Abstract
The origin of intra-ophthalmic artery chemotherapy (SSIOAC) dates back to the 1950s. Cases of advanced intraocular retinoblastoma with (1) large multiple lesions still having enough normal retina so visual preservation was possible, (2) vitreous seeds, or (3) tumors refractory to other treatments presented a challenge for Reese and other early pioneers in the treatment of retinoblastoma, as they unfortunately still do today (Chap. 9).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 Introduction
The origin of intra-ophthalmic artery chemotherapy (SSIOAC) dates back to the 1950s. Cases of advanced intraocular retinoblastoma with (1) large multiple lesions still having enough normal retina so visual preservation was possible, (2) vitreous seeds, or (3) tumors refractory to other treatments presented a challenge for Reese and other early pioneers in the treatment of retinoblastoma, as they unfortunately still do today (Chap. 9).
12.2 Historic Background
For these desperate cases, Reese injected triethylene melamine (TEM) (0.08 mg/kg), a nitrogen mustard analogue, under direct surgical observation into the carotid artery on the side of the affected eye over a 2 minutes period, during which carotid flow was occluded by traction sutures [1]. TEM was chosen based on Reese’s prior experience with its oral and intramuscular forms and the experiences of his antecedent, Kupfer, who paired intravenous nitrogen mustard with external radiation to improve tumor control [2]. External radiation followed the delivery of intracarotid TEM, and a second intracarotid injection was then administered if needed. Reese’s evolution from oral to intramuscular to intracarotid TEM corresponded with a dose reduction in external radiation to 32 gray (Gy). Improved ocular survival rates were reported. Observed toxicities at the time included death (n = 1), seizures (n = 2), bone marrow suppression, and subdural hematomas (n = 1) [1, 3]. Long-term toxicities, however, proved to be unacceptable, and the technique was subsequently abandoned.
Reese’s premise for transitioning to intracarotid injections was that he felt the management of retinoblastoma leant itself to focal treatment, and intra-arterial injections delivered a higher concentration of drug to the eye and tumor with improved efficacy. It is this premise that has fostered continued interest in local drug delivery methods. However, it should be noted that within Reese’s cohort, systemic exposure was comparable to that of intramuscular injections, as the same dose of TEM (0.08 mg/kg) was used.
Kaneko and collaborators adapted Reese’s technique in the 1980s for selective ophthalmic arterial infusion (SOAI) [4, 5]. After accessing the femoral artery, the cervical segment of the internal carotid artery was selectively catheterized, and a micro-balloon was inflated just distal to the orifice of the ophthalmic artery. A dose of 5–7.5 mg/m2 of melphalan was then infused over several seconds. Occlusion of the distal internal carotid artery preferentially directed the melphalan infusion into the ophthalmic artery. Melphalan, also a nitrogen mustard derivative, was chosen based on prior in vitro cytotoxic assays of retinoblastoma cells.
Initial reports of the technique cited 563 infusions in 610 eyes of 187 patients with a technical success rate of 97.5 %. No significant procedural complications were reported from the balloon occlusion technique, and the side effects of systemic chemotherapy were avoided [6, 7]. In a later publication, they detailed 1,469 SAOIs performed between 1987 and 2007 in 408 eyes in 343 patients citing a technical success rate of 98.8 % [8]. Reported ocular complications were negligible, being limited to orbital inflammation (n = 2) and diffuse chorioretinal atrophy (n = 2). Transient periocular swelling and redness occurred in some cases. No adverse systemic events were detected. However, they noted areas of the intracranial vasculature had received high concentrations of chemotherapy despite balloon occlusion. Ocular salvage rates based on the International Classification of Intraocular Retinoblastoma were 100 % in Group A, 88 % in Group B, 65 % in Group C, 45 % in Group D, and 30 % in Group E. In cases without macular tumors, 51 % of eyes had a visual acuity of 0.5 or better, and 36 % had a visual acuity of 1.0 or better [8].
