Abstract
There is clear evidence for the pathogenic role of the renin–angiotensin–aldosterone system (RAAS) in the development and progression of CKD. Treatment with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker has been shown to reduce proteinuria and preserve kidney function in patients with established chronic kidney disease, beyond blood pressure lowering. Such findings have led to widespread recommendations for early and sustained blockade of the RAAS to be considered as the first-line treatment for the management of established CKD. Some of these benefits may reflect the better tolerability, efficacy, and side-effect profile of RAAS blockers, compared to other antihypertensive agents. Specific actions on renal haemodynamics, oxidative stress, inflammation, and other pathogenic elements may also contribute to ‘BP-independent effect’ of RAAS blockade in the setting of CKD. This chapter specifically explores the utility of RAAS blockade to prevent the development and slow the progression of chronic kidney disease, beyond their actions to lower blood pressure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic Kidney Disease
- Mineralocorticoid Receptor
- Sodium Polystyrene Sulfonate
- Angiotensin Receptor Blockade
- Progressive Kidney Disease
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Before You Start: Facts You Need to Know-
Activation of the renin–angiotensin–aldosterone system (RAAS) contributes to the development of hypertension in patients with chronic kidney disease.
-
Blockade of the RAAS is the most widely used strategy to prevent progression of chronic kidney disease, both in the presence and absence of diabetes.
-
Blockade of the RAAS has also been suggested to have pleiotropic effects in the kidney beyond blood pressure lowering, consistent with the role of the RAAS in renal pathophysiology.
-
Clinical trials have demonstrated reduction in proteinuria in patients with chronic kidney disease, beyond that seen with other antihypertensive classes despite comparable efficacy with respect to blood pressure lowering.
1 The Renin–Angiotensin–Aldosterone System (RAAS)
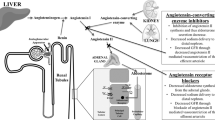
The renin–angiotensin–aldosterone system (RAAS) is a fundamental regulator of vascular homeostasis, mediated directly through its myriad effects on vascular structure and function and indirectly through its effects in the kidney, including sodium and water handling, glomerular filtration pressure, renal blood flow and tubular growth (Box 10.1). The RAAS is a complex multienzymatic hormonal cascade (Fig. 10.1) whose activity is regulated on many levels, with both positive and negative feedback pathways that ensure optimal responsiveness to both physiological and pathogenic stimuli. At its most simplistic, angiotensinogen, the major substrate, is processed in a two-step proteolytic reaction by renin and angiotensin-converting enzyme (ACE), resulting in the generation of angiotensin (Ang) II, the major effector molecule. Other enzymes can also generate Ang II via different enzymatic processing of angiotensinogen (so-called non-ACE pathways) which are more or less important in different tissues and in different states. Ang II is then degraded predominantly by ACE2 and prolylendopetidase (PEP) in the kidney to generate smaller peptides including Ang 1–7 which have vascular and renal actions antagonistic to those of Ang II. The coordinated actions of these opposing pathways provide exquisite control of Ang II levels and its downstream metabolites, allowing for the dynamic responsiveness required to ensure a rapid return to homeostasis.
Box 10.1. Some of the Non-haemodynamic Actions of Angiotensin II in the Kidney
-
Increased sodium retention
-
Tubular hypertrophy and atrophy
-
Epithelial to mesenchymal transition
-
Myofibroblast accumulation
-
Mesangial contraction
-
Foot process effacement (dedifferentiation)
-
Fibrogenesis
-
Renal tubular acidosis
-
NADPH-dependent generation of reactive oxygen species
-
Mitochondrial dysfunction
-
Macrophage accumulation
The most commonly used RAAS blockers inhibit ACE to reduce the synthesis of Ang II or antagonise its actions at the type 1 angiotensin (AT1) receptors (known as angiotensin receptor blockers or ARBs; Fig. 10.2). Both strategies also increase production of Ang 1–7, by reducing its degradation by ACE or AT1-receptor-dependent internalisation, respectively. More recently agents that directly inhibit the enzymatic action of renin or antagonise the effects of aldosterone at the mineralocorticoid receptor have also gained popularity, usually in combination with ACE inhibitors or AT1-receptor blockers. Agents that augment signalling through the AT2 receptor or mimic the actions of Ang 1–7 via the mas receptor or increase ACE2 are also in clinical development for the management of CKD.
