Abstract
Synaptic transmission is a highly dynamic and regulated process in which electrical information is transferred between two neurons by means of a chemical transmitter that diffuses from the presynaptic element to reach, bind, and activate the neurotransmitter receptors located at postsynaptic side. Traditionally, postsynaptic receptors have been considered fixed in front of the releasing site, but over the last decade, compelling evidence has shown that they diffuse in the plane of the neuronal membrane, thus adding a further level of complexity to synaptic neurotransmission. The development of new technologies that allow a close inspection of the diffusive properties of receptors at synapses have revealed that the receptors dynamics is not only part of a “constitutive recycling” but also is responsible for the fast tuning of the receptor number at synapses both in basal conditions and in response to external stimuli, being therefore a major determinant of synaptic plasticity. In this section, we will review the techniques used to study the lateral mobility of individual receptors and the recent advances in the comprehension of the role of receptor diffusion in neuronal synaptic computation.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Although sometimes referred to as a “background noise,” Brownian diffusion is crucial for the actuation of most biological processes. Indeed, many biochemical reactions taking place in the cytosol and in the nucleus of living cells occur thanks to diffusion that ensures the interaction between molecules. At the synapse, for instance, the sequential events of synaptic transmission are dominated by “controlled” diffusive processes. In the presynaptic terminal, after the invasion of the action potential, indeed, the entry and diffusion of calcium ions trigger the release of synaptic vesicles, hence the diffusion of neurotransmitter in the synaptic cleft [5, 17, 25]. The opening of receptor channels induced by neurotransmitter binding, in turn, elicits the diffusion of ions in the postsynaptic element, leading to changes in the postsynaptic membrane potential and/or to the activation of intracellular signaling cascades. More recently, it has been shown that diffusion at the synapse is not limited to ions and small molecules but also involves proteins such as neurotransmitter receptors that are the main players in the transduction between chemical to electrical signals at the postsynaptic element [71]. Neurotransmitter receptors are enriched at postsynaptic sites by means of anchoring proteins that bind receptors to the cytoskeleton. Due to the stability of postsynaptic receptor clusters, receptors were assumed to be immobile and rigidly connected with the neuronal backbone. However, it is now clear that receptors can diffuse at the surface of the neuronal membrane and that scaffolding proteins provide “diffusion traps” that hinder Brownian free diffusion, thus ensuring the formation and maintenance of receptor clusters at the postsynaptic side ([6], 56). The discovery of neurotransmitter receptors diffusion imposed a revision in the existing view of synaptic processes. The traditional vision “receptors are static until external forces change their status” was replaced by “receptors naturally diffuse until external forces limit their mobility.” After the first direct demonstrations of individual surface receptor lateral diffusion and of receptor reversible “stop-and-goes” at synaptic sites ([44], 10), clear hints that receptors exchanges between synaptic and extrasynaptic compartments contributed to the regulation of receptor number at synapses derived from the analysis of the mechanisms underlying the fast remodeling of the postsynaptic receptor organization during synaptic plasticity [14, 41]. In particular, changes of synaptic receptor number observed in response to plasticity induction were demonstrated to depend on the changes of exocytosis and/or endocytosis rates, suggesting the presence of a “dynamic equilibrium” between surface receptors and a pool of intracellular receptors [11, 12, 61]. The observation that both exocytosis and endocytosis occur outside the synaptic area [8, 9, 52] implied that receptors have to laterally diffuse in the plane of the membrane to be inserted to or removed from synapses, thus introducing the concept of “lateral diffusion” in the rapid adjustment of receptor number at synapses [14]. It is now clear that the dynamic interaction of receptors with scaffold proteins underlies several forms of postsynaptic long-term potentiation or depression (LTP, LTD), thus influencing the strength of synaptic contacts and consequently the functioning of brain microcircuits [70, 71]. In addition to its role in long-term plasticity, receptor lateral diffusion has been also implicated in the fast tuning of synaptic signals by modulating the availability of functional receptors at synapses during synaptic transmission in the millisecond range [27, 71]. This highlights the role of receptor diffusion in tuning synaptic transmission and discloses the general paradigm that the dynamic interaction between molecules and the membrane organization at nanoscale range are central determinants of the computational properties of the neuron.
