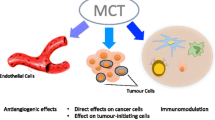
Abstract
Breast cancer is a common disease in women and its incidence is increasing. A proportion of breast cancer patients are metastatic at diagnosis or become metastatic during the follow-up period and need a personalized and/or target treatment approach. Metronomic chemotherapy can be regarded as a multi-targeted therapy for advanced disease, combining effects not only on tumor cells but also on their microenvironment by inhibiting tumor angiogenesis, stimulating anticancer immune response, and potentially inducing tumor dormancy. In the last 10 years, many phase I/II trials with metronomic chemotherapy in advanced breast cancer were published and will be described in details. Although this treatment approach was initially designed to maintain a stable disease as long as possible for metastatic patients that cannot be cured, as results become evident, researchers and clinicians started looking for new applications of this therapeutic strategy. Biomarkers are being developed to identify reliable surrogate markers of response and also to identify the proper patients to be treated. Nowadays, there are several ongoing trials to identify the optimal regimen and schedule of metronomic chemotherapy in the different settings of breast cancer patients. Most trials are aimed at patients with triple negative disease, because in this setting chemotherapy still represents one of the most reliable option. Finally, the potential development of metronomic chemotherapy in breast cancer is still a matter of research with particular attention to identify biomarkers and individual tumor characteristics that can better address the use of this treatment strategy in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Breast Cancer
- Metastatic Breast Cancer
- Advanced Breast Cancer
- Metastatic Breast Cancer Patient
- Pegylated Liposomal Doxorubicin
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Breast cancer is one of the most prevalent malignancies in women in almost all countries. In Europe, women have an 8 % chance to suffer from breast cancer before the age of 75 and a 2 % chance of dying from the disease [1]. Although in the last 20 years the incidence of breast cancer has increased 1.5 % annually, the mortality rates have been progressively decreasing.
Approximately 5–10 % of newly diagnosed breast cancer patients are metastatic at diagnosis, and nearly 10–15 % of breast cancer patients become metastatic in the first 3 years after diagnosis. Nevertheless, a proportion of patients are also likely to develop metastases 10 years or even later after first detection [2].
Metastatic breast cancer remains an incurable but treatable disease. A key component of the approach to this disease is conventional chemotherapy and/or endocrine therapy according to breast cancer biology. In recent years, targeted therapies have been added to various chemotherapy backbones. The median survival rate of the patient with metastasis is within the range of 3 years.
The importance of understanding the mechanisms underlying the metastatic process and the complex interactions between tumor and host during disease progression has been widely recognized. Nevertheless, despite multidisciplinary approaches and novel target treatments, metastatic disease remains the primary cause of death in the majority of patients with breast cancer.
In recent years, clinicians increasingly agree in considering breast cancer not only as one disease; in fact models of breast cancer as a systemic and heterogeneous disease suggest novel ways to target the process of metastasis.
1.1 Breast Cancer Is Not One Disease: Subtypes and Heterogeneity
During the last decade, research has focused in depth on the molecular biology of breast cancer. Particularly, high-throughput approaches allowed researchers to ascertain the nature of breast cancer revealing that this disease is characterized by the interconnection of several signaling pathways. Both the cellular microenvironment and the innate characteristics of the patient might influence pathophysiologic characteristics of breast cancer, its outcome, and treatment response.
These findings led researchers to understand that each patient entails a particular case where personalized medicine could play a crucial role. Clinicians are increasingly seeking to propose a personalized medicine approach, but there are still many unresolved issues to be addressed.
Especially in those individuals who lack a clear therapeutic target, there is a special need to identify and validate new molecular markers. In fact, even if a tumor has a specific druggable pathway, tumor cells often display an unexpected resistance that allows them to escape death.
The hierarchical cluster analysis initially performed by Perou et al. revealed four molecular subtypes: luminal, HER2, basal like, and normal breast [3]. The subsequent expansion of this work in a larger cohort of patients showed that the luminal subgroup could be divided into at least two groups (luminal A and B) and that different molecular subtypes were associated with different prognoses (Fig. 6.1) [4]. This new classification validated by independent groups was based on an unsupervised analysis, grouping tumors according to their biological characteristics regardless of their clinical or prognostic variables.
Survival data of the different molecular subtypes (Adapted from Prat et al. [37])
These molecular classified subgroups correspond reasonably well to clinical-pathological characterization on the basis of estrogen receptor (ER) and HER2 status, as well as proliferation markers or histological grade performed by means of immunohistochemistry (IHC) techniques [5, 6].
During the 2011 St Gallen Consensus Conference, a surrogate definition of intrinsic subtypes of breast cancer was issued for purposes of clinical use [7], and it was further refined in the recent 2013 Consensus Conference [8].
The molecular heterogeneity of breast cancer is reflected in the clinical course of the disease and in responses to treatment.