The levels of Ang II and other angiotensin peptides are higher in the kidney than in any other tissue in the body, reflecting its key role in maintaining healthy kidney function. In patients with chronic kidney disease, activity of the intrarenal RAAS is inappropriately elevated for the elevated volume status of most patients, which under normal circumstances should see suppression of the RAAS and natriuresis. It is thought that this compensation is an adaptation to maintain kidney function in the acute setting but is ultimately maladaptive in the long term. Some patients (especially those with diabetes) may manifest no increase or even suppression of the systemic RAAS, possible because of excessive local activation of the RAAS in the kidney.
It is well established that activation of the RAAS promotes the development and maintenance of hypertension in CKD (see Chap. 5). This is partly mediated by the direct vasoconstrictor actions of Ang II on smooth muscle to increase peripheral vascular resistance. However, salt and water retention, tubular hypertrophy, augmented activation of the sympathetic nervous system, and sensitivity to the effects of noradrenaline in the kidney also play a role [1]. In addition, T-cell activation also appears to be an important driver of angiotensin-dependent hypertension, as the induction of hypertension in mice is prevented by removing the AT1 receptor from T cells.
The key role played by the RAAS in the development of hypertension has meant that blockade of the RAAS has become the most widely used antihypertensive strategy in patients with progressive kidney disease (see Chap. 5). But while the RAAS plays a key role in the pathogenesis of hypertension, it is also recognised that inappropriate or persistent activation can lead to kidney damage over and above its effects on blood pressure. Moreover, it is often suggested that RAAS blockade offers unique renoprotective benefits in patients with chronic kidney disease, beyond blood pressure lowering. These actions are the subject of this chapter.
2 Is It Just Because the Blood Pressure Is Better Controlled when Blocking the RAAS?
Blood pressure control is important to prevent the development and progression of kidney damage. There is no doubt that drugs that block the RAAS are effective antihypertensive agents. However, when used as monotherapy, RAAS blockers achieve blood pressure reductions that are similar to that achieved by other antihypertensive agents. If then additional benefits are observed when using RAAS blockers despite similar blood pressure control, it is common to invoke its many pleiotropic actions. However, RAAS blockers may be different to other strategies in other aspects of blood pressure control, even for the same achieved reduction in mean or systolic blood pressure levels. For example, some researchers have argued that the antiproteinuric benefits of RAAS blockade observed in the micro-HOPE study may simply have reflected the better 24-h and/or night-time control of blood pressure achieved with ramipril rather than any pleiotropic effects arising from RAAS blockade [2].
Another key difference between blood pressure-lowering strategies may be their effects on blood pressure variability, beyond simply lowering of mean blood pressure levels. For example, it is known that visit-to-visit variability in blood pressure is independently associated with the risk of progressive kidney disease, over and above mean blood pressure control [3]. Indeed in the DCCT study, visit-to-visit variability in blood pressure explained as much of the variability in incident nephropathy as differences in mean blood pressure [3]. Notably, some antihypertensive combinations, including some that contain RAAS blockers, result in the lower blood pressure variability than other combinations. This may partly explain why additional renoprotective advantages of RAAS blockade have been largely reported in studies of hypertensive patients, where RAAS blockade is one of usually three or four different antihypertensive agents. Indeed, it may be that the better, more sustained and less variable effects of RAAS blockade on blood pressure may partly explain the so-called ‘independent’ benefits with respect to kidney disease.
3 Is RAAS Blockade Better Because the Patients Take the Pills?
The other key advantage of conventional RAAS blockade is its tolerability and compliance [4]. ARBs appear to be, on average, the best tolerated of all antihypertensive agents. ACE inhibitors are not far behind. Although cough from ACE inhibitor may be troublesome for some individuals, its impact on adherence and compliance is more favourable than seen with oedema and frequency observed with calcium channel blockers and diuretics, respectively. RAAS blockers are generally long acting, taken once a day and can be easily combined with other agents in fixed dose formulations. Taken together, these effects mean that patients prescribed RAAS blockers are generally more likely to be taking them [4]. Again this ultimately translates into better blood pressure control on an intention to treat basis and potentially better kidney outcomes.