12.3 Current Technique
In 2008, Abramson and colleagues continued the evolution of intra-arterial chemotherapy for retinoblastoma when they pioneered the technique we now refer to as super-selective intra-ophthalmic artery chemotherapy (SSIOAC). Abramson and Gobin modified Kaneko’s technique by directly cannulating the ophthalmic artery, thereby obviating the need for distal balloon occlusion of the internal carotid artery and mitigating brain toxicity. In their initial report, eye salvage rates with SSIOAC were encouraging, despite some cases requiring supplemental therapy to achieve disease control [9].
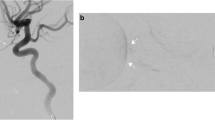
The technique as described in Abramson’s initial paper is as follows [9]. With the patient under general anesthesia, the femoral artery is accessed with a 4 French (F) (1.3 mm) femoral sheath. IV heparin (75 IU/kg) is flushed through the catheter, which is then advanced into the ipsilateral internal carotid artery. Under fluoroscopy, the ophthalmic artery is selectively catheterized using a microcatheter whose distal tip diameter can range between 1.2 and 1.5 F (0.4–0.5 mm). Alternatively, guidewire-directed microcatheters have been used as well. The microcatheter tip is advanced to the ostium of the ophthalmic artery, after which a selective arteriogram is performed (Fig. 12.1). The chemotherapeutic agent (most commonly melphalan 5 mg in 30 mL of normal saline) is infused in a pulsatile fashion over a 30 min time period. After the infusion is complete, the catheters and femoral sheath are withdrawn. Hemostasis of the femoral artery is achieved with manual compression.
Super-selective catheterization of the ophthalmic artery (Reproduced with permission from Jabbour et al. [33])
12.4 Current Results
Abramson’s initial report was that of a phase I/II trial of SSIOAC with melphalan [9]. Ten patients with advanced retinoblastoma (Reese-Ellsworth Group V) who met criteria for enucleation were enrolled in an effort to attain globe salvage. Nine of the ten patients had successful OA cannulation. Clinical response to chemotherapy was seen in all cases (Fig. 12.2), with favorable effects on vitreous seeding noted. The number of treatment sessions varied between 2 and 6 to the same eye. An additional chemotherapeutic agent, carboplatin, was used in combination with the melphalan in selective cases. A total of 27 ophthalmic artery cannulations were performed. At the end of the study, 2 of 9 eyes had been enucleated for suspected tumor recurrence. Median follow-up time was 7.5 months. No adverse anesthesia or vascular complications from the catheterization process were reported.
Before (a) and 3 weeks after (b) a single dose of melphalan (3 mg) delivered via SSIOAC (Reproduced with permission from Abramson et al. [9])
In a subsequent publication from the group, Gobin et al. published the largest series of patients treated with SSIOAC to date, performing a total of 289 catheterizations in 95 eyes – 83 of which were RE Group V (Fig. 12.3) [10]. Only 41 % of these eyes had not received prior therapy. All patients were alive at the end of the study, with two developing systemic metastases. They found ocular event-free survival at 2 years to be 70.0 % (95 % confidence interval (CI), 57.9–82.2 %) in all eyes, 81.7 % (95 % CI, 66.8–96.6) in eyes receiving IAC as primary therapy, and 58.4 % (95 % CI, 39.5–77.2 %) in eyes that had been previously treated with intravenous chemotherapy and/or external beam radiotherapy. In all, 77 % of eyes required additional treatment during or after IAC, with 20 % ultimately having to be enucleated secondary to tumor growth or insufficient tumor regression. Median follow-up time was 13 months.
