Abstract
Pathogenic Leptospira has the capacity to infect a broad range of mammalian hosts. Leptospirosis may appear as an acute, potentially fatal infection in accidental hosts, or progress into a chronic, largely asymptomatic infection in natural maintenance hosts. The course that Leptospira infection follows is dependent upon poorly understood factors, but is heavily influenced by both the host species and bacterial serovar involved in infection. Recognition of pathogen-associated molecular patterns (PAMPs) by a variety of host pattern recognition receptors (PRRs) activates the host immune system. The outcome of this response may result in bacterial clearance, limited bacterial colonization of a few target organs, principally the kidney, or induction of sepsis as the host succumbs to infection and dies. This chapter describes current knowledge of how the host recognizes Leptospira and responds to infection using innate and acquired immune responses. Aspects of immune-mediated pathology and pathogen strategies to evade the host immune response are also addressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Leptospira can cause two distinctly different disease manifestations depending on the mammalian host and infecting serovar. Infection leads to either a chronic, nearly asymptomatic infection or an acute, potentially life-threatening disease. The most severe form, classic Weil’s disease, or acute leptospirosis, occurs most commonly in accidental hosts, including humans, with a wide range of disease manifestations (Faine et al. 1999). In contrast, infection of a normal maintenance host will typically result in a chronic infection with little outward sign of infection (Faine et al. 1999). Maintenance hosts most commonly show evidence of infection during pregnancy, manifested by the appearance of reproductive failure (infertility, abortions, stillbirths, or birth of weak offspring). It is important to note that the same bacterial strain can often cause both acute and chronic infections, depending largely on the mammalian species that is infected. Development of chronic or acute infection is dependent upon poorly understood factors that pair specific mammalian host species with selected Leptospira serovars. Presumably, the interplay between the host immune system and infecting strain of bacteria is critical in directing the outcome of Leptospira infection.
Keeping in mind the dual nature of Leptospira is key to understanding the disease and how the immune system responds to infection. Early work by Adler, Faine, and coworkers clearly established the importance of antibody in providing immune protection in leptospirosis, at least for some host species (Adler and Faine 1977). However, these studies do not tell the full story. Components of both innate and acquired immune systems have been identified that respond to Leptospira infection. Both humoral and cell-mediated immunity (CMI) are needed for immune protection against Leptospira, yet at the same time immunological processes may also contribute to tissue damage during infection. Infected hosts are challenged by a number of bacterial properties that alter the host response or that contribute to immune evasion and persistent infection.
Although Leptospira can infect a wide range of mammalian species, most experimental infection studies have been conducted in golden Syrian hamsters , a species particularly susceptible to acute infection (Morton 1942). However, lack of well characterized immunological reagents for use with hamster tissues has limited our understanding of many aspects of the immune response to Leptospira infection. Although a wide variety of reagents are available for characterization of the mouse immune response, most mouse strains are refractory to infection by pathogenic strains of Leptospira after about 1 month of age (Packchanian 1940). This situation has slowed analysis of many aspects of the host immune response during leptospirosis. Studies involving experimental infection of other mammalian hosts, notably cattle, have helped to establish a prominent role for components of the cellular immune response in development of protective immunity, especially in maintenance hosts. Thus, the overall view of the immune response during leptospirosis draws on knowledge gained from the host responses in widely divergent mammalian genera infected with diverse species of Leptospira.
From the perspective of the host, pathogenic spirochetes, including Leptospira, are difficult adversaries to remove. Pathogenic spirochetes express few proteins on the outer membrane surface (Haake et al. 1991, see the chapter by D.A. Haake and W.R. Zückert, this volume), and may vary expression of surface proteins in response to environmental factors such as iron, temperature, and osmolarity (Lo et al. 2006; Matsunaga et al. 2005, 2007). Limited expression of antigenic surface proteins presents few targets for pathogen recognition and development of a protective immune response by the host. Additionally, spirochete outer membranes are loosely attached to the peptidoglycan layer and are easily removed by mild detergent (Haake et al. 1991; Zuerner et al. 1991). Lateral movement of outer membrane antigens through the lipid bilayer with little or no impediment enables the bacteria freedom of movement when bound by antibody (Charon et al. 1981). Thus, attachment of antibody to surface proteins does not impede motility, often a key factor in tissue penetration. Although leptospiral LPS is less pyrogenic than typical Gram-negative LPS , it stimulates a strong immune response that may, or may not, be important for immunoprotection, depending on the nature of the host–serovar interaction.
This chapter reviews historic and recent findings related to the immune response of the host to Leptospira infection, the bacterial targets of the immune response, and possible role of the immune response in contributing to disease manifestations. Complicating our ability to develop an understanding of how hosts resist Leptospira infection is the variability associated with past experimentation, which has often used different Leptospira species, serovars, and strains, and different mammalian host species. Additionally, a critical flaw in some studies has been the use of strains that had undergone many in vitro passages in bacteriological media without first assessing infectivity (ID50) or lethality (LD50) before animal experimentation. Consequently, findings from different laboratories may appear contradictory, yet could simply represent differences between mammalian hosts or the species, serovar, or strain used in experimentation. Recent development of genetic tools has facilitated construction of defined leptospiral mutants, and this is allowing us to discern the importance of specific bacterial genes in virulence (see the chapter by M. Picardeau, this volume). Likewise, recent development of highly inbred or genetic knockout strains of mice with known genetic deficiencies is now leading to a limited, but growing, understanding of the genetic basis of host susceptibility to infection. It is therefore important to bear in mind that most of the studies described in this chapter are drawn from experiments conducted with widely variable use of hosts and pathogens. Continued development and use of highly infectious (i.e., strains with low ID50) knockout mutants should help to resolve the relative importance of specific genes in host selection and virulence.
2 Animal Models
A wide variety of animal species have been used as hosts for experimental leptospirosis. Early studies used guinea pigs as a preferred animal model host to study acute infection (Noguchi 1918). In the mid-twentieth century, it was discovered that hamsters were particularly susceptible to Leptospira infection (Morton 1942). Owing to their general good health, rapid growth, and lower cost, hamsters are now routinely used as the primary model for acute leptospirosis (Haake 2006). Hamsters retain susceptibility to acute infection with increasing age more than many other animal species and mimic acute infections that share some similarities to clinical disease in humans. Hamsters have been used extensively to test bacterial strain infectivity and virulence, and as a model for testing vaccine efficacy. Indeed, the hamster model of infection is so predictable that many government and international organizations use the hamster model for testing vaccine efficacy.
Common laboratory mice (Mus musculus) and rats (Rattus novegicus) are generally unsuitable as hosts for acute leptospirosis; these species are only susceptible to developing acute leptospirosis within a very short window of time after birth (Packchanian 1940), unless they have specific genetic deficiencies. Infection of mice or rats older than a few weeks of age will more likely lead to development of chronic infections limited to colonization of kidney. However, as will be described in more detail below, infection of cyclophosphamide-treated mice (Adler and Faine 1976) or mice with toll-like receptor (TLR) deficiencies (Pereira et al. 1998) can result in lethal infection. Use of well-defined genetic knockout (KO) mouse strains in leptospirosis research is becoming more common. Rats are also being used as experimental hosts to study chronic leptospirosis (Athanazio et al. 2008; Monahan et al. 2008; Tucunduva de Faria et al. 2007). Use of rats and mice allows access to well-defined tools and the availability of genetically defined strains is leading to new knowledge on components of the immune system that are important for protective immunity.
Although experimental studies using livestock are quite expensive, cattle, goats, and pigs have been used as experimental hosts for infection and vaccination studies for leptospirosis. This is due in large part on the concern for zoonotic transmission between livestock and humans, the impact of leptospirosis on livestock production costs, and the need for effective vaccines in production animals. Research on leptospirosis in cattle has provided new information on the role of CMI in controlling leptospirosis.
Experimental small animals are most often inoculated with Leptospira by intraperitoneal (IP) injection (Haake 2006). Although an unnatural method of disease transmission, IP injection is an efficient, reproducible method for inoculation of small animals such as hamsters and leads to rapid dissemination in the animal. Two alternative methods of experimental inoculation of Leptospira thought to mimic natural routes of infection include conjunctival instillation (Thiermann and Handsaker 1985) and subcutaneous injection (Truccolo et al. 2002).
Virulent leptospires injected into the peritoneum establish a transient peritonitis and can be detected in and around blood vessels within 2 days (Zuerner et al. 2012). In acute leptospirosis, the bacteria may first migrate to the pancreas and potentially reduce insulin production, as seen in some human infections (Spichler et al. 2007). Within 3–4 days enough bacteria can be detected in the kidney to be visualized by microscopic examination of stained sections. If the infection does not progress to fulminant leptospirosis, the bacteria often remain in the kidney with occasional migration to other tissue (including brain and pancreas) (Zuerner et al. 2012). However, in hamsters, many Leptospira strains produce acute infections and the bacteria can be detected in nearly all tissue in the body, including freely swimming in blood. The interval between injection of virulent Leptospira and onset of clinical signs of infection varies, and is dependent upon the strain used, number of in vitro passages, and infectious dose. Standardized tests for vaccine potency or virulence checks for strains are limited to 28 days, by which time most animals should have shown clinical signs of infection.
Many Leptospira infection studies have used death as an endpoint, a practice that has been replaced with use of alternative criteria such as onset of clinical signs of infection, at which point animals are euthanized to avoid pain and distress. This approach leads to calculation of a modified LD50. Determination of ID50 is often more difficult, due to the requirement to detect Leptospira by culture or through direct examination of tissue. There is considerable variation in LD50 values for different strains. Critical points for consideration when planning experimental infections are the age and number of in vitro passes of the culture. Older cultures, or cultures that have been propagated in vitro for several passages, tend to lose virulence. Using hamster virulence as a guide, LD50 values for Leptospira strains may vary from <10 to >108 (Haake 2006).
3 Host Detection of Pathogens
3.1 Pattern Recognition Receptors
How does the immune system detect the presence of microbial pathogens? Mammalian cells display a variety of receptors on the cell surface with the design and purpose to recognize molecular signatures that are characteristic for microbial pathogens; these signatures are referred to as pathogen-associated molecular patterns (PAMPs) (Akira et al. 2006; Iwasaki and Medzhitov 2010). PAMPs include a wide variety of molecules such as bacterial LPS, lipoproteins, peptidoglycan, and flagella proteins. The host receptors, referred to as pattern recognition receptors (PRRs) , interact with PAMPs, initiating a series of intracellular signals that trigger the host response to infection. Host receptors that recognize PAMPs include toll-like receptors (TLRs) and C-type lectin receptors (CLRs) (Akira et al. 2006; Iwasaki and Medzhitov 2010). A related group of receptors that recognize damage associated molecular patterns (DAMPs) , which develop during infection, include receptors for advanced glycosylation end products (RAGE) (Williams et al. 2010). Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) recognize both PAMPs and DAMPs. Interaction of PAMPs and DAMPs with appropriate receptors leads to intracellular signaling cascades that trigger the innate immune response and help direct acquired immune responses (Iwasaki and Medzhitov 2010; Williams et al. 2010). Therefore, understanding these receptors and the responses they initiate are essential in developing an understanding of how the host responds to infection.
