–117
Thomsen MH (2005) Complex media from processing of agricultural crops for microbial fermentation. Appl Microbiol Biotechnol 68:598–606. doi:10.1007/s00253-005-0056-0
Thongchul N (2013) Production of LA and polyLA for industrial applications. In: Yang ST, El-Ensashy H, Thongchul N (eds) Bioprocessing technologies in biorefinery for sustainable production of fuels, chemicals, and polymers. Wiley, Hoboken, NJ, pp 293–316
Trontel A, Batusic A, Gusic I, Slavica A, Santek B, Novak S (2011) Production of D- and L-LA by mono- and mixed cultures of Lactobacillus sp. Food Technol Biotechnol 49(1):75–82
Venus J (2009) Continuous mode lactic acid fermentation based on renewables. Res J Biotechnol 4(2):15–22
Venus J, Richter K (2006) Production of lactic acid from barley: strain selection, phenotypic and medium optimization. Eng Life Sci 6(5):492–500. doi:10.1002/elsc.200520136
Vink ETH, Davies S, Kolstad JJ (2010) The eco-profile for current Ingeo® polylactide production. Ind Biotechnol 6(4):212–224. doi:10.1089/ind.2010.6.212
Vishnu C, Naveena BJ, Altaf M, Venkateshwar M, Reddy G (2006) Amylopullulanase-A novel enzyme of L. amylophilus GV6 in direct fermentation of starch to L(+) LA. Enzyme Microb Technol 38(3–4):545–550. doi:10.1016/j.enzmictec.2005.07.010
Vodnar DC, Venus J, Schneider R, Socaciu C (2010) Lactic acid production by Lactobacillus paracasei 168 in discontinuous fermentation Using Lucerne green juice as nutrient substitute. Chem Eng Technol 33(3):468–474. doi:10.1002/ceat.200900463
Von Wright A, Axelsson L (2012) LA bacteria. In: Lahtinen S, Ouwehand AC, Salminen S, von Wright A (eds) LA bacteria – microbiological and functional aspects, 4th edn. CRC Press/Taylor & Francis Group, Boca Raton, FL, pp 1–16
Walczak P, Oltuszak-Walczak E, Otlewska A, Dybka K, Pietraszek P, Czyzowska A, ygala A (2012) Xylose fermentation to optically pure l-lactate by isolates of Enterococcus faecium. New Biotechnol 29(Suppl):S62
Walkup PC, Rohrmann ChA, Hallen RT, Eakin DE (1991) Production of esters of lactic acid, esters of acrylic acid, lactic acid and acrylic acid. WO 91/11527 08.08.1991
Wang LM, Zhao B, Li FS, Xu K, Ma CQ, Tao F, Li QG, Xu P (2011) Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl Microbiol Biotechnol 89(4):1009–1017. doi:10.1007/s00253-010-2904-9
Wang L, Xue Z, Zhao B, Yu B, Xu P, Ma Y (2012) Jerusalem artichoke powder: a useful material in producing high-optical-purity L-lactate using an efficient sugar-utilizing thermophilic Bacillus coagulans strain. Bioresour Technol 8:174–180. doi:10.1016/j.biortech.2012.11.144
Watson D, Motherway MO, Schoterman MHC, van Neerven RJJ, Nauta A, van Sinderen D (2012) Selective carbohydrate utilization by lactobacilli and bifidobacteria. J Appl Microbiol 114(4):1132–1146. doi:10.1111/jam.12105
Wee YJ, Ryu HW (2009) Lactic acid production by Lactobacillus sp. RKY2 in a cell-recycle continuous fermentation using lignocellulosic hydrolyzates as inexpensive raw materials. Bioresour Technol 100:4262–4270. doi:10.1016/j.biortech.2009.03.074
Whiley RA, Hardie JB (2009) The genus I. Streptococcus. In: Whitman WB, Parte AC (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, NY, pp 655–711
Wood WA (1961) Fermentation of carbohydrates and related compounds. In: Gunsalus IC, Stanier RY (eds) The bacteria. Academic, New York, NY, pp 125–160
Wood BJB, Holzapfel WH (1995) Lactic acid bacteria in contemporary perspective. In: Wood BJB, Holzapfel WH (eds) The lactic acid bacteria, vol 2, The genera of lactic acid bacteria. Academic, London, pp 1–6
Wu X, Jiang S, Liu M, Pan L, Zheng Z, Luo S (2011) Production of L-LA by Rhizopus oryzae using semicontinuous fermentation in bioreactor. J Ind Microbiol Biotechnol 38:565–571. doi:10.1007/s10295-010-0804-8