2 Measuring the Lateral Diffusion of Neurotransmitter Receptors
2.1 Population Diffusion Measurements
Over the last two decades, the detection and the analysis of receptor diffusion at the neuronal surface has been a major technical and intellectual challenge. Converging evidence obtained with different approaches unanimously suggested that neurotransmitter receptors undergo an intense diffusive activity that is much higher and complex than that expected by simple “constitutive receptor recycling.” The first attempts to study the dynamics of receptors at synapses were made by video imaging of receptors tagged with fluorescent proteins. For instance, by performing two-photon imaging of AMPA receptors tagged with GFP in organotypic cultures, Shi and colleagues [61] found that high-frequency stimulation induced the enhancement of GFP fluorescence at glutamatergic spines, thus indicating an activity-dependent AMPA receptors fast redistribution between the synaptic and extrasynaptic areas at the neuronal surface. The fluorescence recovery after photo-bleaching (FRAP) is another approach that allowed studying the diffusion properties of receptors tagged with fluorescent proteins at population level [3]. Indeed, after photo-bleaching a small area containing fluorescent-tagged receptors with a high-intensity laser illumination, neighboring unbleached fluorescent-tagged receptors will diffuse in the bleached area and replenish it with fluorescence ([33, 53], 28). The study of the fluorescence recovery dynamics in the bleached area allows inferring the diffusive properties of the receptors. As a general rule, the fluorescence recovery rate depends on the receptor diffusion coefficient, whereas the fraction of fluorescence recovery at steady state sheds light on the mobile population of the receptors. However, although conceptually straightforward, it has to be pointed out that the “fluorescence replenishment” after the photo-bleaching is a complex phenomenon, not only due to the lateral diffusion of unbleached receptors but also dependent, for instance, on the rate at which the photo-bleached receptors leave the bleached area and/or on possible direct receptor exocytosis in the bleached area [28]. A similar technique to FRAP is the fluorescence loss in photo-bleaching (FLIP), which can be analogously used to monitor the mobility of populations of fluorescence-tagged receptors and exchanges between compartments. With this approach, consequent to a continuous bleaching in a defined area, protein mobility will be deciphered as the fluorescence loss in the surrounding area. The fading of fluorescence outside the bleached area will be due to the invasion by lateral diffusion of bleached proteins [51]. The application of FRAP and FLIP approaches to receptor-tagged pH-sensitive fluorescent proteins, such as pHluorin or SuperEcliptic pHluorin (SEP), represents a technical advantage to restrain the observation to surface receptors, being the fluorescence of these proteins quenched in acidic intracellular compartments [2]. Moreover, the restriction of photo-bleaching to synaptic areas by means of diffraction-limited laser spots allows studying the dynamics of synaptic receptors [27, 53]. More recently, the advent of photo-activated and photo-convertible fluorescent proteins able to change their emission spectral properties upon specific illumination allowed a further advancement in the monitoring of receptor dynamics in living cells [42]. Indeed, by tagging receptors with these proteins, it is possible to “photo-activate” and “photo-convert” them in a specific region of interest and to observe the displacements of “photo-activated/converted receptors” in neighboring areas [42]. These approaches not only allow inferring receptor diffusion properties but also are instrumental for studying the spatial patterns of receptor accumulation in specific subcellular domains [21, 47]. Another “bulk measurement” of molecule diffusion can be achieved by using the fluorescence correlation spectroscopy (FCS), a technique that reveals the mobility of the molecule of interest by its time of residence in small detection volumes [58].
In addition to the aforementioned imaging techniques, pharmacological tools have also proved useful to probe receptor “population diffusion.” For instance, Tovar et al. [68] revealed the diffusion of NMDA receptors at glutamatergic synapses by studying the recovery of NMDA currents following irreversible block of the NMDA receptors by “open-channel” blocker MK-801. This pharmacological agent, indeed, selectively exerts its antagonism on synaptic receptors and not on extrasynaptic ones, since receptor “opening” would selectively occur at synapses. The unexpected current recovery in the presence of MK-801 clearly indicated the replacement of synaptic “blocked NMDA receptors” with naïve extrasynaptic receptors, thus implying the existence of receptor exchanges between synaptic and extrasynaptic compartments by lateral diffusion. With a similar approach applied to GABAergic synapses, Thomas et al. [67] exploited the irreversible and activity-dependent block of mutated GABAA receptors by MTSES to demonstrate the mobility of functional GABAA receptors. This study reported that, at inhibitory synapses of hippocampal pyramidal neurons, the synaptic pool of GABAA receptors is rapidly replaced independently from receptor intracellular trafficking. The increasing development of photo-reactive pharmacological compounds and modifiers of receptor gating combined to electrophysiological approaches represents nowadays a considerable advantage for precise spatial and temporal control of the activation of receptor subpopulations to ultimately provide real-time description of receptor surface diffusion and intracellular trafficking [1].
