Abstract
Microbiologists have historically been struck by both the beautiful pigmentation of phenazine-producing cultures and the high degree of variability in phenazine production among isolates, conditions, and even repeat experiments. Motivated by an interest in controlling phenazine biosynthesis, they have identified many of the factors that affect the regulation of this process. Phenazine production is controlled by complex regulatory networks. The variability of phenazine production can be explained in part by the effects of environmental conditions on these networks and by strain-specific differences in these networks. In this chapter, we describe the components of a common regulatory cascade that is represented in many phenazine-producing pseudomonads. Membrane sensor proteins and two component sensors control the activity of downstream regulators such as quorum sensing systems and RNA-binding proteins and small RNAs; these cytoplasmic regulators then control the production of phenazine biosynthetic proteins. We highlight examples from specific strains and cases where the mechanistic links may vary among them. We also discuss environmental parameters that have been shown to affect phenazine biosynthesis and compare their effects in different isolates. Ongoing work will further elaborate the details of the environmental sensing and regulatory responses that control production of these dramatically colored compounds. New findings have the potential to support enhanced application of phenazine-producing strains in agriculture, where they promote crop health, and the treatment of infections in which phenazines contribute to bacterial pathogenicity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
2.1 Introduction
The dramatic coloration of phenazine-producing bacterial cultures has attracted researchers in many disciplines for over a century (Fordos 1859; Gessard 1894). It may have contributed to the early recognition and classification of Pseudomonas chlororaphis (Guignard and Sauvageau 1894), P. aeruginosa, and other bacteria whose species epithets derive from their pigmentation (Schroeter 1872). Furthermore, it has inspired researchers to ask many different types of questions about the biological relevance of phenazines, and a variety of physiological effects have been demonstrated for these compounds in both the organisms that produce them and the organisms exposed to them (see Chap. 3).
For microbiologists cultivating phenazine-producers, it is apparent that phenazine biosynthesis can vary unpredictably among cultures, suggesting that it is sensitive to subtle environmental variations (Fig. 2.1). Under many conditions, the precise molecular cues that interact with regulatory proteins to control phenazine biosynthetic gene expression are not known. However, downstream mechanisms controlling their expression have been identified in several species, and themes of phenazine regulation have emerged, including control by two component systems, quorum sensing (QS), and small noncoding RNAs (sRNAs) (Fig. 2.2). In this chapter, we will summarize the phylogenetic distribution of phenazine biosynthetic clusters and cite examples from phenazine-producing pseudomonads that illustrate specific regulatory mechanisms. In addition, we will discuss some of the environmental signals that control phenazine production in various isolates. We will focus on the regulation of phenazine biosynthesis in members of the genus Pseudomonas, where the bulk of studies on this topic have been conducted.
2.2 Phylogenetic Distribution and Mechanisms of Phenazine Biosynthesis
Phenazine-producing organisms have been identified that belong to the bacterial phyla Actinobacteria and Proteobacteria and the archaeal phylum Euryarcheota (Mavrodi et al. 2010). The gene cluster encoding biosynthetic enzymes for the archaeal electron carrier methanophenazine, produced by Methanosarcina mazei, and the conditions controlling its biosynthesis are unknown. In bacterial species for which phenazine production has been observed, variation exists at the level of the isolate, such that some strains within a species produce phenazines while others lack the biosynthetic genes. Bacterial phenazine biosynthetic pathways identified to date proceed via chorismate to the formation of the core phenazine structure and ultimately, to production of the common phenazine precursor phenazine-1-carboxylic acid (PCA) (Mentel et al. 2009; Seeger et al. 2011; see also Chap. 1 of this volume). Whether and how PCA is modified to produce other phenazines varies among organisms and depends to some extent on environmental conditions. A variety of functional groups can be added to the core structure to produce phenazines in a range of colors with diverse chemical properties (Turner and Messenger 1986; Laursen and Nielsen 2004; Mavrodi et al. 2006; Pierson and Pierson 2010) (Table 2.1). Many of the decorating enzymes responsible for PCA transformation have been identified and characterized, and regulation of their activities determines the complement of phenazines produced by a given strain under particular conditions.