Kaplan-Meier curve of eye survival until enucleation or external beam radiation therapy in eyes treated with SSIOAC (Reproduced with permission from Gobin et al. [10])
The same group has also reported SSIOAC with three-drug therapy (simultaneous carboplatin, topotecan, and melphalan) in eyes that had tumors refractory to treatment with systemic chemotherapy, SSIOAC with 1 or 2 agents, or external beam radiation [11]. The eye salvage rate was 88 % (23 of 26 eyes) at a mean follow-up of 14 months. Eleven of 26 eyes (35 %) developed disease recurrence and were treated with enucleation (n = 3), or focal therapy (n = 8) including plaque brachytherapy (n = 3).
Shields et al. published a series of 38 catheterizations on an initial cohort of 17 retinoblastoma patients – 13 of which had SSIAOC as their primary treatment [12]. A 5 mg dose of melphalan was used. Ocular salvage was achieved in 8 of the 12 eyes successfully treated with primary SSIOAC. Failures occurred in four of the six Group E eyes (International Classification). Additional therapeutic measures were needed to control disease in some eyes.
Muen et al. reported outcomes of 15 eyes in 14 patients previously treated with systemic chemotherapy or local therapy with refractory or recurrent retinoblastoma [13]. After two or three intra-arterial treatments with melphalan, tumor control was achieved in 12 eyes. Venturi et al. treated 41 eyes in 38 patients with 140 SSIOAC procedures between 2008 and 2010 [14]. Two patients had failed catheterizations and two patients were lost to follow-up. Of the 37 remaining eyes, 8 were enucleated, 7 of which had received no prior treatment. In a subsequent study of 48 eyes in 43 patients, the authors successfully saved 65 % of the eyes; however, only 21 eyes (44 %) were treated with SSIOAC using melphalan alone [15]. Additional chemotherapies – local and systemic – focal therapies, and brachytherapy were needed. Success rates were higher among previously treated eyes as opposed to newly diagnosed eyes, 78 % versus 48 %.
Thampi and colleagues treated 20 eyes in 16 patients; a total of 40 procedures were performed, ranging from 1 to 5 per patient [16]. The dose of melphalan was adjusted based on the age of the patient. At median follow-up of 14.5 months, ranging 1–29 months, radiotherapy and enucleation had been avoided in 86 % (6/7) of Groups A-C eyes and 38 % (5/13) of Groups D and E eyes.
12.5 Visual Outcome
Visual outcomes in treated eyes are of great interest, although little data have been published to date. Brodie et al. have monitored electroretinograms (ERGs) during sequential cycles of SSIOAC as surrogate for visual outcomes in this predominantly preverbal population. Retinal function has been observed to improve initially, remain stable, and later decline [17]. Tsimpida and colleagues looked at 12 eyes with refractory retinoblastoma that had good visual potential based on healthy foveolas as noted by ophthalmoscopy and optical coherence tomography prior to SSIOAC [18]. Five out of 12 eyes (42 %) sustained a reduction in visual acuity, with 2 suffering severe vision loss. Reasons for vision loss included diffuse retinal detachment (n = 1), diffuse choroidal ischemia (n = 2), and sectoral choroidal ischemia involving the fovea (n = 2). Four of the five eyes sustaining vision loss, however, had been previously treated with either EBRT or plaque brachytherapy, suggesting that previous radiation may predispose SSIOAC patients to visual loss. However, two patients with macula-sparing choroidal ischemia had no prior radiation exposure, which supports prior evidence that SSIOAC can lead to vasculopathy. Posttreatment ERGs deteriorated in four of eight eyes. Additional prospective, long-term follow-up studies on survival, metastasis rates, ocular survival, visual outcomes, and ocular toxicities are still needed.
12.6 Confounding Factors
SSIOAC for the treatment of retinoblastoma has quickly disseminated worldwide. Though the outcomes (eye salvage rates) appear promising, the reported case series have many confounding factors that limit the generalizability of this technique. Many patients have been treated previously, concurrently, or subsequently with other therapies. Various doses and agents have been utilized, sometimes with dose titrated to effect. It is difficult to assess the overall efficacy of SSIOAC, and thus, the singular effect of SSIOAC remains unknown.