2.2 Single-particle Tracking Techniques
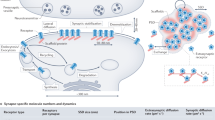
Although the aforementioned techniques are important to estimate the average value of receptor diffusion at population level, a major breakthrough in the study of receptor lateral mobility is represented by the advent of the single-particle tracking (SPT) techniques that allow the direct visualization of receptor diffusion at the single-molecule level. The possibility to study the mobility of individual molecules, indeed, dramatically increased the accuracy of the quantitative description of receptor diffusion. In this way, it was possible to observe that receptor mobility greatly differs among specific receptor subpopulations and among various membrane domains, a heterogeneity that is crucial for the functional role of receptor diffusion in synaptic transmission [37, 57]. The first pioneering studies that visualized and analyzed the trajectories of individual neurotransmitter receptors diffusing on the neuronal surface exploited latex beads coupled to the receptors of interest by means of primary antibodies and visualized by interference contrast microscopy [10, 44, 60]. This approach, first used with glycine, AMPA and mGlu receptors, revealed the fundamental paradigm of surface receptors diffusion that receptors alternate between periods of high and low mobility correlating with the diffusion in the extrasynaptic and synaptic compartments, respectively. Overall, these studies provided the first direct pieces of evidence of receptor fast diffusion at the neuronal surface. Moreover, they highlighted the key concept that receptor Brownian diffusion (induced by thermal agitation) can be “controlled” and “modulated” at the nanoscale level by transient receptor interactions with the scaffold proteins at specific domains of the neuronal plasma membrane. Although the SPT based on the use of latex beads represented a revolution in the study of receptor lateral mobility, the large size of these beacons (500 nm) represented a major limit for a reliable tracking of receptor diffusion at synapses, where the distance between pre- and postsynaptic elements (synaptic cleft) is only 20 nm. For this purpose, a considerable effort has been made to develop SPT approaches based on smaller fluorescent probes as reporters of receptor mobility. Among them, for instance, small organic fluorescent dyes (~1 nm) (such as cyanin dyes and Attodyes) are expected to minimally interfere with receptor diffusion [23, 55, 66]. However, these nanoprobes, recognized by the characteristic one-step photo-bleaching, require strong laser illumination to maximize photon emission and undergo rapid photo-bleaching (<10 s), thus limiting the visualization of the receptor diffusion to short periods [73]. Alternatively, receptor mobility can be probed by using quantum dots (QDs) as reporters. QDs are nanometer-sized semiconductors covered with a ZnS shell and a biocompatible organic coating, giving final fluorescent nanoprobes of 15–25 nm in diameter. Although the QD-based SPT approach can be performed on a limited number of target molecules in each experiment and requires adequate software algorithms for the reconstruction of trajectories (due to the blinking emission of QDs), to date, these semiconductors represent the best available trade-off between size and photo-stability. QDs, indeed, are much smaller than latex beads and, different from organic dyes, they exhibit very low photo-bleaching. The strong photo-stability of QDs allows long-lasting receptor tracking, an essential requirement for the study of receptors diffusion during processes that imply the observation of the same receptor up to 30–40 min such as during long-term synaptic plasticity [70, 71]. Besides their photo-stability, the advantages of QDs for the tracking of receptors are manifold. For instance, QDs possess a very high quantum yield, thus showing an excellent signal-to-noise ratio when illuminated by standard fluorescence lamps. This characteristic is instrumental to achieve considerably high point accuracy in the localization of individual QD-receptor complexes over time. In contrast, as mentioned above, the detection of organic dyes needs strong laser illumination, thus unavoidably determining photo-damage and limiting the observation to brief periods. Importantly, QDs show unique advantageous spectral properties with very broad adsorption and narrow, symmetric emission spectra (the latter depending on the QD characteristics/size). These features make QDs particularly suitable for complex experimental conditions requiring multicolor imaging. An additional advantage of QDs is that they can be easily coupled to biomolecules of interest. Indeed, commercially available QDs can form covalent (or non-covalent) binding to target molecules though surface treatments and functionalization with different reactive groups such as IgGs, biotin, or streptavidin. Functionalization with species-specific IgGs or Fab allows QD binding to primary antibodies targeting the protein to be tracked (Fig. 1). In recent years, streptavidin-functionalized QDs have been extensively used in SPT experiments due to the strong affinity of the biotin–streptavidin interaction that persists during prolonged observation periods. Alternatively, depending on the target molecule and on its engineering with selective tags, functionalization of QDs can include the corresponding binding partner of the tag to achieve a direct and exclusive interaction of QDs with the target molecule. Successful experiments have been performed for instance with scFv, Halotag, polyhistidine, CrAsH, and biotinylated acceptor peptide [19, 29, 32, 36, 63].
Experimental approach of the single-particle tracking technique. a Schematic representation of the SPT experimental procedure. After the acquisition of sequential images of QD-receptor complexes diffusing in a x–y plane, object recognition has to be performed to identify QD and assign them (x, y) coordinates in each plane. Subsequently, QD-receptor trajectories are reconstructed across adjacent planes with a Vogel’s algorithm and then reconnected across QD blinking with a method based on QD maximal allowable displacement during a maximal allowable duration of the dark period. Finally, reconstructed trajectories are distinguished in portions inside and outside compartments of interest (e.g., synapses, EZs, etc.) according to the colocalization of the (x, y) coordinates of the QD on each plane with those of the compartment. b Diagram of surface receptor labeling with a quantum dot (QD) through a specific antibody directed against an extracellular epitope of the receptor of interest. c Visualization of surface GABAA receptor diffusion in synaptic and extrasynaptic areas (red and yellow trajectories, respectively) on the dendrite of a cultured hippocampal neuron (green). Blue spots represent GABAergic synapses visualized by live labeling of vGAT-Oyster550. Scale bar 10 μm. d Up close visualization of the extrasynaptic (yellow) and synaptic (red) reconstructed trajectory of an individual GABAA receptor-QD complex, distinguished according to the juxtaposition to the inhibitory presynaptic terminal labeled with vGAT-Oyster550 (blue). e Mean square displacement curve (MSD) of the receptor trajectory reported in (d) at synaptic (red) and extrasynaptic (yellow) areas. The steady state reached by the red curve indicates that receptors are confined in synaptic areas, while the linear MSD curve describing the receptor mobility in the extrasynaptic space indicates free Brownian diffusion outside synapses
As a result of the QD properties and versatility, the QD-based SPT technique has been intensely used over the last decade, providing most of the current knowledge about the receptor diffusion properties. Technological advances allowed further decreasing the QDs size to 10–12 nm [30] to ensure better access to the highly packed synaptic structure. Additional approaches to tag, label, and track surface proteins at single-molecule level have been developed by introducing the α-bungarotoxin binding site (BBS) in the extracellular portion of surface receptors and exploiting the high affinity binding to fluorescent bungarotoxin [23, 24, 59]. In addition to latex beads, SPT techniques based on non-fluorescent probes have been also attempted. For instance, gold particles of ~5 nm have been used to track AMPA receptor in live neurons [38]. In principle, these probes may perform significantly better than QDs, since they are considerably smaller and do not blink or undergo photo-bleaching. Unfortunately, different from latex beads and fluorescent reporters, they can only be detected by photo-thermal imaging, a technique that requires complex experimental setup [7].