4 How Might RAAS Blockade Actually Slow the Progression of CKD Beyond BP?
An infusion of angiotensin II, even in sub-pressor doses, results in tubular hypertrophy, apoptosis and progressive glomerulosclerosis. The dominant mechanisms by which this occurs include both haemodynamic and non-haemodynamic effects of Ang II (Box 10.1).
The RAAS and renal haemodynamics – Among the earliest changes in the injured kidney is an increase in efferent arteriolar tone leading to an increase in intra-capillary pressure and a loss of auto-regulation. Activation of the RAAS increases the filtration fraction as Ang II constricts the post-glomerular (efferent) arterioles to a greater extent than at the afferent arteriole resulting in an increase intraglomerular pressure (Fig. 10.3). By contrast, blockade of the RAAS with ACE inhibitors or AT1-receptor blockers alleviates hydrostatic ‘stress’ on the glomerulus by causing preferential vasodilatation of the same (post-glomerular) efferent arterioles. This effect on glomerular haemodynamics is most often used to explain why RAAS blockade appears to be more efficacious in preventing proteinuria and renal injury when compared to similar blood pressure reduction using other agents. Moreover, the finding that the slight drop in GFR observed in some patients following the commencement of RAAS blockade (see below) is also associated with a slower decline in renal function suggests that a reduction in intraglomerular pressure plays a key role in both phenomena.
Antiproteinuric effects of RAAS blockade – Proteinuria is not only a marker of renal injury, but also a mediator of progressive renal damage as reabsorption of filtered proteins can injure the tubulointerstitium of the kidney by activating intrACEllular events leading to the release of vasoactive, profibrotic and proinflammatory mediators. In controlled trials in patients with CKD, ACE inhibitors and ARBs reduce urinary protein excretion by approximately 35–40 %, which is greater than other antihypertensive agents, even when the effect of blood pressure reduction on urinary protein excretion has been taken into account. This effect is partly mediated through effects on blood pressure and renal haemodynamics (detailed above), as well as antagonising the direct effects of Ang II on glomerular permselectivity, podocyte structure and function tubular protein handling and the contraction of mesangial cells to decrease the glomerular capillary ultrafiltration coefficient. Post hoc analyses from the RENAAL and IDNT trials showed that the ARB-induced reduction in albuminuria explained most of the long-term renal and cardioprotective effects of ARBs in patients with type 2 diabetes and advanced nephropathy. So important is the antiproteinuric effect of RAAS blockade, that current guidelines strongly recommend the use of RAAS blocker in all forms of proteinuric renal disease, even in the absence of hypertension (Boxes 10.2, 10.3, 10.4, and 10.5). By contrast, the potential utility of RAAS blockade in non-proteinuric renal disease, beyond blood pressure lowering, remains controversial.
Box 10.2. What Do the CARI Guidelines (Australia) Say? [5]
ARB or ACEI should be considered as antihypertensive agents of first choice.
In people with type 2 diabetes and microalbuminuria or macroalbuminuria, angiotensin receptor blocker or angiotensin-converting enzyme inhibitor antihypertensives should be used to protect against progression of kidney disease.
In patients with hypertension associated with renovascular disease, pharmacological inhibition of the renin–angiotensin system effectively and safely lowers blood pressure in most patients (level II evidence).
Box 10.3. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease [6]
ACE inhibitors and ARBs can be used safely in most patients with CKD.
ACE inhibitors and ARBs should be used at moderate to high doses, as used in clinical trials (A).
ACE inhibitors and ARBs should be used as alternatives to each other, if the preferred class cannot be used (B).
ACE inhibitors and ARBs can be used in combination to lower blood pressure or reduce proteinuria (C).
Patients treated with ACE inhibitors or ARBs should be monitored for hypotension, decreased GFR and hyperkalaemia (A).
The interval for monitoring blood pressure, GFR and serum potassium depends on baseline levels (B).
In most patients, the ACE inhibitor or ARB can be continued if:
-
GFR decline over 4 months is <30 % from baseline value (B).
-
Serum potassium is ≤5.5 mEq/l (B).
ACE inhibitors and ARBs should not be used or used with caution in certain circumstances, including women not practising contrACEption, concomitant use of drugs causing hyperkalaemia and bilateral renal artery stenosis.