3.1.1 Toll-like Receptors
Toll-like receptors are the most thoroughly studied PRRs and in many cases initiate the first part of the host response to infection (Aderem and Ulevitch 2000). Most of the published research on recognition of Leptospira by PRRs has focused on TLRs, and therefore this section will focus on TLR detection of PAMPs and subsequent cellular responses. TLRs are a group of related transmembrane proteins with an extracellular pattern recognition domain and a cytoplasmic domain responsible for transmitting the signal generated from the extracellular domain to the host response network (Napetschnig and Wu 2013). The cytoplasmic portions of TLRs share a protein domain with the interleukin 1 receptor (IL-1R). This intracellular toll/interleukin 1 receptor (TIR) domain interacts with cytoplasmic proteins to initiate a signaling cascade that triggers the host cell to respond to the threat of infection.
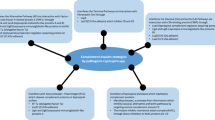
LPS has been used extensively to study the intricacies of TLR signaling and the host response to LPS will be used here to provide an overview of the TLR signaling pathway (Fig. 1 summarizes this process) and to highlight some unusual attributes of the response to Leptospira. Detection of LPS from most Gram-negative bacterial genera involves TLR4, myeloid differentiation protein 2 (MD-2), and CD14. CD14 binds LPS and transfers the molecule to MD-2 (Kawai and Akira 2010). When MD-2 binds the lipid A portion of a LPS molecule, it undergoes a structural change and forms a protein pocket that interacts with TLR4 (Park et al. 2009). This interaction initiates the intracellular TIR domains to come together and form a site where adaptor proteins assemble into oligomeric structures and initiate the intracellular signaling cascade. The TIR domains of most TLRs interact with MyD88 , a protein that forms an oligomeric structure called a Myddosome with IRAK4, IRAK1, and IRAK2 (Napetschnig and Wu 2013). MyD88 forms the top of the structure with IRAK4 forming a layer between MyD88 and IRAK1/IRAK2. MyD88, IRAK1, IRAK2, and IRAK4 are phosphokinases, and one proposed model suggests MyD88 promotes phosphorylation of IRAK4, with subsequent downstream phosphorylation from IRAK4 to IRAK1 and IRAK2 (Napetschnig and Wu 2013). Phosphorylation by the myddosome complex to additional kinases (including p38 MAP kinase) ensues, thereby activating transcriptional regulatory proteins, including nuclear factor kB (NFkB), AP1, and Sp-1 (Napetschnig and Wu 2013), described in more detail below.
Pattern recognition and host response. a Before PAMP (shown here as LPS) interaction with MD-2, the two TIR domains of the TLR1/TLR2 heterodimer or TLR2/TLR2 homodimer are disassociated from each other, and CD14 is not tightly associated with the TLRs. b The MD-2/LPS complex binds the TLR dimer, and CD14 joins the complex; this process leads to structural changes in the TLRs that bring the TIR domains together. c MyD88 binds the TIR domain and forms the myddosome consisting of MyD88 (top), IRAK4 (middle), and IRAK1/2 (bottom). d Phosphorylation from MyD88 through the myddosome is passed to one of several cytoplasmic kinases. e The phosphorylation cycle is propagated through a new cascade, ultimately leading to phosphorylation of IκB. Phosphorylated IκB undergoes ubiquitination, releasing NFκB. f NFκB migrates to the nucleus and activates transcription of immune response genes resulting in induction of the immune response
Although most of the TLR research on Leptospira has focused on TLR2 and 4 (see below), studies on other spirochetes, particularly Borrelia burgdorferi, have shown that TLR5 (Bernardino et al. 2008; Cabral et al. 2006), TLR8 (Cervantes et al. 2011), and TLR7 and TLR9 (Petzke et al. 2009) also play a role in recognizing spirochetes. TLR1, TLR2 and TLR6 are structurally similar and can form heterodimers that are involved in lipoprotein detection, TLR5 detects the presence of flagellin, TLR7 and TLR8 recognize single stranded RNA and imidazoquinoline compounds, and TLR9 recognizes unmethylated CpG motifs in DNA (Akira et al. 2006).
TLRs use a variety of accessory proteins during PAMP recognition and some of these proteins, e.g., MD-2, appear to have multiple functions. For example, LPS-bound MD-2 is also phosphorylated, possibly during endocytosis of the LPS-TLR4-MD-2 complex (Gray et al. 2011). In addition, TLR complexes can also function from within intracellular compartments. TLR4-MD-2 appears to be capable of sensing the presence of LPS; LPS binding through this pathway induces a distinct set of LPS inducible genes (Shibata et al. 2011). While the Myddosomes connect most TLRs to the intracellular signaling network, TIR-domain-containing adapter-inducing interferon-β (TRIF), a protein recognized by TLR3 for signal propagation, is also used for this purpose (Napetschnig and Wu 2013; Petnicki-Ocwieja et al. 2013). Myddosome-mediated signals primarily result in induction of inflammatory cytokine and chemokine expression, whereas TRIF-mediated signals induce type I interferons (Kawai and Akira 2010). TLR2-TRIF mediated signaling was recently demonstrated in response to B. burgdorferi infection in mice (Miller et al. 2010), and may have a role in detecting other spirochetes, including Leptospira. These studies highlight the complexity of signal transduction in recognition of microbial pathogens and rapid growth in our understanding of how host cells sense the presence of microbial pathogens.
3.1.2 Other Pattern Recognition Receptors
NLRs are large oligomeric cytoplasmic proteins that contain the NOD domain, which in NLRs is referred to as NACHT (Leemans et al. 2011). The NOD domain is centrally located, and NLRs also have a carboxy-terminal leucine-rich repeat (LRR) motif. Interaction of NLRs with the appropriate PAMP or DAMP leads to formation of multiprotein structures called inflammasomes. Once formed, inflammasomes activate caspase-1, which in turn activates proinflammatory cytokines IL-1β and IL-8 (Leemans et al. 2011). If the inflammasome is not properly regulated, then a proinflammatory cycle ensues leading to host directed tissue damage (Leemans et al. 2011), for example, during acute lung injury (ALI). Currently, the NLRP3 inflammasome is the best characterized, and is often associated with a variety of inflammatory disorders including chronic kidney disease (Anders and Muruve 2011).
Other PRRs include the mannose receptor, a member of the CLR family. The mannose receptor has been associated with binding of B. burgdorferi to monocyte/macrophages (Cinco et al. 2002). B. burgdorferi interaction with α3β1 integrin also induces proinflammatory cytokine production, independent of TLRs or MyD88 (Behera et al. 2006). The binding to the α3β1 integrin sends a signal to the c-Jun NH(2)-terminal kinase (JNK) pathway and leading to induction of a proinflammatory response (Behera et al. 2006). The roles of these other PRRs in Leptospira infection have not been well defined.
3.1.3 PRR-Mediated Transcription Response
The signaling cascade triggered by interaction of PAMPs with the appropriate receptor results in activation of several transcription factors, most notably NFkB (Napetschnig and Wu 2013). Nonactivated NFkB is located in the cytosol and bound by the inhibitor IkB . A portion of the signaling cascade leads to phosphorylation of IkB kinase (IKK), which in turn phosphorylates IkB. Phosphorylated IkB is recruited to proteosomes and degraded, thereby releasing NFkB (Napetschnig and Wu 2013). IkB-free NFkB migrates to the nucleus where it binds chromosomal DNA at specific sites with the assistance of accessory transcription factors, e.g., activator protein-1 (AP-1), that are also activated by phosphorylation (Napetschnig and Wu 2013). These events lead to transcriptional activation of response genes enabling the activated cells to respond to infection. NFkB can be activated by a variety of molecular signals and pathways, including TNF-α, and IL-1 via TRAF6. This process initiates the innate response to infection, induces the expression of inflammatory cytokines and chemokines, and helps direct development of the acquired immune response.
4 Innate Immunity
4.1 TLR Recognition and Response
The vast majority of research on pattern recognition of Leptospira has focused on the role of TLRs. Although leptospiral LPS has low endotoxicity, it stimulates a strong antibody response during infection (Chapman et al. 1988), or as a result of vaccination with whole-killed cell bacterins (Bolin et al. 1989). As noted above, LPS from most bacteria is predominantly detected using TLR4. However, LPS from Leptospira spp. is primarily recognized in humans by TLR2/TLR1 (Werts et al. 2001). Leptospiral LPS is recognized primarily by TLR2, but TLR4 also contributes to activation of murine cells (Nahori et al. 2005). Information regarding TLR recognition of leptospiral LPS is not available for other mammalian species.
The C3H/HeJ mouse strain lacks functional TLR4. Infection of C3H/HeJ mice with Leptospira interrogans serovar Icterohaemorrhagiae leads to acute leptospirosis and death (Pereira et al. 1998). Subsequent studies have shown that C3H/HeJ mice are also susceptible to infection with L. interrogans serovars Copenhageni and Manilae (Viriyakosol et al. 2006; Koizumi 2003; Nally et al. 2005) indicating that this is not a serovar-specific property. These studies suggest that TLR4 is of critical importance in controlling Leptospira infection in mice. The respective roles of TLR2 and TLR4 were studied using mice with genetically defined mutations in TLR2 and TLR4 (Chassin et al. 2009), and results from this and related studies have helped to define links between innate and adaptive immunity and control of Leptospira infection. In these studies, C57BL/6 J wild type (WT) mice were used as a control group and bred with TLR2−/−, TLR4−/−, and double TLR2/TLR4 knockout (DKO) mice, followed by backcrossing to insure that the TLR mutations were studied in a uniform C57BL/6 J genetic background. The resulting mutant strains and WT were inoculated with L. interrogans serovar Icterohaemorrhagiae and the outcome of infection assessed. Consistent with previous results using TLR4 deficient C3H/HeJ mice as hosts for Leptospira, infection of C57BL/6 J TLR4−/− mice also resulted in lethal infections, as did infections in DKO mice (Chassin et al. 2009). TLR4−/− mice survived longer than DKO mice, a finding that suggests loss of TLR2 increases susceptibility to lethal infection over the TLR4 deficiency alone. The bacterial loads in liver, lung, and kidney were assessed, and compared to WT C57BL/6 J mice in which bacteria were cleared. DKO strains had high bacterial loads in all three organs, but TLR2−/− or TLR4−/− knockout mice had significantly lower bacterial loads in kidney and lung, as compared to DKO mice, and in these two organs resembled WT mice. Infected TLR4−/− mice had high bacterial loads in the liver, whereas TLR2−/− mice resembled WT mice with little or no bacteria detected (Chassin et al. 2009). These studies led to a model where TLR2 and TLR4 have overlapping functions in kidney, but functional TLR4 receptors are needed for bacterial clearance in liver (Chassin et al. 2009). Removal of leptospires from liver in TLR2−/− mice is consistent with the finding that cytokine expression in mouse macrophages involves LPS interaction with TLR4 (Chassin et al. 2009; Viriyakosol et al. 2006). In addition, there is also evidence for TLR-independent induced inflammation (Chassin et al. 2009).
