Abstract
Placebo analgesia has become a well-studied phenomenon that encompasses psychology, physiology and pharmacology. In this chapter we explore the complex interactions between these disciplines in order to argue that the placebo response is more than a simple change in perception but is a cognitive style driven by prior expectations. The expectation of treatment effect is shaped by prior information and prior experience which our brain uses to predict future events. In the case of placebo analgesia the prediction of pain relief overrules the actual feeling of pain leading to a decrease in pain sensation. This altered sensation can be attributed to personality traits, altered error monitoring processes, changes in anticipatory responses to pain and activation of the endogenous opioid system. In conclusion we discuss how altered sensory processing by descending pain modulation may play a part in placebo analgesia and how the loss of the brains prefrontal regions can make it impossible to have a placebo response.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The placebo response, once considered a nuisance in clinical trials, is now being investigated in its own right as a way to enhance treatment effects endogenously. Conditions such as pain and depression, where the outcome measures are continuous, subjective and are based on self-reports, are most likely to be subject to manipulation by placebo (Hrobjartsson and Gotzsche 2010), but the placebo response has also been noted in less subjective disorders such as Parkinson’s disease (Colloca et al. 2004; De La Fuente-Fernandez et al. 2001) and asthma (Kaptchuk et al. 2008; Kemeny et al. 2007). The most studied of these conditions to manipulations by placebo is pain. Studies of placebo analgesia give us great insight into how psychological manipulations can cause physical changes in perception.
Placebo response rates are highly variable, ranging from no response to a full response. A placebo treatment will work if it has “meaning” to the individual receiving it and it is this “meaning” that is thought to cause the variance seen in placebo response rates (Moerman 2002).
Placebo response is highly dependent on prior expectation. Keeping expectations the same leads to a reproducible placebo response (Morton et al. 2009). Conversely, varying expectations, such as altering the name of the placebo, causes the response to become irreproducible (Whalley et al. 2008). Placebo response rates also vary inter-individually when the mode of treatment changes. For example, no relationship was found between subjects’ responses to placebo pills and sham acupuncture (Kong et al. 2013).
At their simplest, the expectations generated by a treatment cause a change in the interpretation of the sensory information which is used to represent and understand the environment. In the case of placebo analgesia this leads to the individual experiencing a decrease in pain perception. However, the variability of the placebo response suggests that the mechanisms behind these changes in perception are much more complex. Here we look at evidence demonstrating that the placebo response is brought about by an enduring cognitive change in information processing.
2 Prior Expectations
If perception is receiving information about your environment, cognition can be viewed as learning or knowing about your environment. Learning is fundamental to placebo responsiveness. For instance, regular use of paracetamol leads to associations with the tablets’ size, shape, colour, packaging and taste with pain relief. In order to learn, one must first receive information and it is this prior information that allows us to generate cognitive factors such as expectations and beliefs regarding future events. Expectations of analgesia are known to modify responses to analgesic treatment, a phenomena that is illustrated by Colloca et al 2004. In this study, patients unaware that they were receiving morphine via a computer-controlled infusion (hidden administration) experienced a significant reduction in analgesia compared to patients explicitly told they would be receiving morphine to help with their pain (open administration) (Colloca et al. 2004). The placebo component of the treatment is thought to be the difference between the open administration of the treatment and the hidden administration of treatment. The strength of these treatment expectations comprises an important component of the placebo response. Parkinson’s patients given varying expectations of receiving active medication when given a placebo only experienced a significant release of dopamine when they were informed that they had a 75 % probability of receiving active medication (Lidstone et al. 2005).
Learning about treatments comes not only through our own experience, but also from knowledge we have gained from others. Gaining information from observing other people can in itself generate expectations of treatment outcome possibly by establishing “a self-projection into the future outcome (pp 33)” (Colloca and Benedetti 2009).
