Abstract
Cnidaria is a large, diverse and ecologically important phylum of marine invertebrates, which includes corals, sea fans, anemones, and jellyfishes. It contains over species, of them belonging to the class Anthozoa. Over marine natural products have been described from this phylum alone, most of them in the twenty-first century. The present work provides an overview of some of the most promising marine bioactive compounds, from a therapeutic point of view, isolated from cnidarians since the year 2000. The order Alcyonacea (class Anthozoa) exhibits the highest number of species yielding promising compounds. Antitumor activity has been the major area of interest in the screening of cnidarian compounds, the most promising ones being terpenoids (monoterpenoids, diterpenoids, and sesquiterpenoids). Future trends and challenges for the bioprospecting of new marine bioactive compounds produced by cnidarians are also discussed, with emphasis on the sustainable production of target cnidarians biomass and the role played by symbiotic microorganisms in the synthesis of important biomolecules.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Soft Coral
- Human Lung Adenocarcinoma
- Human Colon Adenocarcinoma
- Antiplasmodial Activity
- Human Neutrophil Elastase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Cnidarians
The phylum Cnidaria is a large, diverse and ecologically important group of marine invertebrates that contains over extant species (Table 35.1) [1], including hydroids, jellyfish, anemones, and corals, among others (Fig. 35.1 a-d). This group of invertebrate animals is found exclusively in aquatic environments, mostly in marine ecosystems. Cnidarians have simple body forms, usually of a polyp or medusa (Fig. 35.2) [2]. For instance, an anemone is a single polyp, whereas corals are, in general, a colony of individual polyps, which are typically tubular and attached to a surface at their base. Both forms may occur during the life cycle of some cnidarians. All species within the phylum Cnidaria have tentacles surrounding the opening (mouth), with stinging cells in their tips that are used to capture and subdue prey. The stinging cells are coiled structures that shoot out and inject toxins via a dart-like tip [2].
Cnidarians are usually found in a wide geographic range: from deep waters near hydrothermal vents to polar seabeds and tropical reefs. Some cnidarian species, mostly from the class Scyphozoa (jellyfish), are pelagic and live in the water column. Cnidarians lack mechanical means to prey and have optimized their mechanisms to feed throughout evolutionary history, such as stinging cells with powerful toxins that help to disable their prey and drive off predators [3]. In addition to these chemical weapons present in the stinging cells, cnidarians also display other potent compounds that are useful for deterring predators and keeping competitors away [4, 5, 6]. For instance, tropical reefs are ecosystems with a vast biodiversity, where the substrate available for benthic cnidarian species to settle and develop is scarce [7]. Chemical interactions between different species are thus an important mechanism for interspecific competition, which may have dramatic consequences for the organism being outcompeted in this chemical war. Therefore, organisms inhabiting highly biodiverse tropical areas, particularly coral reefs, have developed a large array of chemical compounds that have been the focus of recent bioprospecting efforts [8].
Corals form the structure and foundation of tropical coral reefs and are also important structural elements of some highly diverse deep-sea habitats. Other cnidarian species, such as anemones, are also very diverse and abundant in these tropical ecosystems [9]. These benthic cnidarians display a great variety of molecules that have different biological functions. The harsh chemical and physical environmental conditions of the areas inhabited by these organisms may have been important drivers for the production of a variety of molecules with unique structural features. For instance, the incidence of predation in the majority of these organisms is low due to the toxic compounds they produce to deter predators [10]. Other examples of biological functions of these compounds are defensive functions against pathogens, as well as against fouling organisms, herbivores, and microorganisms [11]. Such chemical compounds have been targeted by scientists searching for new chemical entities from the sea, usually known as marine natural products (GlossaryTerm
MNP
). Although more than compounds have been discovered since the field of GlossaryTermMNP
began in the mid 1960s, only a very limited number have reached the end of the drug discovery pipeline [12].This chapter focuses the compounds that have been discovered from marine cnidarian species, providing a brief overview of this topic and discussing the role of biodiversity and biogeography in the chemical diversity of cnidarians. Cnidarian molecules discovered since the year 2000 that display important bioactivities and promising biotechnological applications are discussed in detail. The rationale for this approach is that the drug discovery pipeline, i. e., the time between discovering a new natural product and the commercialization of that marine drug, is a relatively long process that usually takes between – years. In this view, natural products discovered before 2000 had already had time to go through this pipeline, which means that their biotechnological potential is already fully exploited if the compound did not fail any step of the drug discovery process. Finally, we will discuss the challenges that we foresee for future research on bioactive compounds from cnidarians, as well as their biotechnological application to the industry.
1.1 Overview of Natural Product Discovery from Cnidarians
Research on marine natural products began in the 1950s [13], at a time when important breakthroughs in the taxonomy of marine animals took place [14]. This research field expanded during the 1970s and 1980s. It was only at the end of the 1980s and the beginning of the 1990s that an economically appealing activity started to take shape [15, 6]. Since the beginning of GlossaryTerm
MNP
, sponges (phylum Porifera) have been recognized as the most interesting group of marine invertebrates [16]. However, with growing bioprospecting efforts, and the screening of previously unexplored marine habitats and organisms, the biotechnological potential of other groups of marine invertebrates has also started to become appealing for researchers. The phylum Cnidaria is a large, diverse and ecologically important group of marine invertebrates, which is renowned for the ability to produce powerful toxins and venoms [17]. A total of marine natural products have been described from this phylum alone since 1990 (and until 2011), which shows the importance of cnidarians for marine natural product research. Since the early 1990s, the number of new compounds from marine cnidarians has been higher than the discovery of compounds from sponges [8], and the trend that we currently observe is still a continuous increase of natural product discovery (Fig. 35.3). This shows that bioprospecting efforts on these organisms have been continuously increasing.The quest for new GlossaryTerm
MNP
from cnidarians has benefited from a renaissance since 2005, namely due to the development of new methods in analytical technology, spectroscopy, and high-throughput screening [18]. It has also benefited from the failure to deliver new drug leads in significant numbers by competing technologies, such as chemical synthesis. These two different reasons may support the continuous growth of natural product discovery from cnidarians in the last decade.Bioprospecting efforts have not been evenly distributed among cnidarian taxa. From the new compounds yielded by marine cnidarian species since 1990, were discovered in within class Anthozoa. The remaining is associated with species from class Hydrozoa. Anthozoans display a higher biodiversity, with a higher number of orders (Table 35.1). Nonetheless, of the compounds were discovered in organisms from a single anthozoan order: the Alcyonacea. Only through the analysis of the taxonomic level below order, e. g., the family level, is it possible to observe a more even distribution of new compounds among taxa. Figure 35.4 shows the cumulative number of natural products discovered from alcyonaceans according to family level. It is important to emphasize the Alcyoniidae family due to the continuous increase of new compounds relative to other Aclyonacea families.
Cumulative number of new marine natural products from cnidarians according to the taxonomical level family. The group other refers to the families Acanthogorgiidae, Anthothelidae, Coelogorgiidae, Isididae, Melithaeidae, Nidaliidae, Paragorgiidae, Paralcyoniidae, Primnoidae, Subergorgiidae, and Tubiporidae
The overall increase of new compounds associated with different Cnidaria taxa is displayed in Table 35.2. Of the most representative families from the order Alcyonacea, only the family Briareidae showed a small decrease in the number of new compounds discovered in the last two decades. All other families represented in Table 35.2 showed an increase, which in some particular cases was relatively high (e. g., families Clavulariidae and Neptheidae). These results recorded by Leal etal [8] show that the popularity of cnidarians in bioprospecting efforts continues to increase, with large numbers of new compounds being discovered every year.
The high chemical diversity associated with cnidarians may be related to the high biodiversity displayed by this group. While about cnidarian species are currently known [1], new compounds have only been recorded from species (distributed over genera). This means that only of cnidarian biodiversity has yielded new chemical compounds. This does not necessarily mean that the remaining of cnidarian species do not display any different compounds. Most likely, this is a result of the preference of scientists to search for new chemical entities in a relatively low number of species. For instance, the most popular species among the Alcyoniidae are Clavularia viridis, Briareum excavatum, and Antillogorgia elisabethae (Fig. 35.5), which have been important cnidarians in the history of GlossaryTerm
MNP
research [8]. Diversification of bioprospected species has been relatively low, as can be observed in Fig. 35.5. In this figure, we plot the number of new compounds discovered in cnidarian species since 1990 and sort that information according to the number of new compounds discovered in each species. The uneven result among bioprospected species is clearly observed. As most cnidarian species displaying a high number of new compounds inhabit tropical areas, this may suggest that bioprospecting efforts have been biased toward these particular species, probably driven by previous studies showing the high chemical diversity displayed by such taxa [8]. Although the assumption the all cnidarian species display similar chemical diversity is incorrect, Fig. 35.5 shows that a large number of new molecules associated to other cnidarians are yet to be unveiled. This is particularly evident if we consider that the compounds that are currently known were discovered from only of total cnidarian biodiversity.Another issue that should be noted is biodiscovery hotspots of new cnidarian compounds. Although biogeography itself is a well-studied topic, its investigation in GlossaryTerm
MNP
research is still scarce. This is probably justified by the lack of precise geographical information on collection sites. However, recent studies already started to address this topic and clearly reveal bioprospecting efforts to be biased towards tropical areas [19, 8]. New molecules discovered from cnidarian species over the past decades have mostly resulted from bioprospecting on Asian territories close to tropical areas, particularly in Taiwan, Japan, and China. Remarkably, of such new molecules discovered in cnidarians since 1990 resulted from organisms collected in the marine environment surrounding these two territories.The compounds discovered in cnidarian species belong to various chemical groups, although the majority are terpenoids. Leal etal [19] showed that in the last decade, of the compounds discovered in cnidarians were terpenoids, which contrasts with the relatively low percentage of discovered alkaloids (), steroids (), aliphatic compounds (), and carbohydrates (). This data shows that particular chemical groups, such as terpenoids in the case of cnidarians, have been unquestionably more popular among researchers searching for new compounds. Terpenoids are secondary metabolites that are not directly involved in critical physiological processes. These compounds often play a role in interspecific and other ecological interactions. In terrestrial ecosystems, particularly in plants, terpenoids are known to be very abundant and structurally diverse [20]. Terpenoids are the chemical group that includes most natural products isolated so far from marine environments [21, 22]. This may be related to the large range of structural types that can be included in this group, which is, in part, associated with the fact that their biosynthetic unit can be rearranged and highly oxidized [14, 23]. Terpenoids also display a wide array of known bioactivities and biological functions [24, 25, 26]. Indeed, it was probably because previous studies showed that terpenoids usually display remarkable bioactivities that researchers would increase the chances for successful drug discovery and consequent patenting and commercialization by preferentially targeting molecules of this chemical group [27]. Several examples are already described for the application of terpenoids in the pharmaceutical and food industry due to their potential and effectiveness as medicines and flavor enhancers [20, 27].
Besides bioprospecting efforts directed towards particular groups of organisms and chemical structures, researchers have also been narrowing their searches to particular molecules, with emphasis on the type and relevance of bioactivity displayed, in order to identify the most promising targets for their drug discovery pipelines [28]. These marine molecules display various types of biological activities, such as anticancer, anti-inflammatory, antitumor, antimalarial ones, etc. It is not surprising that over the past years major advances in the discovery of marine drugs have been recorded in clinical trials for cancer [29]. Although there are several marine bioactive compounds in preclinical and clinical trials, only a relatively small number of molecules have reached this stage of the drug discovery pipeline. This process is very complex and encompasses several steps: target identification and validation, assay development, lead identification and optimization, predevelopment, preclinical development, clinical research phase I to phase III, regulatory approval, and phase IV (post-approval studies) [12, 18, 30]. The drug discovery pipeline usually takes – years from the first to the last step.
2 The Most Promising Marine Natural Products from Cnidaria
In this section, we overview the most promising marine bioactive compounds isolated from cnidarians in the twenty-first century. This information was assembled through the survey of the most relevant peer reviewed literature published during this period covering marine natural products [31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52]. Over molecules from cnidarians have been described in the twenty-first century. In order to address only those compounds displaying a high potential for industrial applications, it was decided to use the values of IC (half maximal inhibitory concentration) as guidelines. IC is a quantitative measure that indicates how much of a particular substance (inhibitor) is needed to inhibit a given biological process or component of a process by half. It is important to highlight that the GlossaryTerm
NCI
has renamed the IC to GlossaryTermGI
[53] in order to emphasize the correction for cell count at time zero in cancer cells; in this way, some results of this quantitative measure are now also presented under these directives. Additionally, ED (the median dose that produces the desired effect of a drug in half the test population) was also used to identify promising marine bioactive compounds produced by cnidarians. Only the compounds displaying an IC, or M (except where stated otherwise), and ED were considered in the present review, as these values are commonly used in the surveyed literature to ascertain relevant bioactivity (e. g., [54, 55]). In the few cases where neither IC nor ED values were described for an GlossaryTermMNP
in a study, that compound was selected to be part of the present survey only if either the authors of that study, or those citing it, clearly stated that the results recorded were highly promising for industrial applications. All species producing the compounds selected for the present topic were grouped into classes and orders of the phylum Cnidaria (Table 35.1) (according to the latest classification proposed in GlossaryTermWoRMS
) [1].This approach allowed us to identify which taxonomic groups of cnidarians screened so far display the highest potential to yield new drugs or pharmacological products derived from marine bioactive compounds. Nonetheless, it is important to highlight that the identification of cnidarian species is a challenging task, and it is possible that some of the species (or even genera) referred to in the scientific literature may have been misidentified. In this way, it is of paramount importance that in future work, the authors addressing marine bioactive compounds produced by cnidarians provide a detailed description on how the target species was identified [28].
2.1 Class Anthozoa
The class Anthozoa currently includes orders and over valid species (about of all known cnidarian species; Table 35.1). Within the Anthozoa class, the order Alcyonacea (soft corals and sea fans) is the one that has contributed with the highest number of promising bioactive marine compounds, although other orders, such as Actiniaria (sea anemones) and Scleractinia (hard corals), have also yielded relevant compounds [56, 57, 58, 59].