4.1.1 NLR Mediated Response
Detection of PAMP and DAMP signals by NLRs also occurs during leptospirosis. The NLRP3 inflammasome is primed by TLR2/4 interaction with LPS and activated by a depression of the Na/K-ATPase pump by leptospiral glycolipoprotein (Burth et al. 1997; Lacroix-Lamande et al. 2012). Leptospiral glycoliporotein is a suspected cytotoxic component of the Leptospira outer membrane (Vinh et al. 1986), and has previously been shown to activate peripheral blood mononuclear cells (Diament et al. 2002). Chronic inflammasome activation may be one pathway leading to the development of tissue lesions, especially in kidney; the NLRP3 inflammasome can be triggered by either sterile or infectious stimuli (Anders and Muruve 2011; Vilaysane et al. 2010). Inflammasome activation has been implicated in a variety of chronic kidney diseases (Anders and Muruve 2011), and specific activation of the NLRP3 inflammasome and induction of IL-1β and IL-18 secretion has been associated with the development of chronic kidney disease in a noninfectious mouse model (Vilaysane et al. 2010).
4.1.2 CLR Mediated Response
DC-SIGN and mannose-binding protein are two CLRs that have been identified as having possible roles in Leptospira recognition. The mannose-binding lectin (MBL) is elevated in serum during leptospirosis, with higher serum levels being detected in human patients during an outbreak of more classical Weil’s disease than in an outbreak of more moderate disease manifestations (Miranda et al. 2009). This finding suggests that MBL may be useful as a marker for severe leptospirosis (Miranda et al. 2009). Leptospira detection by human monocyte-derived dendritic cells via DC-SIGN induced secretion of the proinflammatory cytokine TNF-α and IL-12 (which enhances the cytotoxicity of NK and CD8+ T cells ) (Gaudart et al. 2008). However, DC-SIGN induced limited secretion of the anti-inflammatory cytokine IL-10 (Gaudart et al. 2008). Low passage, virulent serovar Pyrogenes strain 2317 induced greater TNF-α and IL-12 secretion than a high passage avirulent derivative of strain 2317 (Gaudart et al. 2008). These results are consistent with studies that show high serum TNF-α correlates with a poor prognosis in human leptospirosis patients (Tajiki and Salomão 1996; Kyriakidis et al. 2011; Wagenaar et al. 2009a). Serum IL-10 levels are also elevated during acute leptospirosis, but this may be in response to high levels of inflammatory cytokines; IL-10 functions to restrain the inflammatory response. The inability of high serum levels of IL-10 to control the inflammation response may lead to immune pathology (see Sect. 6). IL-10 is important for limiting Borrelia turicatae growth in mice, showing a commonality in the need for a balanced immune response to survive infections caused by these spirochetes (Londoño et al. 2008).
4.1.3 Cytokine Induction
The role of inflammatory cytokines in C57BL/6 J mice and assorted mutants was also examined (Chassin et al. 2009). Levels of inflammatory cytokines were highest in DKO mice in kidney and liver as compared to WT, TLR2−/−, or TLR4−/− mice. This result may be due in part to the higher bacterial load in liver and kidney because DKO mutants were unable to clear bacteria during infection, and the observed induction of inflammatory cytokines was by a non-TLR based pathway (Chassin et al. 2009). MyD88−/− mice have approximately the same levels of inflammatory cytokines as TLR2-TLR4 DKO mice, leading to the conclusion that TLRs other than TLR2 and TLR4 do not play a significant role in detecting Leptospira, or for inducing production of IL-1β, IL-6, and chemokines There is no evidence that TLR3, which uses a MyD88-independent signaling pathway, has a significant role in recognition of Leptospira antigens. TNF-α-induced CCL5 (also known as RANTES), CXCL1 (also known as KC) and CXCL2 (also known as MIP-2) (Chassin et al. 2009). This conclusion is inconsistent with another report concluding that TNF-α production is largely TLR5-dependent in human leukocytes (Goris et al. 2011). These differences may indicate either a difference in the relative importance of TLRs in different tissues or the ability of leukocytes from different host species to use different TLRs for pathogen detection. However, a general program of robust production of inflammatory cytokines is consistent across diverse mammalian hosts; the findings cited above are similar to studies conducted in hamsters that also have high inflammatory cytokine production during the acute phase of infection (Lowanitchapat et al. 2010; Marinho et al. 2009; Matsui et al. 2011; Vernel-Pauillac and Merien 2006; Vernel-Pauillac and Goarant 2010).
The array of circulating cytokines and chemokines induced at significant levels in both humans and mice during leptospirosis includes IL1-β , IL-6 , IL-10 , MCP-1 , and TNF-α (Wang et al. 2012). In humans, GM-CSF (granulocyte macrophage colony-stimulating factor) and CCL2 (also known as macrophage chemotactic protein 1, MCP-1) were also found at increased levels in sera (Wang et al. 2012); these two compounds promote granulocyte and monocyte production and recruit leukocytes to sites of inflammation, respectively. Human patients also had elevated IL-11; IL-11 contributes to platelet replenishment due to thromobocytopenia (a frequent complication of leptospirosis) and induction of acute phase proteins (Wang et al. 2012). There is also in vitro evidence of locally produced cytokine and chemokine production; CXCL1/KC , iNOS , and CCL2/MCP-1 , are produced by cultured renal cells using a TLR1/2 driven pathway via MyD88 and a MAP38 kinase pathway (Hung et al. 2006a, b; Yang et al. 2006). The result of CXCL1/KC synthesis in the kidney may help recruit neutrophils to the site of infection. However, another consequence of chemokine/cytokine synthesis in the kidney may be accumulation of fibrous tissue and decreased kidney function (see Sect. 6).
4.2 Cellular Response
Monocytes/macrophages utilize PRR-mediated activation to provide innate immune protection of the host, especially during the early stages of infection. Leptospiral LPS and hemolysins stimulate macrophages to produce IL-1β, IL-6, IFN, and TNF-α (Isogai et al. 1990; Wang et al. 2012). In addition, macrophages treated with leptospiral LPS have enhanced phagocytic activity (Isogai et al. 1990). The role of macrophages in protection against Leptospira infection was suggested in a study where mice were treated with silica to inhibit macrophage function, and it was found that treated mice were impaired in bacterial clearance and had increased susceptibility to infection (Isogai et al. 1986). The role of antibody appears to be important for macrophage-mediated killing of Leptospira; several independent studies have shown that phagocytosis leading to decreased bacterial viability requires opsonization with homologous antibody (Banfi et al. 1982; Cinco et al. 1981; Vinh et al. 1982). Opsonization may overcome an inherent immune evasion mechanism of Leptospira that impairs macrophage function; freshly isolated virulent strains can induce macrophage apoptosis (Merien et al. 1997; Jin et al. 2009) or limit lysosomal fusion with bacteria-laden phagosomes (Toma et al. 2011).
Polymorphonuclear leukocytes (PMNs) include granulocytes, eosinophils, and neutrophils, and these cells comprise important components of the innate immune response, but their role in protection against Leptospira infection is unclear. Two antibacterial peptides produced by bovine neutrophils, Bac5 (also known as cathelicidin-2) and Bac7 (cathelicidin-7), can kill Leptospira (Scocchi et al. 1993). Intact Leptospira, leptospiral peptidoglycan or LPS induce PMN adherence to endothelial cells (Isogai et al. 1989a; Dobrina et al. 1995). However, resistance to PMN phagocytosis has been suggested as a potential virulence factor for Leptospira (Wang et al. 1984). Acute infection with a hamster lethal strain of Leptospira borgpetersenii serovar Hardjo resulted in the in vivo formation in blood of large bacterial–neutrophil aggregates without bacterial clearance (Zuerner et al. 2012). Much like the findings described above noting that macrophages depend on antibody for phagocytosis, PMNs also require assistance from the humoral response to provide immune sera for phagocytosis of Leptospira (McGrath et al. 1984; Wang et al. 1984). These results are similar to those reported for B. burgdorferi, which appears to be more susceptible to PMN attack in the presence, rather than absence, of antibody (Lusitani et al. 2002).
Although platelets have traditionally been associated with hemostasis, recent studies have shown these cells are important components of the innate immune response (Yeaman 2009). Platelets utilize TLRs to detect PAMPs, and produce antimicrobial peptides and cytokines (Cognasse et al. 2008). Activated platelets contribute to the activation of neutrophils and neutrophil extracellular formation (Yeaman 2009). Leptospiral LPS induces platelet aggregation (Isogai et al. 1989b), and thrombocytopenia often occurs in about half of human leptospirosis patients (Edwards et al. 1986). By contributing to platelet removal, Leptospira may be able to subvert part of the innate immune system during the early stages of infection.