The archetypical core phenazine operon is found in pseudomonads such as P. chlororaphis, P. aeruginosa, Pseudomonas sp. CMR12a, and P. fluorescens 2-79 and contains seven genes; the operon structure is more variable in other Proteobacteria and in Actinobacteria (Mavrodi et al. 2010). P. aeruginosa strains appear to be unique among these organisms in that their genomes contain two phenazine biosynthetic operons, which we will refer to as phz1 and phz2 and which are nearly identical at the DNA level. Although the contributions of the core phz operon products to phenazine biosynthesis are generally known, this is an area of active research (see Chap. 1 of this volume). Genes for decorating enzymes can lie adjacent to the core operon or elsewhere in the genome. In some cases, the products of adjacent genes play roles in regulation of the core operon or phenazine transport.
2.3 Mechanisms and Conditions Controlling Phenazine Biosynthesis in Pseudomonads
Researchers in disparate subdisciplines of microbiology have long been interested in elucidating the mechanisms that control phenazine biosynthesis. Phenazine production is critical for the biocontrol properties of certain agriculturally important root-colonizing pseudomonads that protect food crops from attack by pathogenic fungi (Chin-A-Woeng et al. 2003; Haas and Défago 2005; Mavrodi et al. 2006; Mavrodi et al. 2012). In the clinical setting, phenazine production contributes to virulence during acute and chronic P. aeruginosa infections (Lau et al. 2004; Caldwell et al. 2009; Hunter et al. 2012). Regulation of phenazine biosynthesis has therefore been studied in diverse Pseudomonas isolates. Although the precise linkages between regulatory mechanisms may vary among species and even strains, general mechanisms and their overall hierarchy are often shared. We will highlight these commonalities and focus on specific systems that exemplify general principles. Figure 2.3 summarizes the main regulatory systems and molecules that ultimately control phenazine production: two component systems, QS, sRNAs and environmental cues. These mechanisms and cues can act indirectly by influencing activities far upstream of phenazine biosynthetic gene expression or RNA translation, or they can directly control these processes. Additional details regarding the complex relationships between and within these regulatory systems can be found in recent reviews that summarize the literature (Mavrodi et al. 2006; Williams and Camara 2009; Pierson and Pierson 2010; Sonnleitner and Haas 2011; Balasubramanian et al. 2013).
Proteins and sRNAs that control phenazine production, with targets indicated. X indicates which pseudomonad contains a particular element or in which an environmental effect on phenazine production has been observed. Shading in the table indicates the location of each type of element in regulatory cascades; a sample cascade is shown. *Only found in P. aeruginosa
2.3.1 Two Component Systems
In both biocontrol and pathogenic pseudomonads, two component systems were among the first regulatory mechanisms identified that play critical roles in phenazine regulation (Reimmann et al. 1997; Chancey et al. 1999; van den Broek et al. 2003; De Maeyer et al. 2011). They lie conceptually at the top of signaling hierarchies because they have the potential to directly sense environmental cues and then modulate the activities of downstream regulatory mechanisms or directly control gene expression (Fig. 2.4). Such systems typically consist of a membrane-bound sensor protein and a cytoplasmic regulatory protein. The phosphorylation status of the sensor protein is altered through binding of a small molecule or other environmental cue that triggers conformational changes and affects activity. The phosphate group is then transferred to and activates the response regulator protein (Bourret and Silversmith 2010).
GacS/GacA, which is required for wild-type phenazine production in many isolates, is the best-characterized two component system controlling this process (Heeb and Haas 2001; Haas and Defago 2005). In phenazine-producing species, it occupies a position between environmental cues and downstream, intracellular regulatory mechanisms such as those dependent on sRNAs. In describing this system and others below, we will include references to the regulatory cascade in P. fluorescens strains that do not produce phenazines in cases where it is possible that the same cascade operates and affects phz gene expression in P. fluorescens 2-79. In addition, we note here that we use ORF numbers from P. aeruginosa PAO1 (starting with "PA") for some of the proteins described below.