Recently, powerful gene-sequencing techniques have revealed many genetic and epigenetic alterations governing breast cancer [9–14]. The existence of extended genetic variation within a single tumor mass is called intratumoral heterogeneity [15], and new data support the idea that tumor heterogeneity represents a branching pattern of tumor evolution, as opposed to the traditionally accepted linear model. Metastatic disease represents the final stage of this branched tumor evolution [16]. Therefore, in the setting of metastatic breast cancer, this heterogeneity has direct consequences for the emergence of therapy resistance even to targeted agents.
1.2 The Role of Metronomic Chemotherapy
Metronomic chemotherapy exerts both direct and indirect effects not only on tumor cells but also on their microenvironment by inhibiting tumor angiogenesis and stimulating anticancer immune response. In addition, metronomic chemotherapy can directly affect tumor cells through a theoretical drug-driven dependency/deprivation effect and can exert additional anticancer effects and potential (re)induction of tumor dormancy.
New mechanisms have been identified, such as the restoration of the anticancer effect of the immune system. Therefore, metronomic chemotherapy can be regarded as a multi-targeted therapy [17]. Although the rationale of metronomic chemotherapy is yet to be fully elucidated, the use of low-dose oral chemotherapy in the clinic has been initially restricted to palliative purposes.
However, after the publication of several phase I/II trials in metastatic breast cancer (Table 6.1), clinicians are now more inclined to give credit to metronomic chemotherapy.
2 Metronomic Regimens in Breast Cancer: Early Trials
Among conventional cytotoxic agents that may exert a tumor suppressive effect through an antiangiogenic mechanism, cyclophosphamide (CTX) and methotrexate (MTX) were those with a significant bioavailability and were therefore the best candidate for a metronomic regimen to treat advanced breast cancer patients. The first trial in this setting was reported in 2002 in a series of 63 advanced and pretreated breast cancer patients who received metronomic CM (CTX 50 mg/day administered continuously and MTX 2.5 mg bid on days 1 and 2 each week). In this patients’ population, an overall response rate of 19 % (95 % CI 10.2–30.9 %) and an overall clinical benefit (CR + PR + stable disease ≥24 weeks) of 31.7 % (95 % CI 20.6–44.7 %) were reported without significant toxicity [18].
Few years later, the same authors reported results and long-term follow-up for patients with metastatic breast carcinoma who obtained prolonged clinical benefit with CM. One hundred and fifty-three patients who achieved prolonged clinical benefit for a duration of 12 months or more were considered for the analysis. The proportion of patients who achieved prolonged clinical benefit was 15.7 % (95 % confidence interval 9.9–21.4 %). Median time to progression for patients with prolonged clinical benefit was 21 months (range 12–37+ months) [19].
To improve results obtained, a subsequent trial in a similar population of patients investigated the association of metronomic CM with thalidomide [20]. Thalidomide is a derivative of glutamic acid and has immune-modulating activity secondary to inhibition of lymphocyte proliferation [38]. The drug is also able to inhibit tissue tumor necrosis factor-α production by stimulating human monocytes and lymphocytes [39, 40]. In addition to its immune-modulatory activities, oral thalidomide can inhibit angiogenesis induced by basic fibroblast growth factor and vascular endothelial growth factor (VEGF) in the rabbit corneal micropocket assay [41, 42]. Therefore, the activity and biological effects of low-dose oral CTX and MTX was compared with the same combination plus thalidomide. Overall, 171 patients with advanced breast cancer were randomized to receive oral CM or the same regimen plus thalidomide (200 mg daily). Nevertheless, the addition of thalidomide did not improve results previously obtained with the CM regimen.
Preclinical evidence showed a synergism between metronomic chemotherapy and antiangiogenic agents. Moreover, corticosteroids and low-molecular-weight heparins have known antiangiogenic properties [43–45]; therefore, a phase I/II trial combining daily dalteparin, cyclophosphamide, twice-weekly methotrexate, and daily prednisone (dalCMP) was conducted in metastatic breast cancer [21] accruing 41 patients. Median time to progression (TTP) was 10 weeks (95 % CI, 8–17 weeks), and median OS was 48 weeks (95 % CI, 32–79 weeks). VEGF levels decreased but not significantly, whereas sVEGFR-1 and sVEGFR-2 levels increased significantly after 2 weeks of therapy. Authors concluded that metronomic dalCMP was a safe, well-tolerated, and clinically active treatment in this setting of patients.
