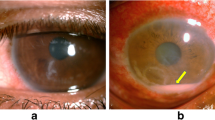
Abstract
An infectious keratitis is a sudden, often life-changing infection most commonly affecting younger, more active individuals. Visual outcomes are primarily dependent on the rapid recognition and effective treatment of the infection as outcomes are sensitive to even small degradations in corneal contour and clarity. While the vast majority of routine corneal infections are amenable to empiric fluoroquinolone therapy, progressive antibiotic resistance and emerging atypical pathogens are challenging this paradigm. An understanding of the risk factors, disease presentation, and disease course are, therefore, critical in the selection of appropriate empiric therapy and diagnostic techniques. Cultures remain the gold standard for identifying responsible pathogens. More rapid and sensitive molecular diagnostics are supplanting cultures for specific diseases such as herpetic keratitis, and confocal microscopy is increasingly utilized for atypical keratitides such as fungal and Acanthamoeba keratitis. Interpretation of these newer modalities is, however, critical in their appropriate use.
The introduction of new, commercial ophthalmic antimicrobials has dwindled in recent years compelling the use of new compounded medications to offset the challenge of resistant and atypical infections. While topical application constitutes the most significant therapeutic advantage for corneal infections, intrastromal, subconjunctival, and intracameral routes have shown promise in more recalcitrant keratitides. As the security of single agent therapy for routine infectious keratitis may be coming to an end, the ophthalmologist will not only need a detailed understanding of potential pathogens and diagnostic options but also to develop the flexibility to apply all available treatment options to insure the best possible visual outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
All forms of infectious keratitis confer a significant personal and economic burden on those afflicted as well as on the society in which they live. While the simple treatment cost for an episode of corneal ulceration varies from less than a US$ 100.00 in India, above the average monthly wage, to over US$ 1,000.00 in Australia, the additive costs of significant visual loss and subsequent attempts for visual rehabilitation drives those costs into the thousands of dollars and beyond (Keay et al. 2008; Prajna et al. 2007). Further, the patients most commonly afflicted are working-age men and women in the third and fourth decades of life (Erie et al. 1993; Jeng et al. 2010) where lost wages and productivity not only impact during their treatment but is compounded for decades if profound visual loss results. Very significant differences exist in the incidence of infectious keratitis primarily dependent on a region’s economic development which in turn affects a number of known risk factors including rates of contact lens wear, occupation, and domestic and environmental sanitation, among others. Regional incidence in the USA has been estimated to have risen from .25 to 1.0 per 10,000 person years in Olmsted county Minnesota from 1950 to 1980, although a recent study by Jeng et al. places the incidence at 2.76 per 10,000 person years in Northern California in 1999 (Erie et al. 1993; Jeng et al. 2010). In contrast, the incidence in South India was estimated to be 11.3 per 10,000 in 1993, potentially affecting more than 800,000 individuals yearly (Gonzales et al. 1996). Further, visual outcomes are highly dependent on prompt diagnosis and treatment with relatively dismal rates in regions with limited health-care resources to relatively reasonable outcomes in developed health-care systems where nearly 90 % will maintain a best corrected vision of better than 20/40 and where blindness is uncommon (Burton et al. 2011; Stapleton et al. 2008).
An understanding of associated risk factors is integral to the diagnosis, management, and prevention of infectious keratitis. In developing countries, trauma is the primary risk factor and combined with environmental exposure leads to a predominance of fungal keratitis (Gopinathan et al. 2009). In developed countries, agricultural and work-related trauma is far less common with contact lens-related keratitis comprising approximately 1/3 of all cases (Ibrahim et al. 2009). Consequently, the more favorable prognosis for these cases of bacterial keratitis and, to a lesser extent, Acanthamoeba keratitis shifts the predominance of corneal blindness to herpetic keratitis in these regions. For nonviral corneal infections, the most important factor in a successful outcome is limitation of corneal scarring through rapid, effective eradication of the offending pathogen and control of inflammation through prompt diagnosis.
2.2 Diagnosis
The most important step in the diagnosis of a potential infectious keratitis is the recognition of the possibility of infection. The history should identify the circumstances of the infection including any specific risk factors that would both suggest an infection as well as a potential etiology primarily for the purpose of identifying the need for special cultures or other diagnostic interventions not routinely performed (Table 2.1). For example, most bacterial infections have a rapid, crescendo clinical course while atypical mycobacterial, fungal, and parasitic infections are usually more slowly progressive but can be more painful in the later stages. Patients with herpes simplex keratitis will often have a history of oral or genital lesions as well as a history of recurrent red eye or keratitis. Failure of prior therapy, especially of antibacterial and antiviral therapy where clinical resistance is uncommon, should direct suspicion to other pathogen classes.
Clinical examination may also be helpful, although acanthamoebal and herpetic infections at various stages may mimic other pathogens as well as noninfectious etiologies of keratitis. Multiple studies have, however, confirmed the pitfalls of basing empiric treatment solely on the history and clinical appearance, but they may offer direction in the absence of positive studies (Dahlgren et al. 2007; Mascarenhas et al. 2012; Dalmon et al. 2012). Prior corticosteroid use will significantly alter the appearance and prognosis of any form of infectious keratitis and should be noted in context to its clinical appearance. Corneal scrapings offer minimal volume for examination and, therefore, need to be apportioned carefully to the studies that offer the highest probability of yield. Standard tests include Gram and Giemsa stains, direct plating of blood agar and chocolate agar for bacterial species, as well as Sabouraud’s dextrose agar (Fig. 2.1) without antifungal additives for fungal isolation and a broth or other media for anaerobic organisms (Bhadange et al. 2013). Superficial scrapings may be obtained either with a moistened calcium alginate swab or metallic instrument such as a platinum spatula or scalpel blade with reasonably equal yields (Benson and Lanier 1992).