2.1.1 The Order Alcyonacea (Soft Corals and Sea Fans)
Soft corals are generally brightly colored and rich in nutritionally important substances. However, the incidence of predation in the majority of these organisms is low due to the toxic compounds they produce to deter predators [60]. Several biosynthetic studies have been carried out on the metabolites of soft corals [61] and some of those compounds have already shown great potential for the development of new pharmaceuticals and antifoulants. Sea fans are also well-known sources of compounds exhibiting significant biological activity [62]. Table 35.3 summarizes the most promising compounds from the order Alcyonacea (class Anthozoa).
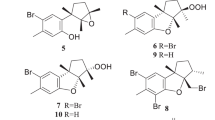
Soft corals are rich sources of secondary metabolites such as diterpenes, sesquiterpenes, furanoditerpenes, terpenoids, capnellene, and steroids (e. g., Lobophytum, Sinularia, Sarcophyton [141], Capnella [142], and Dendronephthya [143]), that have been shown to display GlossaryTerm
HIV
-inhibitory [73], cytotoxic [144, 145], anti-inflammatory [146, 147], anticancer [148, 149], and antimicrobial activity [150], as well as cardiac and vascular responses [151].Chemical investigations on octocorals belonging to the genus Cladiella have resulted in a series of interesting diterpenoids. Terpenoids have been found to display complex structures and various bioactivities [63], especially anti-inflammatory properties. Cladiella australis produces an anti-inflammatory natural product, austrasulfone, which was found to exhibit potent neuroprotective effect against the 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in neuroblastoma SH-SY5Y, a human dopaminergic neuron often used for the study of Parkinson’s disease. The cytotoxicity of 6-OHDA on SH-SY5Y cells was effectively and dose-dependently inhibited by pretreatment at concentrations of –. The ED was [65]. Hirsutalins B-D and H, diterpenoids from Cladiella hirsuta, at a concentration of , displayed significant in vitro anti-inflammatory activity in GlossaryTerm
LPS
(GlossaryTermLPS
)-stimulated GlossaryTermRAW
macrophage cells by inhibiting the expression of the inducible nitric oxide synthase (GlossaryTermiNOS
), with hirsutalin B also effectively reducing the level of GlossaryTermCOX-2
(GlossaryTermCOX
) protein. These compounds, in particular hirsutalin B and D, could be promising anti-inflammatory agents [66]. Cladielloide B and cladieunicellin C, diterpenoids from a non-identified Cladiella, displayed significant inhibitory effects on superoxide anion generation (IC and IC , respectively). The first compound also showed significant inhibitory effects against elastase release (IC ) by human neutrophils at [63, 64]. The same species that produces cladieunicellin C also produces cladieunicellin B and E, two diterpenoids that exhibited significant cytotoxicity against human colorectal adenocarcinoma (GlossaryTermDLD
-1, IC ) and human promyelocytic leukemia (HL-60, IC ) cells, respectively [64].Soft corals of the family Nephtheidae are known for their content of sesquiterpenes and particularly capnellenes [41]. Some sesquiterpenes isolated from Capnella imbricate [142, 152, 153, 154] showed anti-inflammatory activity and a dihydroxycapnellene (capnell-9(12)-ene-8β, 10α-diol) from Dendronephthya rubeola demonstrated antiproliferative activity against murine fibroblasts cell line (L-929, GlossaryTerm
GI
) and cytotoxicity against cancer cell lines implicated in human leukemia (K-562, IC ) and human cervix carcinoma (GlossaryTermHeLa
, IC ) [143]. Capnell-9(12)-ene-8β, 10α-diol strongly inhibits the interaction of the oncogenic transcription factor Myc with its partner protein Max [155, 156], making it a therapeutically interesting compound in oncology [143]. Nephthea chabroli also produces a nor-sesquiterpene compound named chabranol, which displays moderate cytotoxicity against mouse lymphocytic leukemia cells (GlossaryTermP-388
) with an ED [126]. Nephthea erecta produces two proteins in mediated inflammatory responses, the oxygenated ergostanoids 1 and 3. At a concentration of , these two compounds significantly reduced the levels of the GlossaryTermiNOS
( and , respectively) and GlossaryTermCOX-2
protein ( and , respectively), when compared with the control cells stimulated with lipopolysaccharides (GlossaryTermLPS
) [131]. Soft corals of the genus Paralemnalia and Lemnalia have been found to be rich sources of sesquiterpenoids of nardosinane-type. Lemnalia flava produces flavin A, a sesquiterpenoid, that showed significant in vitro anti-inflammatory activity by exhibiting concentration-dependent inhibition of GlossaryTermLPS
-induced GlossaryTermiNOS
and GlossaryTermCOX-2
protein expression (ED values toward both proteins were () and (), respectively). This compound and flavin B also exhibited significant neuroprotective activity [125]. This neuroprotective activity using 6-OHDA-induced neurotoxicity in neuroblastoma SHSY5Y, a human dopaminergic neuron often used for study of Parkinson’s disease, was also demonstrated for paralemnolins Q and S [132, 157] and 2-deoxy-7-O-methyllemnacarnol [157] from Paralemnalia thyrsoides. Nonetheless further investigation for their therapeutic potential against neurodegenerative diseases is suggested.Species in the genus Xenia (family Xeniidae) are a rich source of diterpenoids. Xeniolides I, isolated from Xenia novaebrittanniae demonstrated antibacterial activity at a concentration of in Escherichia coli GlossaryTerm
ATCC
and Bacillus subtilis [140]. Blumiolide C, a diterpenoid from the Xenia blumi (presently accepted as Xenia plicata), exhibited potent cytotoxicity against mouse lymphocytic leukemia (GlossaryTermP-388
, ED ) and human colon adenocarcinoma (GlossaryTermHT-29
, ED ) cells [54].Polyoxygenated cembranoids, crassocolides H–P [84, 85] from Sarcophyton crassocaule, demonstrated cytotoxicity against cancer cell lines of human medulloblastoma (Daoy cells). Crassocolides I, M, and P were found to be more active (IC , , and , respectively). Crassocolide H and N inhibited the growth of human oral epidermoid carcinoma (GlossaryTerm
KB
) cells (IC and , respectively), and crassocolide N and crassocolide L were also active against human cervical epitheloid carcinoma (GlossaryTermHeLa
) cells (IC and , respectively) [84, 85]. Other cembrenoids from the same species, sarcocrassocolides F–L, were found to exhibit anti-inflammatory activities by significantly reducing the levels of GlossaryTermiNOS
protein. Furthermore, sarcocrassocolide I could also effectively reduce GlossaryTermCOX-2
expression with GlossaryTermLPS
treatment. All these compounds might be useful anti-inflammatory agents, sarcocrassocolide I being a promising anti-inflammatory lead compound [86]. Lobophytone Z, from Lobophytum pauciflorum, inhibits GlossaryTermNO
production in mouse peritoneal macrophages induced by GlossaryTermLPS
(IC ) [83]. GlossaryTermNO
is an important signaling molecule that acts in many tissues to regulate a diverse range of physiological and cellular processes. Overproduction of GlossaryTermNO
is associated with various human diseases, including inflammatory and neuronal disorders [158]. The level of GlossaryTermNO
released may reflect the degree of inflammation and provides an indicator to assess inflammatory processes [83].Lobophytum durum and Lobophytum crassum produce durumolides A–C [79], durumhemiketalolide A–C [80], and crassumolides A and C [74], with anti-inflammatory effects. They have been shown to inhibit up-regulation of the proinflammatory GlossaryTerm
iNOS
and GlossaryTermCOX-2
proteins in GlossaryTermLPS
-stimulated murine macrophage cells at IC [74, 79]. From Lobophytum crassum, lobocrassin B displayed significant inhibitory effects on the generation of superoxide anion and the release of elastase by human neutrophils (IC and , respectively). Cytotoxicity of this cembrenoid toward tumor cells showed that it exhibited only modest cytotoxicity against human erythromyeloblastoid leukemia (GlossaryTermK562
), human T-cell acute lymphoblastic leukemia (GlossaryTermCCRF-CEM
), human acute lymphoblastic leukemia (Molt4), and human hepatocellular liver carcinoma (GlossaryTermHepG2
) cells [76].Another example of a potential new therapeutic anticancer agent is a cembranolide diterpene from Lobophytum cristagalli, which has shown a potent inhibitory activity (IC ) [78] over farnesyl protein transferase (GlossaryTerm
FPT
), an important protein in signal transduction and regulation of cell differentiation and proliferation [159]). This type of GlossaryTermFPT
inhibition enhanced interest in this group of metabolites [141]. Other species of this genus also showed cembranoids with significant cytotoxic activity against human lung adenocarcinoma (GlossaryTermA549
, lobophytene [72], lobophytosterol [82], and 13-acetoxysarcophytoxide [75]), human colon adenocarcinoma (GlossaryTermHT-29
, lobophytene [72]; GlossaryTermDLD
-1, culobophylin A [77], and GlossaryTermHCT
-116 lobophytosterol [82]), and human promyelocytic leukemia (HL60, lobophytosterol [82], and culobophylin A and B [77]) cell lines. The diterpenoids, lobohedleolide, (7Z)-lobohedleolide, and 17-dimethylaminolobohedleolide were isolated from the aqueous extract of Lobophytum species and exhibited moderate GlossaryTermHIV
-inhibitory activity (IC approximately –) in a cell-based in vitro anti-GlossaryTermHIV
assay [73]. Additionally, other significant antiviral activity against human cytomegalovirus (IC of ) [81] was described for the compound durumolide Q produced by Lobophytum durum.Eunicellin-based diterpenoids are secondary metabolites often isolated from the genus Klyxum. Klyxum molle has been shown to produce molecules (e. g., klymollin F and G) with interesting bioactivities, such as anti-inflammatory agents [71]. Klyxum simplex also produces diterpene compounds, such as simplexin E, klysimplexins J–N, R, and S, and klysimplexin sulfoxide A–C, which at a concentration of were found to considerably reduce the levels of GlossaryTerm
iNOS
protein. The compounds simplexin E, klysimplexins R and S, and klysimplexin sulfoxide C could also effectively reduce the level of GlossaryTermCOX-2
protein. These results have shown that this compound significantly inhibits the accumulation of the pro-inflammatory GlossaryTermiNOS
and GlossaryTermCOX-2
proteins in GlossaryTermLPS
-stimulated GlossaryTermRAW
macrophage cells, being potential anti-inflammatory agents [67, 69, 70]. This species also produces two diterpenes, klysimplexins B and H, which exhibit moderate cytotoxicity towards human carcinoma cell lines. Klysimplexin B exhibits cytotoxicity toward human hepatocellular carcinoma (Hep G2, IC , and Hep 3B, IC ), human breast carcinoma (GlossaryTermMDA-MB-231
, IC and GlossaryTermMCF
-7, IC ), human lung carcinoma (GlossaryTermA549
, IC ) ,and human gingival carcinoma (GlossaryTermCa9-22
, IC ) cell lines. Metabolite klysimplexin H demonstrated cytotoxicity toward human hepatocellular carcinoma (Hep G2, IC , and Hep 3B, IC ), human breast carcinoma (GlossaryTermMDA-MB-231
, IC , and GlossaryTermMCF
-7, IC ), human lung carcinoma (GlossaryTermA549
, IC ), and human gingival carcinoma (GlossaryTermCa9-22
, IC ) cell lines [68].A tetraprenylated spermine derivative – sinulamide – has been isolated in Sinularia sp., which revealed an H,K-ATPase inhibitory activity. H,K-ATPase is a gastric proton pump of the stomach and is the enzyme primarily responsible for the acidification of the stomach contents. Its inhibition is a very common clinical intervention used in diseases including dyspepsia, peptic ulcer, and gastroesophageal reflux (GlossaryTerm
GORD
/GlossaryTermGERD
). Sinulide is a potential antiulcer drug, as it inhibits production of gastric acid by H,K-ATPase (IC ) [87]. Although it has been chemically synthesized [160], no clinical trials seem to have been reported. The steroid gibberoketosterol [92], isolated from Sinularia gibberosa, and the diterpenoid querciformolide C [93] isolated from Sinularia querciformis, showed significant inhibition of the up-regulation of the pro-inflammatory GlossaryTermiNOS
and GlossaryTermCOX-2
proteins in GlossaryTermLPS
-stimulated murine macrophages at a concentration of [92, 93]. Paralemnalia thyrsoides showed significant inhibition of pro-inflammatory GlossaryTermiNOS
protein expression ( at IC ) [157]. Sinularia species produce significant bioactive molecules. Lipids from Sinularia grandilobata and another unspecified species of Sinularia possess antibacterial and antifungal activity [88]. The diterpene 11-episinulariolide from Sinularia flexibilis is an interesting antifoulant exhibiting strong algacidal properties [91]. This species also produces cembrenoids, named flexilarins, which evidenced cytotoxic activity in cancer cell lines. Flexilarin D exhibited potent cytotoxicity in human hepatocarcinoma (Hep2) cells with IC and moderate cytotoxic activity against human cervical epitheloid carcinoma (GlossaryTermHeLa
, IC ), human medulloblastoma (Daoy, ), and human breast carcinoma (GlossaryTermMCF
-7, ) cell lines [90]. Capilloquinol from Sinularia capillosa displayed cytotoxicity against GlossaryTermP-388
, with an ED of [89].Antifouling agents from natural sources are of increasing interest since the International Maritime Organization (GlossaryTerm
IMO
) banned the use of certain antifouling agents, such as tri-n-butyltin (GlossaryTermTBT