5 Acquired Immunity
5.1 Humoral Response
Antibodies against Leptospira have a key role in providing immune protection against lethal infection in many potential host species. Adler and Faine concluded that antibody to Leptospira is essential for protective immunity with the discovery that mice treated with cyclophosphamide, which preferentially kills B cells , were susceptible to lethal Leptospira infection (Adler and Faine 1976, 1977). Nude mice , which lack a thymus, and therefore cannot produce T cells, were resistant to infection (Adler and Faine 1977). Mice lacking a functional Rag gene are unable to produce functional B and T cells due to an inability to undergo V(D)J recombination in immunoglobulin and T cell receptor genes. Likewise, SCID mice lack an enzyme responsible for DNA recombination needed for maturation of antigen specific B and T cells. μMT mice lack functional B cells. Infection with L. interrogans of Rag−/− , SCID mice, μMT, or nude mice treated with cyclophosphamide results in lethal infection (Adler and Faine 1977; Bandeira et al. 2011; Chassin et al. 2009; Nally et al. 2005). Adler and Faine (1977) clearly demonstrated the importance antibody (and therefore B cells) by injecting cyclophosphamide-treated nude mice with antibody to Leptospira resulting in protection against lethal Leptospira infection. Chassin et al. (2009) reported similar results: μMT mice were protected from lethal challenge using passive transfer of immune sera collected from infected WT mice at 20-days PI. These studies are consistent with an earlier study that showed transfer of maternal antibodies protected mice from becoming chronic carriers of Leptospira (Birnbaum et al. 1972). In contrast, T cells do not appear to have an important role in providing protection from lethal challenge in the mouse model; CD3−/− (T cell deficient) mice are resistant to lethal challenge (Chassin et al. 2009).
A key Leptospira antigen that is important for the development of immune protection in many host species is LPS. Immunization with Leptospira LPS protects hamsters against homologous challenge (Jost et al. 1989). Passive transfer of antibody to LPS has also been used successfully to protect mice, guinea pigs, monkeys, and dogs before lethal infectious challenge (Jost et al. 1986; Challa et al. 2011; Schoone et al. 1989). Indeed, development of antibody to the LPS component of whole cell bacterins is thought to be key for immune protection against lethal infection with several Leptospira serovars in many animal species. Development of agglutinating antibodies, predominantly IgM (Adler and Faine 1978), is important for serological diagnosis of exposure to Leptospira using the microscopic agglutination test (MAT). Antibodies to LPS develop early in infection (Chapman et al. 1991). However, LPS is the serovar-specific antigen in Leptospira and therein lies one of the problems with relying on the LPS component in vaccines for developing protective immunity against a broad spectrum of serovars; antibodies against LPS provide limited cross-protection against other serovars and may provide short duration of immunity. Development of antibody to leptospiral proteins has greater likelihood of cross-protection (Sonrier et al. 2000; Srikram et al. 2011; see the chapters by D.A. Haake and W.R. Zückert and by B. Adler, this volume). The major outer membrane protein of pathogenic Leptospira, LipL32, stimulates a significant early and sustained antibody response during infection (Haake et al. 1991, 2000; Zuerner et al. 1991). LipL32 is proteolytically cleaved in vitro (Haake et al. 2000; Zuerner et al. 1991; Cullen et al. 2002) and the protein is post-translationally modified (Witchell et al. 2014), processes that may limit exposure of antigenic epitopes of this protein on the cell surface (Pinne and Haake 2013). While antibodies to LipL32 are good indicators of infection, development of antibody to this protein does not appear to be protective (Lucas et al. 2011), and LipL32 is not needed for successful infection (Murray et al. 2009). Differential methylation of OmpL32 glutamic residues is another method by which Leptospira may alter exposure of antigenic epitopes during infection (Eshghi et al. 2012b). A more complete review of vaccine development is presented in the chapter by B. Adler, this volume, but, on the whole, the most successful Leptospira vaccines produced to date are composed of whole, killed bacteria (Bey and Johnson 1982), suggesting that a complex mixture of antigens may be required for protection.
5.2 Cell-Mediated Immunity
Although the data described above show the critical importance of a Th2 , or B cell -mediated protective immunity, both B and T lymphocytes have important roles in promoting an immune response to Leptospira infection. Rag−/− mice do not produce significant levels of IFN-γ in liver or kidney, indicating either B or T cells, or both classes of lymphocytes, are required for IFN-γ production during infection (Chassin et al. 2009). B cells appear to be primarily responsible for IFN-γ production and bacterial clearance in the liver, whereas T cells are responsible for these roles in the kidney (Chassin et al. 2009). Furthermore, histological evidence of kidney tissue damage is greater in CD3−/− animals lacking functional T cells, as compared to WT or μMT (B cell deficient) mice. Histological examination of infected kidneys showed evidence of interstitial inflammation and development of nodular infiltrates in T cell deficient mice that were absent from kidneys of infected WT or B cell deficient mice (Chassin et al. 2009). Finally, serological markers of renal damage were elevated in infected T cell deficient mice, but not infected WT or B cell deficient mice. Combined, these data provide a compelling argument that the Th1 , or CMI , response involving T cells is an important component of the immune response as it relates to Leptospira infection.
The studies above focused on protection against lethal Leptospira challenge. There is less information on what components of the immune response are critical to prevent against development of chronic infection by Leptospira. Elimination of chronic leptospirosis, especially in maintenance hosts, is important to reduce the likelihood of disease transmission to accidental hosts. Vaccination and challenge studies using serovar Hardjo infections of cattle have led to a better understanding of the immune response in regard to Leptospira infection of its natural maintenance host. In contrast to the animal studies described above that show antibody to LPS to be protective against lethal leptospirosis, similar studies in cattle have shown that high titers of antibody to LPS fail to protect cattle from infection with serovar Hardjo, the most common serovar associated with chronic bovine leptospirosis (Bolin et al. 1989). Vaccines in this class fail to induce a Th1, or CMI, response in cattle (Naiman et al. 2001b, 2002; Zuerner et al. 2011). Refined whole cell monovalent serovar Hardjo vaccines that protect against significant renal colonization stimulate antibody production, but also induce CMI (Bolin et al. 2000; Bolin and Alt 2001; Ellis et al. 2000). These vaccines stimulate CD4+ and γδ T cells to proliferate and produce IFN-γ in a recall response when exposed to serovar Hardjo antigens (Blumerman et al. 2007a; Naiman et al. 2001b, 2002). In short-term vaccine efficacy studies, where cattle were challenged approximately 4 months after vaccination, these vaccines either prevented renal colonization and urinary shedding of bacteria (Bolin et al. 2000; Bolin and Alt 2001; Ellis et al. 2000) or eliminated urinary shedding within a few weeks after challenge (Zuerner et al. 2011). However, duration of protective immunity remains a problem. Some animals may develop renal colonization with lesion formation if infected 1 year after vaccination (Zuerner et al. 2011). Nevertheless, the general success of these vaccines in limiting renal colonization provides a model to understand the role of CMI in controlling Leptospira infection in cattle, and perhaps other species.
Analysis of how bovine peripheral blood mononuclear cells (PBMCs) respond to vaccination and subsequent infectious challenge has revealed several characteristics of a protective Th1 response to Leptospira infection. Analysis of how cattle respond to L. borgpetersenii serovar Hardjo has played a key role in characterizing bovine γδ T cells because this class of lymphocytes replicate in response to vaccination with a monovalent serovar Hardjo and demonstrate a strong recall response to leptospiral antigens (Baldwin et al. 2002; Naiman et al. 2001a, b; Zuerner et al. 2011). γδ T cells are a unique class of CD4− CD8− T cells that comprise approximately 30 % of the normal adult ruminant PBMC population (Hein and Mackay 1991). This unique class of T cells comprises a smaller percentage of PBMCs in nonruminant species, and is not as well characterized as CD4+ and CD8+ αβ T cells. Most bovine γδ T cells possess the WC1 scavenger receptor on the cell surface (Baldwin et al. 2000). WC1 is one of several members of the scavenger receptor cysteine-rich (SRCR) family (Wang et al. 2011), a group of surface proteins that bind a variety of PAMPs including lipoproteins, lipoteichoic acid, and leucine-rich repeat proteins (Loimaranta et al. 2009). A protective monovalent serovar Hardjo vaccine induces a positive recall response to leptospiral antigens in CD4+ αβ - and γδ-T cells, but the response of CD8+ cells has varied between studies (Baldwin et al. 2002; Naiman et al. 2001b, 2002; Zuerner et al. 2011). Several studies have shown that the phenotype of Leptospira antigen-stimulated WC1+ γδ T cells is consistent with Th1 polarized cells: (1) they express the transcription factors T-bet and GATA-3, as well as IL-12Rβ2 which encodes the high affinity IL-12 receptor (Rogers et al. 2005a, b); (2) they are activated by treatment with IL-12; (3) γδ T cells have elevated transcription of genes associated with cytotoxic activity (Blumerman et al. 2007b); and (4) cells have elevated expression of B-cell activating factor (BAFF, also referred to as BLysS, for B lymphocyte stimulator), and NDFIP2 (Blumerman et al. 2007b), a gene that promotes IFN-γ production in Th1 polarized lymphocytes (Lund et al. 2007). One of the characteristics of CD4+ T cells stimulated with Leptospira antigens is increased transcription of genes encoding cytotoxic functions and CXCL6 (granulocyte chemotactic protein), a chemokine that attracts neutrophils (Blumerman et al. 2007b).
Several studies on human patients have shown that exposure to Leptospira antigens induces a proliferative response in PBMCs. In particular, there is preferential expansion of γδ T cells in leptospirosis patient blood samples stimulated with Leptospira antigens (Barry et al. 2006; Klimpel et al. 2003). However, Tuero et al. (2010) did not detect the presence of a T cell memory response in patient blood following infection. The presence of inflammatory cytokines (de Fost et al. 2003) showed that Leptospira induced significant increases in human PBMC expression of IFN-γ, TNF-α, and IL-12 receptor, consistent with a strong Th1 response to infection. In addition, patients with, or suspected of having, leptospirosis had elevated concentrations of T- and NK-cell derived cytotoxic compounds or chemokines (De Fost et al. 2007).
NK cells are a group of cytotoxic lymphocytes often considered part of the innate immune response. However, recent reports have shown that NK cells have the capacity to mount a recall response to antigens (Cooper et al. 2009; Sun et al. 2009). NK cells, defined as CD335+ (i.e., containing the natural cytotoxicity receptor, NKp46) from cattle vaccinated with a monovalent serovar Hardjo vaccine demonstrated a recall response by expressing IFN-γ when exposed to Leptospira antigens (Zuerner et al. 2011). Surprisingly, NK cells from sham vaccinated cattle also demonstrated an antigen recall response, that by 6 weeks post challenge, was indistinguishable from cells obtained from vaccinated animals (Zuerner et al. 2011). A key difference in the response of lymphocytes from vaccinates versus sham vaccinates may be the ability of the vaccine to induce sustained lymphocyte proliferation with IFN-γ expression following infectious challenge (Naiman et al. 2001b, 2002; Zuerner et al. 2011). The presence of immune cells in vaccinated animals at initiation of infection may limit the initial tissue colonization by Leptospira, and allow the host to eventually resolve the infection. In contrast, infection of nonimmune animals likely allows substantial colonization of host tissue that cannot be removed by the host without additional forms of intervention, e.g., antibiotic treatment.