2.2.1 Bacteria
The most common type of corneal infections in developed countries is caused by bacteria usually presenting as a single, suppurative lesion with significant pain, photophobia, and intraocular inflammation (Fig. 2.2). A history of ocular surface compromise, chronic corticosteroid use, ocular surgery, and/or contact lens wear should suggest first a bacterial etiology. Besides simple detection of bacteria, a Gram stain should be helpful in directing therapy since the activity of most antibacterials act on the bacterial cell wall type identified by a Gram-positive or Gram-negative result. Unfortunately, numerous studies have shown a poor correlation with subsequent culture results making the Gram stain unreliable in this regard (Sharma et al. 2002). In an untreated ulcer, standard testing methods should yield an organism in about 70 % of cases (Bourcier et al. 2003). Acid-fast stains may be more helpful in detecting atypical mycobacteria in the setting of chronic corticosteroid use in ocular surface disease or, more recently, LASIK surgery (Fig. 2.3). These lesions are minimally necrotic with small raised white lesions that are gritty on corneal scraping. Pain is often significant in these patients. Lowenstein-Jensen media and 7H11 agar slants offer an environment conducive to the isolation of these organisms. Nocardia, a filamentous bacterium, is also partially acid fast and is most often detected with Gram or acid-fast stains in about 65 % of cases and grow slowly on charcoal agar with a yeast extract additive (BCYE) (DeCroos et al. 2011). The lesions may have a wreath-like appearance with raised edge and an irregular base. Antibiotic sensitivity testing should be performed on all isolates so that the information is available if a patient does not respond appropriately to empiric therapy.
2.2.2 Fungi
The presentation of fungal keratitis is species dependent with the most common filamentous mold, Fusarium, presenting early with significant pain and proportionally less inflammation than a comparable bacterial ulcer but eventually becoming more infiltrative and characterized by a hypopyon, endothelial plaque, satellite lesions, as well as severe pain and injection (Fig. 2.4). Fusarium may rapidly and directly penetrate into the deep stroma and past an intact Descemet’s membrane to enter the intracameral space. However, slower-growing species of Alternaria or Beauveria may be much more indolent with less inflammation, less pain, and a more superficial appearance. Candida spp. are the most common overall fungal keratitis pathogen in the more temperate climates of North America and present initially with a creamy-white, superficial infiltrate, but its pseudohyphae may penetrate into deep stroma (Figs. 2.5 and 2.6).
Although yeast and filamentous molds may both be detected on Gram stain, molds are more readily identified on stains that highlight cell walls such as a Giemsa stain and KOH preps where hyphae are easily detected (Fig. 2.7). Similarly, acridine orange and calcofluor white stains have been shown to be more sensitive in detecting larger pathogens like fungi and acanthamoeba but require an epifluorescent microscope to excite the stain (Gomez et al. 1988). The majority of ocular fungal pathogens can be isolated with Sabouraud’s dextrose agar or Brain Heart Infusion Agar with non-antifungal additives, e.g., chloramphenicol or gentamicin, since these additives do not affect the saprophytic fungi usually nonpathogenic in other organs (Bhadange et al. 2013). Alternatively, fungal keratitis pathogens will also grow on standard blood and chocolate agar plates incubated at 36 °C. Antifungal sensitivities have been poorly reflective, historically, of clinical drug efficacy, but recent refinement of CLSI testing methods and break points have improved correlation of in vitro and in vivo antifungal activity, the knowledge of which can be critical to a successful outcome (Oechsler et al. 2013).
2.2.3 Virus
A diagnosis of herpetic viral disease is most commonly based on the history and clinical appearance. Herpes simplex keratitis is due more commonly HSV 1, associated with above-the-waist disease, but HSV 2 is also commonly isolated (Fig. 2.8). Patients may have a history of oral or genital herpes simplex as well as a past history of vesicular lesions in and around the eye. Although recurrent disease may surface from latency established from a primary infection from any dermatome served by the trigeminal ganglion, recurrences from prior corneal infection is usual. Epithelial HSV keratitis demonstrates a characteristic dendritic pattern with terminal bulbs, leaving an imprint on the anterior stroma after resolution. Geographic lesions may also occur with both stained by Rose Bengal of fluorescein dyes. Primary disease is almost always epithelial, but recurrences may be an epithelial or stromal interstitial keratitis with a conjunctival limbitis, endotheliitis, or uveitis less common. Patients with atopic keratoconjunctivitis are especially susceptible to severe, bilateral disease. The need for microbiologic confirmation is restricted to neonates and to those patients where the diagnosis is in question. Samples may be obtained through epithelial debridement, swab, or impression cytology of the superficial lesion and submitted for culture, immunofluorescence (IFA), or polymerase chain reaction (PCR) detection. Culture is the least sensitive method while both PCR and IFA have been shown to have similar sensitivities (60–80 %) and high specificities for the detection of HSV-1 in ocular samples (Farhatullah et al. 2004). Serologic testing may be helpful in naïve patients, especially children to rule out HSV as a potential pathogen, but a significant majority of adults have had previous exposure making a positive test unhelpful.