How do we get from an expectation of treatment to an actual placebo response? In the case of experimental placebo analgesia, one would expect that once the subject is exposed to pain after the placebo administration, they would realise that their expectation of pain relief was incorrect and would not experience an analgesic effect. Of course in some instances this is true and is a reason for the variability in magnitude of placebo response. However, in placebo responders this doesn’t happen and may be explained by how our brains process sensory inputs.
3 Signal Detection Errors and Cognitive Bias
In order to quickly interpret the environment our brain constantly generates predictions about what our senses are telling us (Kveraga et al. 2007). These predictions use our past experiences and any prior information of the situation to create a picture of what is actually happening. Changes in our environment produce sensory information that can be incompatible with the model of the environment that has been generated in the brain (Yu and Dayan 2005). If the brain’s predictions (top-down) and the sensory representations (bottom-up) don’t match up, the two sets of information are thought to integrate through an error minimization pathway. A large error signal is then projected to a higher neural region where a new prediction refined by the error signal is generated (Friston 2005). Representing this as a computational model has shown how top-down inputs reduce the uncertainty of the stimulus representation when compared with bottom-up processing alone and leads to faster processing speeds (Siegel et al. 2000). In the placebo response, the expectation of treatment is thought to create uncertainty about incoming sensory information. Siegel’s model shows how top-down/bottom-up synchrony can lead to the biased processing of top-down information.
As individuals our level of cognitive flexibility to error varies (Allan and Siegel 2002). Therefore what is immediately noticeable to one person as violating their prior expectations may be totally overlooked by another. Because there is such variability in placebo response both intra- and inter-individually, researchers are interested in being able to predict placebo responses even before placebo is administered. Studies comparing personality and placebo response indicate that suggestibility (De Pascalis et al. 2002; Morton et al. 2010a, b) and optimism (Geers et al. 2010; Morton et al. 2009) may be important correlates of placebo magnitude. How placebo responders weigh perceptual information against prior expectations has been previously tested. Screening of subjects in a visual perceptual task resulted in an experimental population of which half had a tendency to rely heavily on prior expectations, and half who tended to rely on the current perceptual information (Morton et al. 2011). Individuals who used prior expectations when making perceptual decisions in both the perceptual task and the placebo manipulation were found to have greater magnitude of placebo response (Morton et al. 2010b). These results suggest that placebo responders “ignore” the incoming sensory information to base their decisions on their prior expectations, which creates a conflict between the incoming pain signals and cognitive control. In this scenario, the placebo response should have a direct influence on electrophysiological markers of error processing. This has been shown by Koban et al. (2012) when they hypothesised that placebo analgesia “may induce a transient change in the reactivity of cognitive control networks in order to adjust for the mismatch between predicted and experienced pain” (pp 7). The authors found that placebo analgesia was related to altered error monitoring processes in a go/nogo task. The go/nogo task was specifically designed to cause a large number of response errors and therefore a large event-related potential (ERP) on the EEG that corresponded to error processing and adjustments in behavioural control and error awareness. The error processing potential amplitude was significantly increased for placebo responders in the placebo condition compared to controls. Source reconstructions of the EEG recordings showed that this effect was probably caused by increased activation of specific medial frontal and lateral prefrontal regions, regions previously demonstrated to be vital in placebo analgesia (Krummenacher et al. 2010; Wager et al. 2004). Importantly these areas are also associated with adaptive control brain mechanisms (Botvinick et al. 2001; Ridderinkhof et al. 2004) and adjustments to expectations (Koban et al. 2012; Montague and Lohrenz 2007).