3 Combining Metronomic Chemotherapy with Molecularly Target and Antiangiogenic Target Agents
In order to explore the activity of metronomic chemotherapy plus targeted therapy, 22 patients with metastatic breast cancer and with the overexpression or amplification of HER2-/neu were treated with trastuzumab (6 mg/kg every 3 weeks) in combination with metronomic CM [22]. All patients were already pretreated with trastuzumab plus other cytotoxics. The clinical benefit calculated in all patients and in those with disease resistant to previous trastuzumab therapy was 46 % (95 % CI, 24–68 %) and 27 % (95 % CI, 6–61 %), respectively. Median time to progression was 6 months and median duration of treatment was 5 months (range, 0.7–18.4 months, and range, 1–18 months, respectively). These results showed that the combination of trastuzumab and metronomic chemotherapy is effective and minimally toxic in pretreated advanced breast cancer patients.
There is a rationale for the combination of metronomic chemotherapy and targeted antiangiogenic agents like bevacizumab. In preclinical models, the combination of metronomic chemotherapy with a VEGFR2 antibody resulted in sustained regressions of large tumors, without overt toxicity occurring during the course of treatment [46]. In a randomized phase II trial comparing metronomic CTX and MTX with the same regimen plus bevacizumab in women with pretreated advanced breast cancer, a planned interim analysis after the first 19 patients per arm revealed a significant advantage in favor of the combined arm in terms of objective response (41 %) [23].
Given these premises, 46 patients with advanced breast cancer received metronomic oral capecitabine (500 mg thrice daily) and cyclophosphamide (50 mg daily) plus bevacizumab (10 mg/kg every 2 weeks) within a phase II trial [24]. The overall response rate was 48 % (95 % CI, 33–63 %); long-term disease stabilization (SD ≥24 weeks) occurred in eight patients, for an overall clinical benefit of 68 % (95 % CI, 51–81 %). Median time to progression was 42 weeks (95 % CI, 26–72 weeks). Treatment with metronomic capecitabine and cyclophosphamide in combination with bevacizumab was effective and was minimally toxic. The number of baseline circulating endothelial cells (CECs) significantly correlated with response and outcome, therefore supporting further studies on this surrogate marker for the selection of patients to be candidates for antiangiogenic treatments.
A subsequent trial in the same patient population had the aim to determine the safety and efficacy of metronomic chemotherapy combined with targeted drugs such as bevacizumab and erlotinib [25]. Twenty-six untreated patients with HER2-negative metastatic breast cancer and poor hormone receptor expression received metronomic oral capecitabine (500 mg thrice daily) and cyclophosphamide (50 mg daily) plus bevacizumab (15 mg/kg every 3 weeks) and erlotinib (100 mg daily). The overall clinical benefit was 75 % (95 % CI, 53–90 %). Median time to progression was 43 weeks (95 % CI, 21–69). Patients with low levels of circulating endothelial progenitors (CEPs) at baseline had a significantly improved progression-free survival. Toxicity was generally mild. The analysis of the results suggested that the metronomic chemotherapy combined with bevacizumab and erlotinib is effective and well tolerated in a group of HER2-negative, estrogen receptor, and progesterone receptor-poor advanced breast cancer.
A similar trial explored the activity of cyclophosphamide 50 mg p.o. daily, methotrexate 1 mg/kg i.v. every 14 days, and bevacizumab 10 mg/kg i.v. every 14 days in an anthracycline and taxane refractory metastatic breast cancer patient population [26]. Trastuzumab was added in HER2-overexpressing tumors. Among the 22 patients evaluable for response, the clinical benefit was 63.6 % (95 % CI 40.7–82.8 %). Median progression-free survival was 7.5 months; overall survival was 13.6 months. HER2-overexpressing or high proliferative index tumors had better 6-month PFS (75 % vs. 34 % in HER2-negative tumors, p = 0.043; 67 % vs. 0 % in Ki-67 ≥20 % tumors, p = 0.015).
A recently published phase I study evaluated the safety and tolerability of antiangiogenic therapy using vandetanib and metronomic cyclophosphamide and methotrexate in metastatic breast cancer [27]. Vandetanib is an oral once daily administered inhibitor of VEGFR, EGFR, and RET signaling with activity in combination with chemotherapy in some solid tumors [47]. Twenty-three patients with 0–4 prior chemotherapy regimens were treated. All patients received cyclophosphamide 50 mg daily, methotrexate 2.5 mg days 1–2 weekly, and vandetanib daily in 3 dose-escalation cohorts: 100 mg (C1), 200 mg (C2), and 300 mg (C3). Toxicities were mild and included nausea, vomiting, fatigue, and rash. In all cohorts, a third of patients required vandetanib dose reduction. Of the 20 response-evaluable patients, 10 % had a partial response and 15 % stable disease ≥24 weeks. Proteomic analyses demonstrated changes in platelet content of angiogenesis regulators, including vascular endothelial growth factor and platelet factor 4, with exposure to therapy. This regimen was tolerable at a maximum vandetanib dose of 200 mg; modest clinical activity was observed in this heavily pretreated population. Changes observed in the platelet proteome have been supposed to serve as pharmacodynamic markers of angiogenesis inhibition.