Other herpesviruses including herpes zoster (HZV), Epstein-Barr virus, and cytomegalovirus have been described to cause keratitis. Herpes zoster may manifest as pseudodendritic lesions (poorly branching, absent terminal bulbs), mucoid plaques, stromal keratitis, and/or uveitis characteristically leaving sectoral iris atrophy. Whether some or all of these entities represent immune response or active viral disease is controversial, but virus has been detected in late dendritic disease of HZV which are highly responsive to antiviral therapy (Pavan-Langston et al. 1995). Epstein-Barr virus more commonly causes a nummular keratitis but may also manifest as subepithelial infiltrates or a deep stromal keratitis. Acute and convalescent titers of Epstein-Barr-related IgM and IgG and EBV early antigen may be helpful in this setting. Monoclonal antibodies and PCR of corneal scrapings have been reported but are rarely done (Pflugfelder et al. 1990). Adenoviral epidemic keratoconjunctivitis may similarly be isolated and genotyped from swabs of the ocular surface but is seldom performed except in the setting of tracking large outbreaks of disease. A rapid in office antigen test has been available with a reasonable sensitivity (88 %) and specificity (91 %) to guide treatment and isolation of certain patients (Sambursky et al. 2006).
2.2.4 Parasitic
A number of different parasitic organisms can cause corneal disease. Parasites that are primarily spread to the cornea from endogenous sources include Onchocerca, Leishmania, and Trypanosoma are a devastating source of corneal blindness worldwide. They manifest as peripheral stromal interstitial keratitis at their site of entry, although frank corneal ulceration may sometimes occur. Acanthamoeba represents the most common ocular parasitic pathogen in Western world. Contact lens wear (>90 %) is the strongest risk factor for Acanthamoeba keratitis in that region with nearly 20 cases per million contact lens wearers per year afflicted in the UK with similar numbers now in the USA (Tu and Joslin 2010). Additional risk factors include exposure to contaminated domestic or environmental water while wearing or caring for the lenses and other hygiene related factors (Tu and Joslin 2010). The infection presents in many forms including a diffuse or pseudodendritic epitheliopathy, anterior stromal keratitis, interstitial keratitis, a ring infiltrate, and/or perineural infiltrates leading to confusion with other infectious and noninfectious processes (Fig. 2.9). Pain in the early stages is minimal but increases with the presence of perineural disease or deep stromal keratitis. Corneal scrapings can be subject to Giemsa stain, acridine orange, KOH preps, and calcofluor white for diagnosis with reasonable sensitivity and specificity (Fig. 2.10). Culture on non-nutrient agar with a bacterial overlay (higher yields obtained with Enterobacter than E. coli) or charcoal agar is relatively simple, but sensitivities are generally low between 35 and 50 %. Microsporidia keratitis has been increasingly described in immunocompetent individuals as causing an epithelial/subepithelial keratitis and a stromal interstitial keratitis. These patients usually have some history of exposure to soil or water and have been predominantly described in South Asia. Because it is an obligate intracellular organism, culturing microsporidia is difficult, requiring canine kidney cells for growth. Histologic stains have, therefore, been relied upon to make the diagnosis.
2.2.5 Corneal Imaging and Corneal Biopsy
Confocal microscopy refers to the precise timing of illumination and imaging at a specific focal point or plane to achieve high degrees of magnification, minimizing motion blur and light scatter. The cornea is ideal for this type of imaging because of its relative clarity and lack of reflective elements, to highlight areas of abnormality. Resolution of these instruments are in the 1–2 μm range, making it impossible to image most bacteria and viruses, but specific patterns, e.g., infectious crystalline keratopathy where a biofilm insinuates itself through cornea lamellae, owl eye cells in CMV endotheliitis, and filamentous bacteria (Nocardia) can be recognized (Elmallah et al. 2006; Vaddavalli et al. 2006;. Kobayashi et al. 2012). The greatest utility of the instrument in corneal infectious disorders has been to detect larger pathogens which do not cause dense suppuration such as fungi, acanthamoeba, and, to a lesser extent, Microsporidia (Sagoo et al. 2007). Both cysts and trophozoites can be seen, but the cysts are most characteristic observed as a double-walled cyst or a paired “coffee bean” appearance isolated or in chains (Fig. 2.11). The detection of Acanthamoeba keratitis has been shown in some centers to have a high sensitivity (>90 %) and high specificity ( 100 %) when compared to culture and other microbiologic methods (Tu et al. 2008a) but has also been reported to be significantly lower in other facilities (Hau et al. 2010). Similar results have been reported in the detection of the slender, septate branching of corneal fungal pathogens (Vaddavalli et al. 2011; Tu and Park 2007) as well as yeast. Confocal microscopy is invaluable in imaging deeper lesions without easy access for scraping such as Candida interface keratitis after endothelial keratoplasty (Lee et al. 2011).
Although confocal microscopy has largely supplanted corneal biopsy in many institutions, it remains a relevant technique for refractory or deep keratitides that otherwise defy identification. A number of techniques have been described, but all involve dissection of a sample of solid corneal tissue either from the anterior lamellae or from mid- to deep stroma using a flap created manually or via a femtosecond laser (Kim et al. 2008). Specimens are sent for histopathology and microbiologic studies with a success of greater than 40 % in identifying a causative organism in a recent review (Younger et al. 2012). Corneal scarring or perforation can be significant risks to corneal biopsy.