3 Role of Receptor Diffusion in Long-Term Synaptic Plasticity
The selective and long-lasting tuning of the synaptic strength induced by external stimuli is believed to underlie important brain functions such as learning and memory. Changes in the postsynaptic signaling have been shown to depend upon both pre- and postsynaptic mechanisms. At the postsynaptic side, the amplitude and the kinetics of synaptic currents crucially depend on number of receptors present at the postsynaptic density (PSD) and on the receptor gating properties [41]. Hence, the forms of synaptic potentiation and depression relying on long-lasting changes in the postsynaptic architecture/function are referred to as “postsynaptic plasticity.” As demonstrated in the first studies describing the diffusion of receptors at single-molecule level [10, 44], the lateral mobility of receptors at synapses is strongly influenced by the interactions with scaffold proteins that hinder receptor diffusion by acting as “diffusion traps” [15]. Therefore, the modulation of receptor lateral diffusion via changes of receptor–scaffold interactions is a fundamental mechanism for setting the number of receptors expressed at postsynaptic sites. At glutamatergic synapses, indeed, several studies have highlighted that LTP is largely dictated by lateral diffusion-mediated dendritic redistribution of AMPA receptors that are likely stabilized at synapses through the increased interaction with scaffold proteins at the glutamatergic PSD [40, 48, 49, 53] (Fig. 2). However, it has not been established yet whether after plasticity induction, the increased AMPA receptor anchoring at synapses occurs either through higher availability of “docking sites” and/or increased receptor affinity scaffold molecules. Bats et al. [6] demonstrated that the binding between stargazing (one of the transmembrane AMPA receptor regulatory proteins, TARPs) and PSD-95 (the main component of glutamatergic density) is crucial for the immobilization of AMPA receptors at synapses. More recently, the demonstration that the interaction between stargazing and PSD95 if favored by the stargazing phosphorylation by CaMKII [48] suggested that, during LTP, the stabilization of AMPAR-stargazing onto preexisting “PSD95 slots” is promoted by CaMKII activity [49]. In addition to the interaction with scaffold proteins, AMPA receptor stabilization at synapses can be regulated also by other processes both in basal activity and during synaptic plasticity. Petrini et al. [53] demonstrated that the presence of endocytic zones (EZs) adjacent to glutamatergic synapses allows a “local receptor recycling” that maintains a pool of mobile surface receptors at synapses. Importantly, this pool of mobile receptors is crucial for receptors increase at synapses during synaptic potentiation. Furthermore, by reversibly trapping AMPA receptors, EZs act as diffusional barriers, limiting the dispersion of receptors from glutamatergic synapses (Fig. 2). The role of EZs in modulating receptor lateral diffusion in basal activity and during glutamatergic plasticity highlights the concept that the redistribution of surface receptors crucially depends on the multiscale dynamic interplay among membrane nanodomains that trap surface receptors and between surface and intracellular compartments with specific hierarchy and affinity [15, 50, 71].
Long-term potentiation of glutamatergic synapses. Left during basal activity, intracellular trafficking ensures receptor delivery to and removal from the neuronal membrane. Receptors internalized at endocytic zones through a clathrin-mediated process can then be recycled. Surface receptors laterally diffuse in the plane of the membrane and exchange between extrasynaptic areas, where they are highly mobile, and synaptic compartments, where they are transiently stabilized by the interaction with the PSD. Right during long-term potentiation, AMPA receptor endocytic trafficking is enhanced with promoted exocytosis, leading to a larger number of receptors at the neuronal surface. This pool of activity-dependent exocytosed receptors exhibit higher lateral mobility than preexisting surface receptors. Moreover, upon NMDA receptor activation, Ca2+/calmodulin activates and recruits CaMKII to synapses. CaMKII-dependent phosphorylation of the TARP stargazing and of AMPA receptors reinforces the interaction of the AMPAR-stargazing complex to PSD-95, leading to enhanced accumulation and immobilization of AMPA receptors at synapses
At GABAergic synapses, the role of diffusion on the changes of synaptic strength has been mainly analyzed during inhibitory LTD (iLTD). Sustained neuronal activity, indeed, has been demonstrated to decrease inhibitory synaptic currents due to reduced GABAA receptor and gephyrin clustering [4]. In the same study, this observation was associated with increased mobility and decreased confinement of GABAA receptor at synapses. Similarly, [46] reported that activation of glutamatergic synapses (with consequent Ca2+ entry through NMDA receptors) led to mobilization and dispersal of GABAA receptors from GABAergic synapses. Interestingly, these two studies emphasize the role of the phosphatase calcineurin in this form of inhibitory synaptic depotentiation. In particular, Muir et al. [46] found that the lower interaction of GABAA receptors with the GABAergic PSD and the consequent higher receptor lateral diffusion at inhibitory synapses is due to the dephosphorylation of the residue serine 327 on the γ2 subunit of GABAA receptors, a residue already reported to interfere with GABAA receptor stability at synapses [72]. It is interesting to point out that increased neuronal activity and the resulting rise of intracellular [Ca2+] immobilize synaptic AMPA receptors [10], while increasing the diffusion of GABAA receptors at synapses [4, 46]. This opposite effect of neuronal activity on the regulation of AMPA and GABAAR lateral diffusion at synapses may play an important functional role in the coordination of the activity of excitatory and inhibitory systems. Moreover, the evidence that stimuli-inducing potentiation at glutamatergic synapses also triggers concomitant depression at inhibitory synapses [39], along with the aforementioned opposite modulation of synaptic AMPA and GABAA receptors, suggests that local calcium increase may represent a shared checkpoint to simultaneously orchestrate LTP at glutamatergic synapses and LTD at GABAergic synapses, thus leading to a strong overall unbalance toward excitation.