4 New Uses for Old Drugs
Metronomic cyclophosphamide (CTX) and capecitabine may have a greater potential for treatment of metastatic breast cancer, because of their antiangiogenic activity resulting from the metronomic dosage and upregulation of thymidine phosphorylase by CTX.
Therefore, a phase II trial was conducted in metastatic breast cancer patients receiving oral metronomic CTX 65 mg/m2 daily on days 1–14 plus capecitabine 1,000 mg/m2 twice daily on days 1–14 [28]. The treatment was repeated every 3 weeks. Sixty-six patients were evaluated for efficacy, and after a median follow-up time of 26 months, the median time to progression was 5.2 months (95 % CI, 4.2–6.2 months), and the median overall survival was 16.9 months. The overall response rate was 30.3 % (95 % CI, 20–43 %). Clinical benefit rate was 53.0 % (95 % CI, 38–62 %).
Caelyx is a pegylated liposomal doxorubicin (PLD), used as a single agent in advanced breast cancer at conventional doses ranging from 40 to 50 mg/m2 every 3–4 weeks, with objective response rates ranging from 31 to 33 % [48].
The pharmacokinetics of PLD supports the rationale for using the drug in a metronomic fashion, mainly because of the polyethylene-glycol-coated liposomic coat surrounding the molecule. Liposomes markedly prolong circulation and enhance drug accumulation inside the tumor, retarding uptake by mononuclear phagocytes; PLD achieves a longer half-life than non-pegylated liposomal doxorubicin, as the polyethylene glycol liposome interacts with plasma proteins and inhibits mononuclear phagocytes, consequently prolonging circulation time [49]. The drug is also characterized by a reduced volume of distribution, a long intravascular circulating half-life, and a slow plasma clearance compared with free doxorubicin.
The activity and safety of intravenous PLD 20 mg/m2 biweekly for eight courses in combination with metronomic cyclophosphamide 50 mg/day orally were evaluated in 29 patients with locally advanced breast cancer who were not suitable to receive a standard chemotherapy due to age or comorbidities or who asked for a regimen with low incidence of toxic effects irrespective of age [29]. Eighteen patients (62.1 %) achieved a partial response (including one pathological complete response), ten patients (34.5 %) achieved a stable disease, and one patient experienced a progressive disease. Treatment was well tolerated, with no grade 4 toxicities, and with grade 3 skin toxicity in three patients and hand-foot syndrome in four patients. The rate of breast-conserving surgery was 44.8 %. Although the regimen was well tolerated, this combination chemotherapy showed a limited activity in the preoperative setting.
In a case series report carried out in both anthracycline-naive and pretreated metastatic breast cancer patients, feasibility, clinical efficacy, and tolerability of PLD administered with a the metronomic schedule of 20 mg/m2 i.v. every 2 weeks were tested [30].
Among 52 patients enrolled in the trial, 44 patients were assessed for either response or toxicity. Eight patients (18 %) had partial responses and 17 (39 %) stable disease, with a clinical benefit of 45 % (95 % CI: 30.3–59.7 %). Nineteen patients (43 %) had progressive disease. Neither grade 3 nor grade 4 hematological or clinical side effects were recorded, except for two patients with grade 3 palmar-plantar erythrodysesthesia. No cardiac toxicity was recorded. Metronomic administration of PLD resulted as a feasible and active treatment for extensively pretreated metastatic breast cancer patients, alternative to classic anthracyclines. Overall, a good balancing of clinical efficacy with a good quality of life was reached in terms of reduced side effects and low personal costs for the patient.
Anti-tubulin agents are known to have antiangiogenic effects at doses below that required to induce cytotoxicity, including taxanes, such as paclitaxel [50] and docetaxel [51], and vinca alkaloids such as vinblastine [52].
Most studies evaluating metronomic scheduling of anti-tubulin agents have used weekly drug scheduling. Given the availability of an oral formulation of vinorelbine, which has an oral bioavailability of 43 % and a terminal half-life of approximately 29 h (±7.9 h), permitting more thrice weekly or every other day dosing [53, 54], metronomic oral vinorelbine trials were conducted in a series of patients with advanced breast cancer.
Phase I trials of metronomic oral vinorelbine in patients with advanced cancer indicated that 50 mg given three times a week is the optimal dose for a metronomic schedule, yielding sustainable antitumor activity without overt toxicity [55–57].
Oral vinorelbine at 70 mg/m2, fractionated on days 1, 3, and 5, for 3 weeks on and 1 week off, every 4 weeks was administered to 34 elderly patients with metastatic breast cancer (median age, 74 years; range, 70–84 years) [31]. Patients were treated with for a maximum of 12 cycles. The objective response rate was the primary end point. Two patients achieved complete responses (6 %) and 11 achieved partial responses (32 %). Median progression-free survival and median overall survival were 7.7 months (95 % confidence interval, 6.9–9.05 months) and 15.9 months (95 % CI, 13.1–15.91 months), respectively, for all patients. The fractionated administration of oral vinorelbine is well tolerated with promising activity in elderly metastatic breast cancer patients.