12.7 Complications
Reported complications following SSIOAC are limited to case reports, as this therapy has not yet been evaluated as part of a large prospective retinoblastoma clinical trial. As such, these reports have been sporadic, including sectoral choroidal occlusion, retinal arteriolar emboli, retinal detachment, vitreous hemorrhage, transient retinal ischemia, ophthalmic artery obstruction, and cataract formation. Periocular inflammation and edema, cranial nerve III palsies, strabismus, and eyelash loss are reported orbital and adnexal side effects [10, 13, 14, 19–22]. Additionally, Fallaha et al. documented retinal vascular precipitates during the administration of melphalan into the ophthalmic artery [23].
Histopathology studies of eyes with retinoblastoma treated with SSIOAC have documented findings that have been attributed to the toxicities of SSIAOC. Eagle and coworkers examined eight eyes enucleated after SSIOAC and observed ischemic atrophy involving the outer retina and choroid (n = 4) (Fig. 12.4), orbital vascular occlusion and sub-endothelial smooth muscle hyperplasia (n = 1), and thrombosed blood vessels involving the retrobulbar ciliary arteries (n = 5), scleral emissary canals (n = 1), small choroidal vessels (n = 1), and CRA (n = 1) [24]. Intravascular birefringent foreign material was noted in five eyes and classified as cellulose fibers (n = 3), synthetic fabric fibers (n = 1), or unknown composition (n = 2). Other histopathology studies have focused principally on residual viable tumor [25–27]. In these eyes that were enucleated for progression of disease, partial response to IAC was seen in most cases, although many also had areas of non-necrotic, viable tumor and vitreous seeding still present. None of these papers reported evidence of ocular toxicity to the surrounding tissues from IAC.
Fundus photograph showing chorioretinal atrophy and foci of viable retinoblastoma tumor (a). Histopathology of the same patient showing severe choroidal atrophy and loss of outer nuclear layer, photoreceptors, and retinal pigment epithelium (b) (Reproduced with permission from Eagle et al. [24])
Melphalan, a potent alkylator, has known vascular toxicities [28]. In a 2011 editorial, Wilson et al. attributed the reported vascular complications of SSIOAC to concentration of melphalan being used [29]. A preclinical model was developed to study the vascular sequelae of SSIOAC [30]. Using techniques and protocols similar to those previously published, a cohort of six non-human primates were treated with SSIOAC, and real-time ophthalmoscopic findings were documented during each infusion. Visible pulsatile pallor of the optic nerve, choroidal blanching, and retinal arterial narrowing were observed (Fig. 12.5). Sectoral choroidal non-perfusion and diffuse capillary dropout were seen on fluorescein angiography immediately following chemotherapy infusion (Fig. 12.5). Orbital and ocular histopathology revealed drug-induced toxicity to retinal endothelial cells as well as technique-induced changes to the orbital vasculature, including intimal hyperplasia, fracturing of the internal elastic lamina, and arterial wall dissection involving the ophthalmic, and central retinal arteries [31].
Real-time ophthalmoscopic findings during SSIOAC infusion of melphalan in non-human primate model showing retinal artery narrowing with loss of inferior temporal and nasal arcades (a). Intravenous fluoroscopic angiography of same primate showing delayed, sectoral choroidal perfusion (b) (Reproduced with permission from Wilson et al. [30])
12.8 Current Status
The role and indications for SSIOAC are still evolving as experience with the technique grows. How SSIOAC has been incorporated into the paradigm of retinoblastoma management of retinoblastoma varies from center to center. Most centers will not use SSIOAC on a newly diagnosed Group A patient, although there have been reports of SSIOAC for refractory or partially responsive Group A patients [15, 16]. Some institutions employ SSIOAC only when the tumor is too large to be controlled with focal therapies or to salvage an eye previously destined for enucleation [10]. SSIOAC appears to be commonly used in concert with other local therapies.