4 Anticipatory Responses
The anticipation of less pain during a placebo treatment has been suggested as an important component of placebo analgesia. Imaging a placebo conditioning procedure using fMRI showed activation in the left dorsolateral prefrontal cortex, medial frontal cortex and the anterior mid-cingulate cortex. These same areas were also found to be modulated during the anticipation of placebo analgesia (Watson et al. 2009). Learnt analgesia can have a significant effect on future anticipatory responses to pain. In a repeated placebo paradigm, participants in the placebo group not only anticipated less pain than controls after the administration of placebo but also demonstrated lowered anticipatory responses to pain before placebo administration when the treatment was repeated 2–6 weeks later (Morton et al. 2010a). Using a penalised regression procedure (LASSO-PCR) to create a model of re-analysed data from an earlier experiment (Wager et al. 2004), Wager et al. (2011) were able to predict 12 % of the variance found in the magnitude of placebo analgesia. Large magnitude placebo analgesia was related to increases in anticipatory responses in the prefrontal cortex and correlated with prior expectations of analgesia, and reduced anticipatory responses in somatosensory area 2/temporal regions. The latter probably reflects the shifting of attention away from the painful stimulus (Coghill et al. 1999). Together, these results suggest that an enduring cognitive change in anticipatory pain processing can be produced by placebo analgesia, and the engagement of emotional appraisal pathways is responsible for some of the variation in placebo analgesia.
5 Opioids in Placebo Analgesia and Distraction
Many studies have associated placebo analgesia with the activation of the endogenous opioid system and with brain areas that include the prefrontal, limbic and brainstem regions (Wager et al. 2007; Zubieta et al. 2005). Changes in activity of these brain regions are related to reductions in the physical and emotional aspects of pain experience. Placebo response is most likely initiated in the dorsolateral prefrontal cortex which is regarded as a cognitive-evaluative area. The placebo analgesic effect relies on enhanced functional coupling of the rostral anterior cingulate cortex with the hypothalamus, and brainstem areas such as the opioid receptor-rich periaqueductal grey and rostral ventral medulla (Amanzio and Benedetti 1999; Eippert et al. 2009; Wager et al. 2004, 2007), areas which have consistently shown expectancy-induced increases in relation to placebo analgesia (Atlas et al. 2010; Craggs et al. 2007; Eippert et al. 2009). The activity seen during placebo analgesia within all key regions of the descending pain modulatory system is significantly decreased with naloxone, an opioid antagonist (Amanzio and Benedetti 1999; Eippert et al. 2009; Levine et al. 1979; Zubieta et al. 2005). The placebo-dependent reduction of BOLD responses in fMRI and its reversal by naloxone is most evident in the dorsal anterior cingulate cortex (Eippert et al. 2009). Modulation of this region has been previously demonstrated in expectation manipulations (Keltner et al. 2006). During an fMRI study to image the spinal cord during pain, participants were required to do a continuous performance task (the N-back test) in order to distract them from the painful stimulus. The distraction task significantly reduced spinal responses to painful events whilst administration of naloxone during the task selectively blocked the distraction-induced reductions on reported pain (Sprenger et al. 2012). This indicates that opioids are at least partially required for both placebo responses and distraction effects. However, evidence shows placebo analgesia is not always mediated by opioids with some placebo responses being naloxone insensitive (Amanzio and Benedetti 1999; Vase et al. 2005). For example, Eippert et al. (2009) produced a blockade of placebo-induced decreases in BOLD responses, with naloxone, in regions associated with pain. However, the behavioural response was not completely blocked by naloxone as there was no significant increase in pain ratings after its administration. This suggests that pain self-reports due to placebo can be distinct from the physiological process of nociception which firmly implicates an additional non-opioidergic component to placebo analgesia.