It is worth mentioning that, in addition to modifications of the number of receptors expressed at the PSD, also changes in the receptor gating properties as well as in the receptor subunit composition represent possible mechanisms underlying postsynaptic plasticity. Indeed, both glutamate and GABAA receptors can be expressed in various subtypes with specific gating properties ([69, 13]). Consequently, postsynaptic currents mediated by different receptors isoforms will exhibit distinct amplitude, kinetics and calcium permeability, like, for instance, the GluA2 vs GluA1 containing AMPA receptors or GABAA receptors containing different α1-6 subunits and/or the γ versus δ subunits ([69, 13]). Therefore, significant alterations of the postsynaptic response can occur even in conditions of unchanged number of synaptic receptors through the switch between receptor subtypes. In this context, the stabilization and trapping of diverse receptor subtypes at synapses can be further influenced by the affinity of receptor isoforms for the PSD proteins, following a specific activity-dependent hierarchy.
4 Implications of Receptor Diffusion in Fast Synaptic Signaling
Besides its role in the onset and maintenance of long-term plasticity through the regulation of receptor number at synapses, fast receptor exchange between synaptic and extrasynaptic zones operates a fine tuning of basal synaptic transmission. At conventional central synapses, during repetitive synaptic activation, the postsynaptic response typically decreases in a frequency-dependent manner. At the presynaptic level, indeed, the fatigue of the release machinery depresses the neurotransmitter release, while at the postsynaptic side the accumulation of receptor desensitization may limit receptor activation [74]. After neurotransmitter release, indeed, postsynaptic receptors readily open producing a postsynaptic response. However, following their activation, receptors enter non-conductive (desensitized) state(s) that can persist for hundreds of milliseconds. In this situation, a second event of neurotransmitter release (occurring in tens of millisecond) will generate a smaller response due to the fact that some receptors are non-responsive (Fig. 3). Assuming fast receptor lateral diffusion, after the first release event, desensitized receptors at the synapses would be replaced by extrasynaptic naïve receptors, leading to attenuation of the synaptic response depression [27]. Hence, surface receptor diffusion can modulate the availability of “ready-to-be-activated” receptors at synapses in the millisecond time range. In the current of view of synaptic transmission, the amplitude and duration of synaptic current are determined by the concentration and the release dynamics of neurotransmitter in the synaptic cleft [16]. In addition, lateral diffusion represents a further level of complexity in the modulation of postsynaptic signals at millisecond timescale. Indeed, the level of the steady-state current will be influenced not only by the equilibrium between desensitized and non-desensitized states but also from the rate of exchange between postsynaptic and extrasynaptic receptors. This phenomenon becomes even more pronounced during sustained synaptic activity. In keeping with this, it is interesting to note that the lifetime of AMPA receptors in the desensitized state is compatible with the time needed for the receptor to significantly exchange between synaptic and extrasynaptic space. As a consequence, receptor lateral mobility and receptor gating processes (that determine the receptor intrinsic functional properties) likely cooperate in order to actuate the precise tuning of the postsynaptic current as a function of the frequency of the synaptic activity. In this concern, it could be also hypothesized that receptors showing several kinetically distinct desensitized states (e.g., GABAA receptors) would “modulate” the synaptic current amplitude over a wide range of receptor diffusion rates and repetitive synaptic activation frequencies [34, 35, 45]. In order to better understand the role of receptor diffusion in the fast modulation of synaptic transmission, it will be important to assess the relation between receptor mobility and receptor gating: does the activated/inactivated receptor state interfere with the ability of the receptor to laterally diffuse in the membrane by altered phospholipid and/or protein interactions and/or conformational states? The assessment of the rules by which mobility and gating are “integrated” will be a fundamental step to understand the role of diffusion in synaptic signaling and neuronal computation.