Box 10.4. UK Guidelines for Identification, Management and Referral [7]
ACEIs should be included in the regimen for all patients with proteinuria (urine protein: creatinine ratio >100 mg/mmol), diabetic patients with microalbuminuria, and for patients with heart failure; ARBs may be used as alternatives to ACEIs.
Serum creatinine and potassium concentration should be checked prior to starting ACEIs and/or ARBs, within 2 weeks of starting and within 2 weeks after subsequent increases in dose; during severe intercurrent illness, particularly if there is a risk of hypovolaemia; and at annual intervals thereafter, or more frequently if indicated, according to kidney function. A rise of serum creatinine concentration of >20 % or fall in estimated GFR of >15 % after initiation or dose increase should be followed by further measurements within 2 weeks; if deterioration in kidney function is confirmed, a specialist opinion should be sought (not necessarily by formal referral) on whether the drug treatment should be stopped or the patient subjected to investigation for renal artery stenosis.
Hyperkalaemia (serum potassium >6.0 mmol/l) should result in stopping of concomitant nephrotoxic drugs (e.g. NSAIDs), reduction or cessation of potassium-retaining diuretics (amiloride, triamterene, spironolactone) and reduction of loop diuretic dosage if there is no sign of congestion. If hyperkalaemia persists, the ACEI or ARB should be stopped.
‘Dual blockade’ with combinations of ACEIs and ARBs should usually only be initiated under specialist supervision.
Box 10.5. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease [8]
BP and RAAS Interruption
Individualise BP targets and agents according to age, coexistent cardiovascular disease and other comorbidities, risk of progression of CKD, presence or absence of retinopathy (in CKD patients with diabetes) and tolerance of treatment
We suggest that an ARB or ACEI be used in diabetic adults with CKD and urine albumin excretion 30–300 mg/24 h (or equivalent).
We recommend that an ARB or ACEI be used in both diabetic and nondiabetic adults with CKD and urine albumin excretion >300 mg/24 h (or equivalent).
There is insufficient evidence to recommend combining an ACEI with ARBs to prevent progression of CKD.
We recommend temporary discontinuation of potentially nephrotoxic and renally excreted drugs in people with a GFR <60 ml/min/1.73 m2 who have serious intercurrent illness that increases the risk of AKI. These agents include, but are not limited to, RAAS blockers (including ACEIs, ARBs, aldosterone inhibitors, direct renin inhibitors).
Interactions with kidney disease pathways – Ang II is also an important stimulus for inflammation, oxidative stress and fibrogenesis in the kidney (Box 10.1). Each of these represents important pathogenic pathways involved in the development and progression of CKD. For example, the formation of reactive oxygen species (ROS) as a result of oxidative stress is recognised as a key component in the progression of chronic kidney disease. ROS are directly cytotoxic and up-regulate inflammation and fibrosis. The expression and activity of NADPH oxidase represents the major source of ROS in the kidney and NADPH oxidase is directly stimulated by Ang II via activation of the AT1 receptor. This pro-oxidant action may independently contribute to the renal consequences of activation of the AT1 receptor and therein the benefits arising from its blockade in the setting of kidney disease. Ang II is also able to modulate immune responses relevant to scarring, inflammation and hypertension in progressive kidney disease. Indeed, immunosuppression during Ang II-induced hypertension is able to reduce albuminuria, inflammatory cell infiltration and structural damage in the kidney, suggesting that changes in immune functioning play a vital role in determining the actions of RAAS activation.
5 Is There Evidence that Blocking the RAAS Really Prevents Kidney Disease Beyond BP?
Although there is a strong physiological rationale for early blockade of the RAAS in patients at risk of kidney disease, the utility of RAAS blockade for primary prevention beyond blood pressure lowering continues to be debated. Certainly, lowering blood pressure is effective in preventing diabetic kidney disease (see Chap. 6) and many trials have demonstrated renal benefits using RAAS blockers in hypertensive patients while at the same time lowering blood pressure levels. However, the utility of RAAS blocker in normotensive individuals is variable at best. For example, the ACE inhibitor ramipril (10 mg/day) did not reduce the incidence of new onset microalbuminuria in normotensive patients with type 2 diabetes from the micro-HOPE study [9]. Similarly, in type 2 diabetic patients enrolled in the DIRECT study, the ARB, candesartan (16 mg/day), failed to reduce the development of microalbuminuria, despite lower blood pressure levels in the candesartan-treated group [10].