During opioid analgesia and placebo analgesia there is consistent overlapping of brain regions involved in pain. In opioid analgesia there is more activation in the rostral anterior cingulate cortex and the anterior insula, whilst placebo analgesia generates greater responses in the lateral orbitofrontal cortex and ventrolateral prefrontal cortex. It is thought that this difference can be accounted for by the error signal generated by the discrepancy between actual pain and expectations of pain relief in placebo analgesia that is not present in opioid analgesia (Petrovic et al. 2010; Wager and Roy 2010). Colloca et al. (2004) open/hidden paradigm discussed earlier demonstrated that expectations of pain relief influence the magnitude of analgesia. Using this same paradigm to test the relationship between expectations and the opiate remifentanil, Atlas et al. (2012) showed that a hidden administration of remifentanil (no expectation of analgesia) influenced different brain regions when compared to an open administration of remifentanil (expectation of analgesia). Expectation of analgesia activated lateral and ventromedial prefrontal cortices and caused reduced responses in amygdala and pain-processing thalamic and somatosensory regions whereas analgesia caused by remifentanil without expectation of analgesia produced strong decreases in the anterior cingulate cortex and the weakest effects on somatosensory areas (S2/dorsal posterior insula). This suggests expectation operated independently but alongside remifentanil to reduce pain sensation. What these studies show us is that opioids, distraction, and placebo may have a common effect on pain, but they involve dissociable brain regions.
6 Altered Sensory Processing
As discussed in the previous section, the periaqueductal grey and the rostral ventral medulla are important in the production of the opioid-mediated placebo response. These same areas are also involved in the descending inhibition of pain by diffuse noxious inhibitory control (DNIC). DNIC was first described by LeBars et al. (1979) and is an endogenous pain-modulating system which includes descending inhibitory projections coordinated in the rostral ventral medulla. DNIC is a mechanism by which the response to painful stimulation by dorsal horn wide dynamic range neurons is inhibited by a second painful stimulus (counter-irritation). This response has been previously shown to reduce the amplitude of a spinal/nociceptive flexion reflex (RIII) (Willer et al. 1989, 1990). It has been suggested that the opioid-dependent placebo response may be attributed to, or work in parallel with, the inhibition of nociceptive processing in the dorsal horn of the spinal cord. Experimentally, expectations of hyperalgesia (nocebo) have been shown to block the normal decrease in both pain perception and the nociceptive reflex activity that is usually seen during counter-irritation (Goffaux et al. 2007). In contrast, fMRI imaging of the cervical spinal cord during painful heat together with placebo treatment significantly reduced spinal activity in response to heat compared to no treatment (Eippert et al. 2009). These findings suggest that the modulation of pain by placebo affects nociceptive signal processing at the earliest stage of the central nervous system.
7 Loss of Prefrontal Regions
The activation of opioid transmission has been seen in prefrontal brain areas (Eippert et al. 2009; Zubieta et al. 2005). In neurodegenerative conditions such as Alzheimer’s disease, loss of prefrontal lobes can have severe implications for treatment effects. Benedetti et al. (2006) applied a local anaesthetic either openly or covertly to the skin of Alzheimer’s patients to reduce burning pain after venipuncture. In this paradigm, as in Colloca et al. (2004), the placebo component of the treatment was shown by the difference in analgesia after expected and unexpected application of the anaesthetic. Frontal lobe damage often seen in Alzheimer’s can be assessed using the frontal assessment battery, a series of simple tests which identifies impairments in cognition and motor behaviour. Patients with reduced frontal assessment battery scores showed a reduced placebo component of treatment and the reduction in placebo response was correlated with reduced cognitive status and the reduced functional connectivity of the frontal lobes to the rest of the brain. Losing the placebo component reduced the effectiveness of the treatment so much that a dose increase was needed to ensure sufficient analgesia.
Of particular interest in placebo analgesia is the involvement of the dorsolateral prefrontal cortex, an area known for cognitive and attention-related pain regulation (Lorenz et al. 2003; Miller and Cohen 2001; Peyron et al. 2000) that has been repeatedly identified in expectation-related placebo analgesia (Wager et al. 2004; Zubieta et al. 2005). Disruption of the dorsolateral prefrontal cortex has been shown to interfere with placebo analgesia. Krummenacher et al. (2010) used sham repetitive transcranial magnetic stimulation (rTMS) and an expectation of pain relief to induce an increase in pain threshold and pain tolerance indicative of a placebo response. Then using low-frequency rTMS, they artificially inhibited the function of the dorsolateral prefrontal cortex, which disrupted the placebo response and decreased pain threshold and pain tolerance. Previously, the dorsolateral prefrontal cortex has been related to the generation, maintenance and manipulation of cognitive representations (Miller and Cohen 2001; Pacheco-Lopez et al. 2006) and it has also been implicated in general attentional processes (Miller and Cohen 2001). The authors suggest that the loss of placebo analgesia after rTMS can be explained by the effects of disrupting the cognitive representation of analgesia and the directing of attention towards the painful stimulus.