), due to the ecological impacts of these biocides in the marine environment. Several studies have demonstrated that soft corals can yield large quantities of promising antifouling metabolites [161, 162]. In fact, of potential antifouling natural compounds are from cnidarians (e. g., soft corals) [163]. One of the most promising natural antifouling agents identified so far is an isogosterone isolated from an unspecified Dendronephthya [128]. Also 3β-methoxyguaian-10(14)-en-2β-ol, a sesquiterpene from the gorgonian Echinogorgia pseudossapo, was evaluated for its antilarval activity against Amphibalanus amphitrite and Bugula neritina larvae. The results showed that this compound had significant antilarval activity towards Amphibalanus amphitrite larvae with an EC50 value of (), and showed inhibition towards the settlement of Bugula neritina larvae at concentration of . This EC value is lower than the standard requirement of an EC of established by the US Navy program as an efficacy level for natural antifoulants, indicating that 3β-methoxyguaian-10(14)-en-2β-ol is a potential natural antifouling agent [133].Most of the bioactive substances from the family Clavulariidae with promising biotechnological potential are antitumor molecules from the genus Clavularia. Even so, Carijoa sp. produces a sterol glycoside, carijoside A, that displayed significant inhibitory effects on superoxide anion generation (IC ) and elastase release (IC ) by human neutrophils in anti-inflammatory activity testing [104]. As was previously mentioned, genus Clavularia contains promising secondary metabolites with unique structures and remarkable biological activities. Some of the species in this genus produce prostanoids (icosanoids) [101, 103, 164, 165, 166, 55], steroids [102], and diterpenoids [167, 99]. The bioactive marine diterpene, stolonidiol, isolated from an unidentified Clavularia, showed potent choline acetyltransferase (GlossaryTerm
ChAT
) inducible activity in primary cultured basal forebrain cells and clonal septal SN49 cells, suggesting that it may act as a potent neurotrophic factor-like agent on the cholinergic nervous system [98]. Cholinergic neurons in the basal forebrain innervate the cortex and hippocampus, and their function may be closely related to cognitive function and memory. The degeneration of neuronal cells in this brain region is considered to be responsible for several types of dementia, including Alzheimer’s disease. One of the neurotransmitters, acetylcholine, is synthesized from acetyl coenzyme A and choline by the action of GlossaryTermChAT
. Therefore, induction of GlossaryTermChAT
activity in cholinergic neurons may improve the cognitive function in diseases exhibiting cholinergic deficits [168, 169, 170].Prostanoids (claviridic acid) isolated from Clavularia viridis exhibited potent inhibitory effects on phytohemagglutinin-induced proliferation of peripheral blood mononuclear cells (GlossaryTerm
PBMC
, ), as well as significant cytotoxic activity against human gastric cancer cells (GlossaryTermAGS
, IC –) [100]. Claviridenone extracts also showed potent cytotoxicity against mouse lymphocytic leukemia (GlossaryTermP-388
) and human colon adenocarcinoma (GlossaryTermHT-29
), and exceptionally powerful cytotoxicity against human lung adenocarcinoma (GlossaryTermA549
) cells, with ED between and [55]. Claviridins A–D exhibited potent cytotoxicity against four human cancer cell lines: Hep2 (ED –), Doay (ED –), colon adenocarcinoma (WiDr, ED –), and GlossaryTermHeLa
(ED –) [103]. Halogenated prostanoids also showed cytotoxic activity against human T lymphocyte leukemia cells (GlossaryTermMOLT
-4, IC ), human colorectal adenocarcinoma (GlossaryTermDLD
-1, IC ), and human diploid lung fibroblast (IMR-90, IC ) cells [101]. The cyclopentenone prostanoid, bromovulone III is a promising marine natural compound for the treatment of prostate, colon, and hepatocellular carcinoma, which showed antitumor activity against human prostate (GlossaryTermPC
-3) and human colon (HT29) cancer cells at an IC of [164], and induced apoptotic signaling in a sequential manner in GlossaryTermHep3B
cells [171]. In the case of prostate cancer cells, this compound displayed an antitumor activity – times more effective than cyclopentenone prostaglandins (known to suppress tumor cell growth and to induce apoptosis in prostate cancer cells), by causing a rapid redistribution and clustering of Fas (member of the tumor necrosis factor (GlossaryTermTNF
) receptor superfamily). Apoptotic stimulation of Fas by specific ligand or antibodies caused the formation of a membrane-associated complex comprising Fas clustering) in GlossaryTermPC
-3 cells [172]. Clavularia viridis also produces steroids that show cytotoxic activity against human colorectal adenocarcinoma (GlossaryTermDLD
-1, IC) and also against human T lymphocyte leukemia cells (GlossaryTermMOLT
-4, IC ) in the case of yonarasterols [102]. Additionally, stoloniferone displayed potent cytotoxicity against mouse lymphocytic leukemia (GlossaryTermP-388
), human colon adenocarcinoma (GlossaryTermHT-29
), and human lung adenocarcinoma (GlossaryTermA549
) cells [55]. This species produces several compounds with antitumor activity in different types of human tumors, although more in vitro studies are needed to determine which compounds are potential anticancer agents. Clavularia koellikeri produces diterpenoids as secondary metabolites, which display cytotoxic activity against human colorectal adenocarcinoma (GlossaryTermDLD
-1, IC ) and strong growth inhibition against human T lymphocyte leukemia cells (GlossaryTermMOLT
-4, IC ) [99].In the genus Cespitularia, several interesting diterpenes of cembrane and neodolabellane skeletons have been identified. In Cespitularia hypotentaculata (family Xeniidae) a significant production of diterpenoids was detected. Cespitularin C exhibited potent cytotoxicity against mouse lymphocytic leukemia (GlossaryTerm
P-388
, ED ) and human lung adenocarcinoma (GlossaryTermA549
, ED ) cells, while cespitularin E exhibited potent cytotoxicity against human lung adenocarcinoma (GlossaryTermA549
, ED ) cell cultures [139]. A less active diterpene, Asterolaurin A, from Asterospicularia laurae (a species from the same family) exhibited cytotoxicity against human hepatocellular carcinoma (GlossaryTermHepG2
) cells with an IC of [138].Telesto riisei produces punaglandins, highly functional cyclopentadienone and cyclopentenone prostaglandins. Cyclopentenone prostaglandins have unique antineoplastic activity and are potent growth inhibitors in a variety of cultured cells. These punaglandins have been shown to inhibit P53 accumulation (a tumor suppressor protein) and ubiquitin isopeptidase activity (IC between and ) (enzyme involved in protein degradation system) in vitro and in vivo [105]. Since these proteasome inhibitors exhibit higher antiproliferative effects than other prostaglandins [173], they may represent a new class of potent cancer therapeutics.
Sea fans are well-known sources of compounds exhibiting significant biological activity [62]. Studies on Isis hippuris resulted in the isolation of a series of novel metabolites such as sesquiterpenes [124], steroids [174], A-nor-hippuristanol [127], and isishippuric acid B [127]. These compounds exhibit potent cytotoxicity against cancer cell lines of human hepatocellular carcinoma (GlossaryTerm
HepG2
and GlossaryTermHep3B
, IC –, and –, respectively) [127, 175], human breast carcinoma (GlossaryTermMCF
-7, IC – and GlossaryTermMDA-MB-231
, IC –) [175], mouse lymphocytic leukemia (GlossaryTermP-388
), human lung adenocarcinoma (GlossaryTermA549
), and human colon adenocarcinoma (GlossaryTermHT-29
) with ED of values less than [127, 174] and IC of [124]. Polyoxygenated steroids were also isolated from this species and showed moderate cytotoxicity against cultured NBT-T2 rat bladder epithelial cells (IC between and ) [122], GlossaryTermP-388
and GlossaryTermA549
cell lines (ED and , respectively), and inhibitory activity against GlossaryTermHCMV
(EC ) [123].Species from the genus Pseudopterogorgia (currently accepted as Antillogorgia sp.) are a rich source of unusual biologically active diterpenoids, sesquiterpenes, and polyhydroxylated steroids, which exhibit diverse structures [109, 176, 177]. A sample of the organic extract of Antillogorgia bipinnata (formerly Pseudopterogorgia bipinnata) was included in an initial screening carried out as part of an effort in the discovery of new antimalarial agents. This extract was found to be active in inhibiting the growth of Plasmodium falciparum (a protozoan parasite responsible for the most severe forms of malaria). Caucanolide A and D demonstrated significant in vitro antiplasmodial activity against chloroquine-resistant P. falciparum W2 (IC and IC , respectively) [110]. Three secosterols isolated from an unidentified gorgonian from genus Pseudopterogorgia inhibited human protein kinase C (GlossaryTerm
PKC
) α, βI, βII, γ, δ, , η, and ζ, with IC values in the range – [121]. GlossaryTermPKC
is a key player in cellular signal transduction and has been implicated in cancer, cardiovascular and renal disorders, immunosuppression, and autoimmune diseases such as rheumatoid arthritis [159]. Semisynthetic derivatives also showed a similar activity [121]. Promising antimicrobial substances were also reported from Antillogorgia rigida (formerly Pseudopterogorgia rigida) (e. g., curcuphenol) [118] and from Antillogorgia elisabethae (formerly Pseudopterogorgia elisabethae) (e. g., pseudopterosin X and Y) [111]. Ileabethoxazole, homopseudopteroxazole, caribenols A and B and elisapterosin B from A. elisabethae and bipinnapterolide B from Antillogorgia bipinnata inhibit Mycobacterium tuberculosis H37Rv at a concentration of [113, 115] (for elisapterosin B and homopseudopteroxazole) and at a concentration range of – [112, 114, 178] (for other compounds). In fact, the inhibition of Micobacterium tuberculosis H37Rv is within the range recorded for rifampin [112]. A. elisabethae and A. bipinnata also produce antituberculosis compounds. Bielschowskysin, a naturally occurring diterpene isolated from Antillogorgia kallos (formerly Pseudopterogorgia kallos) [117] and aberrarone isolated from A. elisabethae [116] exhibited antiplasmodial activity (IC ) when tested against P. falciparum. The first compound was also found to display strong and specific in vitro cytotoxicity against the EKVX non-small cell lung cancer (GlossaryTermGI
) and CAKI-1 renal cancer (GlossaryTermGI
) [117]. Bis(pseudopterane) amine from Antillogorgia acerosa (formerly Pseudopterogorgia acerosa) was found to exhibit selective activity against GlossaryTermHCT116
(IC ) cell lines [108]. Astrogorgol F, a secosteroid produced by Astrogorgia sp., showed significant inhibition against protein kinases IGF-1R (insulin-like growth factor receptor-1), SRC and GlossaryTermVEGF
-R2 with IC of , , and , respectively. These kinases are currently regarded as very important therapeutic targets for cancer. Protein IGF-1R activates crucial signaling pathways that benefit cancer cells. Inhibition of this protein function has shown to significantly decrease cancer cell proliferation and increase sensitivity to chemotherapy and radiation treatment. Kinase GlossaryTermVEGF
-R2 plays an important role in tumor angiogenesis, and its relevance as pharmacological target for the treatment of a large variety of solid cancers has been extensively described in the literature. The potent inhibitory activity of secosteroids toward GlossaryTermVEGF
-R2 indicates that they may induce the inhibition of tumor angiogenesis. SRC family kinases play a critical role in cell adhesion, invasion, proliferation, survival, and angiogenesis during tumor development. It was reported that the three kinds of kinases (SRC, GlossaryTermVEGF
-R2, and IGF-1R) involved in the different signaling pathways are influenced by crosstalk and interaction with each other [129].Fuscosides, originally isolated from Eunicea fusca [135], selectively and irreversibly inhibited leukotriene synthesis. Leukotrienes are molecules of the immune system that contribute to inflammation in asthma and allergic rhinitis and their production is usually related to histamine release [179]. Pharmacological studies indicated that fuscoside B inhibits the conversion of arachidonic acid (GlossaryTerm
AA
) to leukotriene B4 and C4 (LTB4 and LTC4) [135, 180] by inhibiting the 5-lipoxygenase (5-LO), in the case of LTB4 with an IC of [180]. These selective inhibitors of lipoxygenase isoforms can be useful as pharmacological agents, as nutraceuticals, or as molecular tools [159]. Fuscoside B and E were also used in the classical experiment of acute inflammation, the GlossaryTermTPA
-induced ear edema model, which allows evaluation of the anti-inflammatory properties of some natural products. The topical application on the mouse ear edema of the of these two compounds extracts showed high inflammation inhibition levels of and , respectively, when compared to the activity shown by the anti-inflammatory commercial drug indomethacin () used as reference [136]. A diterpenoid, dolabellane, and sesquiterpenoids metabolites isolated from Eunicea sp. displayed antiplasmodial activity against the malaria parasite P. falciparum W2 (chloroquine-resistant) strain, with IC values of , and IC values ranging from –, respectively [130, 134].The gorgonian Junceella fragilis produces secondary metabolites, frajunolides B and C, with anti-inflammatory effects towards superoxide anion generation and elastase release by human neutrophils, with an IC [106]. When properly stimulated, activated neutrophils secrete a series of cytotoxins, such as the superoxide anion (), a precursor of other reactive oxygen species (GlossaryTerm
ROS
), granule proteases, and bioactive lipids [181, 182]. The production of the superoxide anion is linked to the killing of invading microorganisms, but it can also directly or indirectly damage surrounding tissues. In contrast, neutrophil elastase is a major secreted product of stimulated neutrophils and a major contributor to the destruction of tissue in chronic inflammatory disease [183]. The anti-inflammatory butenolide lipide [184] from the gorgonian Euplexaura flava [137] can be currently synthesized, which opens the possibility of advancing into a new level of anti-inflammatory pharmaceuticals.Some of the most interesting compounds identified so far in the on-going search for new antifouling agents have been recorded in the order Gorgonacea. Notable examples of such compounds are juncin ZII from Junceella juncea [107], homarine from Leptogorgia virgulata and Leptogorgia setacea [119], and pukalide and epoxypukalide so far only recorded from Leptogorgia virgulata [120].