6 Immune Pathology
Some of the manifestations of acute leptospirosis may be the result of unrestrained activation of the host immune response. Several lines of evidence suggest that robust activation of inflammasomes may contribute to tissue damage, particularly lung and kidney. The two triggers (e.g., LPS and potassium efflux) needed to obtain high-level activation of inflammasomes resulting in robust production of IL-1β (Mariathasan and Monack 2007) are present during acute leptospirosis. LPS is an outer membrane component and potassium efflux is induced by the leptospiral glycolipoprotein (Lacroix-Lamande et al. 2012). Overstimulation of the inflammasome can contribute to tissue damage to lung (Xiang et al. 2011) and kidney (Vilaysane et al. 2010; Anders and Muruve 2011). Consistent with this hypothesis, evidence for “massive overexpression” of proinflammatory cytokines in hamsters experiencing severe leptospirosis has been presented (Matsui et al. 2011). Other studies have found high inflammatory cytokine levels associated with lethal infection of hamsters (Marinho et al. 2009; Vernel-Pauillac and Goarant 2010). Human patients suffering from acute leptospirosis also have evidence of overexpression of inflammatory cytokines , and soluble ST2 and long pentraxin PTX3 have been indicated as possible markers for acute infection (Wagenaar et al. 2009a, b). Elevated expression of neutrophil chemokines CXCL1 (Hung et al. 2006a, b) and CXCL6 (Blumerman et al. 2007b) may promote PMN migration, cell activation, and inflammation, all of which are associated with acute lung injury (Li et al. 2009). The presence of neutrophil–bacterial aggregates during acute infection (Zuerner et al. 2011), coupled with extracellular net formation can result in release of neutrophil granules and tissue damage (Abraham 2003; Lee and Downey 2001). Several immune receptors were upregulated in lung tissue from human patients that had died from acute leptospirosis and experienced pulmonary involvement (Del Carlo Bernardi et al. 2012).
Pathogenic Leptospira binds to chondroitin sulfate B, a proteoglycan of host cells (Breiner et al. 2009). Interaction of pathogenic Leptospira with endothelial cells induces cellular changes that are consistent with disruption of endothelial barriers, thereby aiding bacterial invasion (Martinez-Lopez et al. 2010). Interestingly, this process, at least in vitro, can be blocked using the ACE inhibitor lysinopril, suggesting that pharmaceutical treatments that supplement antibiotic therapy may be useful in limiting tissue damage (Martinez-Lopez et al. 2010). Tissue from infected animals often shows evidence of tubulointerstitial nephritis, an accumulation of extracellular matrix (ECM ) proteins in the kidney (Araujo et al. 2010), a process that can be replicated with mouse proximal tubule cells treated with Leptospira OMP preparation, which may well contain LPS (Yang et al. 2000). Evidence for a TLR2-driven signaling cascade stimulating ECM production in response to exposure to Leptospira outer membrane proteins, especially LipL32, was recently shown using immortalized human kidney cells (Hsu et al. 2010; Tian et al. 2011). Production of CXCL1/KC attracts neutrophils and monocytes (Hsu et al. 2010), and production TGF-B1 likely drives synthesis of type I and type IV collagen (Tian 2006), leading to fibrosis. In situ expression of inflammatory cytokines has been suggested as contributing to tissue damage in acute lung injury and pulmonary fibrosis (Kolb et al. 2001), and a similar mechanism of tissue damage and errant repair may also occur in renal tissue following infection with Leptospira and contribute to the development of tubulointerstitial nephritis.
iNOS expression may present a double-edged sword with respect to protecting the host and damaging tissue. Production of iNOS is important for host survival (Prêtre et al. 2011), but two studies have suggested that iNOS activity may contribute to damage observed during Leptospira-induced pulmonary hemorrhage (Chen et al. 2007; Yang and Hsu 2005).
Leptospirosis induced autoimmunity may contribute to the development of uveitis and loss of eyesight in horses (Faber et al. 2000; Verma and Stevenson 2012) and humans (Chu et al. 1998; Mancel et al. 1999). Antibodies from uveitis horses recognize two Leptospira proteins, LruA and LruB that share antigenic cross reactivity with eye proteins in horses (Verma et al. 2005). Sera from human uveitis patients also recognize LruA and LruB (Verma et al. 2008). Development of antibodies to other host proteins in leptospirosis patients has been reported, for example antibodies to host cardiolipin following infection (Rugman et al. 1991), but this has not been associated with development of autoimmune disease.
Autoimmunity, in the form of Goodpasture’s syndrome, has also been suggested as contributing to the development of pulmonary hemorrhage in a guinea pig model (Nally et al. 2004). In that model it was proposed that autoantibodies to connective tissue triggered by Leptospira infection were the cause of pulmonary damage. This hypothesis has since been disproven (Craig et al. 2009).
7 Immune Evasion
Leptospira has several proteins that have the capacity to bind components of the complement system, including factor H (fH) and factor H -like (fHl) proteins. By binding components of the complement system, Leptospira avoids complement activation and cell damage. Among the leptospiral proteins capable of binding components of the complement system are two endostatin-like proteins LenA (LfhA) and LenB (Stevenson et al. 2007; Verma et al. 2010), LigA and LigB (Castiblanco-Valencia et al. 2012), and LIC11834, and LIC12253 (Domingos et al. 2012). Some of these proteins, as well as many other Leptospira proteins including other members of the endostatin-like proteins (Stevenson et al. 2007) and LipL32 (Choy et al. 2011), bind a variety of host proteins, thereby masking bacterial antigens and contributing to evasion from the host immune system.
The major outer membrane protein of pathogenic Leptospira, LipL32, induces a strong antibody response and is a TLR2 agonist (Hsu et al. 2010). However, mutants in LipL32 are still infectious, and LipL32 monovalent vaccines are not protective (Lucas et al. 2011), findings consistent with a recent report that challenges the long-held belief that LipL32 is exposed on the bacterial surface (Pinne and Haake 2013). If the subsurface localization of this protein is confirmed, then perhaps the robust antigenic activity of LipL32 serves to divert the immune response away from effective targets for immune clearance and instead direct the immune response to antigens for which antibodies do not impair in vivo bacterial survival.
Leptospira may also mask antigenic sites on outer membrane proteins. Amino acids in OmpL32 are methylated, and the pattern of methylation changes depending on various environmental factors (Eshghi et al. 2012b). This process likely alters antibody access to this protein, thereby interfering with opsonization. As noted above, LipL32 is also post-translationally modified in a way that may alter antibody binding to antigenic domains on the major outer membrane protein (Witchell et al. 2014).
Leptospira is also able to penetrate host cells, including macrophages, and induce apoptosis (Merien et al. 1997, 1998; Jin et al. 2009). This function likely abrogates the normal role of macrophages in innate immunity and bacterial clearance. Expression of catalase may contribute to intracellular survival of Leptospira (Eshghi et al. 2012a).
8 Conclusions
We are gaining new insight into the complexities involved as mammalian hosts respond to Leptospira infection. The infection is a perpetual battle between host and bacterium, each with its own arsenal of weapons to attack the other. On the host side, an array of inflammatory cytokines and chemokines is used to stimulate cells to resist infection and kill the bacteria. The bacteria present an array of molecules to the host immune system that may initiate a destructive immune response leading to sepsis and death, or in a more limited setting, chronic tissue damage as the bacteria establishes a persistent infection. Information is needed on how to avoid over induction of a proinflammatory response and instead develop a measured response that limits host tissue damage while contributing to bacterial death. Identification of bacterial evasion strategies and key bacterial antigens is needed to enable development of safe, effective vaccines that promote clearance of bacteria from the host. These findings provide a basis for future study on the immune response to Leptospira infection. Future work may involve genetic determinants of susceptibility; several genetic polymorphisms that may predispose humans to severe infection have been identified (Fialho et al. 2009), and may be important for anticipating treatment strategies, especially in areas where humans are chronic carriers of endemic strains of Leptospira.