In a recently published trial, escalated doses of oral metronomic vinorelbine (starting dose 30 mg) every other day continuously and capecitabine (starting dose 800 mg/m2 bid) on days 1–14 every 21 days were administered [32]. Thirty-six women were enrolled at eight escalating dose levels. For 24 patients, treatment was first line, for 8 second line, and for 4 third line. The dose-limiting toxicity (DLT) level was reached at oral metronomic vinorelbine 70 mg and capecitabine 1250 mg/m2, and the recommended maximum tolerated doses (MTD) were vinorelbine 60 mg and capecitabine 1,250 mg/m2. DLTs were febrile neutropenia grades 3 and 4, diarrhea grade 4, and treatment delays due to unresolved neutropenia. There was no treatment-related death. The main toxicities were grade 2–3 neutropenia in 16.6 % of patients each, grade 2–3 anemia 16.5 %, grade 2–4 fatigue 27.5 %, grade 2–3 nausea/vomiting 11 %, and grade 3–4 diarrhea 8.2 %. Two complete and ten partial responses were documented. Therefore, oral metronomic vinorelbine with capecitabine was deemed as a well-tolerated and feasible regimen that merits further evaluation in this patients’ setting.
The role of metronomic chemotherapy was further explored in the setting of patients with brain metastases from breast cancer [33]. Thirty-six patients with newly diagnosed brain metastases were treated with temozolomide (TMZ) orally administered at a dose of 75 mg/m2 during whole-brain radiotherapy, followed by 4 weeks off-therapy and a subsequent administration of oral 70 mg/m2 vinorelbine fractionated in days 1, 3, and 5, weekly for 3 consecutive weeks plus TMZ at 75 mg/m2 on days 1–21. Cycles were repeated every 4 weeks for up to 12 additional cycles. The primary end point was the evaluation of the objective response rate (ORR). Three complete responses and 16 partial responses have been achieved with an ORR of 52 % (95 % CI 38–67 %) that exceeded the target activity per study design. The median progression-free survival and overall survival were 8 and 11 months, respectively. The schedule appeared to be well tolerated, and side effects reported were generally mild. Authors concluded that the treatment was safe and a significant number of objective responses were observed with a significant improvement in quality of life in this particular setting of breast cancer patients.
5 Combining Metronomic Chemotherapy with Endocrine Therapy
The activity of oral metronomic CM combined with fulvestrant was retrospectively assessed in two cohorts of heavily pretreated estrogen receptor-positive advanced breast cancer patients [34]. A series of 33 postmenopausal patients received fulvestrant 250 mg via i.m. injection every 28 days. Twenty patients in the first cohort added metronomic CM after disease progression, continuing fulvestrant at the same dose. Thirteen patients in the second cohort started fulvestrant plus metronomic CM upfront. Clinical benefit for both cohorts was 56 % (95 % CI 38–74 %). The addition of metronomic CM did not determine relevant toxicities. Treatment with fulvestrant plus metronomic CM was effective in this group of patients and was minimally toxic providing long-term disease control in a high proportion of them.
To investigate the activity of letrozole with or without oral metronomic cyclophosphamide as primary systemic treatment in elderly breast cancer patients, 114 consecutive elderly women with T2–4 N0–1 and estrogen receptor-positive breast cancer were randomly assigned to primary letrozole therapy (2.5 mg daily for 6 months) or a combination of letrozole plus oral cyclophosphamide (50 mg/daily for 6 months) in an open-labeled, randomized phase II trial [35]. Overall response rate was 71.9 % (95 % CI, 60.0–83.8) in the 57 patients randomly assigned to receive primary letrozole and 87.7 % (95 % CI, 78.6–96.2) in the 57 patients randomly assigned to receive letrozole plus cyclophosphamide.
The safety and antitumor activity of the metronomic chemo-hormonal therapy with daily cyclophosphamide and twice daily megestrol acetate (mCM regimen) were investigated in patients with metastatic pretreated breast cancer [36]. This phase II study enrolled 29 pretreated postmenopausal patients with multiple metastatic sites. Four patients had a triple negative status, 19 a positive hormonal ER and PgR status, and 3 HER-2 overexpression. Patients received treatment with cyclophosphamide (50 mg/daily day 1–21/q28) and fractionated megestrol acetate (80 mg twice a day). The overall objective response rate was 31.0 %, disease control rate 41.3 %, mean time to tumor progression 7.4 months (CI 95 %, 3.8–10.88, range 1–48 months), and mean overall survival 13.4 months (CI 95 %, 7.24–17.18, range 1–53 months).
6 Tolerability of Metronomic Chemotherapy
Despite patients treated within clinical trials of metronomic chemotherapy are often heavily pretreated or elderly, toxicities and long-term effects reported in the majority of trials showed that metronomic chemotherapy, alone or in combination, is generally well tolerated.