2.2.6 Molecular Diagnosis
Molecular diagnosis refers to identification of pathogens from their molecular signature, primarily DNA or RNA which by definition is unique to each organism. Utilizing primers either specific to an organism, a class of organisms, or universal primers, a single copy of DNA can be multiplied a million times over a period of hours, allowing its easy sequencing. Once its sequence has been determined, it can be matched fully or partially to previously sequenced organisms for identification. Because of its high sensitivity, however, false-positive results can be seen because of contamination at any stage of specimen recovery or with organisms that may be present or nonviable but not pathogenic. Extraneous compounds such as topical anesthetic or fluorescein can decrease the sensitivity of the assays (Goldschmidt et al. 2006). Quantitative and real-time PCR with appropriate validation can reduce the number of false-positive test results. It is most useful in detecting organisms not normally present in the eye and/or is difficult to confirm by other means. For example, PCR of tears or corneal scrapings directed toward the HSV polymerase gene has largely supplanted culture for herpes simplex ocular disease because of culture’s low sensitivity for an organism which is not normally found in the eye (Farhatullah et al. 2004; Satpathy et al. 2011). Identification of bacterial pathogens is based on their 16 s ribosomal DNA sequences (Prabhasawat et al. 2010), while a more significant experience with the 18 s ribosomal RNA region for the diagnosis of fungal keratitis has proven its utility (Vengayil et al. 2009; Ferrer and Alio 2011). Recent development of acanthamoebal PCR should lead to its increasing study and validation (Thompson et al. 2008; Goldschmidt et al. 2009).
2.3 Management
Unlike many other organ systems, comparatively small alterations in corneal structure and clarity can profoundly affect lifelong visual function. This combined with the relative inability of the corneal immune response to limit corneal infection makes it imperative to rapidly eliminate pathogens and modulate the corneal immune response to retain as much vision as possible. Empiric therapy is, therefore, directed not only against the most likely responsible pathogens but also against those pathogens most rapidly destructive of the corneal stroma specifically Gram-positive and Gram-negative bacteria. Although other pathogens may be more common in certain regions and mechanisms of injury, atypical bacteria, acanthamoeba, microsporidia, filamentous molds, and yeast are all comparatively slow growing, whereas bacteria may destroy the cornea in a matter of hours.
Since the availability and success of single-drug empiric therapy in the early 1990s, corneal scrapings for culture and smear are no longer routinely employed as part of the initial management of a routine bacterial corneal ulcer. However, microbiologic yields are highest before initiation of any antimicrobial therapy and should be strongly considered in large or deep ulcers, ulcers near the visual axis, ulcers failing prior therapy, and ulcers with either an atypical appearance or history at presentation. Suspicion for a larger, atypical organism at any stage should prompt consideration of confocal microscopy, although corneal biopsy should be reserved for later in the process because of its more invasive nature and higher risk of complication. Ongoing therapy should be directed by initial response or lack of response to initial therapy and directed by microbiologic examination when available.
2.3.1 Bacteria
Since the commercial availability of broad-spectrum fluoroquinolones in the early 1990s, single-drug therapy of presumed bacterial keratitides has been shown to have similar outcomes to multiple, fortified drug therapy (O’Brien et al. 1995; Constantinou et al. 2007). Single-drug therapy has the advantage of availability, easier compliance, and less time off of work, but some studies have pointed to a higher rate of corneal perforation presumably related to its activation of matrix metalloproteinases (Constantinou et al. 2007; Mallari et al. 2001). Although they are considered broad spectrum, having activity against both Gram-positive and Gram-negative bacteria, fluoroquinolones are commonly divided into second (ciprofloxacin, ofloxacin), third (levofloxacin), and fourth (moxifloxacin, gatifloxacin, besifloxacin) generations which predicts their specific antibacterial efficacy. Of these, only besifloxacin has pursued the FDA indication, but more extensive clinical studies of infectious keratitis have sufficiently established the particular spectrums of efficacy of the other fluoroquinolones in bacterial corneal ulceration. In general, the best Gram-negative activity, including Pseudomonas, is seen with the second-generation fluoroquinolones, while the fourth-generation fluoroquinolones have added some Gram-positive and atypical mycobacterial coverage. Other antibiotics need to be “fortified” to achieve effective corneal concentrations and combined together to afford the broad-spectrum coverage needed for empiric therapy. This usually consists of topical vancomycin or cephalosporin prescribed with an aminoglycoside like gentamicin or tobramycin.
Inducible antibiotic resistance is increasingly seen in both systemic and ocular infections. For corneal isolates specifically, methicillin-resistant Staphylococcus aureus (MRSA) has been increasingly reported in postsurgical corneal ulceration, and the laboratory resistance of Pseudomonas sp. to fluoroquinolones is rising secondary to both local and systemic use (Ray et al. 2013; Fintelmann et al. 2011). Despite laboratory resistance, topical ophthalmic antibiotics generally deliver sufficient concentrations of drugs to be clinically effective, but infections may be slower to resolve (Wilhelmus et al. 2003). While MRSA demonstrates high levels of fluoroquinolone resistance, vancomycin still remains almost universally effective against ocular pathogens. Vancomycin-resistant enterococcus is a rare but increasingly reported pathogen for infectious keratitis but may be sensitive to newer antibiotics like linezolid (Tu and Jain 2013). Similarly, 4th-generation fluoroquinolones have some activity against atypical mycobacteria, but primary therapeutic options remain topical fortified amikacin or clarithromycin with recent reports of linezolid sensitivity. Nocardia spp. are sensitive to fluoroquinolones but may also respond to amikacin and topical sulfamethoxazole-trimethoprim.