References
Abraham E (2003) Neutrophils and acute lung injury. Crit Care Med 31:S195–S199
Aderem A, Ulevitch RJ (2000) Toll-like receptors in the induction of the innate immune response. Nature 406:782–787
Adler B, Faine S (1976) Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans Serovar Pomona. Infect Immun 14:703–708
Adler B, Faine S (1977) Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect Immun 17:67–72
Adler B, Faine S (1978) The antibodies involved in the human immune response to leptospiral infection. J Med Microbiol 11:387–400
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–802
Anders H-J, Muruve DA (2011) The inflammasomes in kidney disease. J Am Soc Nephrol 22:1007–1018
Araujo ER, Seguro AC, Spichler A, Magaldi AJ, Volpini RA, De Brito T (2010) Acute kidney injury in human leptospirosis: an immunohistochemical study with pathophysiological correlation. Virchows Arch 456:367–375
Athanazio DA, Santos CS, Santos AC, McBride FWC, Reis MG (2008) Experimental infection in tumor necrosis factor alpha receptor, interferon gamma and interleukin 4 deficient mice by pathogenic Leptospira interrogans. Acta Trop 105:95–98
Baldwin CL, Sathiyaseelan T, Rocchi M, McKeever D (2000) Rapid changes occur in the percentage of circulating bovine WC1(+)gamma delta Th1 cells. Res Vet Sci 69:175–180
Baldwin CL, Sathiyaseelan T, Naiman B, White AM, Brown R, Blumerman S, Rogers A, Black SJ (2002) Activation of bovine peripheral blood gammadelta T cells for cell division and IFN-gamma production. Vet Immunol Immunopathol 87:251–259
Bandeira M, Santos CS, de Azevedo EC, Soares LM, Macedo JO, Marchi S, da Silva CLR, Chagas-Junior AD, McBride AJA, McBride FWC, Reis MG, Athanazio DA (2011) Attenuated nephritis in inducible nitric oxide synthase knockout C57BL/6 mice and pulmonary hemorrhage in CB17 SCID and aecombination activating gene 1 knockout C57BL/6 mice infected with Leptospira interrogans. Infect Immun 79:2936–2940
Banfi E, Cinco M, Bellini M, Soranzo MR (1982) The role of antibodies and serum complement in the interaction between macrophages and leptospires. Microbiol 128:813–816
Barry M, Wisnewski AV, Matthias MA, Inouye SK, Vinetz JM (2006) Suburban leptospirosis: atypical lymphocytosis and γ-δ T cell response. Clin Infect Dis 43:1304–1307
Behera AK, Hildebrand E, Uematsu S, Akira S, Coburn J, Hu LT (2006) Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin alpha 3 beta 1. J Immunol 177:657–664
Bernardino ALF, Myers TA, Alvarez X, Hasegawa A, Philipp MT (2008) Toll-like receptors: insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infect Immun 76:4385–4395
Bey RF, Johnson RC (1982) Immunogenicity and humoral and cell-mediated immune responses to leptospiral whole cell, outer envelope, and protoplasmic cylinder vaccines in hamsters and dogs. Am J Vet Res 43:835–840
Birnbaum S, Shenberg E, Torten M (1972) The influence of maternal antibodies on the epidemiology of leptospiral carrier state in mice. Am J Epidemiol 96:313–317
Blumerman SL, Herzig CTA, Baldwin CL (2007a) WC1+ gammadelta T cell memory population is induced by killed bacterial vaccine. Eur J Immunol 37:1204–1216
Blumerman SL, Herzig CTA, Wang F, Coussens PM, Baldwin CL (2007b) Comparison of gene expression by co-cultured WC1+ gammadelta and CD4+ alphabeta T cells exhibiting a recall response to bacterial antigen. Mol Immunol 44:2023–2035
Bolin CA, Zuerner RL, Trueba G (1989) Effect of vaccination with a pentavalent leptospiral vaccine containing Leptospira interrogans serovar Hardjo type hardjo-bovis on type hardjo-bovis infection of cattle. Am J Vet Res 50:2004–2008
Bolin CA, Alt DP, Zuerner RL (2000) Protection of cattle from renal and genital tract colonization with Leptospira borgpetersenii serovar Hardjo. In: Proceedings of the 21st world buiatrics congress, Punte del Este, Uruguay
Bolin CA, Alt DP (2001) Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar Hardjo. Am J Vet Res 62:996–1000
Breiner DD, Fahey M, Salvador R, Novakova J, Coburn J (2009) Leptospira interrogans binds to human cell surface receptors including proteoglycans. Infect Immun 77:5528–5536
Burth P, Younes-Ibrahim M, Gonçalez FH, Costa ER, Faria MV (1997) Purification and characterization of a Na+, K+ ATPase inhibitor found in an endotoxin of Leptospira interrogans. Infect Immun 65:1557–1560
Cabral ES, Gelderblom H, Hornung RL, Munson PJ, Martin R, Marques AR (2006) Borrelia burgdorferi lipoprotein-mediated TLR2 stimulation causes the down-regulation of TLR5 in human monocytes. J Infect Dis 193:849–859
Castiblanco-Valencia MM, Fraga TR, Silva LBd, Monaris D, Abreu PAE, Strobel S, Józsi M, Isaac L, Barbosa AS (2012) Leptospiral immunoglobulin-like proteins interact with human complement regulators factor H, FHL-1, FHR-1, and C4BP. J Infect Dis 205:995–1004
Cervantes JL, Dunham-Ems SM, La Vake CJ, Petzke MM, Sahay B, Sellati TJ, Radolf JD, Salazar JC (2011) Phagosomal signaling by Borrelia burgdorferi in human monocytes involves Toll-like receptor (TLR) 2 and TLR8 cooperativity and TLR8-mediated induction of IFN-beta. Proc Nat Acad Sci USA 108:3683–3688
Challa S, Nally JE, Jones C, Sheoran AS (2011) Passive immunization with Leptospira LPS-specific agglutinating but not non-agglutinating monoclonal antibodies protect guinea pigs from fatal pulmonary hemorrhages induced by serovar Copenhageni challenge. Vaccine 29:4431–4434
Chapman AJ, Adler B, Faine S (1988) Antigens recognised by the human immune response to infection with Leptospira interrogans serovar Hardjo. J Med Microbiol 25:269–278
Chapman AJ, Everard CO, Faine S, Adler B (1991) Antigens recognized by the human immune response to severe leptospirosis in Barbados. Epidemiol Infect 107:143–155
Charon N, Lawrence C, O’Brien S (1981) Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc Natl Acad Sci USA 78:7166
Chassin C, Picardeau M, Goujon J-M, Bourhy P, Quellard N, Darche S, Badell E, d’ Andon MF, Winter N, Lacroix-Lamande S, Buzoni-Gatel D, Vandewalle A, Werts C (2009) TLR4- and TLR2-mediated B cell responses control the clearance of the bacterial pathogen, Leptospira interrogans. J Immunol 183:2669–2677
Chen HI, Kao SJ, Hsu YH (2007) Pathophysiological mechanism of lung injury in patients with leptospirosis. Pathology 39:339–344
Choy HA, Kelley MM, Croda J, Matsunaga J, Babbitt JT, Ko AI, Picardeau M, Haake DA (2011) The multifunctional LigB adhesin binds homeostatic proteins with potential roles in cutaneous infection by pathogenic Leptospira interrogans. PLoS ONE 6:e16879
Chu KM, Rathinam R, Namperumalsamy P, Dean D (1998) Identification of Leptospira species in the pathogenesis of uveitis and determination of clinical ocular characteristics in South India. J Infect Dis 177:1314–1321
Cinco M, Banfi E, Soranzo MR (1981) Studies on the interaction between macrophages and leptospires. Microbiol 124:409–413
Cinco M, Cini B, Perticarari S, Presani G (2002) Leptospira interrogans binds to the CR3 receptor on mammalian cells. Microb Pathog 33:299–305
Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, Garraud O (2008) Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol 141:84–91
Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM (2009) Cytokine-induced memory-like natural killer cells. Proc Nat Acad Sci USA 106:1915–1919
Craig SB, Graham GC, Burns M-A, Dohnt MF, Wilson RJ, Smythe LD, Jansen CC, McKay DB (2009) Leptospirosis and Goodpasture’s syndrome: testing the aetiological hypothesis. Ann Trop Med Parasitol 103:647–651
Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler B (2002) Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun 70:2311–2318
de Fost M, Hartskeerl RA, Groenendijk MR, van der Poll T (2003) Interleukin 12 in part regulates gamma interferon release in human whole blood stimulated with Leptospira interrogans. Clin Diagn Lab Immunol 10:332–335
de Fost M, Chierakul W, Limpaiboon R, Dondorp A, White NJ, van der Poll T (2007) Release of granzymes and chemokines in Thai patients with leptospirosis. Clin Microbiol Infect 13:433–436
Del Carlo Bernardi F, Ctenas B, da Silva LFF, Nicodemo AC, Saldiva PHN, Dolhnikoff M, Mauad T (2012) Immune receptors and adhesion molecules in human pulmonary leptospirosis. Hum Pathol 43:1601–1610
Diament D, Brunialti MKC, Romero EC, Kallas EG, Salomão R (2002) Peripheral blood mononuclear cell activation induced by Leptospira interrogans glycolipoprotein. Infect Immun 70:1677–1683
Dobrina A, Nardon E, Vecile E, Cinco M, Patriarca P (1995) Leptospira icterohemorrhagiae and leptospire peptidolgycans induce endothelial cell adhesiveness for polymorphonuclear leukocytes. Infect Immun 63:2995–2999
Domingos RF, Vieira ML, Romero EC, Goncales AP, Morais ZM, Vasconcellos SA, Nascimento AL (2012) Features of two proteins of Leptospira interrogans with potential role in host-pathogen interactions. BMC Microbiol 12:50
Edwards CN, Nicholson GD, Hassell TA, Everard CO, Callender J (1986) Thrombocytopenia in leptospirosis: the absence of evidence for disseminated intravascular coagulation. Am J Trop Med Hyg 35:352–354
Ellis W, McDowell S, Mackie D, Pollock M, Taylor M (2000). Immunity to bovine leptospirosis. In: Proceedings of the 21st world buiatrics congress, Punte del Este, Uruguay
Eshghi A, Lourdault K, Murray GL, Bartpho T, Sermswan RW, Picardeau M, Adler B, Snarr B, Zuerner RL, Cameron CE (2012a) Leptospira interrogans catalase is required for resistance to H2O2 and for virulence. Infect Immun 80:3892–3899
Eshghi A, Pinne M, Haake DA, Zuerner RL, Frank A, Cameron CE (2012b) Methylation and in vivo expression of the surface-exposed Leptospira interrogans outer-membrane protein OmpL32. Microbiol 158:622–635
Faber NA, Crawford M, Lefebvre RB, Buyukmihci NC, Madigan JE, Willits NH (2000) Detection of Leptospira spp. in the aqueous humor of horses with naturally acquired recurrent uveitis. J Clin Microbiol 38:2731–2733
Faine S, Adler B, Perolat P, Bolin C (1999) Leptospira and leptospirosis. MediSci, Melbourne
Fialho RN, Martins L, Pinheiro JP, Bettencourt BF, Couto AR, Santos MR, Peixoto MJ, Garrett F, Leal J, Tomás AM, Bruges-Armas J (2009) Role of human leukocyte antigen, killer-cell immunoglobulin-like receptors, and cytokine gene polymorphisms in leptospirosis. Hum Immunol 70:915–920