Species of the genus Briareum (family Briareidae) (which commonly exhibit an incrusting appearance rather than the fan-like shape of many gorgonians) are widely abundant in Indo-Pacific and Caribbean coral reefs. These organisms have been recognized as a valuable source of bioactive compounds with novel structural features. Briarane-related natural products are a good example of such promising compounds due to their structural complexity and biological activity [185, 186]. Briaexcavatin E, from Briareum excavata, also occasionally referred to as Briarium excavatum, inhibited human neutrophil elastase (GlossaryTerm
HNE
) release with an IC between – [96]. Briaexcavatolides L and P, diterpenoids from the same species, exhibited significant cytotoxicity against mouse lymphocytic leukemia (GlossaryTermP-388
) tumor cells with ED of [97] and [187], respectively. Brialalepolides B and C, from a non-identified Briarium species, reduced the expression of GlossaryTermCOX-2
in human colon adenocarcinoma (GlossaryTermRAW
) cells, as well as in murine macrophage cells. This is significant because the metabolic products of GlossaryTermCOX-2
have been implicated in the pathogenesis of colon cancer and other diseases. Additionally, mouse macrophages cells are used to test the effects of drugs on inflammation pathways. These data support the idea that briaranes such as brialalepolides B and C might be interesting candidates for therapeutic consideration as dual-acting cancer cell cytotoxins and inflammatory response inhibitors [94]. Diterpenoids produced from Briareum polyanthes (presently accepted as Briareum asbestinum), namely Briarellin D, K, and L, exhibited antimalarial activity against P. falciparum with an IC between – [95].2.1.2 Other Orders
Sea anemones (order Actiniaria) are a rich source of biologically-active proteins and polypeptides. Several cytolytic toxins, neuropeptides, and protease inhibitors have been identified from this group of organisms [56]. In addition to several equinatoxins, potent cytolytic proteins and an inhibitor of papain-like cysteine proteinases (equistatin) were isolated from the sea anemone Actinia equina [188]. Equistatin has been shown to be a very potent inhibitor of papain and a specific inhibitor of the aspartic proteinase cathepsin D [189]. While papain-like cysteine proteases have been implicated in various diseases of the central nervous system, such as brain tumors, Alzheimer’s disease, stroke, cerebral lesions, neurological autoimmune diseases, and certain forms of epilepsy [190], aspartic proteinase cathepsin D is involved in the pathogenesis of breast cancer [191] and possibly Alzheimer’s disease [192]. An acylamino acid, bunodosine (GlossaryTerm
BDS
) was isolated from the venom of the sea anemone, Bunodosoma cangicum. Intraplantar injection of GlossaryTermBDS
into the hind paw of a rat induced a potent analgesic effect. This effect was not altered by naloxone (an opioid receptor antagonist), but was completely reversed by methysergide (a serotonin receptor antagonist), indicating that the effect is mediated by activation of serotonin receptors [193].Cycloaplysinopsin C, a bis(indole) alkaloid isolated from Tubastraea sp. (order Scleractinia), was found to inhibit growth of two strains of P. falciparum, one chloroquine-sensitive (F32/Tanzania) and another chloroquine-resistant (FcB1/Colombia) with IC and , respectively [59]. Cladocorans A and B, isolated from Cladocora caespitosa (order Scleractinia) [57], are marine sesterterpenoids that possess a γ-hydroxybutenolide moiety, which is thought to be responsible for the biological activity of these compounds. The potent anti-inflammatory activity of these natural metabolites was attributed to the inhibition of secretory phospholipase A2 (sPLA2, IC –). Given the general role of inflammation in diseases that include bronchial asthma and rheumatoid arthritis, the identification and development of potent inhibitors of sPLA2 continues to be of great importance for the pharmaceutical industry, with this type of metabolite being of paramount importance for future research [58].
A sphingolipid, 2S*,3S*,(4E,8E)-2N-[35-tetradecanoyl]-4(E),8(E)-icosadiene-1, 3-diol and a steroid (22E)-methylcholesta-5,22-diene-1a,3b,7a-triol, were isolated from the black coral Antipathes dichotoma (order Antipatharia) and screened for antibacterial activity against Gram-positive and Gram-negative bacteria at concentration. Data obtained showed that the sphingolipid exhibits potent activity against Bacillus subtilis and Pseudomonas aeruginosa (GlossaryTerm
MIC
and , respectively), while the trihy droxy steroid showed potent activity against B. subtilis (GlossaryTermMIC
) [194].2.2 Class Hydrozoa
The class Hydrozoa includes 7 orders and nearly valid species (Table 35.1), some of which are solitary while others are colonial. Among the most emblematic species are probably hydroids and the Portuguese man-o-war (Physalia physalis). Despite the large number of species in the class Hydrozoa, only a few have yielded interesting marine natural products in the last decade.
Immune escape plays an important role in cancer progression and, although not completely understood, it has been proposed that indoleamine 2,3-dioxygenase (GlossaryTerm
IDO
) plays a central role in the evasion of T-cell-mediated immune rejection [195]. GlossaryTermIDO
catalyzes the oxidative cleavage of the ,-bond of tryptophan, which is the first and rate-limiting step in the kynurenine pathway of tryptophan catabolism in mammalian cells [196]. The polyketides annulins A, B, and C, purified from the marine hydroid Garveia annulata (order Anthoathecata), potently inhibited GlossaryTermIDO
in vitro (Ki –) [197]. These annulins are more powerful than most tryptophan analogs known to be GlossaryTermIDO
inhibitors. These compounds are active at concentrations higher than and are, therefore, more effective than 1-methyltryptophan (Ki ), one of the most potent GlossaryTermIDO
inhibitors currently available [198]. Solandelactones C, D, and G are cyclopropyl oxylipins isolated from the hydroid Solanderia secunda (order Anthoathecata) and exhibit moderate inhibitory activity against GlossaryTermFPT
(, , and inhibition, respectively) at a concentration of [199]. Note that GlossaryTermFPT
is associated with cell differentiation, and proliferation and its inhibition may be a target for novel anticancer agents (as was already mentioned above for the soft coral Lobophytum cristagalli).2.3 Class Scyphozoa
Approximately species are currently classified in the three orders of the class Scyphozoa (Table 35.1). However, in the last decade, only a single marine natural product was purified from the mesoglea of the jellyfish Aurelia aurita (order Semaeostomeae) and considered to be promising enough to be included in the present overview. This compound, aurelin, is a novel endogenous antibacterial peptide that exhibited activity against Gram-positive and Gram-negative bacteria. As an example, aurelin displayed an IC of for Escherichia coli (Gram-negative bacteria) [200].
2.4 Other Classes
Staurozoa, Cubozoa, and Polypodiozoa are the classes with the least number of species in the phylum Cnidaria (Table 35.1). This fact may explain the current lack of data on secondary metabolites produced by these organisms. It is possible that with growing bioprospecting efforts new compounds may be revealed once these cnidarian species are screened. Cubozoa (box jellies), for example, produce some of the most harmful cnidarian toxins known to humans [201].
3 Concluding Remarks and Future Challenges
The intense pressure to find and develop more profitable molecules for all sorts of industries continues to fuel bioprospecting efforts of marine invertebrates. While the phylum Cnidaria is not the most significantly bioprospected at present, this chapter shows that some cnidarian species are promising sources of marine bioactive compounds of medical, economic, and scientific interest. Green fluorescent protein (GlossaryTerm
GFP
), GPF-like proteins, red fluorescent, and orange fluorescent protein (GlossaryTermOPF
) are good examples of biotechnological metabolites derived from cnidarians that are currently employed as molecular biomarkers. They were first purified from a fluorescent hydrozoan medusa [202] and have since been recorded in other cnidarian species [203, 204, 205, 206, 207, 208].In the present survey of the most promising bioactive marine natural products from cnidarians, only about of extant cnidarian species are represented, with the class Anthozoa displaying by far the highest number of promising bioactive marine natural products () (Fig. 35.6). This result is probably due to the fact that this class displays the highest number of species in the phylum (Table 35.1). Additionally, many anthozoans occupy marine habitats that can be readily accessed for the collection of biomass (e. g., coral reefs and intertidal regions), which facilitates bioprospecting. Of all the compounds presented in this review, were detected in cnidarians collected from tropical waters (mostly from Southeast Asia and the Caribbean Sea), with the remaining recorded from species that are mostly present in temperate waters (e. g., European countries and Japan).
Marine bioactive compounds with high biotechnological potential studied from the phylum Cnidaria in the twenty-first century (after [28])
Antitumor drugs are the main area of interest in the screening of marine natural products from cnidarians (, Fig. 35.7). This is not surprising, as the major financial effort for the screening of new marine compounds is made by cancer research [209]. Terpenoids (terpenoids, diterpenoids, sesquiterpenoids, sesterterpenoids, cembranoids) [14] (Fig. 35.8) are the main chemistry group in the GlossaryTerm
MNP
s analyzed in this survey.Distribution in drug classes of marine bioactive compounds with high biotechnological potential studied from cnidarian species in the twenty-first century (after [28])
Distribution of chemistry classes of marine bioactive compounds with high biotechnological potential studied from cnidarian species in the twenty-first century (after [28])
Even though most pharmaceutical industries abandoned their natural product-based discovery programs over a decade ago, the lack of new compounds in their pipelines in some strategic areas (e. g., antibiotics) suggests that a renewed interest in this field is imminent. The establishment of small biotechnology companies can play a decisive role in the initial discovery of promising marine bioactive compounds, as these enterprises will work closely together with academics and governmental agencies to take the initial steps in the discovery of new chemical entities. Collaboration between private companies and public institutions can be of paramount importance for financial support in the discovery process. On the other hand, crude extracts and pure compounds produced by academic laboratories may be screened by diverse bioassays as part of broader collaboration programs, nationally and internationally, with private biotechnology companies. One challenge for universities is to devise mechanisms that protect intellectual property and simultaneously encourage partnerships with the private sector, by recognizing that the chances of a major commercial pay-off are small if drug discovery is pursued by a single institution [29].
The commercial use of some promising marine bioactive compounds isolated from cnidarians may still be several years away. However, new compounds other than toxins and venoms produced by members of this highly diverse group of marine invertebrates may soon be discovered in the ongoing quest for new GlossaryTerm
MNP
.As with most marine organisms, the bioprospecting of cnidarians has been mostly limited to shallow habitats accessible either by foot, snorkeling, or GlossaryTerm
SCUBA
diving. In recent years, with the advent of deep-sea exploration it has been possible to bioprospect several unique ecosystems that had remained inaccessible to researchers [210, 211]. Deep-sea habitats (including hydrothermal vents and cold seeps), as well as seamounts, are commonly colonized by unique cnidarian species [212, 213, 214] that exhibit remarkable adaptation to extreme environments [215] and are promising candidates for the discovery of new GlossaryTermMNP
s [216]. Marine biodiversity conservation has been capturing the growing attention of nations worldwide. The growing concerns towards the conservation of marine habitats are already conditioning the bioprospecting of coral reefs, deep sea and other endangered marine habitats [217, 218, 219]. Even so, it is likely that in the years to come bioprospecting for new marine natural products will continue to grow and that new cnidarian groups will be targeted by researchers.Modern screening techniques rely on the use of a significantly lower amount of biomass (micrograms) than what was required a decade ago for the discovery of new chemical compounds [18]. The incorporation of modern molecular biology and bioinformatics to complement the use of chemical approaches in the study of biosynthetic pathways has allowed researchers to make significant breakthroughs in the production of marine drugs [220]. Nonetheless, the production of such compounds at a commercial level is still a remarkable challenge. Large-scale production of a given compound can be possible either through chemical synthesis or through its extraction from the source organism. Unfortunately, the first option is not always possible, as several complex molecules are simply impossible to produce or incur production costs that cannot be afforded by commercial applications [221, 222]. The harvest of the source animal from the wild for the extraction of a compound is invariably an unsustainable practice and rarely a long-term option [219]. On the other hand, the production of source organism biomass (either in situ or ex situ) has been considered as a potential alternative to the collection of wild specimens [223]. Additionally, the production of source organisms under controlled conditions may help to control the ecophysiological diversity promoted by environmental interactions and maximize the production of target marine molecules. Unfortunately, the culture of most target organisms has turned out to be more technically challenging and significantly more expensive than initially assumed [224, 225].