Topical corticosteroids should reduce inflammatory damage by modulating corneal damage in infectious keratitis but theoretically has the potential to worsen infections that require an intact immune response for clearance of the pathogen. Multiple studies have demonstrated a worsening of outcomes in vitro and in vivo when corticosteroid administration precedes effective antibacterial therapy but were less definitive when administered with or after initiation of antibiotics (Wilhelmus 2002). A recent prospective, randomized trial of corticosteroid administration 48 h after antibiotic initiation demonstrated no increase in complications with a trend toward improved visual acuity in those patients presenting with severe visual loss (Srinivasan et al. 2012). Future studies should help define the optimal indications and timing for administration of corticosteroids for bacterial keratitis.
2.3.2 Fungi
The treatment of fungal keratitis is significantly more challenging than bacterial keratitis because of the nature of fungal infections of the cornea and the limited options for topical and systemic therapy. The recognition of fungal keratitis is usually delayed, and fungal pathogens are often more deeply infiltrative which further hampers sufficient delivery of poorly penetrating antifungals to the deeper cornea (Table 2.2). Natamycin, an ophthalmic suspension of the polyene class drug pimaricin, is the only commercially available antifungal in commercial production. It is most efficacious against filamentous molds and was recently shown to be superior to voriconazole in the treatment of Fusarium keratitis, the most common fungal corneal pathogen, and similarly effective for other molds (Prajna et al. 2013). Topical amphotericin B 0.15 %, a compounded polyene antifungal, is effective against most fungi and was considered the drug of choice for yeast pathogens. Both drugs are associated with considerable ocular surface toxicity and irritation with topical use. Systemic use of amphotericin B, 1st- and 2nd-generation triazoles such as ketoconazole, fluconazole, and itraconazole have been described, but penetration into ocular tissues for the azoles is limited and severe side effects from all of the drugs limit their utility in all but the more refractory cases, especially amphotericin B. More recent preparations are much better tolerated. Compounded topical formulations of clotrimazole, itraconazole, and fluconazole have also been described for specific uses (Tu 2009). Because of the long turnaround time from order to result, all suspected fungal ulcers should be isolated, definitive identification pursued and antifungal sensitivities obtained without regard to the initial clinical response so that therapeutic options are available if and when treatment fails.
A number of new antifungals from the triazole (voriconazole, posaconazole, ravuconazole) and echinocandin (caspofungin, micafungin, anidulafungin) classes have been recently introduced. The triazoles are better absorbed, achieve higher tissue levels, and have a broader spectrum than previous azoles. Multiple reports of clinical resolution of keratitis and consecutive intracameral fungal endophthalmitis have been reported with systemic administration of these drugs (Tu and Park 2007; Tu et al. 2007). Although topical voriconazole was shown to be inferior to natamycin in Fusarium solani keratitis, it has been reported to be successful in a number of atypical fungal keratitides highlighting the utility of antifungal sensitivity testing for individual isolates. Since it is highly aqueous soluble, voriconazole as well as amphotericin B has been delivered intrastromally in recalcitrant fungal ulcers with sometimes dramatic resolution after a single injection (Garcia-Valenzuela and Song 2005; Prakash et al. 2008). Unlike bacterial keratitis, a certain number of fungal corneal infections will not be amenable to medical cure either due to pan-resistance to antifungals or because of the extent of infection. Circumscribable lesions which are responding poorly to medical therapy should be considered for early corneal transplantation. The lack of highly effective antifungals and the potentiation of fungal proliferation makes the use of corticosteroids at any point during routine treatment inadvisable. For this reason, alternative immune suppressants such as topical cyclosporine have been utilized post-keratoplasty.
2.3.3 Virus
In immunocompetent individuals, herpes simplex epithelial keratitis is a self-limited disease. Visual disability from a single episode is uncommon but increases significantly with reactivation of the virus from its latent state in the trigeminal ganglia resulting in repeated episodes epithelial keratitis, stromal or endothelial involvement, and/or uveitis (Young et al. 2010). Therapy is, therefore, directed against primary involvement of the cornea, although infection in any dermatome of CN V may result in corneal recurrence and prevention of recurrent disease. Although topical and systemic therapy is often prescribed to patients with periocular outbreaks, avoidance of direct inoculation is critically important. For corneal epithelial disease, commercially available ophthalmic treatments include topical trifluridine, the most epithelially toxic, and ganciclovir in the USA and topical acyclovir abroad which can reduce the duration of epithelial disease by 1–2 days (Table 2.3). Use of topical drugs should be limited to a 10-day period before reassessment for potential toxicity simulating active disease. The Herpetic Eye Disease Study did not find that a 3-week course of oral acyclovir (400 mg 5×/day) prevented progression of an epithelial HSV keratitis to stromal keratitis or iritis, but did find a suggestion that oral acyclovir may be beneficial in treating HSV iritis. Although topical corticosteroids did not affect final outcome, resolution of HSV keratitis symptoms was significantly more rapid when used in conjunction with an antiviral. Importantly, the study did find that long-term prophylaxis with oral acyclovir (400 mg 2×/day) reduced by half the number of recurrences of HSV ocular disease. This benefit has been confirmed in subsequent series (Young et al. 2010). Valacyclovir is a prodrug which is converted to acyclovir in vivo and demonstrates oral absorption ~5 times greater than oral acyclovir. This results in lower-frequency dosing which may be better tolerated and improve compliance with long-term prophylaxis. Long-term prophylaxis, however, may select for acyclovir-resistant strains, already high in patients with HSV keratitis, which would also be resistant to the common systemic alternatives of valacyclovir, famciclovir, or ganciclovir (van Velzen et al. 2013). The development of Herpes simplex vaccines has the potential to reduce both primary infection as well as suppress viral reactivation but has yet to be realized.