The cue that activates GacS has not been identified, but additional membrane proteins that control GacS activity in some isolates are known. These include RetS and LadS, hybrid sensor kinases that contain the unusual arrangement of periplasmic sensor domains linked to cytoplasmic histidine kinase and response regulator receiver domains (Goodman et al. 2004; Ventre et al. 2006). In strains of P. fluorescens and P. aeruginosa, RetS interacts with GacS and inhibits the phosphorelay (Goodman et al. 2009; Workentine et al. 2009; Vincent et al. 2010). A physical interaction between LadS and GacS is also predicted, however, this hybrid sensor potentiates phosphotransfer from GacS to GacA (Workentine et al. 2009). Although the GacS/GacA system does not directly control expression of phz genes, it does modulate the activities of sRNA- and QS-dependent regulatory mechanisms, which can then directly interact with phz gene promoters or transcripts (Fig. 2.4). These systems are discussed in further detail below.
The two component system CzcS/CzcR was recently implicated in regulation of phz gene expression in P. aeruginosa PAO1 (Dieppois et al. 2012). CzcS/CzcR is activated by zinc, cadmium, and cobalt and induces expression of an efflux pump that confers resistance to these metals. Dieppois et al. (2012) observed that mutants lacking functional CzcS/CzcR overproduce the phenazine pyocyanin (5-N-methyl-1-hydroxyphenazine, PYO), despite the fact that this two component system positively regulates QS. Chromatin immunoprecipitation assays suggest that CzcR binds to the phz1 promoter when the system is activated by zinc. In this way, CzcR could inhibit phz1 expression directly, negating the positive control of this operon by QS. This mechanism would constitute an unusual example of a direct link between a two component system and transcriptional control of phz genes.
Several other two component systems have been shown to affect phenazine production in various isolates, and evidence reported thus far indicates that this regulation is mediated via additional proteins and mechanisms. The RpeB/RpeA system positively regulates phenazine production in P. chlororaphis 30-84, and homologues to this system are present in other biocontrol strains but not in P. aeruginosa (Wang et al. 2012a). Proteins identified that positively regulate, or would be expected to regulate, phenazine production or phz genes in P. aeruginosa include the sensor/regulator pair BfiS/BfiR (acting via an indirect effect on levels of the sRNA RsmZ) (Petrova and Sauer 2010); the sensor/regulator pair CbrA/CbrB, which induces the expression of sRNA CrcZ in response to nonpreferred carbon sources (Sonnleitner et al. 2009); the sensor PA2573, which affects PYO production through an unknown regulator (McLaughlin et al. 2012); and the individual sensors PA1611, PA1976, and PA2824, which can all control the activation state of the response regulator HptB (Hsu et al. 2008). HptB would be expected to affect phenazine production indirectly through a complex regulatory cascade that ultimately controls expression of the sRNA RsmY. The positions of RsmZ and RsmY in the regulatory network controlling phenazine production are discussed further below.
2.3.2 Quorum Sensing
Phenazine production in liquid batch cultures typically occurs after the period of most rapid growth, and phenazines accumulate in the culture in stationary phase. This is in part due to the regulation of phz gene expression by QS. In QS, bacteria excrete small molecule or peptide signals into the environment which can then affect gene expression in the producer. Their regulatory effects become apparent after they have reached a minimum concentration, often after a decrease in culture growth rate. Molecules with diverse structures have been implicated in this behavior, but acyl homoserine lactones (AHLs) and quinolone derivatives in particular are most relevant for phz gene expression.