Gaudart N, Ekpo P, Pattanapanyasat K, van Kooyk Y, Engering A (2008) Leptospira interrogans is recognized through DC-SIGN and induces maturation and cytokine production by human dendritic cells. FEMS Immunol Med Microbiol 53:359–367
Goris MGA, Wagenaar JFP, Hartskeerl RA, van Gorp ECM, Schuller S, Monahan AM, Nally JE, van der Poll T, van ‘t Veer C (2011) Potent innate immune response to pathogenic Leptospira in human whole blood. PLoS ONE 6:e18279
Gray P, Dagvadorj J, Michelsen KS, Brikos C, Rentsendorj A, Town T, Crother TR, Arditi M (2011) Myeloid Differentiation Factor-2 interacts with lyn kinase and is tyrosine phosphorylated following lipopolysaccharide-induced activation of the TLR4 signaling pathway. J Immunol 187:4331–4337
Haake DA, Walker EM, Blanco DR, Bolin CA, Miller MN, Lovett MA (1991) Changes in the surface of Leptospira interrogans serovar Grippotyphosa during in vitro cultivation. Infect Immun 59:1131–1140
Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA (2000) The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun 68:2276–2285
Haake DA (2006) Hamster model of leptospirosis. Curr Protoc Microbiol 12:Unit 12E
Hein W, Mackay C (1991) Prominence of gamma delta T cells in the ruminant immune system. Immunol Today 12:30–34
Hsu S-H, Lo Y-Y, Tung J-Y, Ko Y-C, Sun Y-J, Hung C-C, Yang CW, Tseng F-G, Fu C-C, Pan R-L (2010) Leptospiral outer membrane lipoprotein LipL32 binding on toll-like receptor 2 of renal cells as determined with an atomic force microscope. Biochemistry 49:5408–5417
Hung C-C, Chang C-T, Chen K-H, Tian Y-C, Wu MS, Pan MJ, Vandewalle A, Yang C-W (2006a) Upregulation of chemokine CXCL1/KC by leptospiral membrane lipoprotein preparation in renal tubule epithelial cells. Kidney Int 69:1814–1822
Hung C-C, Chang C-T, Tian Y-C, Wu MS, Yu C-C, Pan MJ, Vandewalle A, Yang CW (2006b) Leptospiral membrane proteins stimulate pro-inflammatory chemokines secretion by renal tubule epithelial cells through toll-like receptor 2 and p38 mitogen activated protein kinase. Nephrol Dial Transplant 21:898–910
Isogai E, Kitagawa H, Isogai H, Kurebayashi Y, Ito N (1986) Phagocytosis as a defense mechanism against infection with leptospiras Zentralbl Bakteriol Mikrobiol Hyg A 261:65–74
Isogai E, Isogai H, Wakizaka H, Miura H, Kurebayashi Y (1989a) Chemiluminescence and phagocytic responses of rat polymorphonuclear neutrophils to leptospires. Zentralbl Bakteriol 272:36–46
Isogai E, Kitagawa H, Isogai H, Matsuzawa T, Shimizu T, Yanagihara Y, Katami K (1989b) Effects of leptospiral lipopolysaccharide on rabbit platelets. Zentralbl Bakteriol Mikrobiol Hyg A 271:186–196
Isogai E, Isogai H, Fujii N, Oguma K (1990) Macrophage activation by leptospiral lipopolysaccharide. Zentralbl Bakteriol 273:200–208
Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327:291–295
Jin D, Ojcius DM, Sun D, Dong H, Luo Y, Mao Y, Yan J (2009) Leptospira interrogans induces apoptosis in macrophages via caspase-8- and caspase-3-dependent pathways. Infect Immun 77:799–809
Jost BH, Adler B, Vinh T, Faine S (1986) A monoclonal antibody reacting with a determinant on leptospiral lipopolysaccharide protects guinea pigs against leptospirosis. J Med Microbiol 22:269–275
Jost BH, Adler B, Faine S (1989) Experimental immunisation of hamsters with lipopolysaccharide antigens of Leptospira interrogans. J Med Microbiol 29:115–120
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Klimpel GR, Matthias MA, Vinetz JM (2003) Leptospira interrogans activation of human peripheral blood mononuclear cells: preferential expansion of TCR gamma delta+ T cells vs TCR alpha beta+ T cells. J Immunol 171:1447–1455
Koizumi N (2003) Identification of a novel antigen of pathogenic Leptospira spp. that reacted with convalescent mice sera. J Med Microbiol 52:585–589
Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J (2001) Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 107:1529–1536
Kyriakidis I, Samara P, Papa A (2011) Serum TNF-α, sTNFR1, IL-6, IL-8 and IL-10 levels in Weil’s syndrome. Cytokine 54:117–120
Lacroix-Lamande S, d’Andon MF, Michel E, Ratet G, Philpott DJ, Girardin SE, Boneca IG, Vandewalle A, Werts C (2012) Downregulation of the Na/K-ATPase pump by leptospiral glycolipoprotein activates the NLRP3 inflammasome. J Immunol 188:2805–2814
Lee WL, Downey GP (2001) Neutrophil activation and acute lung injury. Curr Opin Crit Care 7:1–7
Leemans JC, Cassel SL, Sutterwala FS (2011) Sensing damage by the NLRP3 inflammasome. Immunol Rev 243:152–162
Li Y, Xiang M, Yuan Y, Xiao G, Zhang J, Jiang Y, Vodovotz Y, Billiar TR, Wilson MA, Fan J (2009) Hemorrhagic shock augments lung endothelial cell activation: role of temporal alterations of TLR4 and TLR2. Am J Physiol Regul Integr Comp Physiol 297:R1670–R1680
Lo M, Bulach DM, Powell DR, Haake DA, Matsunaga J, Paustian ML, Zuerner RL, Adler B (2006) Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect Immun 74:5848–5859
Loimaranta V, Hytönen J, Pulliainen AT, Sharma A, Tenovuo J, Strömberg N, Finne J (2009) Leucine-rich repeats of bacterial surface proteins serve as common pattern recognition motifs of human scavenger receptor gp340. J Biol Chem 284:18614–18623
Londoño D, Marques A, Hornung RL, Cadavid D (2008) IL-10 helps control pathogen load during high-level bacteremia. J Immunol 181:2076–2083
Lowanitchapat A, Payungporn S, Sereemaspun A, Ekpo P, Phulsuksombati D, Poovorawan Y, Chirathaworn C (2010) Expression of TNF-alpha, TGF-beta, IP-10 and IL-10 mRNA in kidneys of hamsters infected with pathogenic Leptospira. Comp Immunol Microbiol Infect Dis 33:423–434
Lucas DSD, Cullen PA, Lo M, Srikram A, Sermswan RW, Adler B (2011) Recombinant LipL32 and LigA from Leptospira are unable to stimulate protective immunity against leptospirosis in the hamster model. Vaccine 29:3413–3418
Lund RJ, Löytömäki M, Naumanen T, Dixon C, Chen Z, Ahlfors H, Tuomela S, Tahvanainen J, Scheinin J, Henttinen T, Rasool O, Lahesmaa R (2007) Genome-wide identification of novel genes involved in early Th1 and Th2 cell differentiation. J Immunol 178:3648–3660
Lusitani D, Malawista SE, Montgomery RR (2002) Borrelia burgdorferi are susceptible to killing by a variety of human polymorphonuclear leukocyte components. J Infect Dis 185:797–804
Mancel E, Merien F, Pesenti L, Salino D, Angibaud G, Perolat P (1999) Clinical aspects of ocular leptospirosis in New Caledonia (South Pacific). Aust N Z J Ophthalmol 27:380–386
Mariathasan S, Monack DM (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7:31–40
Marinho M, Oliveira-Júnior IS, Monteiro CMR, Perri SH, Salomão R (2009) Pulmonary disease in hamsters infected with Leptospira interrogans: histopathologic findings and cytokine mRNA expressions. Am J Trop Med Hyg 80:832–836
Martinez-Lopez DG, Fahey M, Coburn J (2010) Responses of human endothelial cells to pathogenic and non-pathogenic Leptospira species. PLoS Negl Trop Dis 4:e918
Matsui M, Rouleau V, Bruyère-Ostells L, Goarant C (2011) Gene expression profiles of immune mediators and histopathological findings in animal models of leptospirosis: comparison between susceptible hamsters and resistant mice. Infect Immun 79:4480–4492
Matsunaga J, Sanchez Y, Xu X, Haake DA (2005) Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect Immun 73:70–78
Matsunaga J, Medeiros MA, Sanchez Y, Werneid KF, Ko AI (2007) Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiol 153:3390–3398
McGrath H, Adler B, Vinh T, Faine S (1984) Phagocytosis of virulent and avirulent leptospires by guinea-pig and human polymorphonuclear leukocytes in vitro. Pathology 16:243–249
Merien F, Baranton G, Perolat P (1997) Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect Immun 65:729–738
Merien F, Truccolo J, Rougier Y, Baranton G, Perolat P (1998) In vivo apoptosis of hepatocytes in guinea pigs infected with Leptospira interrogans serovar Icterohaemorrhagiae. FEMS Microbiol Lett 169:95–102
Miller JC, Maylor-Hagen H, Ma Y, Weis JH, Weis JJ (2010) The Lyme disease spirochete Borrelia burgdorferi utilizes multiple ligands, including RNA, for interferon regulatory factor 3-dependent induction of type I interferon-responsive genes. Infect Immun 78:3144–3153
Miranda KA, Vasconcelos LRS, Coelho LCBB, Lima Filho JL, Cavalcanti MSM, Moura P (2009) High levels of serum mannose-binding lectin are associated with the severity of clinical signs of leptospirosis. Braz J Med Biol Res 42:353–357
Monahan AM, Callanan JJ, Nally JE (2008) Proteomic analysis of Leptospira interrogans shed in urine of chronically infected hosts. Infect Immun 76:4952–4958
Morton HE (1942) Susceptibility of Syrian hamsters to leptospirosis. Proc Soc Exp Biol Med (New York, NY) 49:566–568
Murray GL, Srikram A, Hoke DE, Wunder EA, Henry R, Lo M, Zhang K, Sermswan RW, Ko AI, Adler B (2009) Major surface protein LipL32 is not required for either acute or chronic infection with Leptospira interrogans. Infect Immun 77:952–958
Nahori M-A, Fournié-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CRH, Saint Girons I, Werts C (2005) Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol 175:6022–6031
Naiman BM, Alt D, Bolin CA, Zuerner R, Baldwin CL (2001a) Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infect Immun 69:7550–7558
Naiman BM, Alt DP, Bolin CA, Zuerner RL, Baldwin CL (2001b) Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infect Immun 69:7550–7558
Naiman BM, Blumerman S, Alt D, Bolin CA, Brown R, Zuerner RL, Baldwin CL (2002) Evaluation of type 1 immune response in naïve and vaccinated animals following challenge with Leptospira borgpetersenii serovar Hardjo: involvement of WC1(+) gammadelta and CD4 T cells. Infect Immun 70:6147–6157
Nally JE, Chantranuwat C, Wu X-Y, Fishbein MC, Pereira MM, da Silva JJP, Blanco DR, Lovett MA (2004) Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am J Pathol 164:1115–1127
Nally JE, Fishbein MC, Blanco DR, Lovett MA (2005) Lethal infection of C3H/HeJ and C3H/SCID Mice with an isolate of Leptospira interrogans serovar Copenhageni. Infect Immun 73:7014–7017