A number of trials have attempted to specifically explore the unique renoprotective utility of RAAS blockade beyond blood pressure lowering in patients with diabetes. However, with few exceptions these studies have largely failed to demonstrate a clear and independent efficacy for the primary prevention of microalbuminuria. Put together with observational findings in a meta-analysis, Casas et al. controversially concluded that ACE or ARBs provided no renoprotective effect beyond BP control [11]. This study has been widely criticised because of ‘methodological flaws’ and, in particular, the inclusion of post hoc renal data from the ALLHAT study, which because of its size, dominated the outcome analysis. This study included a large proportion of black patients in whom RAAS blockade is often considered to be less effective, and patients in the RAAS treatment arm were limited in their access to diuretics.
Although some subsequent clinical studies have observed some renoprotective effects from RAAS blockade, many of these studies deliberately included hypertensive patients and/or achieved greater blood pressure lowering with the RAAS blocker. Consequently, whether RAAS blockade truly offers additional benefits for primary prevention over and above blood pressure control remains contentious. At best, any ‘independent effects’ on primary prevention achieved by RAAS blockers beyond blood pressure lowering are modest, and certainly not the panACEa envisaged by many practitioners.
6 Does Blocking the RAAS Slow the Progression of Established Kidney Disease Beyond BP Lowering?
While data in primary prevention are controversial, there is unequivocal evidence that blocking the RAAS has beneficial actions in the kidney in patients with incipient or established kidney disease, beyond its effects to lower pressure. In patients with diabetes, there is strong evidence that RAAS blockade reduce the risk of progression from microalbuminuria to macroalbuminuria by at least one third and increased likelihood of regression from microalbuminuria to normoalbuminuria by two- to threefold when compared to standard (non-RAAS) antihypertensive therapy [12]. Doubling of serum creatinine and/or progression to end-stage renal disease (ESRD) is also reduced by RAAS blockade. Importantly, in each case, these renoprotective benefits in the response to RAAS blockade is independent on baseline blood pressure levels such that relative efficacy is similar in hypertensive and normotensive patients.
7 Is the Effect of RAAS Blockade on the Kidneys Sustained?
Although RAAS blockade is effective in patients with CKD, most of the separation in outcomes between patients on RAAS blockers and those receiving standard therapy occurs early, within the first 18 months. After this time, the (time-to-event) lines appear to run in parallel. Moreover, if or when RAAS blocking agents are discontinued, albuminuria often increases. These observations call into question the durability of the treatment effect on the RAAS and/or the underlying disease processes. This may be because the RAAS relies on feedback regulation to achieve and sustain the delicate balance required for vascular function and this feedback regulation is intrinsically antagonistic to the therapeutic goal of blocking the RAAS (Fig. 10.3). The blockade achieved by ACE inhibitors and ARBs is only partial and short lived, even when used in combination [13]. In fact, in a third to a half of all patients treated with ACE inhibitors, there is a paradoxical overshoot in aldosterone concentrations after 12 months of treatment (known as aldosterone escape). This escape phenomenon also occurs with ARBs possibly due to activation of the AT2 receptor [13]. Indeed equal rates of elevated aldosterone levels are observed among subjects on ACE inhibitors, ARBs, or a combination of both [13], which may explain the lack of additive effect observed in some clinical studies.
8 Are ACE Inhibitors Better Than ARBs, or Is It the Other Way Round?
The antihypertensive effects of ACE inhibitors and ARBs are not significantly different, although individuals’ responses vary. Similarly, the antiproteinuric response appears to be roughly equivalent in magnitude. It has been argued that ARBs may have several advantages for renoprotection in kidney disease, as compared to ACE inhibitors. Although both agents attenuate signalling through the RAAS, by directly antagonising AT1 receptors, ARBs also block the signalling of angiotensin II generated via non-ACE-dependent pathways (e.g. chymase). ARBs also lead to the preferential stimulation of the unblocked AT2 receptor, which may contribute to the prevention of hypertrophic effects of RAAS activation and might provide further end-organ protection [14]. However, in head-to-head trails, similar effects have been observed when using ACE inhibitors and ARBs. Data from the Diabetics Exposed to Telmisartan and Enalapril (DETAIL) study also supports this assertion, finding that enalapril and telmisartan had similar renoprotective actions in patients with type 2 diabetes and early kidney disease [15]. However, a most recent meta-analysis suggested that while ACE inhibitors reduced the risk of new onset of microalbuminuria, macroalbuminuria or both by 29 % when compared to plACEbo (RR 0.71, 95 % CI 0.56–0.89), no effect was observed for angiotensin receptor blockers (RR 0.90, 95 % CI 0.68–1.19). Controversially, this led some guidelines to the recommendation that ACE inhibitors and not ARBs be used for renoprotection, despite a higher incidence of side effects and comparable BP control. However, the confidence intervals overlap significantly, and it is more likely that each produces a similar, albeit modest effect on renal progression.
