Abstract
Chemoprevention refers to the use of pharmacologic interventions to delay, prevent, or reverse carcinogenesis with the ultimate goal of reducing cancer incidence. Two large, population-based, phase 3 prostate cancer prevention trials reported that 5-alpha reductase inhibitors significantly reduce prostate cancer risk. However, this class of agents were also associated with increased detection of high-grade prostate cancer. Another large, phase 3 prostate cancer prevention clinical trial showed no benefit for long-term supplementation with the trace element Se, given in the form of selenomethionine, or vitamin E, either individually or in combination. Paradoxically, a significant increase in prostate cancer was observed among men randomized to receive vitamin E alone. A great deal of progress had been made in the field of prostate cancer prevention over the past decade. Future studies will focus on prevention of disease progression in men on Active Surveillance, immunotherapy, mechanistically based drug combinations, and novel biomarkers of risk and benefit.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Prostate Cancer
- Gleason Score
- Prostate Cancer Incidence
- Prostate Cancer Prevention
- Prostate Cancer Prevention Trial
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
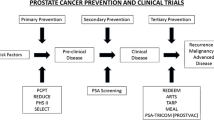
1 The Prostate Cancer Prevention Trial (PCPT)
1.1 Rationale and Study Design
The PCPT was a phase III, double-blind, placebo-controlled trial of finasteride for the primary prevention of prostate cancer. Finasteride belongs to a class of agents (5-alpha-reductase inhibitors, 5-ARIs), which converts testosterone (T) into the more potent androgen, di-hydrotestosterone (DHT) (Bartsch et al. 2000; Tindall and Rittmaster 2008). 18,882 men age 55 and older with a PSA of ≤3 ng/ml and a normal digital rectal examination (DRE) were enrolled on this clinical trial between 1993 and 1996 and followed with yearly PSAs and digital rectal examinations. The primary study endpoint was the 7-year period prevalence of biopsy-proven prostate cancer; biopsy Gleason Score was a secondary endpoint.
1.2 Results
The primary finding of the PCPT was a 24.8 % relative reduction in prostate cancer prevalence among men randomized to the finasteride arm (24.4 vs. 18.4 %, p < 0.001) among the 9,060 men in whom a prostate biopsy or trans-urethral resection of the prostate had been performed during the study (Thompson et al. 2003). Finasteride was associated with decreased prostate cancer prevalence regardless of a priori risk level based on age, race, family history, and baseline PSA (Thompson et al. 2003). However, despite a clear decrease in the prevalence of prostate cancer, finasteride was associated with a small but statistically significant increase in the prevalence of high-grade prostate cancer. Overall, 43 more cancers with a Gleason score (GS) of 7–10 were diagnosed in men on the finasteride than on the placebo arm (280 vs. 237 men) representing 6.4 and 5.1 % of men on the two study arms, respectively (p = 0.005). Among these men, 90 on the finasteride arm and 53 on the placebo arm had a GS 8–10 prostate cancer (Thompson et al. 2003).
1.3 High-Grade Prostate Cancer in PCPT
The observation that the excess of high-grade cancer observed on the finsteride arm of the PCPT occurred early and did not increase over the course of the 7-year trial suggests that the association between finasteride and high-grade prostate cancer may not have been causal (Thompson et al. 2003). Alternatively, finasteride may have simply increased the detection of previously existing high-grade cancer and, in fact, two forms of detection bias appear to have been operative in the PCPT.
Reliance on biopsies to address the secondary Gleason score endpoint, a practical necessity as not all men diagnosed with prostate cancer undergo prostatectomy, coupled with the effects of finasteride on gland volume, clearly introduced an element of “volume bias.” Ultrasound examinations at the time of prostate biopsy confirmed a nearly 25 % decrease in median prostate volume among men treated with finasteride (Thompson et al. 2003). As the extent of biopsy sampling was similar on the two study arms, there was relatively greater sampling of the smaller, finasteride-treated glands, increasing the likelihood of detecting high-grade prostate cancer, if present, among men on finasteride.