Wu X, Altman R, Eiteman MA, Altman E (2013) Effect of overexpressing nhaA and nhaR on sodium tolerance and lactate production in Escherichia coli. J Biol Eng 7:3. doi:10.1186/1754-1611-7-3
Xiaodong W, Xuan G, Rakshit SK (1997) Direct fermentative production of lactic acid on cassava and other starch substrates. Biotechnol Lett 19:841–843. doi:10.1023/A:1018321200591
Yadav AK, Chaudhari AB, Kothari RM (2011) Bioconversion of renewable resources into LA: an industrial view. Crit Rev Biotechnol 1:1–19. doi:10.3109/07388550903420970
Yamane T, Tanaka R (2013) Highly accumulative production of L (+)-lactate from glucose by crystallization fermentation with immobilized Rhizopus oryzae. J Biosci Bioeng 115(1):90–95. doi:10.1016/j.jbiosc.2012.08.005
Yen HW, Kang JL (2010) LA production directly from starch in a starch-controlled fed-batch operation using Lactobacillus amylophilus. Bioprocess Biosyst Eng 33(9):1017–1723. doi:10.1007/s00449-010-0426-6
Yin PM, Nishina N, Kosakai Y, Yahiro K, Park Y, Okabe M (1997) Enhanced production of (L)-LA from corn starch in a culture Rhizopus oryzae using an air-lift bioreactor. J Ferment Bioeng 84:249–253. doi:10.1016/S0922-338X(97)82063-6
Zacharof MP, Lovitt RW (2013) Modelling and simulation of cell growth dynamics, substrate consumption, and LA production kinetics of Lactococcus lactis. Biotechnol Bioprocess Eng 8(1):52–64. doi:10.1007/s12257-012-0477-4
Zhang DX, Cheryan M (1991) Direct fermentation of starch to LA by Lactobacillus amylovorus. Biotechnol Lett 13(10):733–738. doi:10.1007/BF01088178
Zhang Z, Jin B (2010) L(+)-lactic acid production using sugarcane molasses and waste potato starch: an alternative approach. Int Sugar J 112:17–22
Zhao J, Xu L, Wang Y, Zhao X, Wang J, Garza E, Manow R, Zhou S (2013a) Homofermentative production of optically pure L-LA from xylose by genetically engineered Escherichia coli B. Microb Cell Fact 12:57. doi:10.1186/1475-2859-12-57
Zhao K, Qiao Q, Chu D, Gu H, Dao TH, Zhang J, Bao J (2013b) Simultaneous saccharification and high titer LA fermentation of corn stover using a newly isolated LA bacterium Pediococcus acidilactici DQ2. Bioresour Technol 135:481–489. doi:10.1016/j.biortech.2012.09.063
Zheng L, Bai ZZ, Xu TT, He BF (2012) Glucokinase contributes to glucose phosphorylation in D-LA production by Sporolactobacillus inulinus Y2-8. J Ind Microbiol Biotechnol 39:1685–1692. doi:10.1007/s10295-012-1176-z
Zhou S, Causey TB, Hasona A, Shanmugam KT, Ingram LO (2003a) Production of optically pure D-LA in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69(1):399–407. doi:10.1128/AEM.69.1.399-407.2003
Zhou S, Shanmugam KT, Ingram LO (2003b) Functional replacement of the Escherichia coli D-(−)-Lactate dehydrogenase gene (ldhA) with the L-(+)-Lactate dehydrogenase 2326 gene (ldhL) from Pediococcus acidilactici. Appl Environ Microbiol 69(4):2237–2244. doi:10.1128/AEM.69.4.2237-2244.2003
Zhou L, Niu DD, Tian KM, Chen XZ, Prior BA, Shen W, Shi GY, Singh S, Wang ZX (2012) Genetically switched d-lactate production in Escherichia coli. Metab Eng 14(5):560–568. doi:10.1016/j.ymben.2012.05.004
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Idler, C., Venus, J., Kamm, B. (2015). Microorganisms for the Production of Lactic Acid and Organic Lactates. In: Kamm, B. (eds) Microorganisms in Biorefineries. Microbiology Monographs, vol 26. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45209-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-45209-7_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45208-0
Online ISBN: 978-3-662-45209-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)