Functional role of lateral diffusion in the fast modulation of synaptic response. Upper panels diagram of fast AMPA receptor exchange at synapses during synaptic transmission. Following glutamate release (left), some AMPA receptors undergo desensitization (middle) while glutamatergic currents are elicited (middle inset). After 50 ms (right), lateral mobility allows desensitized receptors to be partially replaced by naïve extrasynaptic receptors, thus limiting the depression of synaptic currents due to the accumulation of desensitized receptors (right inset) [27]. Lower panels when receptor lateral diffusion is impaired (left), the receptors desensitized by a presynaptic release (middle) persist at the synapse. As a consequence, a second presynaptic event will elicit a more depressed postsynaptic current (right inset) due to the persistence of non-activatable desensitized receptors at synapses (right)
5 Future Perspectives
One of the clearest demonstrations that receptor lateral diffusion is a highly regulated phenomenon that absolves to precise functional and physiological roles in synaptic transmission is the differential modulation of AMPA and GABAA receptors mobility at glutamatergic and GABAergic synapses in response to sustained neuronal activity. Indeed, the same change in neuronal activity that immobilizes synaptic AMPA receptors at excitatory synapses also increases GABAA receptor mobility at inhibitory synapses [4, 53]. Interestingly, this opposite regulation of the mobility of excitatory and inhibitory receptors is associated to glutamatergic LTP and GABAergic iLTD, respectively. Although the role of Ca2+-dependent intracellular signaling has been highlighted in this process, the exact mechanisms of such opposite modulation are still unclear. Further investigations to clarify the mechanisms underlying the coordinated mobility of receptors at both glutamatergic and GABAergic synapses will be crucial to understand the rules of dendritic E/I balance. In addition, it will be a major intellectual challenge to understand how lateral diffusion at synapses can simultaneously operate long-term variations of synaptic strength and short-term fine tuning of synaptic signaling, two processes that coexist at synapses but whose duration differs by several orders of magnitude. Similarly, it will be also important to establish the hierarchy of “diffusive interactions” between diverse membrane nanodomains in relation to their distance and/or spatial organization.
In order to answer these fundamental questions, it will be important to refine our conceptual and technological approach to the study of the multiscale dynamic interaction between proteins. Recent advances in the super-resolution microscopy techniques allowed “counting” molecules at postsynaptic density, thus establishing the precise ratio between receptors and scaffolding proteins [26, 64]. This methodology has to be extended to the manifold players taking places in the transient stabilization and anchoring of receptors at postsynaptic densities [31]. In addition, it will be also important to consider the diffusion and the interactions of the anchoring proteins in relation to their posttranslational modifications. Ultimately, these processes have to be clarified in conditions of synaptic plasticity in which the synapse stability is perturbed to reach a novel level of stability.
The major technological advance that allowed the fine characterization of receptor mobility at the neuronal surface has been the possibility to analyze the behavior of individual receptors. Importantly, the SPT technique revealed that the diffusive properties of receptors show a substantial variability, ranging from immobility to relatively high mobility (up to 1 μm2/s). This huge variability suggests that the different lateral diffusion observed among specific receptor fractions are at the base of synapse formation, functioning, and plasticity. However, although the detection of receptor diffusion can be now visualized at super-resolution level [20, 43, 62], the techniques that allow controlling the activity of individual receptors (or very small groups of receptors) are not available yet. The best spatial resolution for the activation/inactivation of ligand-gated receptors, indeed, is diffraction-limited (~200 nm) being obtained with optical techniques such as laser photo-release of caged compounds (neurotransmitter, antagonists, etc.). Moreover, when using these techniques, the diffusion front generated by the photo-release makes the effective area invaded by the neurotransmitter be even larger than 250–300 nm, thus making impossible to reach the single-protein resolution or to selectively stimulate specific membrane nanodomains. It is also questionable whether diffraction-limited neurotransmitter uncaging really limits the neurotransmitter activation to individual synapses that range from 100 to 300 nm. In order to overcome these technical limitations, we are developing at IIT a new illumination device capable of focusing light in subdiffraction-limited spots. The methodology for constructing a highly focused beam of light is based on the increment of the localized electric field occurring when a laser beam interacts with a metallic surface with a sharp nanostructure. This phenomenon is at the basis of plasmon polariton technology [54]. The methodological innovation consists in the combination and utilization of nanofabrication techniques to develop structures with spatial control at the nanoscale, such as piezo manipulators or AFM scanning probes. The spatial confinement of light in the near field is comparable to the radius of curvature of tapered nanowires, generating a highly localized beam of light in the order of 20–30 nm (Fig. 4) [18, 22]. By exploiting these tapered nanoprobes, it will be possible, for the first time, to restrict the illumination to individual synapses and/or nanodomains of synapses. In order to be relevant for the investigation of the receptor function at the nanoscale level, this tool has to be used in combination with adequate light-sensitive effectors. For instance, the light-gated ionotropic glutamate receptor (LiGluK2) represents a convenient optogenetic tool that can be effectively switched to either the open/desensitized or closed state by illuminating with 380 nm or >460 nm light, respectively [65]. By studying the diffusion of light-gated ionotropic receptors such as LiGluK2, indeed, it will be possible to explore the receptor diffusion in “controlled conformational states” and to test the functional effects of their transitions between synaptic nanodomains.