Herpes zoster keratitis is also usually self-limited and is not as prone to frequent recurrences, although chronic stromal keratitis can be blinding. Topical antivirals are usually unnecessary except in late pseudodendritic disease or mucoid plaques. Inflammatory stromal keratitis can be treated with topical corticosteroids. Both herpes simplex and herpes zoster keratitis, however, may develop blinding complications related to neurotrophic keratitis and/or chronic stromal inflammation. Persistent epithelial defects may lead to corneal melting and scarring, metaherpetic lesions may mimic persistent infection but require corticosteroids for resolution, and chronic stromal keratitis may lead to vascularization and corneal opacification. Because the prognosis for corneal transplantation is dismal in this setting, every effort should be taken to preserve sufficient clarity of the patient’s cornea with adequate immunosuppression, treating the corticosteroid side effects of cataract formation and glaucoma as needed. The effect of the increasing use of herpes zoster vaccines on recurrent ocular disease is yet unknown, but selective application of the vaccine may have unintended consequences in increasing reactivations in patients currently excluded from vaccination windows.
Although adenoviral disease is normally of minimal visual impact, the sheer number of sufferers and the uncommon moderate visual disability caused by EKC attracts attempts at treatment. Several promising antiviral compounds have been found to be of limited benefit and have been abandoned. Topical betadine corticosteroid combinations are currently being studied. Despite the availability of topical ganciclovir, no specific justification for its use in this disorder has yet surfaced.
2.3.4 Parasitic
The management options for Acanthamoeba keratitis are limited, and visual outcomes are highly dependent on early recognition and initiation of effective therapy (Tu et al. 2008b). A high index of suspicion should be held in all atypical keratitides especially in contact lens wearers. Once a diagnosis is made, debridement of all involved epithelium to debulk the infection is performed both for therapeutic and diagnostic purposes. The mainstay of therapy are the biguanides, either chlorhexidine 0.02 % or polyhexamethylene biguanide (PHMB) 0.02 % administered hourly for the first several days followed by a slow taper usually over several months based on clinical response (Table 2.4) (Dart et al. 2009). Adjunctive use of diamidines, propamidine or hexamidine, can be helpful in the early stages of treatment to inhibit encystment and kill active trophozoites but exhibit cumulative toxicity over several weeks. A minority of cases may be recalcitrant requiring increased biguanide concentrations, increased frequency of administration, and/or the addition of systemic medications. Topical neomycin and clotrimazole have also been described. Other previous generation azoles have been added systemically with limited evidence of benefit. The successful adjunctive use of topical and the sole use of oral voriconazole have been reported in the cure of Acanthamoeba keratitis (Tu et al. 2010; Bang et al. 2010). Acanthamoeba keratitis may also cause adnexal disease consisting of limbitis, scleritis, and dacryoadenitis as well as late complications of glaucoma, cataract, and permanent mydriasis. Corneal melting, persistent epithelial defects, and persistent stromal disease can severely complicate treatment. No definitive evidence yet exists for a detrimental effect of corticosteroid therapy on Acanthamoeba keratitis, and many of the corneal and extracorneal complications are immune in nature but still should be avoided if not needed. Selected cases may benefit from immune suppression either with corticosteroids or other systemic drugs to preserve ocular function (Dart et al. 2009). Similarly, the prognosis for corneal transplantation in active corneal disease has improved significantly with the use of effective anti-acanthamoebal therapy and should be considered a well-circumscribed, recalcitrant disease (Kitzmann et al. 2009). Microsporidial disease remains rarely described in the USA. Increased incidence of disease in Asia is a considerably different disease entity than previously described in immunosuppressed patients, occurring in immunocompetent individuals and responding to various commercially available topical antibacterials as well as observation only (Tu and Joslin 2012). Some patients will still progress to corneal transplantation, however.
Collagen cross-linking, a therapeutic intervention designed to “harden” the cornea with riboflavin-assisted UV irradiation for cases of corneal ectasia, has been recently described for a number of cases of infectious keratitis of varying etiologies. In vitro studies, however, show no effect on acanthamoebal cysts or fungi and a variable effect on most bacteria with the concentrations of riboflavin and UV exposures utilized for cross-linking. The depth of penetration of standard treatments is also limited to the anterior cornea. Recent studies have demonstrated a reduction in corneal necrosis and overall improvement in bacterial ulcers and some acanthamoebal ulcers as an adjunctive treatment to medical therapy with fungal ulcers being the least responsive (Price et al. 2012; Makdoumi et al. 2010; Alio et al. 2013). There is little agreement on the mechanism, indications, or complications for collagen cross-linking and will be the continued subject of studies for the near future.
2.4 Conclusions
Infectious keratitis can lead to significant ocular morbidity and loss of overall function and represents both a diagnostic and management challenge. Prevention and prompt diagnosis relies on an understanding of the prevalent risk factors for corneal infection which vary from contact lens wear in more economically developed areas to trauma in less developed areas. Common pathogens will vary based on these risk factors as well as climate, local immunosuppression, and mechanism of injury. While almost all initial empiric therapy for infectious keratitis must target common bacterial pathogens because of their propensity for rapid corneal destruction, a high index of suspicion should be held for unresponsive or atypical appearing keratitides. All of these patients should be subject to culture and sensitivity testing. With all forms of infectious keratitis, final visual outcomes are highly dependent on minimizing delay in the administration of effective antimicrobial treatment. Topical ophthalmic administration provides a highly concentrated route of drug delivery. Treatment efficacy should be judged by frequent observation regardless of culture results and changed if found to be ineffective. A flexible approach to utilizing available diagnostic techniques and treatment options alongside innovative drugs, alternative routes of administration, and judicious use of immunosuppressants will improve long-term visual outcomes.