High-grade toxic effects were either rare or absent, and the most common toxic effects were grade 1 nausea and/or vomiting, grade 1 and 2 anemia, neutropenia, leucopenia, as well as low-grade fatigue. Alopecia grade 1 was rarely reported.
Some toxic effects were observed when metronomic chemotherapy was combined with other agents, such as bevacizumab, or when combined with standard doses of chemotherapy.
Nevertheless, clinicians should bear in mind that prolonged metronomic chemotherapy may lead to high total cumulated doses of anticancer agents, which can be associated with secondary diseases. For instance, high cumulated dose of etoposide [58] or temozolomide [59] can lead to the development of secondary leukemia. However, the long-term effect of prolonged exposure to long-term chemotherapy on normal endothelial and vascular tissues is unknown.
7 Biomarkers of Clinical Response
Following the preclinical observation that maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial cells (CECs) [60] and that CEC kinetics correlates well with more invasive biomarker of angiogenesis [61, 62], CECs and their progenitor counterpart (CEPs) were measured in the blood of breast cancer patients enrolled in a variety of clinical trials involving metronomic chemotherapy alone or in combination with other drugs. CECs were found to be dynamic markers of clinical response in breast cancer patients receiving CTX and MTX [63], and baseline CECs and CEPs were found to be predictive markers in clinical trials where metronomic chemotherapy was administered along with the anti-VEGF monoclonal antibody bevacizumab [24, 64]. CECs and CEPs have been found to have predictive and dynamic prognostic potential in several other types of cancer in addition to breast cancer, but the wide application of this measurement is still hampered by the lack of simple and standardized procedures [65]. An international effort towards the standardization of CEC and CEP enumeration is currently ongoing. Finally, the study of Dellapasqua et al. [66] has shown that in advanced breast cancer patients, an increase in mean corpuscular volume of red blood cells may predict response to metronomic capecitabine and cyclophosphamide in combination with bevacizumab. This finding needs to be confirmed in larger clinical trials.
8 Conclusion and Future Directions
Metronomic chemotherapy demonstrated activity and provided disease control for patients with metastatic breast cancer. This treatment approach was initially designed to maintain a stable disease as long as possible for metastatic patients that cannot be cured. In fact, the low burden of personal costs for the patient and the possibility to continue the treatment for several months supported the use of metronomic chemotherapy as an additional therapeutic tool for metastatic and pretreated patients with breast cancer. Either elderly patients or those who prefer relatively nontoxic regimens also benefited from this therapeutic option.
However, as results became evident and research for elucidating some conceivably novel mechanisms of action were intriguing, researchers and clinicians started looking for new applications of this therapeutic strategy. On the other hand, not all tumors and especially not all patients derive the same benefit from metronomic chemotherapy. Large and highly aggressive tumors may limit this treatment option, favoring conventional chemotherapy or targeted agents.
Biomarkers are being developed to identify reliable surrogate markers of response and also to identify the proper patients to be treated. Among these, kinetics and viability of circulating endothelial cells (CECs) and progenitor endothelial cells (CEPs) are deemed to be potent predictive tools for patient stratification and treatment monitoring, although the best methods of identification and measurement are still a matter of research.
In fact, it seems unlikely that a single metronomic regimen may have the same efficacy in all patients; the optimal combination regimens, dosing and scheduling of metronomic chemotherapy, remains to be determined for specific breast cancer conditions.
In recent years, the role of metronomic chemotherapy is being studied in the adjuvant setting after a standard adjuvant chemotherapy regimen in the category of ER-negative patients. The CM Maintenance Trial (IBCSG 22-00) investigated a tailored chemotherapy approach for patients with endocrine nonresponsive tumors to reduce the risk of relapse and improve survival. Unlike patients with endocrine responsive disease, who benefit from at least 5 years of endocrine therapy after chemotherapy, patients with endocrine nonresponsive disease do not have the same opportunity.
In the abovementioned trial, 1 year of CM is compared with no further therapy beyond the standard adjuvant program. The trial recently concluded with the enrolment of 1086 patients and the results are eagerly awaited.
As shown in Table 6.2, there are still several ongoing trials to identify the optimal regimen and schedule of metronomic chemotherapy in different settings of patients. Many of them are still investigating specific metronomic regimens in the metastatic setting, and most trials are aimed at patients with triple negative disease, because in this setting chemotherapy still represents one of the most reliable option. Nevertheless, there are also trials exploring the role of metronomic chemotherapy in the adjuvant and post-neoadjuvant setting, being this scheduling of chemotherapy possibly useful when target agents are lacking.
A special consideration should be paid to the economic aspects. Given the time being, we cannot avoid to consider the economic impact that the costs of cancer treatments have on public health. In fact, the new targeted treatments often have very high costs. Therefore, therapies such as metronomic CM and similar regimens position themselves as potentially significantly cost-effective palliative treatments for metastatic breast cancer when compared with other novel therapeutic strategies [67].