A second form of bias is known as “PSA bias.” Subsequent analyses of the PCPT showed that finasteride increases the sensitivity of PSA testing for the detection of prostate cancer, in general, and high-grade prostate cancer, in particular (Thompson et al. 2006). As approximately 50 % of the cancers diagnosed in the PCPT were prompted by PSA tests, this finasteride-induced increase in PSA sensitivity would be expected to have resulted in both an overestimate of high-grade prostate cancer and an underestimate in the reduction of nonhigh-grade cancer among men on the finasteride arm. Supporting the hypothesis that detection bias accounted, at least in part, for the observed increase in high-grade cancer on the finasteride arm of the PCPT is the observation that patients in whom high-grade disease was documented at prostatectomy (the gold-standard for determining Gleason score) were significantly more likely to have had their high-grade cancer correctly identified on biopsy if they had been on finasteride than if they had been on placebo (70 vs. 51 %, p = 0.01) (Lucia et al. 2007).
Another relevant issue is whether reducing a man’s risk of being diagnosed with low-risk prostate cancer confers true clinical benefit given the indolent natural history of the majority of such cancers. The answer to this question is related to the aggressiveness with which the disease is treated. As recently as 2004–2006, approximately 85 % of the men in the CaPSURE registry with low-risk prostate cancer received definitive therapy (usually surgery or radiation) with their attendant morbidities, including impotence, urinary incontinence, and rectal injury (Cooperberg et al. 2007). This underscores the substantial burden of disease imposed even by low-risk prostate cancer.
Finally, it is important to consider the degree to which adverse consequences (such as a true increase in high-grade cancer) are acceptable in the cancer prevention setting. The tolerance for such events must be balanced against an individual’s risk of being diagnosed with and subsequently treated for cancer in the absence of the preventive intervention. In the case of prostate cancer, a man’s risk of diagnosis (and hence treatment) is highly dependent on whether he chooses to undergo regular screening. Therefore, 5-alpha-reductase inhibitors would have a more favorable risk–benefit ratio in men committed to regular screening than in non-screened populations.
2 The Selenium and Vitamin E Cancer Prevention Trial (SELECT)
SELECT was a phase III randomized, placebo-controlled trial of selenium (200 mg/day, L-selenomethionine), and/or vitamin E (400 IU/day) supplementation for prostate cancer prevention (Lippman et al. 2005). The rationale for studying selenium and vitamin E was based on secondary endpoints from two earlier phase III, placebo-controlled, randomized, cancer prevention trials: the alpha-Tocopherol beta-Carotene Study (ATBC) and the Nutritional prevention of cancer Study (NPC). Although both studies were negative with regard to their primary endpoints, lung cancer and non-melanoma skin cancer incidence, respectively, men randomized to the vitamin E arm of ATBC had a 40 % reduction in prostate cancer mortality (Heinonen et al. 1998) and men randomized to the selenium arm of NPC had an approximately two-thirds reduction in prostate cancer incidence (Clark et al. 1996).
The major eligibility requirements for SELECT were age ≥55 years for non-African American men (≥50 years for African American men), serum PSA ≤4 ng/ml, and a non-suspicious DRE. SELECT accrued 35,533 participants between July 2001 and July 2004; participants were seen every 6 months throughout the trial (initially planned for 7–12 years) for adherence and adverse events monitoring (Lippman et al. 2005). The primary endpoint was the clinical incidence of prostate cancer; secondary endpoints included lung, colon, and total cancer incidence, cardiovascular events, death from any cause and toxicity. In addition, four prospectively conducted sub-studies addressing the usefulness of selenium and vitamin E in the prevention of macular degeneration, chronic obstructive lung disease, Alzheimer’s disease, and colon polyps were performed in men already accrued to the parent study.