There is growing evidence that microorganisms associated with marine invertebrates may be the true producers of some of the GlossaryTerm
MNP
s isolated from these animals and may even be responsible for the variation of chemical diversity at species level [226, 227, 228]. The microbiome present in marine invertebrates is likely to shift geographically [229, 230], which may enhance the production of different secondary metabolites. Whether this is also the case of most cnidarians remains to be confirmed [231]. Nonetheless, it has already been recognized that one of the promising compounds recorded from the gorgonian Antillogorgia elisabethae is, in fact, produced by its symbiotic dinoflagellates [232]. In this way, it is possible that future bioprospecting efforts may shift from invertebrate hosts towards symbiotic microorganisms. Under this scenario another constraint for the commercial use of these compounds must be overcome, as the culture of symbiotic microorganisms is generally not possible using classic/standardized methodologies. Once isolated from their host symbiotic microorganisms rarely thrive in vitro or no longer produce the desired compound [223, 224, 225, 226, 227, 228].The early impetuous of natural product-based discovery programs by pharmaceutical industries has decreased in the twenty-first century [233]. Nonetheless, the lack of new compounds in some of the strategic pipelines for drug discovery impels researchers to consider the new tools available for blue biotechnology [234] and the importance of joining efforts with academic institutions for the early stages of marine organisms bioprospecting. New drugs derived from GlossaryTerm
MNP
isolated from cnidarians may still be several years away, but it is unquestionable that more chemical entities, besides toxins and venoms, will be recorded from cnidarians [28]. In conclusion, this diverse group of marine invertebrates is destined to play a major role in the pursuit of new drugs from the sea.Abbreviations
- A549:
-
human alveolar epithelial cells
- AA:
-
arachidonic acid
- AGS:
-
human gastric cancer cell
- ATCC:
-
American Type Culture Collection
- BDS:
-
bunodosine
- CCRF-CEM:
-
human T-cell lymphoblast-like cell line
- COX-2:
-
cyclooxygenase-2
- COX:
-
cyclooxygenase
- Ca9-22:
-
human gingival carcinoma
- ChAT:
-
choline acetyltransferase
- DLD:
-
dihydrolipoamide dehydrogenase
- FPT:
-
farnesyl protein transferase
- GERD:
-
gastroesophageal reflux disease
- GFP:
-
green fluorescent protein
- GI :
-
growth inhibition
- GORD:
-
gastroesophageal reflux disease
- HCMV:
-
human cytomegalovirus
- HCT116:
-
human colorectal carcinoma cell line
- HCT:
-
human colorectal tumor
- HIV:
-
human immunodeficiency virus
- HNE:
-
human neutrophil elastase
- HT-29:
-
human colon adenocarcinoma
- HeLa:
-
human cervix carcinoma cells
- Hep3B:
-
human liver carcinoma
- HepG2:
-
hepatoma cell line
- IDO:
-
indoleamine 2,3-dioxygenase
- IMO:
-
International Maritime Organization
- K562:
-
human chronic myeloid leukemia cells
- KB:
-
human epidermoid carcinoma
- LPS:
-
lipopolysaccharide
- MCF:
-
Michigan Cancer Foundation-7
- MDA-MB-231:
-
human breast carcinoma
- MIC:
-
minimum inhibitory concentration
- MNP:
-
marine natural product
- MOLT:
-
molybdate uptake transporter
- NCI:
-
National Cancer Institute
- NO:
-
nitric oxide
- OPF:
-
orange fluorescent protein
- P-388:
-
mouse lymphocytic leukemia
- PAN:
-
plane polyacrylonitrile
- PBMC:
-
peripheral blood mononuclear cells
- PC:
-
phycocyanin
- PKC:
-
protein kinase C
- RAW:
-
ATCC cell line
- ROS:
-
reactive oxygen species
- SCUBA:
-
self-contained under water breathing apparatus
- TBT:
-
tri-n-butyltin
- TNF:
-
tumor necrosis factor
- TPA:
-
12-O-tetradecanoylphorbol-13-acetate
- VEGF:
-
vascular endothelial growth factor
- WoRMS:
-
World Register of Marine Species
- iNOS:
-
inducible nitric oxide synthase
References
W. Appeltans, P. Bouchet, G.A. Boxshall, C. De Broyer, N.J. de Voogd, D.P. Gordon, B.W. Hoeksema, T. Horton, M. Kennedy, J. Mees, G.C.B. Poore, G. Read, S. Stohr, T.C. Walter, M.J. Costello: World Register of Marine Species (2012), available online at http://www.marinespecies.org, last accessed: 14.02.2013
C.P. Hickman, L.S. Roberts, A. Larson, H. I'Anson: Integrated Principles of Zoology, 13th edn. (McGraw-Hill Education, Dubuque, Iowa 2007)
P. Tardent: The cnidarian cnidocyte, a hightech cellular weaponry, Bioessays 17, 351–362 (1995)
V.J. Paul, R. Ritson-Williams, K. Sharp: Marine chemical ecology in benthic environments, Nat. Prod. Rep. 28, 345–388 (2011)
B. Haefner: Drugs from the deep: Marine natural products as drug candidates, Drug Discov. Today 8, 536–544 (2003)
D.J. Faulkner: Highlights of marine natural products chemistry (1972–1999), Nat. Prod. Rep. 17, 1–6 (2000)
M.E. Hay: Marine Chemical Ecology: Chemical signals and cues structure marine populations, communities, and ecosystems, Ann. Rev. Mar. Sci. 1, 193–212 (2009)
M.C. Leal, J. Puga, J. Serôdio, N.C.M. Gomes, R. Calado: Trends in the discovery of new marine natural products from invertebrates over the last two decades – where and what are we bioprospecting?, PLoS One 7, e30580 (2012)
K.P. Sebens: Biodiversity of coral reefs: what are we losing and why?, Am. Zool. 34, 115–133 (1994)
N. Lindquist: Palatability of invertebrate larvae to corals and sea anemones, Mar. Biology 126, 745–755 (1996)
V.J. Paul, M.P. Puglisi: Chemical mediation of interactions among marine organisms, Nat. Prod. Rep. 21, 189–209 (2004)
A.M. Mayer, K.B. Glaser, C. Cuevas, R.S. Jacobs, W. Kem, R.D. Little, J.M. McIntosh, D.J. Newman, B.C. Potts, D.E. Shuster: The odyssey of marine pharmaceuticals: A current pipeline perspective, Trends Pharmacol. Sci. 31, 255–265 (2010)
W. Bergmann, D. Burke: Contributions to the study of marine products. XXXIX. The nucleosides of sponges. III. Spongothymidine and spongouridine, J. Org. Chem. 20, 1501–1507 (1955)
J.W. Blunt, M.H.G. Munro (Eds.): Dictionary of Marine Natural Products: Dictionary of Marine Natural Products (Chapman Hall/CRC, Boca Raton 2008), pp. 2108, 119
C. Avila, S. Taboada, L. Núñez-Pons: Antarctic marine chemical ecology: What is next?, Marine Ecol. 29, 1–71 (2008)
R. Osinga, J. Tramper: Cultivation of marine sponges for metabolite production: Applications for biotechnology?, Trends Biotechnol. 16, 130–134 (1998)
T. Turk, W.R. Kem: The phylum Cnidaria and investigations of its toxins and venoms until 1990, Toxicon 54, 1031–1037 (2009)
T.F. Molinski, D.S. Dalisay, S.L. Lievens, J.P. Saludes: Drug development from marine natural products, Nat. Rev. Drug Discov. 8, 69–85 (2009)
M.C. Leal, C. Madeira, C.A. Brandão, J. Puga, R. Calado: Bioprospecting of marine invertebrates for new natural products – a zoogeographical and chemical perspective, Molecules 17, 9842–9854 (2012)
S. Zwenger, C. Basu: Plant terpenoids: Applications and future potentials, Biotechnol. Mol. Biol. Rev. 3, 001–007 (2008)
M. Gavagnin, A. Fontana: Diterpenes from marine opisthobranch molluscs, Curr. Org. Chem. 4, 1201–1248 (2000)
G.-P. Hu, J. Yuan, L. Sun, Z.-G. She, J.-H. Wu, X.-J. Lan, X. Zhu, Y.-C. Lin, S.-P. Chen: Statistical research on marine natural products based on data obtained between 1985 and 2008, Mar. Drugs 9, 514–525 (2011)
T. Maimone, P. Baran: Modern synthetic efforts toward biologically active terpenes, Nat. Chem. Biol. 3, 396–407 (2007)
V.J. Paul: Ecological Roles of Marine Natural Products (Comstock Publications Association, Ithaca, New York 1992)
J. McClintock, B.J. Baker (Eds.): Marine Chemical Ecology (CRC, Boca Raton 2001)
V.J. Paul, K.E. Arthur, R. Ritson-Williams, C. Ross, K. Sharp: Chemical defenses: From compounds to communities, Biol. Bulletin 213, 226–251 (2007)
M. Munro, J.W. Blunt, E. Dumdei, S. Hickford, R. Lill, S. Li, C. Battershill, A. Duckworth: The discovery and development of marine compounds with pharmaceutical potential, J. Biotechnol. 70, 15–25 (1999)
J. Rocha, L. Peixe, N.C.M. Gomes, R. Calado: Cnidarians as a source of new marine bioactive compounds-an overview of the last decade and future steps for bioprospecting, Mar. Drugs 9, 1860–1886 (2011)
R.T. Hill, W. Fenical: Pharmaceuticals from marine natural products: Surge or ebb?, Curr. Opin. Biotechnol. 21, 777–779 (2010)
S.M. Paul, D.S. Mytelka, C.T. Dunwiddie, C.C. Persinger, B.H. Munos, S.R. Lindborg, A.L. Schacht: How to improve R&D productivity: The pharmaceutical industry's grand challenge, Nat. Rev. Drug Discov. 9, 203–214 (2010)
D.J. Faulkner: Marine natural products, Nat. Prod. Rep. 17, 7–55 (2000)
D.J. Faulkner: Marine natural products, Nat. Prod. Rep. 18, 1–49 (2001)
D.J. Faulkner: Marine natural products, Nat. Prod. Rep. 19, 1–48 (2002)
J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 20, 1–48 (2003)
J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 21, 1–49 (2004)
J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 22, 15–61 (2005)
J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 23, 26–78 (2006)
J.W. Blunt, B.R. Copp, W.P. Hu, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 24, 31–86 (2007)
J.W. Blunt, B.R. Copp, W.P. Hu, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 25, 35–94 (2008)
J.W. Blunt, B.R. Copp, W.P. Hu, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 26, 170–244 (2009)
J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 27, 165–237 (2010)
J.W. Blunt, B.R. Copp, M.H.G. Munro, P.T. Northcote, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 28, 196–268 (2011)
J.W. Blunt, B.R. Copp, R.A. Keyzers, M.H.G. Munro, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 29, 144–222 (2012)
J.W. Blunt, B.R. Copp, R.A. Keyzers, M.H.G. Munro, M.R. Prinsep: Marine natural products, Nat. Prod. Rep. 30, 237–323 (2013)
A. Mayer, M. Hamann: Marine Pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action, Mar. Biotechnol. 6, 37–52 (2004)
A.M.S. Mayer, M.T. Hamann: Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action, Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 140, 265–286 (2005)
A.M.S. Mayer, K.R. Gustafson: Marine pharmacology in 2001–2002: Antitumour and cytotoxic compounds, Eur. J. Cancer 40, 2676–2704 (2004)
A.M.S. Mayer, K.R. Gustafson: Marine pharmacology in 2003–2004: Anti-tumour and cytotoxic compounds, Eur. J. Cancer 42, 2241–2270 (2006)
A.M.S. Mayer, A.D. Rodríguez, R.G.S. Berlinck, M.T. Hamann: Marine pharmacology in 2003–2004: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action, Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 145, 553–581 (2007)
A.M.S. Mayer, K.R. Gustafson: Marine pharmacology in 2005–2006: Antitumour and cytotoxic compounds, Eur. J. Cancer 44, 2357–2387 (2008)
A.M.S. Mayer, A.D. Rodriguez, R.G.S. Berlinck, M.T. Hamann: Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action, Biochim. Biophys. Acta - Gen. Subj. 1790, 283–308 (2009)
A.M.S. Mayer, A.D. Rodríguez, R.G.S. Berlinck, N. Fusetani: Marine pharmacology in 2007–2008: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action, Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 153, 191–222 (2011)
M.R. Boyd, K.D. Paull, L.R. Rubinstein: Data display and analysis strategies for the NCI disease-oriented in vitro antitumor drug screen. In: Cytotoxic Anticancer Drugs: Models and Concepts for Drug Discovery and Development, (Springer, Dordrecht 1992) p. 20