References
Alio JL, Abbouda A, Valle DD, Del Castillo JM, Fernandez JA. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect. 2013;3(1):47.
Bang S, Edell E, Eghrari AO, Gottsch JD. Treatment with voriconazole in 3 eyes with resistant Acanthamoeba keratitis. Am J Ophthalmol. 2010;149(1):66–9.
Benson WH, Lanier JD. Comparison of techniques for culturing corneal ulcers. Ophthalmology. 1992;99(5):800–4.
Bhadange Y, Sharma S, Das S, Sahu SK. Role of liquid culture media in the laboratory diagnosis of microbial keratitis. Am J Ophthalmol. 2013;156:745–51.
Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834–8.
Burton MJ, Pithuwa J, Okello E, et al. Microbial keratitis in East Africa: why are the outcomes so poor? Ophthalmic Epidemiol. 2011;18(4):158–63.
Constantinou M, Daniell M, Snibson GR, Vu HT, Taylor HR. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007;114(9):1622–9.
Dahlgren MA, Lingappan A, Wilhelmus KR. The clinical diagnosis of microbial keratitis. Am J Ophthalmol. 2007;143(6):940–4.
Dalmon C, Porco TC, Lietman TM, et al. The clinical differentiation of bacterial and fungal keratitis: a photographic survey. Invest Ophthalmol Vis Sci. 2012;53(4):1787–91.
Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487–99.e482.
DeCroos FC, Garg P, Reddy AK, et al. Optimizing diagnosis and management of nocardia keratitis, scleritis, and endophthalmitis: 11-year microbial and clinical overview. Ophthalmology. 2011;118(6):1193–200.
Elmallah MK, Munir WM, Janda WM, Tu EY. Gemella haemolysans infectious crystalline keratopathy. Cornea. 2006;25(10):1245–7.
Erie JC, Nevitt MP, Hodge DO, Ballard DJ. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Arch Ophthalmol. 1993;111(12):1665–71.
Farhatullah S, Kaza S, Athmanathan S, Garg P, Reddy SB, Sharma S. Diagnosis of herpes simplex virus-1 keratitis using Giemsa stain, immunofluorescence assay, and polymerase chain reaction assay on corneal scrapings. Br J Ophthalmol. 2004;88(1):142–4.
Ferrer C, Alio JL. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J Ophthalmic Inflamm Infect. 2011;1(1):15–22.
Fintelmann RE, Hoskins EN, Lietman TM, et al. Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch Ophthalmol. 2011;129(4):399–402.
Garcia-Valenzuela E, Song CD. Intracorneal injection of amphothericin B for recurrent fungal keratitis and endophthalmitis. Arch Ophthalmol. 2005;123(12):1721–3.
Gomez JT, Robinson NM, Osato MS, Wilhelmus KR. Comparison of acridine orange and Gram stains in bacterial keratitis. Am J Ophthalmol. 1988;106(6):735–7.
Goldschmidt P, Rostane H, Saint-Jean C, et al. Effects of topical anaesthetics and fluorescein on the real-time PCR used for the diagnosis of Herpesviruses and Acanthamoeba keratitis. Br J Ophthalmol. 2006;90(11):1354–6.
Goldschmidt P, Degorge S, Benallaoua D, et al. New tool for the simultaneous detection of 10 different genotypes of Acanthamoeba available from the American Type Culture Collection. Br J Ophthalmol. 2009;93(8):1096–100.
Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3(3):159–66.
Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57(4):273–9.
Hau SC, Dart JK, Vesaluoma M, et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol. 2010;94(8):982–7.
Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–24.
Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128(8):1022–8.
Keay L, Edwards K, Dart J, Stapleton F. Grading contact lens-related microbial keratitis: relevance to disease burden. Optom Vis Sci. 2008;85(7):531–7.
Kim JH, Yum JH, Lee D, Oh SH. Novel technique of corneal biopsy by using a femtosecond laser in infectious ulcers. Cornea. 2008;27(3):363–5.
Kitzmann AS, Goins KM, Sutphin JE, Wagoner MD. Keratoplasty for treatment of Acanthamoeba keratitis. Ophthalmology. 2009;116(5):864–9.
Kobayashi A, Yokogawa H, Higashide T, Nitta K, Sugiyama K. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. Am J Ophthalmol. 2012;153(3):445–53.
Lee WB, Foster JB, Kozarsky AM, Zhang Q, Grossniklaus HE. Interface fungal keratitis after endothelial keratoplasty: a clinicopathological report. Ophthalmic Surg Lasers Imaging. 2011;42:e44–8.
Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010;29(12):1353–8.
Mallari PL, McCarty DJ, Daniell M, Taylor H. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am J Ophthalmol. 2001;131(1):131–3.
Mascarenhas J, Srinivasan M, Chen M, et al. Differentiation of etiologic agents of bacterial keratitis from presentation characteristics. Int Ophthalmol. 2012;32(6):531–8.
O’Brien TP, Maguire MG, Fink NE, Alfonso E, McDonnell P. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group. Arch Ophthalmol. 1995;113(10):1257–65.
Oechsler RA, Feilmeier MR, Miller D, Shi W, Hofling-Lima AL, Alfonso EC. Fusarium keratitis: genotyping, in vitro susceptibility and clinical outcomes. Cornea. 2013;32(5):667–73.