Conclusion
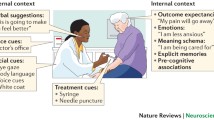
If perception is the information we receive about a stimulus, cognition is how we have learnt to deal with that information. In the context of a placebo response, the stimulus information we receive is not variable but how we have learnt to deal with it using the expectations we have formed from our prior experiences is. To suggest that placebo response is due to a simple change in perception is to suggest that the placebo response is formed by a simple mechanism. Instead we see that a network of brain areas is responsible for the formation of a response, and that the frontal cortex, particularly the dorsolateral prefrontal cortex, is the core area for the cognitive modulation of pain. With no prefrontal cortex there can be no cognitive input, and with no cognitive input there can be no placebo response (Fig. 1).
References
Allan LG, Siegel S (2002) A signal detection theory analysis of the placebo effect. Eval Health Prof 25(4):410–420
Amanzio M, Benedetti F (1999) Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 19(1):484–494
Atlas LY, Bolger N, Lindquist MA, Wager TD (2010) Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977
Atlas LY, Whittington R, Lindquist MA, Wielgosz J, Sonty N, Wager TD (2012) Dissociable influences of opiates and expectations on pain. J Neurosci 32(23):8053–8064
Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, Asteggiano G (2006) Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain 121(1–2):133–144
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652
Coghill RC, Sang CN, Maisog JM, Iadarola MJ (1999) Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 82(4):1934–1943
Colloca L, Benedetti F (2009) Placebo analgesia induced by social observational learning. Pain 144(1–2):28–34
Colloca L, Lopiano L, Lanotte M, Benedetti F (2004) Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol 3(11):679–684
Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM (2007) Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. NeuroImage 38(4):720–729
De La Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ (2001) Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science 293(5532):1164–1166
De Pascalis V, Chiaradia C, Carotenuto E (2002) The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain 96(3):393–402
Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C (2009) Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63(533):543
Friston KJ (2005) A theory of cortical responses. Philos Trans R Soc B 360:815–836
Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR (2010) Dispositional optimism predicts placebo analgesia. J Pain 11(11):1165–1171
Goffaux P, Redmond WJ, Rainville P, Marchand S (2007) Descending analgesia - when the spine echoes what the brain expects. Pain 130:137–143
Hrobjarrtsson A, Gotzsche PC (2010) Placebo interventions for all clinical conditions. Cochrane Database Syst Rev 1:CD003974. doi:10.1002/14651858.CD003974.pub3
Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, Kirsch I, Wechsler ME (2008) Do “placebo responders” exist? Contemp Clin Trials 29(4):587–595
Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL (2006) Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 26(16):4437–4443
Kemeny ME, Rosenwasser LJ, Panetierri RA, Rose RM, Berg-Smith SM, Kline JN (2007) Placebo response in asthma: a robust and objective phenomenom. J Allergy Clin Immunol 199(6):1375–1381
Koban L, Brass M, Lynn MT, Pourtois G (2012) Placebo analgesia affects brain correlates of error processing. PLoS ONE 7(11):e49784
Kong J, Spaeth R, Cook A, Kirsch I, Claggett B, Vangel M, Gollub RL, Smoller JW, Kaptchuk TJ (2013) Are all placebo effects equal? Placebo pills, sham accupuncture, cue condtioning and their association. PLoS ONE 8(7):e67485
Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G (2010) Prefrontal cortex modulates placebo analgesia. Pain 148(3):368–374
Kveraga K, Boshyan B, Bar B (2007) Top-down predicitons in the cognitive brain. Brain Cogn 65:145–168