A.A.H. El-Gamal, C.Y. Chiang, S.H. Huang, S.K. Wang, C.Y. Duh: Xenia diterpenoids from the Formosan soft coral Xenia blumi, J. Nat. Prod. 68, 1336–1340 (2005)
C.Y. Duh, A.A.H. El-Gamal, C.J. Chu, S.K. Wang, C.F. Dai: New cytotoxic constituents from the Formosan soft corals Clavularia viridis and Clavularia violacea, J. Nat. Prod. 65, 1535–1539 (2002)
B. Strukelj, B. Lenarcic, K. Gruden, J. Pungercar, B. Rogelj, V. Turk, D. Bosch, M.A. Jongsma: Equistatin, a protease inhibitor from the sea anemone Actinia equina, is composed of three structural and functional domains, Biochem. Biophys. Res. Commun. 269, 732–736 (2000)
A. Fontana, M.L. Ciavatta, G. Cimino: Cladocoran A and B: Two novel γ-hydroxybutenolide sesterterpenes from the Mediterranean coral cladocora cespitosa, J. Org. Chem. 63, 2845–2849 (1998)
H. Miyaoka, M. Yamanishi, H. Mitome: PLA${}_{{2}}$ inhibitory activity of marine sesterterpenoids cladocorans, their diastereomers and analogues, Chem. Pharm. Bull. 54, 268–270 (2006)
M. Meyer, F. Delberghe, F. Liron, M. Guillaume, A. Valentin, M. Guyot: An antiplasmodial new (bis)indole alkaloid from the hard coral Tubastraea sp., Nat. Prod. Res. 23, 178–182 (2009)
G.J. Hooper, M.T. Davies-Coleman: New metabolites from the south african soft coral capnella thyrsoidea, Tetrahedron 51, 9973–9984 (1995)
D.S. Bhakuni, D.S. Rawat: Bioactive Marine Natural Products (Springer, New York 2005) p. 396
X.Y. Chai, J.F. Sun, L.Y. Tang, X.W. Yang, Y.Q. Li, H. Huang, X.F. Zhou, B. Yang, Y.H. Liu: A novel cyclopentene derivative and a polyhydroxylated steroid from a South China sea gorgonian Menella sp., Chem. Pharm. Bull. 58, 1391–1394 (2010)
Y.-H. Chen, C.-Y. Tai, T.-L. Hwang, C.-F. Weng, J.-J. Li, L.-S. Fang, W.-H. Wang, Y.-C. Wu, P.-J. Sung: Cladielloides A and B: New eunicellin-type diterpenoids from an Indonesian octocoral cladiella sp, Mar. Drugs 8, 2936–2945 (2010)
Y.-H. Chen, C.-Y. Tai, Y.-H. Kuo, C.-Y. Kao, J.-J. Li, T.-L. Hwang, L.-S. Fang, W.-H. Wang, J.-H. Sheu, P.-J. Sung: Cladieunicellins A-E, new eunicellins from an Indonesian soft coral cladiella sp, Chem. Pharm. Bull. 59, 353–358 (2011)
Z.-H. Wen, C.-H. Chao, M.-H. Wu, J.-H. Sheu: A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue, Eur. J. Med. Chem. 45, 5998–6004 (2010)
B.-W. Chen, S.-M. Chang, C.-Y. Huang, C.-H. Chao, J.-H. Su, Z.-H. Wen, C.-H. Hsu, C.-F. Dai, Y.-C. Wu, J.-H. Sheu: Hirsutalins A–H, eunicellin-based diterpenoids from the soft coral cladiella hirsuta, J. Nat. Prod. 73, 1785–1791 (2010)
S.-L. Wu, J.-H. Su, Z.-H. Wen, C.-H. Hsu, B.-W. Chen, C.-F. Dai, Y.-H. Kuo, J.-H. Sheu: Simplexins A-I, eunicellin-based diterpenoids from the soft coral Klyxum simplex, J. Nat. Prod. 72, 994–1000 (2009)
B.W. Chen, Y.C. Wu, M.Y. Chiang, J.H. Su, W.H. Wang, T.Y. Fan, J.H. Sheu: Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex, Tetrahedron 65, 7016–7022 (2009)
B.-W. Chen, C.-H. Chao, J.-H. Su, Z.-H. Wen, P.-J. Sung, J.-H. Sheu: Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex, Org. Biomol. Chem. 8, 2363–2366 (2010)
B.-W. Chen, C.-H. Chao, J.-H. Su, C.-W. Tsai, W.-H. Wang, Z.-H. Wen, C.-Y. Huang, P.-J. Sung, Y.-C. Wu, J.-H. Sheu: Klysimplexins I-T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex, Org. Biomol. Chem. 9, 834–844 (2011)
F.-J. Hsu, B.-W. Chen, Z.-H. Wen, C.-Y. Huang, C.-F. Dai, J.-H. Su, Y.-C. Wu, J.-H. Sheu: Klymollins A-H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle, J. Nat. Prod. 74, 2467–2471 (2011)
H.T. Nguyen, V.M. Chau, V.K. Phan, T.H. Hoang, H.N. Nguyen, X.C. Nguyen, H.Q. Tran, X.N. Nguyen, J.H. Hyun, H.K. Kang, Y.H. Kim: Chemical components from the Vietnamese soft coral lobophytum sp., Arch. Pharm. Res. 33, 503–508 (2010)
M.A. Rashid, K.R. Gustafson, M.R. Boyd: HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species, J. Nat. Prod. 63, 531–533 (2000)
C.-H. Chao, Z.-H. Wen, Y.-C. Wu, H.-C. Yeh, J.-H. Sheu: Cytotoxic and anti-inflammatory cembranoids from the soft coral lobophytum crassum, J. Nat. Prod. 71, 1819–1824 (2008)
S.T. Lin, S.K. Wang, C.Y. Duh: Cembranoids from the dongsha atoll soft coral lobophytum crassum, Mar. Drugs 9, 2705–2716 (2011)
C.-Y. Kao, J.-H. Su, M.-C. Lu, T.-L. Hwang, W.-H. Wang, J.-J. Chen, J.-H. Sheu, Y.-H. Kuo, C.-F. Weng, L.-S. Fang, Z.-H. Wen, P.-J. Sung: Lobocrassins A-E: New cembrane-type diterpenoids from the soft coral lobophytum crassum, Mar. Drugs 9, 1319–1331 (2011)
N.-L. Lee, J.-H. Su: Tetrahydrofuran cembranoids from the cultured soft coral lobophytum crassum, Mar. Drugs 9, 2526–2536 (2011)
S.J. Coval, R.W. Patton, J.M. Petrin, L. James, M.L. Rothofsky, S.L. Lin, M. Patel, J.K. Reed, A.T. McPhail, W.R. Bishop: A cembranolide diterpene farnesyl protein transferase inhibitor from the marine soft coral lobophytum cristagalli, Bioorg. Med. Chem. Lett. 6, 909–912 (1996)
S.-Y. Cheng, Z.-H. Wen, S.-F. Chiou, C.-H. Hsu, S.-K. Wang, C.-F. Dai, M.Y. Chiang, C.-Y. Duh: Durumolides A-E, anti-inflammatory and antibacterial cembranolides from the soft coral Lobophytum durum, Tetrahedron 64, 9698–9704 (2008)
S.-Y. Cheng, Z.-H. Wen, S.-K. Wang, S.-F. Chiou, C.-H. Hsu, C.-F. Dai, M.Y. Chiang, C.-Y. Duh: Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral lobophytum durum, J. Nat. Prod. 72, 152–155 (2009)
S.-Y. Cheng, P.-W. Chen, H.-P. Chen, S.-K. Wang, C.-Y. Duh: New cembranolides from the dongsha atoll soft coral lobophytum durum, Mar. Drugs 9, 1307–1318 (2011)
Q. Tran Hong, H. Tran Thu, M. Van Chau, K. Van Phan, H. Hoang Thanh, N. Nguyen Thi Thanh, N. Nguyen Xuan, T. Nguyen Huu, T. Nguyen Phuong, T. Dinh Thi Thu, S.B. Song, H.-J. Boo, H.-K. Kang, Y.H. Kim: Cytotoxic and PPARs transcriptional activities of sterols from the Vietnamese soft coral Lobophytum laevigatum, Bioorg. Med. Chem. Lett. 21, 2845–2849 (2011)
P. Yan, Z. Deng, L. van Ofwegen, P. Proksch, W. Lin: Lobophytones U-Z(1), Biscembranoids from the Chinese soft coral lobophytum pauciflorum, Chem. Biodivers. 8, 1724–1734 (2011)
H.-C. Huang, C.-H. Chao, Y.-H. Kuo, J.-H. Sheu: Crassocolides G-M, cembranoids from the Formosan soft coral sarcophyton crassocaule, Chem. Biodiversity 6, 1232–1242 (2009)
G.-H. Wang, H.-C. Huang, J.-H. Su, C.-Y. Huang, C.-H. Hsu, Y.-H. Kuo, J.-H. Sheu: Crassocolides N-P, three cembranoids from the Formosan soft coral Sarcophyton crassocaule, Bioorg. Med. Chem. Lett. 21, 7201–7204 (2011)
W.-Y. Lin, Y. Lu, J.-H. Su, Z.-H. Wen, C.-F. Dai, Y.-H. Kuo, J.-H. Sheu: Bioactive cembranoids from the dongsha atoll soft coral sarcophyton crassocaule, Mar. Drugs 9, 994–1006 (2011)
N. Fusetani: Research toward drugs from the sea, New J. Chem. 14, 721–728 (1990)
A.S. Dmitrenok, P. Radhika, V. Anjaneyulu, S. Subrahmanyam, P.V.S. Rao, P.S. Dmitrenok, V.M. Boguslavsky: New lipids from the soft corals of the Andaman Islands, Russ. Chem. Bull. 52, 1868–1872 (2003)
S.-Y. Cheng, K.-J. Huang, S.-K. Wang, C.-Y. Duh: Capilloquinol: A novel farnesyl quinol from the dongsha atoll soft coral Sinularia capillosa, Mar. Drugs 9, 1469–1476 (2011)
Y.-S. Lin, C.-H. Chen, C.-C. Liaw, Y.-C. Chen, Y.-H. Kuo, Y.-C. Shen: Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis, Tetrahedron 65, 9157–9164 (2009)
K. Michalek, B.F. Bowden: A natural algacide from soft coral Sinularia flexibilis (Coelenterata, Octocorallia, Alcyonacea), J. Chem. Ecol. 23, 259–273 (1997)
A.F. Ahmed, Y.-T. Hsieh, Z.-H. Wen, Y.-C. Wu, J.-H. Sheu: Polyoxygenated sterols from the Formosan soft coral Sinularia gibberosa, J. Nat. Prod. 69, 1275–1279 (2006)
Y. Lu, C.Y. Huang, Y.F. Lin, Z.H. Wen, J.H. Su, Y.H. Kuo, M.Y. Chiang, J.H. Sheu: Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa, J. Nat. Prod. 71, 1754–1759 (2008)
P.M. Joyner, A.L. Waters, R.B. Williams, D.R. Powell, N.B. Janakiram, C.V. Rao, R.H. Cichewicz: Briarane diterpenes diminish cox-2 expression in human colon adenocarcinoma cells, J. Nat. Prod. 74, 857–861 (2011)
C.A. Ospina, A.D. Rodriguez, E. Ortega-Barria, T.L. Capson: Briarellins J-P and polyanthellin A: New eunicellin-based diterpenes from the gorgonian coral Briareum polyanthes and their antimalarial activity, J. Nat. Prod. 66, 357–363 (2003)
P.-J. Sung, Y.-P. Chen, T.-L. Hwang, W.-P. Hu, L.-S. Fang, Y.-C. Wu, J.-J. Li, J.-H. Sheu: Briaexcavatins C-F, four new briarane-related diterpenoids from the Formosan octocoral Briareum excavatum (Briareidae), Tetrahedron 62, 5686–5691 (2006)
P.J. Sung, J.H. Su, C.Y. Duh, M.Y. Chiang, J.H. Sheu: Briaexcavatolides K-N, new briarane diterpenes from the gorgonian Briareum excavatum, J. Nat. Prod. 64, 318–323 (2001)
T. Yabe, H. Yamada, M. Shimomura, H. Miyaoka, Y. Yamada: Induction of choline acetyltransferase activity in cholinergic neurons by stolonidiol: Structure-activity relationship, J. Nat. Prod. 63, 433–435 (2000)
M. Iwashima, Y. Matsumoto, H. Takahashi, K. Iguchi: New marine cembrane-type diterpenoids from the Okinawan soft coral Clavularia koellikeri, J. Nat. Prod. 63, 1647–1652 (2000)
Y.S. Lin, A.T. Khalil, S.H. Chiou, Y.C. Kuo, Y.B. Cheng, C.C. Liaw, Y.C. Shen: Bioactive marine prostanoids from octocoral clavularia viridis, Chem. Biodiversity 5, 784–792 (2008)
K. Watanabe, M. Sekine, H. Takahashi, K. Iguchi: New halogenated marine prostanoids with cytotoxic activity from the Okinawan soft coral Clavularia viridis, J. Nat. Prod. 64, 1421–1425 (2001)
M. Iwashima, K. Nara, Y. Nakamichi, K. Iguchi: Three new chlorinated marine steroids, yonarasterols G, H and I, isolated from the Okinawan soft coral, clavularia viridis, Steroids 66, 25–32 (2001)
Y.-C. Shen, K.-L. Lo, J.-Y. Chang, Y.-S. Lin, K. Mendbayar, Y.-H. Kuo, Y.-C. Lin: New cytotoxic prostanoids from Taiwanese soft coral clavularia viridis, Chem. Biodiversity 7, 2702–2708 (2010)
C.-Y. Liu, T.-L. Hwang, M.-R. Lin, Y.-H. Chen, Y.-C. Chang, L.-S. Fang, W.-H. Wang, Y.-C. Wu, P.-J. Sung: Carijoside A, a bioactive sterol glycoside from an octocoral carijoa sp. (Clavulariidae), Mar. Drugs 8, 2014–2020 (2010)
S.M. Verbitski, J.E. Mullally, F.A. Fitzpatrick, C.M. Ireland: Punaglandins, chlorinated prostaglandins, function as potent Michael receptors to inhibit ubiquitin isopeptidase activity, J. Med. Chem. 47, 2062–2070 (2004)
Y.-C. Shen, Y.-H. Chen, T.-L. Hwang, J.-H. Guh, A.T. Khalil: Four new briarane diterpenoids from the gorgonian coral junceella fragilis, Helv. Chim. Acta 90, 1391–1398 (2007)
S.H. Qi, S. Zhang, P.Y. Qian, H.H. Xu: Antifeedant and antifouling briaranes from the South China Sea gorgonian junceella juncea, Chem. Nat. Compd. 45, 49–54 (2009)
A.S. Kate, J.K. Pearson, B. Ramanathan, K. Richard, R.G. Kerr: Isolation, biomimetic synthesis, and cytotoxic activity of bis(pseudopterane) amines, J. Nat. Prod. 72, 1331–1334 (2009)
C.A. Ospina, A.D. Rodriguez, H. Zhao, R.G. Raptis: Bipinnapterolide B, a bioactive oxapolycyclic diterpene from the Colombian gorgonian coral Pseudopterogorgia bipinnata, Tetrahedron Lett. 48, 7520–7523 (2007)
C.A. Ospina, A.D. Rodríguez, J.A. Sánchez, E. Ortega-Barria, T.L. Capson, A.M.S. Mayer: Caucanolides A$-$F, Unusual Antiplasmodial Constituents from a Colombian Collection of the Gorgonian Coral Pseudopterogorgia bipinnata, J. Nat. Prod. 68, 1519–1526 (2005)
A. Ata, H.Y. Win, D. Holt, P. Holloway, E.P. Segstro, G.S. Jayatilake: New antibacterial diterpenes from pseudopterogorgia elisabethae, Helv. Chim. Acta 87, 1090–1098 (2004)