9 What Is the Best Dose to Use?
Although therapy should be initiated at a low dose to reduce the risk of side effects, the best renoprotective outcomes appear to be achieved by titration up to maximum approved dose of ACE inhibitors and ARBs, even without additional blood pressure-lowering efficacy. There also is some evidence that megadoses of ACE inhibitors or ARBs exceed the effectiveness of conventional doses in experimental models of chronic kidney disease and clinical observations have suggested that conventional doses should be exceeded if proteinuria remains substantial. This paradigm remains to be formally tested clinical trials.
10 What Are the Potential Drawbacks of RAAS Blockade?
Although RAAS blockers have many potential benefits, treatment with ACE inhibitors and ARBs may also result in adverse effects, which are more common in patients with CKD (Box 10.6). Apart from cough with ACE inhibitors, the most common side effects leading to modification or discontinuation of therapy include an early decrease in GFR, hyperkalaemia and hypotension.
Box 10.6. Side Effects Arising from Blockade of the RAAS
Related to RAAS Blocking Activities
-
Hypotension
-
Acute decline in GFR/kidney failure
-
Hypokalaemia
-
Foetal toxicity
Unrelated to RAAS Blocking Activity
-
Cough (10–20 % of those taking ACE inhibitors, minimal with ARBs)
-
Rash/urticaria/itch (especially with captopril)
-
Angioedema
-
Neutropaenia/agranulocytosis
-
Dysgeusia (abnormal taste sensation; especially with captopril)
Many clinicians are concerned that RAAS blockade might be contraindicated in the presence of CKD or might cause kidney damage because of an acute decline in glomerular filtration rate observed with both ACE inhibitors and/or ARBs. In fact, this fall is a common dose-related adverse effect related to reduce efferent arterial tone following blockade of the RAAS. In all patients starting RAAS blockers, renal function should be checked 4 weeks after initiation. An acute fall in estimated GFR of more than 15 % occurs in approximately 10 % of patients following initiation of RAAS blockade. It is not diagnostic of bilateral renal artery stenosis, which may be present only in a very small number of cases. It is more commonly related volume status, dose at initiation and pressure dependence of renal function in any one individual. This risk of declining renal function should be reduced by optimised volume status prior to initiation (e.g. reducing diuretics, controlling hyperglycaemia or heart failure) and slow dose titration. Nonetheless, an acute fall in GFR that stabilises within the first 2 months actually predicts a slower decrease in long-term renal function. If GFR decreases by more than 30 % over baseline, the dose of ACE inhibitor or ARB should be reduced, and the GFR reassessed frequently until kidney function has stabilised. In many cases, the ACE inhibitor or ARB can be managed without discontinuation.
It is well known that RAAS blockade may precipitate acute renal failure in patients with bilateral critical renal vascular disease, as GFR is maintained in this state by heightened activity of the intrarenal RAAS. However, such events are uncommon and reversible (if detected early). Most patients with established renovascular disease do not experience acute renal failure when treated with a RAAS blocker. Even amongst patients with known renal vascular disease, the use of RAAS blockade is actually associated with an improved kidney and cardiovascular outcomes.
Hyperkalaemia may also be induced following RAAS blockade in patients with CKD due to inhibition of aldosterone production. It may be modestly more common with ACE inhibitors than ARBs. Increases in serum potassium are also more common in kidney patients with diabetes, interstitial nephritis, heart failure and acidosis and those taking NSAIDs, beta-blockers, potassium-sparing diuretics or potassium supplements. Again, this can usually be managed without discontinuation with dose reduction, initiating a ‘low-potassium diet’ of ≤2–3 g/day (approximately 50–75 mEq/day), loop diuretics and alkali replACEment (if metabolic acidosis, serum bicARBonate concentration <21 mEq/l) or using binding resins like sodium polystyrene sulfonate.