Pavan-Langston D, Yamamoto S, Dunkel EC. Delayed herpes zoster pseudodendrites. Polymerase chain reaction detection of viral DNA and a role for antiviral therapy. Arch Ophthalmol. 1995;113(11):1381–5.
Pflugfelder SC, Huang A, Crouse C. Epstein-Barr virus keratitis after a chemical facial peel. Am J Ophthalmol. 1990;110(5):571–3.
Prabhasawat P, Leelaporn A, Tesavibul N, Uiprasertkul M, Chirapapaisan C. Molecular identification by 16S rDNA sequencing using excised corneal tissues: a useful diagnostic tool for refractory keratitis. Jpn J Ophthalmol. 2010;54(1):97–100.
Prajna VN, Nirmalan PK, Saravanan S, Srinivasan M. Economic analysis of corneal ulcers in South India. Cornea. 2007;26(2):119–22.
Prajna NV, Krishnan T, Mascarenhas J, et al. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–9.
Prakash G, Sharma N, Goel M, Titiyal JS, Vajpayee RB. Evaluation of intrastromal injection of voriconazole as a therapeutic adjunctive for the management of deep recalcitrant fungal keratitis. Am J Ophthalmol. 2008;146(1):56–9.
Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price Jr FW. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012;28(10):706–13.
Ray KJ, Prajna L, Srinivasan M, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131(3):310–3.
Sagoo MS, Mehta JS, Hau S, et al. Microsporidium stromal keratitis: in vivo confocal findings. Cornea. 2007;26(7):870–3.
Sambursky R, Tauber S, Schirra F, Kozich K, Davidson R, Cohen EJ. The RPS adeno detector for diagnosing adenoviral conjunctivitis. Ophthalmology. 2006;113(10):1758–64.
Satpathy G, Mishra AK, Tandon R, et al. Evaluation of tear samples for Herpes Simplex Virus 1 (HSV) detection in suspected cases of viral keratitis using PCR assay and conventional laboratory diagnostic tools. Br J Ophthalmol. 2011;95(3):415–8.
Sharma S, Kunimoto DY, Gopinathan U, Athmanathan S, Garg P, Rao GN. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: a survey of eight years of laboratory experience. Cornea. 2002;21(7):643–7.
Srinivasan M, Mascarenhas J, Rajaraman R, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT). Arch Ophthalmol. 2012;130(2):143–50.
Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115(10):1655–62.
Thompson PP, Kowalski RP, Shanks RM, Gordon YJ. Validation of real-time PCR for laboratory diagnosis of Acanthamoeba keratitis. J Clin Microbiol. 2008;46(10):3232–6.
Tu EY. Alternaria keratitis: clinical presentation and resolution with topical fluconazole or intrastromal voriconazole and topical caspofungin. Cornea. 2009;28(1):116–9.
Tu EY, Jain S. Topical linezolid 0.2% for the treatment of vancomycin-resistant or vancomycin-intolerant gram-positive bacterial keratitis. Am J Ophthalmol. 2013;155(6):1095–8.e1091.
Tu EY, Joslin CE. Recent outbreaks of atypical contact lens-related keratitis: what have we learned? Am J Ophthalmol. 2010;150(5):602–8.e602.
Tu EY, Joslin CE. Microsporidia and Acanthamoeba: the role of emerging corneal pathogens. Eye (Lond). 2012;26(2):222–7.
Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592). Am J Ophthalmol. 2007;143(2):222–7.
Tu EY, Joslin CE, Sugar J, Booton GC, Shoff ME, Fuerst PA. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea. 2008a;27(7):764–72.
Tu EY, Joslin CE, Sugar J, Shoff ME, Booton GC. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008b;115(11):1998–2003.
Tu EY, Joslin CE, Shoff ME. Successful treatment of chronic stromal acanthamoeba keratitis with oral voriconazole monotherapy. Cornea. 2010;29(9):1066–8.
Tu EY, Park AJ. Recalcitrant Beauveria bassiana keratitis: confocal microscopy findings and treatment with posaconazole (Noxafil). Cornea. 2007;26(8):1008–10.
Vaddavalli PK, Garg P, Sharma S, Thomas R, Rao GN. Confocal microscopy for Nocardia keratitis. Ophthalmology. 2006;113(9):1645–50.
Vaddavalli PK, Garg P, Sharma S, Sangwan VS, Rao GN, Thomas R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology. 2011;118(1):29–35.
van Velzen M, van de Vijver DA, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis. 2013;208:1359–65.
Vengayil S, Panda A, Satpathy G, et al. Polymerase chain reaction-guided diagnosis of mycotic keratitis: a prospective evaluation of its efficacy and limitations. Invest Ophthalmol Vis Sci. 2009;50(1):152–6.
Wilhelmus KR. Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology. 2002;109(5):835–42; quiz 843.
Wilhelmus KR, Abshire RL, Schlech BA. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003;121(9):1229–33.
Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128(9):1178–83.
Younger JR, Johnson RD, Holland GN, et al. Microbiologic and histopathologic assessment of corneal biopsies in the evaluation of microbial keratitis. Am J Ophthalmol. 2012;154(3):512–9.e512.
Compliance with Ethical Requirements
Elmer Tu declares that he has no financial interests in the subject matter of the manuscript.
No animal or human studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tu, E.Y. (2014). Advances in the Diagnosis and Management of Infectious Keratitis. In: Jeng, B. (eds) Advances in Medical and Surgical Cornea. Essentials in Ophthalmology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44888-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-662-44888-5_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44887-8
Online ISBN: 978-3-662-44888-5
eBook Packages: MedicineMedicine (R0)