On September 15, 2008, following the second of five planned interim analyses, the Data and Safety Monitoring Committee recommended that the study supplements, vitamin E and selenium, be discontinued due to lack of efficacy. In addition, vitamin E was associated with a nonsignificant 13 % increase in prostate cancer incidence (p = 0.06, not corrected for multiple comparisons). This trend was not seen in the combined vitamin E + selenium arm. No significant differences were observed in any of the prespecified secondary endpoints, including lung and colon cancer, overall cancer, cardiovascular events, and toxicity (Lippman et al. 2009). A follow-up analysis including 54,464 additional person-years of follow-up and 521 additional cases of prostate cancer reported a statistically significant 17 % increase in prostate cancer incidence on the vitamin E alone arm, p = 0.008 (Klein et al. 2011).
These findings show the importance of conducting adequately powered, controlled, clinical trials to determine the true risks and benefits of products with healthcare claims, including nonprescription nutritional supplements. An important component of SELECT was the creation of a biorepository of prediagnostic specimens (both serum and DNA) from all participants. These biospecimens, which are linked to a clinical database, provide a powerful tool to explore the biology of prostate cancer and other diseases through the conduct of correlative studies. Details regarding procedures for gaining access to these samples can be found at the Southwest Oncology Group (SWOG) website, http://www.swog.org.
3 Conclusions
While selenium and vitamin E, in the doses and formulations tested, were ineffective for the prevention, and vitamin E appeared to increase the risk of prostate cancer, finasteride was definitively shown to reduce a man’s risk of this disease. Whether the potential benefits of 5-alpha-reductase inhibitors, both in terms of overall risk reduction and enhanced detection of high-grade disease, are outweighed by the possibility of a small increase in the risk of high-grade cancer remains controversial and these drugs are currently not FDA approved for prostate cancer prevention. Given the substantial resources needed to conduct large-scale, phase III cancer prevention trials, it is important that the future trials be well supported by mechanistic, preclinical, and phase II clinical data. The National Cancer Institute’s division of cancer prevention is committed to supporting chemoprevention agent development research with these goals in mind.
References
Bartsch G, Rittmaster RS, Klocker H (2000) Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. Eur Urol 37:367–380
Clark LC, Combs GF Jr, Turnbull BW et al (1996) Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional prevention of cancer study group. JAMA 276:1957–1963
Cooperberg MR, Broering JM, Kantoff PW, Carroll PR (2007) Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol 178:S14–S19
Heinonen OP, Albanes D, Virtamo J et al (1998) Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 90:440–446
Klein EA, Thompson IM Jr, Tangen CM et al (2011) Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT). JAMA 306:1549–1556
Lippman SM, Goodman PJ, Klein EA et al (2005) Designing the selenium and vitamin E cancer prevention trial (SELECT). J Natl Cancer Inst 97:94–102
Lippman SM, Klein EA, Goodman PJ et al (2009) Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA 301:39–51
Lucia MS, Epstein JI, Goodman PJ et al (2007) Finasteride and high-grade prostate cancer in the prostate cancer prevention trial. J Natl Cancer Inst 99:1375–1383
Thompson IM, Goodman PJ, Tangen CM et al (2003) The influence of finasteride on the development of prostate cancer. N Engl J Med 349:215–224
Thompson IM, Chi C, Ankerst DP et al (2006) Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst 98:1128–1133
Tindall DJ, Rittmaster RS (2008) The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer. J Urol 179:1235–1242
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Parnes, H.L., Brawley, O.W., Minasian, L.M., Ford, L.G. (2014). Phase III Prostate Cancer Chemoprevention Trials. In: Cuzick, J., Thorat, M. (eds) Prostate Cancer Prevention. Recent Results in Cancer Research, vol 202. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-45195-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-45195-9_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-45194-2
Online ISBN: 978-3-642-45195-9
eBook Packages: MedicineMedicine (R0)