11 Is There Any Advantage for Combined RAAS Blockade?
Direct renin inhibition – The feedback induction of prorenin may provide one explanation why renal function and/or albuminuria may still deteriorate in the face of maximally recommended doses of RAAS blockade. As a result, renin inhibition had emerged as a possible target for the management of CKD, both as a means for reducing escape from conventional RAAS blockade and amplifying the clinical response to these agents. Despite exciting data suggesting an additive antiproteinuric effect, the prospective, randomised Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Disease Endpoints (ALTITUDE) study was stopped by the Data Monitoring Committee in late 2011, because of futility (i.e. no prospect of demonstrating the treatment benefit anticipated in the protocol) as well as safety concerns including an excess of renal dysfunction, hyperkalaemia, hypotension and strokes [16].
Combined ACE inhibition and angiotensin receptor blockade – Another potential strategy to achieve better inhibition of RAAS has been to combine ACE inhibition with angiotensin receptor blockade in so-called dual therapy. A number of studies have reported additive antiproteinuric effects of combination therapy, although this may partly reflect the suboptimal doses used of either or both components when used on their own. More recently, the ONTARGET trial, which used high doses of one or both ramipril and/or telmisartan, did demonstrate that albuminuria fell more from baseline with dual therapy compared with monotherapy at 2 years. But again whether this was due to blood pressure lowering or better blockade is uncertain. Moreover, combination therapy was associated with an increased risk of hyperkalaemia and kidney failure.
Mineralocorticoid receptor blockade – The addition of a mineralocorticoid receptor antagonist to an ACE inhibitor or ARB has also been studied as a potential means to achieve better RAAS blockade. Some (but not all) short-term studies have suggested additive antiproteinuric effects, but it is unclear whether the benefits of combination therapy are specifically enhanced in patients with aldosterone escape, or simply because of better blood pressure control with enhanced diuresis. The long-term effects of combination therapy on hard clinical end points remain unknown. Moreover, hyperkalaemia is a significant risk with this strategy in patients with CKD. There may also be unforeseen problems with combination therapy, with one study reporting higher levels of Ang II and an increased incidence of escape in patients receiving spironolactone in combination with conventional RAAS blockade [17].
12 Does an Effect Beyond BP Really Matter?
Although it is widely publicised that RAAS blockade has unique renoprotective benefits for patients with CKD, in modern clinical practice such arguments are largely moot. Given the better tolerability, efficacy and side-effect profile of RAAS blockers over other antihypertensive agents [4], as well as added beneficial effects on retinal and cardiovascular disease [18], heart failure and other end-organ damage [10], most patients with or at risk of CKD currently receive RAAS blockers as first-line antihypertensive agents. Indeed, most patients will initially or ultimately need combination antihypertensive therapy to control their blood pressure, in which case RAAS blockade will almost always be utilised in routine clinical practice. Patients without hypertension or proteinuria generally have a low risk of adverse renal outcomes such that even if there was some renoprotective effect in these patients the number need to treat would be large to afford any benefit while at the same time exposing patients to unnecessary treatment.
Finally, it is important to note that despite its benefits, RAAS blockade even in optimal combination with other interventions is not enough to prevent progressive kidney disease. At its best it achieves a modest and temporary slowing of renal decline in some patients. So while it important to use RAAS blockade in our patients, it is also important to acknowledge that more must be done to preserve kidney function and health in our patients with CKD (Box 10.7).
Box 10.7. Relevant Guidelines
-
1.
KDIGO Guideline
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(Suppl):1–150.
http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf
-
2.
CARI Guideline
CARI Guidelines. Concord, NSW: Caring for Australasians with Renal Impairment; 2004. Updated 9 Jan 2013, cited 12 Aug 2013.
Available from: http://www.cari.org.au
-
3.
The Renal Association Guideline
Clinical Practice Guidelines for the Care of Patients with Chronic Kidney Disease
-
4.
Japanese Society of Nephrology Guideline
Evidence-based practice guideline for the treatment of CKD. Clin Exp Nephrol. 2009;13:533–66.
-
5.