LeBars D, Dickenson AH, Besson JM (1979) Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6(3):283–304
Levine JD, Gordon NC, Fields HL (1979) Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature 278(5706):740–741
Lidstone SC, De La Fuente-Fernandez R, Stoessl AJ (2005) The placebo response as a reward mechanism. Sem Pain Med 3(1):37–42
Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126(5):1079–1091
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202
Moerman D (2002) Meaning, medicine, and the “placebo effect”. Cambridge University Press, Cambridge
Montague PR, Lohrenz T (2007) To detect and correct: norm violations and their enforcement. Neuron 56:14–18
Morton DL, Watson A, El-Deredy W, Jones AKP (2009) Reproducibility of placebo analgesia: effect of dispositional optimism. Pain 146(1):194–198
Morton DL, Brown CA, Watson A, El-Deredy W, Jones AKP (2010a) Cognitive changes as a result of a single exposure to placebo. Neuropsychologia 48(7):1958–1964
Morton DL, El-Deredy W, Watson A, Jones AKP (2010b) Placebo analgesia as a case of a cognitive style driven by prior expectation. Brain Res 1359:137–141
Morton DL, El-Deredy W, Morton AS, Elliott R, Jones AKP (2011) Optimism facilitates the utilisation of prior cues. Eur J Pers 25(6):424–430
Pacheco-Lopez G, Engler H, Niemi MB, Schedlowski M (2006) Expectations and associations that heal: immunomodulatory placebo effects and its neurobiology. Brain Behav Immun 20(5):430–446
Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M (2010) A prefrontal non-opioid mechanism in placebo analgesia. Pain 150(1):59–65
Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Clin Neurophysiol 30(5):263–288
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306:443–447
Siegel M, Kording KP, Konig P (2000) Integrating top-down and bottom-up sensory processing by somato-dendritic interactions. J Comput Neurosci 8:161–173
Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Bnchel C (2012) Attention modulates spinal cord responses to pain. Curr Biol 22(11):1019–1022
Vase L, Robinson ME, Verne GN, Price DD (2005) Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain 115(3):338–347
Wager TD, Roy M (2010) Separate mechanisms for placebo and opiate analgesia? Pain 150(1):8–9
Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD (2004) Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 303(5661):1162–1167
Wager TD, Scott DJ, Zubieta JK (2007) Placebo effects on human {micro}-opioid activity during pain. Proc Natl Acad Sci USA 104:11056–11061
Wager TD, Atlas LY, Leotti LA, Rilling JK (2011) Predicting individual differences in placebo analgesia: contributions of brain activity during pain anticipation and pain experience. J Neurosci 31(2):439–452
Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I (2009) Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145:24–30
Whalley B, Hyland ME, Kirsch I (2008) Consistency of the placebo effect. J Psychosom Res 64:537–541
Willer JC, De Broucker T, Le Bars D (1989) Encoding of nociceptive thermal stimuli by diffuse noxious inhibitory controls in humans. J Neurophysiol 62(5):1028–1038
Willer JC, Le Bars D, De Broucker T (1990) Diffuse noxious inhibitory controls in man: Involvement of an opioidergic link. Eur J Pharmacol 182(2):347–355
Yu AJ, Dayan P (2005) Uncertainty, neuromodulation, and attention. Neuron 46(4):681–692
Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS (2005) Placebo effects mediated by endogenous opioid activity on {micro}-opioid receptors. J Neurosci 25(34):7754–7762
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Morton, D.L., El-Deredy, W., Jones, A.K.P. (2014). Placebo Analgesia: Cognition or Perception. In: Benedetti, F., Enck, P., Frisaldi, E., Schedlowski, M. (eds) Placebo. Handbook of Experimental Pharmacology, vol 225. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44519-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-44519-8_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44518-1
Online ISBN: 978-3-662-44519-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)