The spatial confinement of light in the near field. This illumination device is composed by a single nanowire excited from one end by a highly focused laser beam that, through a “backfire coupling,” generates a highly localized optical field at the other end of the nanowire. This focused light acts as optical exciting source of light-sensitive receptors expressed at synapses
References
Adesnik H, Nicoll RA, England PM (2005) Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron 48(6):977–985
Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM (2004) Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci 24(22):5172–5176
Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW (1976) Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J 16(9):1055–1069
Bannai H, Lévi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A (2009) Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62(5):670–682
Barberis A, Petrini EM, Mozrzymas JW (2011) Impact of synaptic neurotransmitter concentration time course on the kinetics and pharmacological modulation of inhibitory synaptic currents. Front Cell Neurosci 5:6
Bats C, Groc L, Choquet D (2007) The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53(5):719–734
Berciaud S, Cognet L, Blab GA, Lounis B (2004) Photothermal heterodyne imaging of individual nonfluorescent nanoclusters and nanocrystals. Phys Rev Lett 93(25):257402
Blanpied TA, Scott DB, Ehlers MD (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36(3):435–449
Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ (2006) Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J 25:4381–4389
Borgdorff AJ, Choquet D (2002) Regulation of AMPA receptor lateral movements. Nature 417(6889):649–653
Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40(2):361–379
Carroll RC, Beattie EC, von Zastrow M, Malenka RC (2001) Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci 2(5):315–324
Cherubini E, Conti F (2001) Generating diversity at GABAergic synapses. Trends Neurosci 24(3):155–162
Choquet D, Triller A (2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4(4):251–265
Choquet D, Triller A (2013) The dynamic synapse. Neuron 80(3):691–703. doi:10.1016/j.neuron.2013.10.013
Clements JD (1996) Transmitter time course in the synaptic cleft: its role in central synaptic function. Trends Neurosci 19:163–171
Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL (1992) The time course of glutamate in the synaptic cleft. Science 258:1498–1501
De Angelis F, Liberale C, Coluccio ML, Cojoc G, Di Fabrizio E (2011) Emerging fabrication techniques for 3D nano-structuring in plasmonics and single molecule studies. Nanoscale 3(7):2689–2696
Genin E, Carion O, Mahler B, Dubertret B, Arhel N, Charneau P, Doris E, Mioskowski C (2008) CrAsH-quantum dot nanohybrids for smart targeting of proteins. J Am Chem Soc 130(27):8596–8597. doi: 10.1021/ja802987q (Epub 13 Jun 2008)
Giannone G, Hosy E, Levet F, Constals A, Schulze K, Sobolevsky AI, Rosconi MP, Gouaux E, Tampé R, Choquet D, Cognet L (2010) Dynamic superresolution imaging of endogenous proteins on living cells at ultra-high density. Biophys J 99(4):1303–1310. doi:10.1016/j.bpj.2010.06.005
Giannone G, Hosy E, Sibarita JB, Choquet D, Cognet L (2013) High-content super-resolution imaging of live cell by uPAINT. Methods Mol Biol 950:95–110
Giugni A, Torre B, Toma A, Francardi M, Malerba M, Alabastri A, Proietti Zaccaria R, Stockman MI, Di Fabrizio E (2013) Hot-electron nanoscopy using adiabatic compression of surface plasmons. Nat Nanotechnol 8(11):845–852
Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L (2007) Surface trafficking of neurotransmitter receptor: comparison between single-molecule/quantum dot strategies. J Neurosci 27(46):12433–12437
Hannan S, Wilkins ME, Thomas P, Smart TG (2013) Tracking cell surface mobility of GPCRs using α-bungarotoxin-linked fluorophores. Methods Enzymol 521:109–129
Harata NC, Aravanis AM, Tsien RW (2006) Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J Neurochem 97(6):1546–1570
Hastie P, Ulbrich MH, Wang HL, Arant RJ, Lau AG, Zhang Z, Isacoff EY, Chen L (2013) AMPA receptor/TARP stoichiometry visualized by single-molecule subunit counting. Proc Natl Acad Sci USA 110(13):5163–5168. doi:10.1073/pnas.1218765110 (Epub 11 Mar 2013)
Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D (2008) Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320:201–205
Holcman D, Triller A (2006) Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys J 91(7):2405–2415
Howarth M, Takao K, Hayashi Y, Ting AY (2005) Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci USA 102(21):7583–7588 (Epub 16 May 2005)
Howarth M, Liu W, Puthenveetil S, Zheng Y, Marshall LF, Schmidt MM, Wittrup KD, Bawendi MG, Ting AY (2008) Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat Methods 5(5):397–399
Hoze N, Nair D, Hosy E, Sieben C, Manley S, Herrmann A, Sibarita JB, Choquet D, Holcman D (2012) Heterogeneity of AMPA receptor trafficking and molecular interactions revealed by superresolution analysis of live cell imaging. Proc Natl Acad Sci USA 109(42):17052–17057. doi: 10.1073/pnas.1204589109 (Epub 3 Oct 2012)
Iyer G, Michalet X, Chang YP, Pinaud FF, Matyas SE, Payne G, Weiss S (2008) High affinity scFv-hapten pair as a tool for quantum dot labeling and tracking of single proteins in live cells. Nano Lett 8(12):4618–4623. doi:10.1021/nl8032284
Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ (2005) Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci 25:10469–10478
Jones MV, Sahara Y, Dzubay JA, Westbrook GL (1995) Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15(1):181–191
Jones MV, Westbrook GL (1998) Defining affinity with the GABAA receptor. J Neurosci 18(21):8590–8604
Kim J, Park HY, Kim J, Ryu J, Kwon do Y, Grailhe R, Song R (2008) Ni-nitrilotriacetic acid-modified quantum dots as a site-specific labeling agent of histidine-tagged proteins in live cells. Chem Commun (Camb) (16):1910–1912. doi: 10.1039/b719434j (Epub 19 Feb 2008)
