Abstract
A novel experimental approach combining high-resolution cryoelectron microscopy of vitreous tissue section (CEMOVIS) defocus series with electron microscopy simulation was employed to show that the stratum corneum lipid matrix is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. The new knowledge may serve as a molecular platform for in silico approaches to identify molecules for enhancing skin penetration for percutaneous drug delivery as well as for tightening a leaking barrier in dry skin conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Terrestrial life was only made possible through the adaptive evolution of a waterproof barrier in the integument of organisms. In man, this barrier is constituted by a uniquely organized lipid material situated between the cells of the horny layer of the skin (Breathnach et al. 1973; Elias and Friend 1975). Recently, the lipid material’s molecular organization was determined in situ with the aid of a novel experimental approach: high-resolution cryoelectron microscopy of vitreous tissue section (CEMOVIS) defocus series combined with molecular modeling and electron microscopy simulation (Iwai et al. 2012). The lipid material is organized in an arrangement not previously described in a biological system – stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety (Iwai et al. 2012). This organization not only rationalizes the low permeability of the skin barrier but also its robustness. The new knowledge may serve as a molecular platform for in silico approaches to identify molecules for enhancing skin penetration for percutaneous drug delivery.
Below follows a brief account of the structure-function relationships of the human skin barrier.
2 Skin Lipid Composition and Phase State
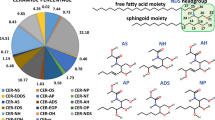
The horny layer lipids consist of a heterogeneous mixture of saturated, long-chain ceramides, free fatty acids, and cholesterol in a roughly 1:1:1 molar ratio (Wertz and Norlén 2003). More than 300 different species have been identified in the ceramide fraction alone (Masukawa et al. 2009).
The most characteristic features of the horny layer lipid composition (Wertz and Norlén 2003) are (1) extensive compositional heterogeneity with broad, but invariable, chain length distributions (20–32 C; peaking at 24 C) in the ceramide fatty acid and free fatty acid fractions, (2) almost complete dominance of saturated very long hydrocarbon chains (C20:0–C32:0), and (3) large relative amounts of cholesterol (about 30 mol%).
These compositional features are typically those stabilizing lipid gel phases. It has therefore been proposed that the horny layer lipid structure exists as a single and coherent gel phase (Norlén 2001b). The viscous gel-like behavior of the lipid structure has recently been demonstrated by its remarkable malleability in situ (Iwai et al. 2012).
3 Skin Lipid Structure
CEMOVIS has recently shown that the extracellular lipid matrix of the horny layer is organized as a bilayer structure of fully extended (splayed chain) ceramides with the sphingoid moieties interfacing. Both cholesterol and the free fatty acids are distributed selectively: cholesterol at the ceramide sphingoid end and the free fatty acid at the ceramide fatty acid end (Iwai et al. 2012) (Figs. 4.1 and 4.2). A unique feature of the horny layer lipid organization is that the lipid molecules are arranged in the splayed chain conformation, with the two hydrocarbon tails pointing in opposite directions, contrary to conventional biological membranes where the lipids are arranged in the hairpin conformation with the two hydrocarbon tails pointing in the same direction. Further, the skin barrier organization differs from conventional fat crystals arranged in the splayed chain conformation, as the lipid layers in the skin are stacked in an alternate fashion as bilayers rather than as stacked monolayers.
Molecular organization of the skin barrier. The stratum corneum lipid layer is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety (Iwai et al. 2012). Green spheres represent hydrogen and carbon atoms in ceramides, cholesterol, and free fatty acids. Red spheres represent oxygen atoms
Schematic drawing of the skin. Left part schematic cellular-scale drawing of epidermis. Middle part molecular-scale drawing of the lamellar lipid structure occupying the space between the cells of the stratum corneum. Right part atomic model of the lipid structure’s repeating unit, composed of two mirrored subunits, each composed of one fully extended ceramide (CER), one cholesterol (CHOL), and one free fatty acid (FFA) molecule (Adapted from Norlén (2012), with permission)
4 Skin Lipid Formation
In order to appreciate the structure-function relationships of the skin barrier in vivo, it is of value to understand horny layer lipid formation, as the horny layer’s lipid structure may represent a “frozen-in” or “immobilized” open biological system rather than a primary minimum energy order equilibrium system. Skin lipid formation is also central from a dermatological standpoint, since barrier malformation may be an etiological factor in barrier-deficient skin conditions such as eczema, psoriasis, and “dry skin.”
It has recently been proposed that skin lipid formation proceeds via (1) membrane synthesis in the trans-Golgi of a membrane system with cubic-like symmetry, followed by (2) morphologically continuous (non-fusion-dependent) secretion of the cubic-like membrane system into the extracellular space, (3) phase transition from cubic-like to lamellar membrane morphology, (4) dehydration, (5) condensation, and (6) lipid chain rearrangement from a folded (hairpin) to an extended (splayed chain) stacked bilayer conformation (Norlén 2001a; Iwai et al. 2012). CEMOVIS supports the proposed continuity of the lipid secretion system as well as the proposed structural association of non-lamellar and lamellar lipid morphologies (Norlén et al. 2003; Al-Amoudi et al. 2005). However, structure determination of the intermediate stages of skin lipid formation may require access to native molecular resolution tomographic 3D data in situ (molecular tissue TOVIS (cf. Norlén et al. 2009)), a developing technology that may not yet have reached its full potential.