Finally, the potential development of metronomic chemotherapy in breast cancer cannot disregard the development of research to identify biomarkers and individual tumor characteristics that can better address the use of this treatment strategy in the future.
References
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J et al (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403
Brunner WN, Stephens RW, Dano K (2009) Control of invasion and metastasis. In: Harris JR, Lippman ME, Morrow M, Osborne CK (eds) Diseases of the breast, 4th edn. Lippincott Williams and Wilkins, Philadelphia, pp 367–376
Perou CM, Sørlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Sørlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98:10869–10874
Sotiriou C, Pusztai L (2009) Gene-expression signatures in breast cancer. N Engl J Med 360:790–800
Cheang MC, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736–1747
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223
Stephens PJ, Tarpey PS, Davies H et al (2012) The landscape of cancer genes and mutational processes in breast cancer. Nature 486:400–404
Shah SP, Roth A, Goya R et al (2012) The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486:395–399
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490:61–70
Kan Z, Jaiswal BS, Stinson J et al (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466:869–873
Ellis MJ, Ding L, Shen D et al (2012) Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486:353–360
Banerji S, Cibulskis K, Rangel-Escareno C et al (2012) Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486:405–409
Yap TA, Gerlinger M, Futreal PA et al (2012) Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med 4:127ps10
Sethi N, Kang Y (2011) Unravelling the complexity of metastasis – molecular understanding and targeted therapies. Nat Rev Cancer 11:735–748
Pasquier E, Kavallaris M, André N (2010) Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol 7:455–465
Colleoni M, Rocca A, Sandri MT et al (2002) Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 13(1):73–80
Orlando L, Cardillo A, Rocca A et al (2006) Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anti-Cancer Drugs 17:961–967
Colleoni M, Orlando L, Sanna G et al (2006) Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol 17(2):232–238
Wong NS, Buckman RA, Clemons M et al (2010) Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol 28((5):723–730
Orlando L, Cardillo A, Ghisini R et al (2006) Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer 6:225
Burstein HJ, Spigel D, Kindsvogel K et al (2005) Metronomic chemotherapy with and without bevacizumab for advanced breast cancer: a randomized phase II study. In: 28th annual San Antonio breast cancer symposium, San Antonio, 8–11 Dec 2005, (abstr 4)
Dellapasqua S, Bertolini F, Bagnardi V et al (2008) Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 26(30):4899–4905
Montagna E, Cancello G, Bagnardi V et al (2012) Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin Breast Cancer 12(3):207–214
García-Sáenz JA, Martín M, Calles A et al (2008) Bevacizumab in combination with metronomic chemotherapy in patients with anthracycline- and taxane-refractory breast cancer. J Chemother 20(5):632–639
Mayer EL, Isakoff SJ, Klement G et al (2012) Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res Treat 136(1):169–178
Wang Z, Lu J, Leaw S et al (2012) An all-oral combination of metronomic cyclophosphamide plus capecitabine in patients with anthracycline- and taxane-pretreated metastatic breast cancer: a phase II study. Cancer Chemother Pharmacol 69(2):515–522
Dellapasqua S, Mazza M, Rosa D et al (2011) Pegylated liposomal doxorubicin in combination with low-dose metronomic cyclophosphamide as preoperative treatment for patients with locally advanced breast cancer. Breast 20(4):319–323
Munzone E, Di Pietro A, Goldhirsch A et al (2010) Metronomic administration of pegylated liposomal-doxorubicin in extensively pre-treated metastatic breast cancer patients: a mono-institutional case-series report. Breast 19(1):33–37
Addeo R, Sgambato A, Cennamo G et al (2010) Low-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancer. Clin Breast Cancer 10(4):301–306
Saridaki Z, Malamos N, Kourakos P et al (2012) A phase I trial of oral metronomic vinorelbine plus capecitabine in patients with metastatic breast cancer. Cancer Chemother Pharmacol 69(1):35–42
Addeo R, Sperlongano P, Montella L et al (2012) Protracted low dose of oral vinorelbine and temozolomide with whole-brain radiotherapy in the treatment for breast cancer patients with brain metastases. Cancer Chemother Pharmacol 70(4):603–609
Aurilio G, Munzone E, Botteri E et al (2012) Oral metronomic cyclophosphamide and methotrexate plus fulvestrant in advanced breast cancer patients: a mono-institutional case-cohort report. Breast J 18(5):470–474
Bottini A, Generali D, Brizzi MP et al (2006) Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol 24(22):3623–3628
Licchetta A, Correale P, Migali C et al (2010) Oral metronomic chemo-hormonal-therapy of metastatic breast cancer with cyclophosphamide and megestrol acetate. J Chemother 22(3):201–204