I.I. Rodríguez, A.D. Rodríguez, Y. Wang, S.G. Franzblau: Ileabethoxazole: A novel benzoxazole alkaloid with antimycobacterial activity, Tetrahedron Lett. 47, 3229–3232 (2006)
I.I. Rodriguez, A.D. Rodriguez: Homopseudopteroxazole, a new antimycobacterial diterpene alkaloid from Pseudopterogorgia elisabethae, J. Nat. Prod. 66, 855–857 (2003)
X. Wei, I.I. Rodriguez, A.D. Rodriguez, C.L. Barnes: Caribenols A and B, sea whip derived norditerpenes with novel tricarbocyclic skeletons, J. Org. Chem. 72, 7386–7389 (2007)
A.D. Rodriguez, C. Ramirez, I.I. Rodriguez, C.L. Barnes: Novel terpenoids from the West Indian sea whip Pseudopterogorgia elisabethae (Bayer). Elisapterosins A and B: Rearranged diterpenes possessing an unprecedented cagelike framework, J. Org. Chem. 65, 1390–1398 (2000)
I.I. Rodriguez, A.D. Rodriguez, H. Zhao: Aberrarone: A Gorgonian-Derived Diterpene from Pseudopterogorgia elisabethae, J. Org. Chem. 74, 7581–7584 (2009)
J. Marrero, A.D. Rodriguez, P. Baran, R.G. Raptis, J.A. Sanchez, E. Ortega-Barria, T.L. Capson: Bielschowskysin, a gorgonian-derived biologically active diterpene with an unprecedented carbon skeleton, Org. Letters 6, 1661–1664 (2004)
F.J. McEnroe, W. Fenical: Structures and synthesis of some new antibacterial sesquiterpenoids from the gorgonian coral Pseudopterogorgia rigida, Tetrahedron 34, 1661–1664 (1978)
N.M. Targett, S.S. Bishop, O.J. McConnell, J.A. Yoder: Antifouling agents against the benthic marine diatom, Navicula salinicola: homarine from the gorgonians Leptogorgia virgulata and L. setacea and analogs, J. Chem. Ecol. 9, 817–829 (1983)
D.J. Gerhart, D. Rittschof, S.W. Mayo: Chemical ecology and the search for marine antifoulants – Studies of a predatory-prey symbiosis, J. Chem. Ecol. 14, 1905–1917 (1988)
H.Y. He, P. Kulanthaivel, B.J. Baker, K. Kalter, J. Darges, D. Cofield, L. Wolff, L. Adams: New antiproliferative and antiinflammatory 9,11-secosterols from the gorgonian Pseudopterogorgia sp, Tetrahedron 51, 51–58 (1995)
M.H. Uddin, N. Hanif, A. Trianto, Y. Agarie, T. Higa, J. Tanaka: Four new polyoxygenated gorgosterols from the gorgonian Isis hippuris, Nat. Prod. Res. 25, 585–591 (2011)
W.-H. Chen, S.-K. Wang, C.-Y. Duh: Polyhydroxylated steroids from the octocoral Isis hippuris, Tetrahedron 67, 8116–8119 (2011)
J.-H. Sheu, K.-C. Hung, G.-H. Wang, C.-Y. Duh: New cytotoxic sesquiterpenes from the gorgonian isis hippuris, J. Nat. Prod. 63, 1603–1607 (2000)
Y. Lu, P.J. Li, W.Y. Hung, J.H. Su, Z.H. Wen, C.H. Hsu, C.F. Dai, M.Y. Chiang, J.H. Sheu: Nardosinane sesquiterpenoids from the Formosan soft coral lemnalia flava, J. Nat. Prod. 74, 169–174 (2011)
S.-Y. Cheng, K.-J. Huang, S.-K. Wang, Z.-H. Wen, C.-H. Hsu, C.-F. Dai, C.-Y. Duh: New terpenoids from the soft corals Sinularia capillosa and nephthea chabroli, Org. Lett. 11, 4830–4833 (2009)
J.H. Sheu, C.H. Chao, G.H. Wang, K.C. Hung, C.Y. Duh, M.Y. Chiang, Y.C. Wu, C.C. Wu: The first A-nor-hippuristanol and two novel 4,5-secosuberosanoids from the gorgonian isis hippuris, Tetrahedron Lett. 45, 6413–6416 (2004)
Y. Tomono, H. Hirota, N. Fusetani: Isogosterones A-D, antifouling 13,17-Secosteroids from an octocoral Dendronephthya sp, J. Org. Chem. 64, 2272–2275 (1999)
D. Lai, S. Yu, L. van Ofwegen, F. Totzke, P. Proksch, W. Lin: 9,10-Secosteroids, protein kinase inhibitors from the Chinese gorgonian astrogorgia sp, Bioorg. Med. Chem. 19, 6873–6880 (2011)
S.P. Garzón, A.D. Rodríguez, J.A. Sánchez, E. Ortega-Barria: Sesquiterpenoid metabolites with antiplasmodial activity from a Caribbean gorgonian coral, eunicea sp, J. Nat. Prod. 68, 1354–1359 (2005)
S.-Y. Cheng, Z.-H. Wen, S.-K. Wang, M.Y. Chiang, A.A.H. El-Gamal, C.-F. Dai, C.-Y. Duh: Revision of the absolute configuration at C(23) of lanostanoids and isolation of secondary metabolites from Formosan soft coral nephthea erecta, Chem. Biodiversity 6, 86–95 (2009)
C.-Y. Huang, J.-H. Su, B.-W. Chen, Z.-H. Wen, C.-H. Hsu, C.-F. Dai, J.-H. Sheu, P.-J. Sung: Nardosinane-type sesquiterpenoids from the Formosan soft coral paralemnalia thyrsoides, Mar. Drugs 9, 1543–1553 (2011)
C.-H. Gao, Y.-F. Wang, S. Li, P.-Y. Qian, S.-H. Qi: Alkaloids and sesquiterpenes from the South China Sea gorgonian echinogorgia pseudossapo, Mar. Drugs 9, 2479–2487 (2011)
X. Wei, A.D. Rodriìguez, P. Baran, R.G. Raptis: Dolabellane-type diterpenoids with antiprotozoan activity from a southwestern Caribbean gorgonian octocoral of the genus eunicea, J. Nat. Prod. 73, 925–934 (2010)
J.H. Shin, W. Fenical: Fuscosides A-D: Antinflammatory diterpenoid glycosides of new structural classes from the Caribbean gorgonian Eunicea fusca, J. Org. Chem. 56, 3153–3158 (1991)
E. Reina, C. Puentes, J. Rojas, J. Garcia, F.A. Ramos, L. Castellanos, M. Aragon, L.F. Ospina: Fuscoside E: A strong anti-inflammatory diterpene from Caribbean octocoral eunicea fusca, Bioorg. Med. Chem. Lett. 21, 5888–5891 (2011)
H. Kikuchi, Y. Tsukitani, H. Nakanishi, I. Shimizu, S. Saitoh, K. Iguchi, Y. Yamada: New butenolides from the gorgonian euplexaura flava (Nutting), Chem. Lett. 11, 233–236 (1982)
Y.-C. Lin, M.H. Abd El-Razek, T.-L. Hwang, M.Y. Chiang, Y.-H. Kuo, C.-F. Dai, Y.-C. Shen: Asterolaurins A-F, xenicane diterpenoids from the Taiwanese soft coral asterospicularia laurae, J. Nat. Prod. 72, 1911–1916 (2009)
C.Y. Duh, A.A.H. El-Gamal, S.K. Wang, C.F. Dai: Novel terpenoids from the Formosan soft coral cespitularia hypotentaculata, J. Nat. Prod. 65, 1429–1433 (2002)
A. Bishara, A. Rudi, I. Goldberg, Y. Benayahu, Y. Kashman: Novaxenicins A-D and xeniolides I-K, seven new diterpenes from the soft coral Xenia novaebrittanniae, Tetrahedron 62, 12092–12097 (2006)
G.M. Konig, A.D. Wright: New cembranoid diterpenes from the soft coral Sarcophyton ehrenbergi, J. Nat. Prod. 61, 494–496 (1998)
C.H. Chang, Z.H. Wen, S.K. Wang, C.Y. Duh: Capnellenes from the Formosan soft coral capnella imbricata, J. Nat. Prod. 71, 619–621 (2008)
D. Grote, F. Hanel, H.M. Dahse, K. Seifert: Capnellenes from the soft coral Dendronephthya rubeola, Chem. Biodiversity 5, 1683–1693 (2008)
C.Y. Duh, R.S. Hou: Cytotoxic cembranoids from the soft corals Sinularia gibberosa and sarcophyton trocheliophorum, J. Nat. Prod. 59, 595–598 (1996)
J.H. Su, A.F. Ahmed, P.J. Sung, C.H. Chao, Y.H. Kuo, J.H. Sheu: Manaarenolides A-I, diterpenoids from the soft coral Sinularia manaarensis, J. Nat. Prod. 69, 1134–1139 (2006)
R.S. Norton, R. Kazlauskas: C-13 NMR-study of flexibilide, an anti-inflammatory agent from a soft cora, Experientia 36, 276–278 (1980)
D.H. Williams, D.J. Faulkner: Two practical syntheses of an anti-inflammatory sesquiterpene furoic acid from Sinularia spp., Tetrahedron 52, 4245–4256 (1996)
A.J. Weinheimer, J.A. Matson, M.B. Hossain, D. van der Helm: Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral, Sinularia flexibilis, Tetrahedron Lett. 18, 2923–2926 (1977)
G.Q. Li, Y.L. Zhang, Z.W. Deng, L.P. van Ofwegen, P. Proksch, W.H. Lin: Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa, J. Nat. Prod. 68, 649–652 (2005)
T.L. Aceret, J.C. Coll, Y. Uchio, P.W. Sammarco: Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis quoy and gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia), Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 120, 121–126 (1998)
T.L. Aceret, L. Brown, J. Miller, J.C. Coll, P.W. Sammarco: Cardiac and vascular responses of isolated rat tissues treated with diterpenes from Sinularia flexibilis (Coelenterata: Octocorallia), Toxicon 34, 1165–1171 (1996)
Y.M. Sheikh, G. Singy, M. Kaisin, H. Eggert, C. Djerassi, B. Tursch, D. Daloze, J.C. Braekman: Terpenoids–LXXI: Chemical studies of marine invertebrates–XIV. Four representatives of a novel sesquiterpene class–the capnellane skeleton, Tetrahedron 32, 1171–1178 (1976)
M. Kaisin, Y.M. Sheikh, L.J. Durham, C. Djerassi, B. Tursch, D. Daloze, J.C. Braekman, D. Losman, R. Karlsson: Capnellane – a new tricyclic sesquiterpene skeleton from the soft coral Capnella imbricata, Tetrahedron Lett. 15, 2239–2242 (1974)
M. Kaisin, J.C. Braekman, D. Daloze, B. Tursch: Novel acetoxycapnellenes from the alcyonacean capnella imbricata, Tetrahedron 41, 1067–1072 (1985)
K. Peukert, P. Staller, A. Schneider, G. Carmichael, F. Hanel, M. Eilers: An alternative pathway for gene regulation by Myc, EMBO Journal 16, 5672–5686 (1997)
H. Hermeking: The MYC oncogene as a cancer drug target., Curr. Cancer Drug Targets 3, 163–175 (2003)
H.C. Huang, Z.H. Wen, C.H. Chao, A.F. Ahmed, M.Y. Chiang, Y.H. Kuo, C.H. Hsu, J.H. Sheu: Novel sesquiterpenoids from the Formosan soft coral paralemnalia thyrsoides, Tetrahedron Lett. 47, 8751–8755 (2006)
S. Cheenpracha, E.-J. Park, B. Rostama, J. Pezzuto, L.C. Chang: Inhibition of Nitric Oxide (NO) Production in Lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells by the norsesterterpene peroxide, epimuqubilin A, Mar. Drugs 8, 429–437 (2010)
Y. Nakao, N. Fusetani: Enzyme inhibitors from marine invertebrates, J. Nat. Prod. 70, 689–710 (2007)
N.U. Sata, M. Sugano, S. Matsunaga, N. Fusetani: Sinulamide: an H,K-ATPase inhibitor from a soft coral Sinularia sp., Tetrahedron Lett. 40, 719–722 (1999)
J.C. Coll: The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia), Chem. Rev. 92, 613–631 (1992)
M. Maida, P.W. Sammarco, J.C. Coll: A diffusion chamber for assessing efficacy of natural anti-fouling defenses in marine organisms, J. Exp. Mar. Biol. Ecol. 337, 59–64 (2006)
L.D. Chambers, K.R. Stokes, F.C. Walsh, R.J.K. Wood: Modern approaches to marine antifouling coatings, Surf. Coat. Technol. 201, 3642–3652 (2006)
Y.C. Shen, Y.B. Cheng, Y.C. Lin, J.H. Guh, C.M. Teng, C.L. Ko: New prostanoids with cytotoxic activity from Taiwanese octocoral clavularia viridis, J. Nat. Prod. 67, 542–546 (2004)
K. Watanabe, M. Iwashima, K. Iguchi: New marine prostanoid carboxylate salts from the Okinawan soft coral Clavularia viridis, J. Nat. Prod. 59, 980–982 (1996)
M. Iwashima, I. Terada, K. Okamoto, K. Iguchi: Tricycloclavulone and clavubicyclone, novel prostanoid-related marine oxylipins, isolated from the Okinawan soft coral Clavularia viridis, J. Org. Chem. 67, 2977–2981 (2002)
T. Kusumi, T. Hamada, M. Hara, M.O. Ishitsuka, H. Ginda, H. Kakisawa: Structure and absolute-configuration of Isoclavukerin-A, a component from an Okinawan soft coral, Tetrahedron Lett. 33, 2019–2022 (1992)
P. Davies, A.J.F. Maloney: Selective loss of central cholinergic neurons in Alzheimer's disease, The Lancet 2, 1403–1403 (1976)
P.J. Whitehouse, D.L. Price, R.G. Struble, A.W. Clark, J.T. Coyle, M.R. Delong: Alzheimer's disease and senile dementia: Loss of neurons in the basal forebrain, Science 215, 1237–1239 (1982)
R.T. Bartus, R.L. Dean, B. Beer, A.S. Lippa: The cholinergic hypothesis of geriatric memory dysfunction, Science 217, 408–417 (1982)
P.C. Chiang, C.L. Chien, S.L. Pan, W.P. Chen, C.M. Teng, Y.C. Shen, J.H. Guh: Induction of endoplasmic reticulum stress and apoptosis by a marine prostanoid in human hepatocellular carcinoma, J. Hepatol. 43, 679–686 (2005)
P.C. Chiang, F.L. Kung, D.M. Huang, T.K. Li, J.R. Fan, S.L. Pan, Y.C. Shen, J.H. Guh: Induction of Fas clustering and apoptosis by coral prostanoid in human hormone-resistant prostate cancer cells, Eur. J. Pharm. 542, 22–30 (2006)
S. Tsukamoto, H. Yokosawa: Inhibition of the ubiquitin-proteasome system by natural Ppoducts for cancer therapy, Planta Medica 76, 1064–1074 (2010)