5 Skin Lipid Function
Current knowledge suggests that a stacked, fully extended (splayed chain) ceramide bilayer arrangement (Figs. 4.1 and 4.2) with a high cholesterol content and a heterogeneous, saturated, long-chain lipid composition represents an optimized barrier organization for skin. This is because it renders skin largely impermeable to water as well as to both hydrophilic and lipophilic substances due to its condensed chain packing and its alternating lipophilic (alkyl chain) and hydrophilic (headgroup) regions. Likewise, it is resistant to both hydration and dehydration because of its lack of exchangeable water between lipid leaflets. It is also resistant towards temperature and pressure changes because of its heterogeneous lipid composition and high cholesterol content, which stabilize gel-like chain packing and thereby prevent both lateral domain formation and induction of “pores” or non-lamellar morphologies. Further, this bilayer arrangement accounts for stratum corneum cell cohesion without advocating specialized intercellular adhesion structures such as desmosomes. The arrangement hence allows for sliding of stratum corneum cells to accommodate skin bending. Finally, as the interaction between the individual layers of the lipid structure involves only hydrocarbons, the layers may be relatively free to slide with respect to one another, making the lipid structure pliable. The fully extended ceramide bilayer arrangement with high cholesterol content and heterogeneous saturated long-chain lipid composition thus meets the barrier needs of the skin by being simultaneously impermeable and robust.
6 Conclusions
It was recently shown that the human skin barrier is organized as stacked bilayers of fully extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety.
The physical state of the skin’s lipid structure has been proposed to be that of a single and coherent gel phase. Further, the lipid structure may be formed via a phase transition from cubic-like to stacked lamellar morphology followed by a flip of the constituent lipid components from a folded (hairpin) to an extended (splayed chain) ceramide bilayer conformation.
The skin’s lipid structure is responsible for both the skin’s low permeability towards water and hydrophilic and lipophilic substances and the barrier’s robustness towards environmental stress, such as hydration and dehydration, temperature and pressure changes, stretching, compression, bending, and shearing.
The new molecular description of the skin barrier may serve as a molecular platform for in silico approaches such as molecular simulations, as well as for in vitro modeling, to underpin interactions of the lipid matrix with drugs and other chemicals. For example, it is foreseeable that this knowledge will now enable in silico screening to identify molecules for enhancing skin penetration for percutaneous drug delivery.
References
Al-Amoudi A, Dubochet J, Norlén L (2005) Nanostructure of the epidermal extracellular space as observed by cryo-electron microscopy of vitreous sections of human skin. J Invest Dermatol 124:764–777
Breathnach AS, Goodman T, Stolinski C, Gross M (1973) Freeze fracture replication of cells of stratum corneum of human epidermis. J Anat 114:65–81
Elias PM, Friend DS (1975) The permeability barrier in mammalian epidermis. J Cell Biol 65:180–191
Iwai I, Han H, den Hollander L, Svensson S, Öfverstedt LG, Anwar J, Brewer J, Bloksgaard Mølgaard M, Laloeuf A, Nosek D, Masich S, Bagatolli L, Skoglund U, Norlén L (2012) The human skin barrier is organized as stacked bilayers of fully-extended ceramides with cholesterol molecules associated with the ceramide sphingoid moiety. J Invest Dermatol 132:2215–2225. doi:10.1038/jid.2012.43
Masukawa Y, Narita H, Sato H, Naoe A, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Tagaki Y, Kitahara T (2009) Comprehensive quantification of ceramide species in human stratum corneum. J Lipid Res 50:1708–1719
Norlén L (2001a) Skin barrier formation: the membrane folding model. J Invest Dermatol 117(4):823–829
Norlén L (2001b) Skin barrier structure and function: the single gel-phase model. J Invest Dermatol 117(4):830–836
Norlén, L (2012) Skin Lipids. In Gordon C. K. Roberts (ed.) Encyclopedia of Biophysics, Springer-Verlag Berlin Heidelberg, Vol 5, pp. 2368–2373
Norlén L, Al-Amoudi A, Dubochet J (2003) A cryo-transmission electron microscopy study of skin barrier formation. J Invest Dermatol 120:555–560
Norlén L, Öktem O, Skoglund U (2009) Molecular cryo-electron tomography of vitreous tissue sections: current challenges. J Microsc 235:293–307
Wertz P, Norlén L (2003) “Confidence intervals” for the “true” lipid compositions of the human skin barrier? In: Forslind B, Lindberg M (eds) Skin, hair, and nails. Structure and function. Marcel Dekker Inc, New York, pp 85–106, Biochim Biophys Acta 304:265–275
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Norlén, L. (2015). Molecular Structure and Function of the Skin Barrier. In: Dragicevic, N., Maibach, H. (eds) Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45013-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-45013-0_4
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45012-3
Online ISBN: 978-3-662-45013-0
eBook Packages: MedicineMedicine (R0)