National Institute for Health and Clinical Excellence (NICE) Guideline
Chronic Kidney Disease. National clinical guideline for early identification and management in adults in primary and secondary care. 2008, Royal College of Physicians.
-
6.
Canadian Society of Nephrology Guideline
Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–62 [15].
-
7.
National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) Clinical Practice Guidelines
NKF KDOQI guidelines on hypertension and antihypertensive agents in chronic kidney disease [internet]. New York: National Kidney Foundation; 2013. Cited 12 Aug 2013.
http://www.kidney.org/professionals/kdoqi/guidelines_bp/guide_11.htm
Before You Finish: Practice Pearls for the Clinician
-
Blockade of the RAAS is an effective strategy to reduce blood pressure in patients with CKD, but no more so than other antihypertensive strategies.
-
RAAS blockers have a more favourable side-effect profile than other antihypertensive agents, meaning that patients are generally more likely to be taking them.
-
Any ‘independent’ effect of RAAS blockade for the primary prevention of diabetic nephropathy, beyond blood pressure control, remains to be clearly established.
-
Clear benefits have been observed in proteinuric renal disease, while renoprotective actions in the absence of proteinuria remain controversial.
-
Combination strategies using dual blockade, renin inhibitors or aldosterone antagonists to achieve a more complete RAAS blockade have not improved renal outcomes in patients with diabetes.
References
Price DA, De’Oliveira JM, Fisher ND, Williams GH, Hollenberg NK. The state and responsiveness of the renin-angiotensin-aldosterone system in patients with type II diabetes mellitus. Am J Hypertens. 1999;12:348–55.
Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the hope study and micro-hope substudy. Heart outcomes prevention evaluation study investigators. Lancet. 2000;355:253–259.
Kilpatrick ES, Rigby AS, Atkin SL. The role of blood pressure variability in the development of nephropathy in type 1 diabetes. Diabetes Care. 2010;33:2442–7.
Kronish IM, Woodward M, Sergie Z, Ogedegbe G, Falzon L, Mann DM. Meta-analysis: impact of drug class on adherence to antihypertensives. Circulation. 2011;123:1611–21.
CARI Guidelines [internet]. Concord: caring for Australasians with renal impairmen; 2004. Available from: http://www.cari.org.au. Updated 9 Jan 2013, cited 12 Aug 2013.
National Kidney Foundation: Kidney Disease Outcomes Quality Initiative (NKF KDOQI) clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease [internet]. New York: National Kidney Foundation; 2013. Available from: http://www.kidney.org/professionals/kdoqi/guidelines_bp/guide_11.htm. Cited 12 Aug 2013.
Royal College of Physicians of London. Chronic kidney disease in adults: UK guidelines for identification, management and referral [internet]. London: Royal College of Physicians of London; 2006. Available at: http://www.renal.org/CKDguide/full/CKDprintedfullguide.pdf. Cited 12 Aug 2013.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(Suppl):1–150. http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf.
Gerstein HC, Mann JF, Pogue J, Dinneen SF, Halle JP, Hoogwerf B, Joyce C, Rashkow A, Young J, Zinman B, Yusuf S. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the heart outcomes prevention evaluation study. The hope study investigators. Diabetes Care. 2000;23 Suppl 2:B35–9.
Bilous RCN, Sjølie AK, Fuller J, Klein R, Orchard T, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes. Three randomized trials. Ann Intern Med. 2009;151:11–20.
Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–33.
Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin ii receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ. 2004;329:828.
Horita Y, Taura K, Taguchi T, Furusu A, Kohno S. Aldosterone breakthrough during therapy with angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers in proteinuric patients with immunoglobulin a nephropathy. Nephrology (Carlton). 2006;11:462–6.
Hollenberg NK. Renal implications of angiotensin receptor blockers. Am J Hypertens. 2001;14:237S–41.
Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–61.
Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13.
van de Wal RMPH, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, van Gilst WH, Voors AA. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367–72.
Strippoli G. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2011;26:2827–47.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Thomas, M.C. (2014). Preventing Progression of Chronic Kidney Disease: Renin–Angiotensin–Aldosterone System Blockade Beyond Blood Pressure. In: Arici, M. (eds) Management of Chronic Kidney Disease. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54637-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-54637-2_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54636-5
Online ISBN: 978-3-642-54637-2
eBook Packages: MedicineMedicine (R0)