The ocular oncology community, in concert with our pediatric oncology counterparts, must determine which patients and eyes will benefit from SSIOAC. All would agree that International Classification Group A should not be treated in such a manner, but there remains debate over Groups B, C, and D. Unilateral Group E eyes, where the extensive nature of disease precludes any significant visual potential, may uncommonly be treated by SSIOAC; enucleation in such patients with advanced intraocular disease remains common despite the advent of SSIOAC.
Of note, not all retinoblastoma centers have adopted SSIOAC in the treatment of retinoblastoma. The trepidation is, in part, twofold. First, there is the need to provide patient-centric care, ensuring the entire patient is adequately treated. Approximately 10 % of International Classification Group D and 50 % of Group E eyes will have at least one high-risk histopathology feature pertaining to the chance of developing metastatic disease [32]. Thus, if the eye is solely treated and the risks to the entire patient are dismissed, the likelihood of resurgent metastatic retinoblastoma becomes very real. Secondly, there is the need to further delineate and quantify the toxicities, acute and long term, associated with SSIOAC.
12.9 Future Studies
The need for prospective evaluation of this technique is well understood. A single institution phase II trial of intra-arterial chemotherapy for retinoblastoma (NCT01293539) will provide initial prospective evaluation of this technique. The feasibility and toxicity of SSIOAC will also be addressed in an upcoming prospective, multi-institution clinical trial developed by the Children’s Oncology Group (ARET12P1) (Chap. 21). Patients with unilateral Group D retinoblastoma will be eligible for treatment with three infusions of intra-arterial melphalan. Local therapy will be withheld until after the first treatment. Catheterizations and ocular outcomes will be submitted for centralized review, and toxicities will be prospectively recorded. Target accrual is 44 patients over 28 months. We anticipate the immediate and long-term follow-up of these patients, along with continued preclinical modeling, will provide further insight into the optimal utilization of this local delivery technique in our retinoblastoma population.
References
Reese AB, Hyman GA, Tapley ND, Forrest AW. The treatment of retinoblastoma by x-ray and triethylene melamine. AMA Arch Ophthalmol. 1958;60:897–906.
Kupfer C. Retinoblastoma treated with intravenous nitrogen mustard. Am J Ophthalmol. 1953;36:1721–3.
Retinoblastoma. In: Reese AB, editor. Tumors of the eye. 2nd ed. New York: Harper and Row; p. 84–161.
Kaneko A, Takayama J, Matsuoka H, et al. Chemotherapy was successful in two cases of recurrence of intraocular retinoblastoma after irradiation [in Japanese]. Rinsho Ganka. 1990;44:289–92.
Mohri M. The technique of selective ophthalmic arterial infusion for conservative treatment of recurrent retinoblastoma [in Japanese]. Keio Igaku (J Keio Med Soc). 1993;70:679–87.
Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9:69–73.
Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured human retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78:858–68.
Suzuki S, Yamane T, Mohri M, Kaneko A. Selective ophthalmic artery injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology. 2011;118:2081–7.
Abramson DH, Dunkel IJ, Brodie SE, et al. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–404. 1404.e1.
Gobin YP, Dunkel IJ, Marr BP, et al. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129(6):732–7.
Marr BP, Brodie SE, Dunkel IJ, et al. Three-drug intra-arterial chemotherapy using simultaneous carboplatin, topotecan and melphalan for intraocular retinoblastoma: preliminary results. Br J Ophthalmol. 2012;96:1300–3.
Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma report no. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129(11):1399–406.
Muen WJ, Kingston JE, Robertson F, et al. Efficacy and complications of super-selective intra-ophthalmic artery melphalan for the treatment of refractory retinoblastoma. Ophthalmology. 2012;119(3):611–6.
Venturi C, Bracco S, Cerase A, et al. Superselective ophthalmic artery infusion of melphalan for intraocular retinoblastoma: preliminary results from 140 treatments. Acta Ophthalmol. 2013;91(4):335–42.