Napetschnig J, Wu H (2013) Molecular Basis of NF-κB Signaling. Annu Rev Biophys 42:443–468
Noguchi H (1918) A comparative study of experimental prophylactic inoculation against Leptospira icterohaemorrhagiae. J Exp Med 28:561–570
Packchanian A (1940) Susceptibility and resistance of certain species of american deer mice, genus Peromyscus, and other rodents to Leptospira icterohaemorrhagiae. Public Health Rep 55:1389–1402
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195
Pereira MM, Andrade J, Marchevsky RS, Ribeiro dos Santos R (1998) Morphological characterization of lung and kidney lesions inC3H/HeJ mice infected with Leptospira interrogans serovar icterohaemorrhagiae: Defect of CD4+ and CD8+ T-cells are prognosticators of the disease progression. Exp Toxicol Pathol 50:191–198
Petnicki-Ocwieja T, Chung E, Acosta DI, T RL, Shin OS, Ghosh S, Kobzik L, Li X, Hu LT (2013) TRIF mediates Toll-Like Receptor 2-dependent inflammatory responses to Borrelia burgdorferi. Infect Immun 81:402–410
Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I (2009) Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol 183:5279–5292
Pinne M, Haake DA (2013) LipL32 is a subsurface lipoprotein of Leptospira interrogans: presentation of new data and reevaluation of previous studies. PLoS ONE 8:e51025
Prêtre G, Olivera N, Cédola M, Haase S, Alberdi L, Brihuega B, Gómez RM (2011) Role of inducible nitric oxide synthase in the pathogenesis of experimental leptospirosis. Microb Pathog 51:203–208
Rogers AN, VanBuren DG, Hedblom E, Tilahun ME, Telfer JC, Baldwin CL (2005a) Function of ruminant gammadelta T cells is defined by WC1.1 or WC1.2 isoform expression. Vet Immunol Immunopathol 108:211–217
Rogers AN, VanBuren DG, Hedblom EE, Tilahun ME, Telfer JC, Baldwin CL (2005b) Gammadelta T cell function varies with the expressed WC1 coreceptor. J Immunol 174:3386–3393
Rugman FP, Pinn G, Palmer MF, Waite M, Hay CR (1991) Anticardiolipin antibodies in leptospirosis. J Clin Pathol 44:517–519
Schoone GJ, Everard COR, Korver H, Carrington DG, Inniss VA, Baulu J, Terpstra WJ (1989) An immunoprotective monoclonal antibody directed against Leptospira interrogans serovar Copenhageni. Microbiol 135:73–78
Scocchi M, Romeo D, Cinco M (1993) Antimicrobial activity of two bactenecins against spirochetes. Infect Immun 61:3081–3083
Shibata T, Motoi Y, Tanimura N, Yamakawa N, Akashi-Takamura S, Miyake K (2011) Intracellular TLR4/MD-2 in macrophages senses Gram-negative bacteria and induces a unique set of LPS-dependent genes. Int Immunol 23:503–510
Sonrier C, Branger C, Michel V, Ruvoën-Clouet N, Ganière JP, André-Fontaine G (2000) Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine 19:86–94
Spichler A, Spichler E, Moock M, Vinetz JM, Leake JAD (2007) Acute pancreatitis in fatal anicteric leptospirosis. Am J Trop Med Hyg 76:886–887
Srikram A, Zhang K, Bartpho T, Lo M, Hoke DE, Sermswan RW, Adler B, Murray GL (2011) Cross-protective immunity against leptospirosis elicited by a live, attenuated lipopolysaccharide mutant. J Infect Dis 203:870–879
Stevenson B, Choy HA, Pinne M, Rotondi ML, Miller MC, Demoll E, Kraiczy P, Cooley AE, Creamer TP, Suchard MA, Brissette CA, Verma A, Haake DA (2007) Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE 2:e1188
Sun JC, Beilke JN, Lanier LL (2009) Adaptive immune features of natural killer cells. Nature 457:557–561
Tajiki H, Salomão R (1996) Association of plasma levels of tumor necrosis factor alpha with severity of disease and mortality among patients with leptospirosis. Clin Infect Dis 23:1177–1178
Thiermann AB, Handsaker AL (1985) Experimental infection of calves with Leptospira interrogans serovar Hardjo: conjunctival versus intravenous route of exposure. Am J Vet Res 46:329–331
Tian Y-C (2006) Leptospiral outer membrane protein induces extracellular matrix accumulation through a TGF-beta1/smad-dependent pathway. J Am Soc Nephrol 17:2792–2798
Tian Y-C, Hung C-C, Li Y-J, Chen Y-C, Chang M-Y, Yen T-H, Hsu H-H, Wu MS, Phillips A, Yang CW (2011) Leptospira santorosai Serovar Shermani detergent extract induces an increase in fibronectin production through a Toll-like receptor 2-mediated pathway. Infect Immun 79:1134–1142
Toma C, Okura N, Takayama C, Suzuki T (2011) Characteristic features of intracellular pathogenic Leptospira in infected murine macrophages. Cell Microbiol 13:1783–1792
Truccolo J, Charavay F, Merien F, Perolat P (2002) Quantitative PCR assay to evaluate ampicillin, ofloxacin, and doxycycline for treatment of experimental leptospirosis. Antimicrob Agents Chemother 46:848–853
Tucunduva de Faria M, Athanazio D, Goncalves Ramos E, Silva E, Reis M, Ko A (2007) Morphological alterations in the kidney of rats with natural and experimental Leptospira infection. J Comp Pathol 137:231–238
Tuero I, Vinetz JM, Klimpel GR (2010) Lack of demonstrable memory T cell responses in humans who have spontaneously recovered from leptospirosis in the Peruvian Amazon. J Infect Dis 201:420–427
Verma A, Artiushin S, Matsunaga J, Haake DA, Timoney JF (2005) LruA and LruB, novel lipoproteins of pathogenic Leptospira interrogans associated with equine recurrent uveitis. Infect Immun 73:7259–7266
Verma A, Rathinam SR, Priya CG, Muthukkaruppan VR, Stevenson B, Timoney JF (2008) LruA and LruB antibodies in sera of humans with leptospiral uveitis. Clin Vacc Immunol 15:1019–1023
Verma A, Brissette CA, Bowman AA, Shah ST, Zipfel PF, Stevenson B (2010) Leptospiral endostatin-like protein a is a bacterial cell surface receptor for human plasminogen. Infect Immun 78:2053–2059
Verma A, Stevenson B (2012) Leptospiral uveitis–there is more to it than meets the eye! Zoonoses Public Health 59:132–141
Vernel-Pauillac F, Merien F (2006) Proinflammatory and immunomodulatory cytokine mRNA time course profiles in hamsters infected with a virulent variant of Leptospira interrogans. Infect Immun 74:4172–4179
Vernel-Pauillac F, Goarant C (2010) Differential cytokine gene expression according to outcome in a hamster model of leptospirosis. PLoS Negl Trop Dis 4:e582
Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA (2010) The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21:1732–1744
Vinh T, Adler B, Faine S (1982) The role of macrophages in the protection of mice against leptospirosis: in vitro and in vivo studies. Pathology 14:463–468
Vinh T, Adler B, Faine S (1986) Glycolipoprotein cytotoxin from Leptospira interrogans serovar Copenhageni. J Gen Microbiol 132:111–123
Viriyakosol S, Matthias MA, Swancutt MA, Kirkland TN, Vinetz JM (2006) Toll-Like Receptor 4 protects against lethal Leptospira interrogans serovar icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect Immun 74:887–895
Wagenaar JFP, Gasem MH, Goris MGA, Leeflang M, Hartskeerl RA, van der Poll T, van ‘t Veer C, van Gorp ECM (2009a) Soluble ST2 levels are associated with bleeding in patients with severe leptospirosis. PLoS Negl Trop Dis 3:e453
Wagenaar JFP, Goris MGA, Gasem MH, Isbandrio B, Moalli F, Mantovani A, Boer KR, Hartskeerl RA, Garlanda C, van Gorp ECM (2009b) Long pentraxin PTX3 is associated with mortality and disease severity in severe leptospirosis. J Infect 58:425–432
Wang B, Sullivan JA, Sullivan GW, Mandell GL (1984) Role of specific antibody in interaction of leptospires with human monocytes and monocyte-derived macrophages. Infect Immun 46:809–813
Wang F, Herzig CTA, Chen C, Hsu H, Baldwin CL, Telfer JC (2011) Scavenger receptor WC1 contributes to the γδ T cell response to Leptospira. Mol Immunol 48:801–809
Wang H, Wu Y, Ojcius DM, Yang XF, Zhang C, Ding S, Lin X, Yan J (2012) Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2-and 4-mediated JNK and NF-κB signaling pathways. PLoS ONE 7:e42266
Werts C, Tapping RI, Mathison JC, Chuang T-H, Kravchenko V, Saint Girons I, Haake DA, Godowski PJ, Hayashi F, Ozinsky A, Underhill DM, Kirschning CJ, Wagner H, Aderem A, Tobias PS, Ulevitch RJ (2001) Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol 2:346–352
Williams A, Flavell RA, Eisenbarth SC (2010) The role of NOD-like receptors in shaping adaptive immunity. Curr Opin Immunol 22:34–40
Witchell TD, Eshghi A, Nally JE, Hof R, Boulanger MJ, Wunder EA, Ko AI, Haake DA, Cameron CE (2014) Post-translational modification of LipL32 during Leptospira interrogans infection. PLoS Negl Trop Dis. Accepted 19 Sept 2014
Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Scott MJ, Billiar TR, Wilson MA, Fan J (2011) Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol 187:4809–4817
Yang C-W, Wu MS, Pan MJ, Hong JJ, Yu CC, Vandewalle A, Huang CC (2000) Leptospira outer membrane protein activates NF-kappaB and downstream genes expressed in medullary thick ascending limb cells. J Am Soc Nephrol 11:2017–2026
Yang C-W, Hung C-C, Wu MS, Tian Y-C, Chang C-T, Pan MJ, Vandewalle A (2006) Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int 69:815–822
Yang G-G, Hsu Y-H (2005) Nitric oxide production and immunoglobulin deposition in leptospiral hemorrhagic respiratory failure. J Formos Med Assoc 104:759–763
Yeaman MR (2009) Platelets in defense against bacterial pathogens. Cell Mol Life Sci 67:525–544
Zuerner RL, Knudtson W, Bolin CA, Trueba G (1991) Characterization of outer membrane and secreted proteins of Leptospira interrogans serovar Pomona. Microb Pathog 10:311–322
Zuerner RL, Alt DP, Palmer MV, Thacker TC, Olsen SC (2011) A Leptospira borgpetersenii serovar Hardjo vaccine induces a Th1 Response, activates NK cells, and reduces renal colonization. Clin Vacc Immunol 18:684–691
Zuerner RL, Alt DP, Palmer MV (2012) Development of chronic and acute golden Syrian hamster infection models with Leptospira borgpetersenii serovar Hardjo. Vet Pathol 49:403–411
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Zuerner, R.L. (2015). Host Response to Leptospira Infection. In: Adler, B. (eds) Leptospira and Leptospirosis. Current Topics in Microbiology and Immunology, vol 387. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45059-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-45059-8_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45058-1
Online ISBN: 978-3-662-45059-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)