Prat A, Parker AS, Karginova O et al (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12:R68
Coulson AS, Summers LJ, Lindahl-Kiessling K et al (1970) The effect of two soluble thalidomide derivatives on lymphocytes stimulation. Clin Exp Immunol 7:241–247
Nogueira AC, Neubert R, Helge H, Neubert D (1994) Thalidomide and the immune system: Simultaneous up and down regulation of different integrin receptor on human white blood cells. Life Sci 55:77–92
Sampaio EP, Sarno EN, Galilly R et al (1991) Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 173:699–703
D'Amato RJ, Loughnan MS, Flynn E, Folkman J (1994) Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A 91:4082–4085
Kruse FE, Joussen AM, Rohrschneider K et al (1998) Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch Clin Exp Ophthalmol 236:461–466
Wolff JE, Molenkamp G, Hotfilder M et al (1997) Dexamethasone inhibits glioma-induced formation of capillary like structures in vitro and angiogenesis in vivo. Klin Paediatr 209:275–277
Wolff JE, Guerin C, Laterra J et al (1993) Dexamethasone reduces vascular density and plasminogen activator activity in 9 L rat brain tumors. Brain Res 604:79–85
Nauck M, Karakiulakis G, Perruchoud AP et al (1998) Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol 341:309–315
Klement G, Baruchel S, Rak J (2000) Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 105:R15–R24
Holden SN, Eckhardt SG, Basser R et al (2005) Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol 16(8):1391–1397
O’Brien ME (2008) Single-agent treatment with pegylated liposomal doxorubicin for metastatic breast cancer. Anticancer Drugs 9(1):1–7
Park JW (2002) Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res 4(3):95–99
Belotti D, Vergani V, Drudis T et al (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 2:1843–1849
Hotchkiss KA, Ashton AW, Mahmood R et al (2002) Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther 1:1191–1200
Vacca A, Iurlaro M, Ribatti D et al (1999) Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood 94:4143–4155
Rowinsky EK, Noe DA, Trump DL et al (1994) Pharmacokinetic, bioavailability, and feasibility study of oral vinorelbine in patients with solid tumors. J Clin Oncol 12:1754–1763
Depierre A, Freyer G, Jassem J et al (2001) Oral vinorelbine: feasibility and safety profile. Ann Oncol 12:1677–1681
Briasoulis E, Aravantinos G, Kouvatseas G et al (2013) Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study. BMC Cancer 13(1):263
Rajdev L, Negassa A, Dai Q et al (2011) Phase I trial of metronomic oral vinorelbine in patients with advanced cancer. Cancer Chemother Pharmacol 68(5):1119–1124
Briasoulis E, Pappas P, Puozzo C et al (2009) Dose-ranging study of metronomic oral vinorelbine in patients with advanced refractory cancer. Clin Cancer Res 15(20):6454–6461
Le Deley MC, Vassal G, Taïbi A et al (2005) High cumulative rate of secondary leukemia after continuous etoposide treatment for solid tumors in children and young adults. Pediatr Blood Cancer 45:25–31
De Vita S, De Matteis S, Laurenti L et al (2005) Secondary Ph+ acute lymphoblastic leukemia after temozolomide. Ann Hematol 84:760–762
Bertolini F, Paul S, Mancuso P et al (2003) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63:4342–4346
Shaked Y, Bertolini F, Man S et al (2005) Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: Implications for cellular surrogate markers and analysis of antiangiogenesis. Cancer Cell 7:101–111
Shaked Y, Emmenegger U, Man S et al (2005) The optimal biological dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood 106:3058–3061
Mancuso P, Colleoni M, Calleri A et al (2006) Circulating endothelial cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 108:452–459
Torrisi R, Bagnardi V, Cardillo A et al (2008) Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER- and/or PgR-positive breast cancer: clinical and biological activity. Br J Cancer 99:1564–1571
Bertolini F, Marighetti P, Shaked Y (2010) Cellular and soluble markers of tumor angiogenesis: from patient selection to the identification of the most appropriate post-resistance therapy. Biochim Biophys Acta Rev Cancer 1806:131–137
Dellapasqua S, Bagnardi V, Bertolini F et al (2012) Increased mean corpuscular volume of red blood cells predicts response to metronomic capecitabine and cyclophosphamide in combination with bevacizumab. Breast 21:209–213
Bocci G, Tuccori M, Emmenegger U et al (2005) Cyclophosphamide-methotrexate ‘metronomic’ chemotherapy for the palliative treatment of metastatic breast cancer. A comparative pharmacoeconomic evaluation. Ann Oncol 16(8):1243–1252
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Munzone, E., Bertolini, F., Colleoni, M. (2014). Metronomic Chemotherapy in Breast Cancers. In: Bocci, G., Francia, G. (eds) Metronomic Chemotherapy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43604-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-662-43604-2_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43603-5
Online ISBN: 978-3-662-43604-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)