Bracco S, Leonini S, de Francesco S, et al. Intra-arterial chemotherapy with melphalan for intraocular retinoblastoma. Br J Ophthalmol. 2013;97(9):1219–21.
Thampi S, Hetts S, Cooke D, et al. Superselective intra-arterial melphalan therapy for newly diagnosed and refractory retinoblastoma: results from a single institution. Clin Ophthalmol. 2013;7:981–9.
Brodie SE, Gobin YP, Dunkel IJ, et al. Persistence of retinal function after selective ophthalmic artery chemotherapy infusion for retinoblastoma. Doc Ophthalmol. 2009;119:13–22.
Tsimpida M, Thompson DA, Liasis A, et al. Visual outcomes following intraophthalmic artery melphalan for patients with refractory retinoblastoma and age appropriate vision. Br J Ophthalmol. 2013;97:1464–70.
Munier FL, Beck-Popovic M, Balmer A, et al. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31(3):566–73.
Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol. 2010;21(3):203–12.
Vajzovic LM, Murray TG, Aziz-Sultan MA. Supraselective intra-arterial chemotherapy: evaluation of treatment related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–6.
Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma: report no. 2, treatment complications. Arch Ophthalmol. 2011;129(11):1407–15.
Fallaha N, Dubois J, Carret AS, et al. Real-time ophthalmoscopic findings of intraophthalmic artery chemotherapy in retinoblastoma. Arch Ophthalmol. 2012;130:1075–7.
Eagle RC, Shields CL, Bianciotto C, et al. Histopathologic observations after intra-arterial chemotherapy for retinoblastoma. Arch Ophthalmol. 2011;129(11):1416–21.
Vajzovic LM, Murray TG, Aziz-Sultan MA, et al. Clinicopathologic review of enucleated eyes after intra-arterial chemotherapy with melphalan for advanced retinoblastoma. Arch Ophthalmol. 2010;128(12):1619–23.
Graeber CP, Gobin YP, Marr BP, et al. Histopathologic findings of eyes enucleated after treatment with chemosurgery for retinoblastoma. Open Ophthalmol J. 2011;5:1–5.
Kim J, Do H, Egbert P. Enucleated eyes after failed intra-arterial infusion of chemotherapy for unilateral retinoblastoma: histopathologic evaluation of vitreous seeding. Clin Ophthalmol. 2011;5:1655–8.
Samuels BL, Bitran JD. High-dose intravenous melphalan: a review. J Clin Oncol. 1995;13:1786–99.
Wilson MW, Haik BG, Dyer MA. Superselective intraophthalmic artery chemotherapy what we do not know. Arch Ophthalmol. 2011;129(11):1490–1.
Wilson MW, Jackson JS, Phillips BX, et al. Real-time ophthalmoscopic findings of superselective intraophthalmic artery chemotherapy in a nonhuman primate model. Arch Ophthalmol. 2011;129(11):1458–65.
Tse BC, Steinle JJ, Johnson D, et al. Superselective intraophthalmic artery chemotherapy in a nonhuman primate model. JAMA Ophthalmol. 2013;131(7):903–11.
Wilson MW, Qaddoumi I, Billups C, et al. A clinic-pathological correlation of 67 eyes primarily enucleated for advanced intraocular retinoblastoma. Br J Ophthalmol. 2011;95(4):553–8.
Jabbour P, Chalouhi N, Tjoumakaris S. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma: a review. J Neurosurg Pediatr. 2012;10:127–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tse, B.C., Brennan, R.C., Wilson, M.W. (2015). Intra-ophthalmic Artery Chemotherapy for Retinoblastoma. In: Singh, A., Murphree, A., Damato, B. (eds) Clinical Ophthalmic Oncology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43451-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-662-43451-2_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43450-5
Online ISBN: 978-3-662-43451-2
eBook Packages: MedicineMedicine (R0)