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 34:351–378
Lasne D, Blab GA, Berciaud S, Heine M, Groc L, Choquet D, Cognet L, Lounis B (2006) Single nanoparticle photothermal tracking (SNaPT) of 5-nm gold beads in live cells. Biophys J 91(12):4598–4604
Lu YM, Mansuy IM, Kandel ER, Roder J (2000) Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron 26(1):197–205
Makino H, Malinow R (2009) AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64(3):381–390
Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126 (Epub 4 Mar 2002)
Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E (2008) Lippincott-Schwartz High-density mapping of single-molecule trajectories with photoactivated localization microscopy. J. Nat Methods. 5(2):155–157
Manley S, Gillette JM, Lippincott-Schwartz J (2010) Single-particle tracking photoactivated localization microscopy for mapping single-molecule dynamics. Methods Enzymol 475:109–120. doi:10.1016/S0076-6879(10)75005-9
Meier J, Vannier C, Sergé A, Triller A, Choquet D (2001) Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci 4(3):253–260
Mott DD, Rojas A, Fisher JL, Dingledine RJ, Benveniste M (2010) Subunit-specific desensitization of heteromeric kainate receptors. J Physiol 588(Pt 4):683–700
Muir J, Arancibia-Carcamo IL, MacAskill AF, Smith KR, Griffin LD, Kittler JT (2010) NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc Natl Acad Sci USA 107(38):16679–16684
Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB (2013) Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci 33:13204–13224
Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D (2010) CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67(2):239–252
Opazo P, Sainlos M, Choquet D (2012) Regulation of AMPA receptor surface diffusion by PSD-95 slots. Curr Opin Neurobiol 22(3):453–460
Owen DM, Williamson D, Rentero C, Gaus K (2009) Quantitative microscopy: protein dynamics and membrane organisation. Traffic. 10(8):962–971
Perestenko PV, Henley JM (2003) Characterization of the intracellular transport of GluR1 and GluR2 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem 278(44):43525–43532 Epub 2003 Aug 8
Petralia RS, Wang YX, Wenthold RJ (2003) Internalization at glutamatergic synapses during development. Eur J Neurosci 18(12):3207–3217
Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D (2009) Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron 63(1):92–105
Raether H (1988) Surface plasmons. Springer, New York
Renner M, Choquet D, Triller A (2009) Control of the postsynaptic membrane viscosity. J Neurosci 29(9):2926–2937. doi:10.1523/JNEUROSCI.4445-08.2009
Saliba RS, Kretschmannova K, Moss SJ (2012) Activity-dependent phosphorylation of GABAA receptors regulates receptor insertion and tonic current. EMBO J 31(13):2937–2951. doi: 10.1038/emboj.2012.109
Saxton MJ, Jacobson K (1997) Single-particle tracking: applications to membrane dynamics. Annu Rev Biophys Biomol Struct 26:373–399
Schwille P, Haupts U, Maiti S, Webb WW (1999) Molecular dynamics in living cells observed by fluorescence correlation spectroscopy with one- and two-photon excitation. Biophys J 77(4):2251–2265
Sekine-Aizawa Y, Huganir RL (2004) Imaging of receptor trafficking by using alpha-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci USA 101(49):17114–17119 (Epub 24 Nov 2004)
Sergé A, Fourgeaud L, Hémar A, Choquet D (2002) Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci 22(10):3910–3920
Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284(5421):1811–1816
Shrivastava AN, Rodriguez PC, Triller A, Renner M (2013) Dynamic micro-organization of P2X7 receptors revealed by PALM based single particle tracking. Front Cell Neurosci 26(7):232. doi:10.3389/fncel.2013.00232
So MK, Yao H, Rao J (2008) HaloTag protein-mediated specific labeling of living cells with quantum dots. Biochem Biophys Res Commun 374(3):419–423
Specht CG, Izeddin I, Rodriguez PC, El Beheiry M, Rostaing P, Darzacq X, Dahan M, Triller A (2013) Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron 79(2):308–321. doi:10.1016/j.neuron.2013.05.013
Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, Flannery J, Baier H, Trauner D, Isacoff EY (2007) Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54(4):535–545
Tardin C, Cognet L, Bats C, Lounis B, Choquet D (2003) Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J 22(18):4656–4665
Thomas P, Mortensen M, Hosie AM, Smart TG (2005) Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neurosci 8:889–897
Tovar KR, Westbrook GL (2002) Mobile NMDA receptors at hippocampal synapses. Neuron 34(2):255–264
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62(3):405–496. doi:10.1124/pr.109.002451
Triller A, Choquet D (2005) Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci 28(3):133–139
Triller A, Choquet D (2008) New concepts in synaptic biology derived from single-molecule imaging. Neuron 59(3):359–374
Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT (2003) Control of synaptic strength, a novel function of Akt. Neuron 38(6):915–928
Weiss S (1999) Fluorescence spectroscopy of single biomolecules. Science 283(5408):1676–1683
Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Petrini, E.M., Barberis, A. (2014). Probing the Lateral Diffusion of Individual Neurotransmitter Receptors. In: Benfenati, F., Di Fabrizio, E., Torre, V. (eds) Novel Approaches for Single Molecule Activation and Detection. Advances in Atom and Single Molecule Machines. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43367-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-662-43367-6_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43366-9
Online ISBN: 978-3-662-43367-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)