N. González, M.A. Barral, J. Rodríguez, C. Jiménez: New cytotoxic steroids from the gorgonian Isis hippuris. Structure-activity studies, Tetrahedron 57, 3487–3497 (2001)
C.H. Chao, L.F. Huang, Y.L. Yang, J.H. Su, G.H. Wang, M.Y. Chiang, Y.C. Wu, C.F. Dai, J.H. Sheu: Polyoxygenated steroids from the gorgonian Isis hippuris, J. Nat. Prod. 68, 880–885 (2005)
A.D. Rodríguez: The natural products chemistry of west indian gorgonian octocorals, Tetrahedron 51, 4571–4618 (1995)
W. Fenical: Marine soft corals of the genus pseudopterogorgia: A resource for novel anti-inflammatory diterpenoids, J. Nat. Prod. 50, 1001–1008 (1987)
J. Marrero, C.A. Ospina, A.D. Rodríguez, P. Baran, H. Zhao, S.G. Franzblau, E. Ortega-Barria: New diterpenes of the pseudopterane class from two closely related Pseudopterogorgia species: Isolation, structural elucidation, and biological evaluation, Tetrahedron 62, 6998–7008 (2006)
P. Martelletti, E. Adriani, S. Bonini, D. Celestino, L. Lenti, C. Armaleo, A. Dipastena, R. Misasi, M. Giacovazzo: Basophil histamine-release and leukotriene (LTB4-LTC4) production in cluster headache, Headache 29, 46–48 (1989)
P.B. Jacobson, R.S. Jacobs: Fuscoside – An antiinflammatory marine natural product which selectively inhibits 5-lipoxygenase. Biochemical-studies in the human neutrophil, J. Pharmacol. Exp. Ther. 262, 874–882 (1992)
C. Nathan: Neutrophils and immunity: Challenges and opportunities, Nat. Rev. Immunol. 6, 173–182 (2006)
P. Lacy, G. Eitzen: Control of granule exocytosis in neutrophils, Front. Biosci. 13, 5559–5570 (2008)
C.T.N. Pham: Neutrophil serine proteases: Specific regulators of inflammation, Nat. Rev. Immunol. 6, 541–550 (2006)
J. Boukouvalas, R.P. Loach: General, regiodefined access to alpha-substituted butenolides through metal-halogen exchange of 3-bromo-2-silyloxyfurans. Efficient synthesis of an anti-inflammatory gorgonian lipid, J. Org. Chem. 73, 8109–8112 (2008)
P.J. Sung, J.H. Sheu, J.P. Xu: Survey of briarane-type diterpenoids of marine origin, Heterocycles 57, 535–579 (2002)
P.J. Sung, P.C. Chang, L.S. Fang, J.H. Sheu, W.C. Chen, Y.P. Chen, M.R. Lin: Survey of briarane-related diterpenoids - part II, Heterocycles 65, 195–204 (2005)
S.L. Wu, P.J. Sung, M.Y. Chiang, J.Y. Wu, J.H. Sheu: New polyoxygenated briarane diterpenoids, briaexcavatolides O-R, from the gorgonian briareum excavatum, J. Nat. Prod. 64, 1415–1420 (2001)
B. Lenarcic, A. Ritonja, B. Strukelj, B. Turk, V. Turk: Equistatin, a new inhibitor of cysteine proteinases from actinia equina, is structurally related to thyroglobulin type-1 domain, J. Biol. Chem. 272, 13899–13903 (1997)
B. Lenarcic, V. Turk: Thyroglobulin type-1 domains in equistatin inhibit both papain-like cysteine proteinases and cathepsin D, J. Biol. Chem. 274, 563–566 (1999)
D. Brömme, S. Petanceska: Papain-like cysteine proteases and their implications in neurodegenerative diseases. In: Role of Proteases in the Pathophysiology of Neurodegenerative Diseases, ed. by A. Lajtha, N.L. Banik (Springer, New York, NY, USA 2002) pp. 47–61
M. Wolf, I. Clark-Lewis, C. Buri, H. Langen, M. Lis, L. Mazzucchelli: Cathepsin D specifically cleaves the chemokines macrophage inflammatory protein-la, macrophage inflammatory protein-1 beta, and SLC that are expressed in human breast cancer, Am. J. Pathol. 162, 1183–1190 (2003)
NCBI: CTSD cathepsin D [Homo sapiens]. available online: http://www.ncbi.nlm.nih.gov/gene/1509, last accessed 03.01.2013
A.J. Zaharenko, G. Picolo, W.A. Ferreira Jr., T. Murakami, K. Kazuma, M. Hashimoto, Y. Cury, J.C. de Freitas, M. Satake, K. Konno: Bunodosine 391: An analgesic acylamino acid from the venom of the sea anemone bunodosoma cangicum, J. Nat. Prod. 74, 378–382 (2011)
S.S. Al-Lihaibi, S.-E.N. Ayyad, F. Shaher, W.M. Alarif: Antibacterial sphingolipid and steroids from the black coral antipathes dichotoma, Chem. Pharm. Bull. 58, 1635–1638 (2010)
A.J. Muller, J.B. DuHadaway, P.S. Donover, E. Sutanto-Ward, G.C. Prendergast: Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy, Nat. Med. 11, 312–319 (2005)
U. Grohmann, F. Fallarino, P. Puccetti: Tolerance, DCs and tryptophan: Much ado about IDO, Trends Immunol. 24, 242–248 (2003)
A. Pereira, E. Vottero, M. Roberge, A.G. Mauk, R.J. Andersen: Indoleamine 2,3-Dioxygenase inhibitors from the northeastern Pacific marine hydroid garveia annulata, J. Nat. Prod. 69, 1496–1499 (2006)
A.J. Muller, W.P. Malachowski, G.C. Prendergast: Indoleamine 2,3-dioxygenase in cancer: Targeting pathological immune tolerance with small-molecule inhibitors, Expert Opin. Ther. Targets 9, 831–849 (2005)
Y.W. Seo, K.W. Cho, J.R. Rho, J.H. Shin, B.M. Kwon, S.H. Bok, J.I. Song: Solandelactones A-I, lactonized cyclopropyl oxylipins isolated from the hydroid solanderia secunda, Tetrahedron 52, 10583–10596 (1996)
T.V. Ovchinnikova, S.V. Balandin, G.M. Aleshina, A.A. Tagaev, Y.F. Leonova, E.D. Krasnodembsky, A.V. Men'shenin, V.N. Kokryakov: Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins, Biochem. Biophys. Res. Commun. 348, 514–523 (2006)
D.L. Brinkman, J.N. Burnell: Biochemical and molecular characterisation of cubozoan protein toxins, Toxicon 54, 1162–1173 (2009)
O. Shimomura, F.H. Johnson, Y. Saiga: Extraction, purification and properties of aequorin, a bioluminescent protein from luminous hydromedusan, Aequorea, J. Cell, Comp. Physiol. 59, 223 (1962)
C.E. Schnitzler, R.J. Keenan, R. McCord, A. Matysik, L.M. Christianson, S.H.D. Haddock: Spectral diversity of fluorescent proteins from the anthozoan Corynactis californica, Marine Biotechnol. 10, 328–342 (2008)
D.T.M. Ip, K.B. Wong, D.C.C. Wan: Characterization of novel orange fluorescent protein cloned from cnidarian tube anemone Cerianthus sp, Marine Biotechnol. 9, 469–478 (2007)
M.C.Y. Chan, S. Karasawa, H. Mizuno, I. Bosanac, D. Ho, G.G. Prive, A. Miyawaki, M. Ikura: Structural characterization of a blue chromoprotein and its yellow mutant from the sea anemone Cnidopus japonicus, J. Biol. Chem. 281, 37813–37819 (2006)
H.W. Ai, J.N. Henderson, S.J. Remington, R.E. Campbell: Directed evolution of a monomeric, bright and photostable version of clavularia cyan fluorescent protein: Structural characterization and applications in fluorescence imaging, Biochem. J. 400, 531–540 (2006)
A. Goulding, S. Shrestha, K. Dria, E. Hunt, S.K. Deo: Red fluorescent protein variants with incorporated non-natural amino acid analogues, Protein Eng. Design Sel. 21, 101–106 (2008)
H.B. Tu, Q. Xiong, S.L. Zhen, X.F. Zhong, L.H. Peng, H.P. Chen, X.Y. Jiang, W.H. Liu, W.L. Yang, J.W. Wei, M.L. Dong, W.Y. Wu, A.L. Xu: A naturally enhanced green fluorescent protein from magnificent sea anemone (Heteractis magnifica) and its functional analysis, Biochem. Biophys. Res. Commun. 301, 879–885 (2003)
S.A. Pomponi: The oceans and Human health: The discovery and development of marine-derived drugs, Oceanography 14, 78–87 (2001)
M. Synnes: Bioprospecting of organisms from the deep sea: Scientific and environmental aspects, Clean Technol. Environ. Policy 9, 53–59 (2007)
C.C. Thornburg, T.M. Zabriskie, K.L. McPhail: Deep-sea hydrothermal vents: Potential hot spots for natural products discovery?, J. Nat. Prod. 73, 489–499 (2010)
S. Roberts, M. Hirshfield: Deep-sea corals: Out of sight, but no longer out of mind, Front. Ecol. Environ. 2, 123–130 (2004)
S.D. Cairns: Deep-water corals: An overview with special reference to diversity and distribution of deep-water scleractinian corals, Bull. Mar. Sci. 81, 311–322 (2007)
M. Daly, M.R. Brugler, P. Cartwright, A.G. Collins, M.N. Dawson, D.G. Fautin, S.C. France, C.S. McFadden, D.M. Opresko, E. Rodriguez, S.L. Romano, J.L. Stake: The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after linnaeus, Zootaxa 1668, 127–182 (2007)
E. Rodríguez, M. Daly: Phylogenetic relationships among deep-sea and chemosynthetic sea anemones: Actinoscyphiidae and actinostolidae (Actiniaria: Mesomyaria), PLoS One 5, 10958e (2010)
D. Skropeta: Deep-sea natural products, Nat. Prod. Rep. 25, 1131–1166 (2008)
D.G. Kingston: Modern natural products drug discovery and its relevance to biodiversity conservation, J. Nat. Prod. 74, 496–511 (2011)
A.L. Harvey, N. Gericke: Bioprospecting: Creating a value for biodiversity. In: Research in Biodiversity – Models and Applications, ed. by I.Y. Pavlinov (InTech, Rijeka 2011)
J.M. Arrieta, S. Arnaud-Haond, C.M. Duarte: What lies underneath: Conserving the oceans' genetic resources, Proc. Nat. Acad. Sci. 107, 18318–18324 (2010)
A.L. Lane, B.S. Moore: A sea of biosynthesis: Marine natural products meet the molecular age, Nat. Prod. Rep. 28, 411–428 (2011)
P. Proksch, R. Edrada-Ebel, R. Ebel: Drugs from the sea – Opportunities and obstacles, Mar. Drugs 1, 5–17 (2003)
P.-Y. Qian, Y. Xu, N. Fusetani: Natural products as antifouling compounds: Recent progress and future perspectives, Biofouling 26, 223–234 (2009)
K.J. Schippers, D. Sipkema, R. Osinga, H. Smidt, S.A. Pomponi, D.E. Martens, R.H. Wijffels: Cultivation of sponges, sponge cells and symbionts: Achievements and future prospects, Adv. Mar. Biol. 62, 273–337 (2012)
M.J. Page, S.J. Handley, P.T. Northcote, D. Cairney, R.C. Willan: Successes and pitfalls of the aquaculture of the sponge Mycale hentscheli, Aquaculture 312, 52–61 (2011)
D. Mendola: Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: Process developments and economics, Biomolecular Eng. 20, 441–458 (2003)
O.K. Radjasa, Y.M. Vaske, G. Navarro, H.C. Vervoort, K. Tenney, R.G. Linington, P. Crews: Highlights of marine invertebrate-derived biosynthetic products: Their biomedical potential and possible production by microbial associants, Bioorg. Med. Chem. 19, 6658–6674 (2011)
D.J. Newman, G.M. Cragg: Natural products as sources of new drugs over the last 25 years, J. Nat. Prod. 70, 461–477 (2007)
J. Piel: Bacterial symbionts: Prospects for the sustainable production of invertebrate-derived pharmaceuticals, Curr. Med. Chem. 13, 39–50 (2006)
U. Hentschel, J. Hopke, M. Horn, A.B. Friedrich, M. Wagner, J. Hacker, B.S. Moore: Molecular evidence for a uniform microbial community in sponges from different oceans, Appl. Environ. Microbiol. 68, 4431–4440 (2002)
M. Hay, W. Fenical: Chemical ecology and marine biodiversity: Insights and products from the sea, Oceanography 9, 10–20 (1996)
J. Piel: Metabolites from symbiotic bacteria, Nat. Prod. Rep. 26, 338–362 (2009)
N.C. Newberger, L.K. Ranzer, J.M. Boehnlein, R.G. Kerr: Induction of terpene biosynthesis in dinoflagellate symbionts of Caribbean gorgonians, Phytochemistry 67, 2133–2139 (2006)
J.W.-H. Li, J.C. Vederas: Drug discovery and natural products: End of an era or an endless frontier?, Science 325, 161–165 (2009)
J.G. Burgess: New and emerging analytical techniques for marine biotechnology, Curr. Opin. Biotechnol. 23, 29–33 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rocha, J., Calado, R., Leal, M. (2015). Marine Bioactive Compounds from Cnidarians. In: Kim, SK. (eds) Springer Handbook of Marine Biotechnology. Springer Handbooks. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-53971-8_35
Download citation
DOI: https://doi.org/10.1007/978-3-642-53971-8_35
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-53970-1
Online ISBN: 978-3-642-53971-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)