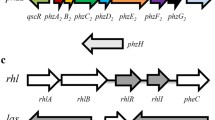
Systems that support AHL-dependent QS control of phz gene expression contain homologues of LuxR, a DNA binding sensory protein whose activity is controlled by AHL, and, typically, homologues of LuxI, an AHL synthase. These proteins were first identified in the recently reclassified luminescent bacterium Aliivibrio fischeri (Meighen 1991) and their homologues have since been characterized in a broad diversity of species. LuxR homologues vary in their specificity for AHL derivatives, with some proteins requiring a single signal for activation and others responding to several variations on a core structure. In P. chlororaphis strains 30-84 and PCL1391, P. fluorescens 2-79, and Pseudomonas sp. CMR12a, the LuxR/I homologues PhzR and PhzI are encoded by ORFs that lie adjacent to the phenazine biosynthetic genes but are each individually transcribed (Pierson et al. 1994; Wood and Pierson 1996; Chin-A-Woeng et al. 2001; Khan et al. 2005; De Maeyer et al. 2011). The PhzR/I systems in these strains produce and are controlled by N-(3-hydroxy-hexanoyl)-HSL (3–OH–C6–HSL). Although additional LuxR/I type systems in some of these strains produce and respond to other AHLs, 3–OH–C6–HSL is the main such signal relevant for phz gene regulation (Khan et al. 2007). The promoter regions of the phz operons in the root-colonizing strains that have phzR and phzI contain canonical binding sites for LuxR-type regulators; these are near-perfect inverted repeats that can be referred to as lux, las/rhl, or phz boxes (Egland and Greenberg 1999; Chin-A-Woeng et al. 2001; Khan et al. 2005).
The P. aeruginosa genome encodes at least three LuxR homologues called LasR, RhlR, and QscR. The cognate AHL synthases for LasR and RhlR produce N-(3-oxododecanoyl) homoserine lactone (3–O–C12–HSL) and N-butanoyl-L-homoserine lactone (C4–HSL), respectively (Pearson et al. 1994; Pearson et al. 1995). Interestingly, QscR has no obvious cognate AHL synthase, but it responds most effectively to 3–O–C12–HSL (Lee et al. 2006). Whether these LuxR homologues activate or repress gene expression depends on the location of the binding site relative to the transcription start site of the target gene. In contrast to the QS circuits in root-colonizing pseudomonads that control phenazine production, which are not known to regulate loci other than the phz operons, the AHL-controlled regulatory networks in P. aeruginosa affect expression of countless targets (Whiteley et al. 1999; Wagner et al. 2003).
Although their genomes share high sequence similarity, the P. aeruginosa strains PAO1, PA14, and M18 exhibit strain-dependent differences with respect to QS-dependent regulation of phz gene expression, and in many cases the mechanisms underlying these activities have not been thoroughly characterized. Often, the PCA derivative PYO is used as an indicator molecule in studies evaluating phenazine production because it is easier to detect than the other P. aeruginosa phenazines. In PAO1 and PA14, Las- and Rhl-defective mutant strains lose the ability to produce PYO, while in M18, the Las and Rhl systems are apparently negative regulators of phenazine production. Recently, Wurtzel et al. (2012) used gel mobility shift assays to confirm the presence of a LasR/RhlR binding site in the promoter region of phz1 in PA14. This binding site also influences expression of phzM, which encodes an enzyme that catalyzes the first step in the transformation of PCA to PYO, and is divergently transcribed from the phz1 operon. No las/rhl box has been identified in the promoter region of phz2, although interestingly, the gene encoding QscR lies adjacent to this operon. QscR is a negative regulator of phz1 and phz2 expression and appears to act through repression of lasI (Chugani et al. 2001; Ledgham et al. 2003).
Many additional regulators have been identified that affect QS, thereby altering phenazine production; in some cases they may affect phenazine production both indirectly through QS and through direct regulation of phz gene expression (Beatson et al. 2002; Juhas et al. 2004; Xu et al. 2005; Liang et al. 2009; Rampioni et al. 2009; Siehnel et al. 2010). An important class of regulators that can influence transcription are sigma factors, which associate with RNA polymerase and control preferences for specific promoters. The sigma factors σ S (RpoS) and σ 54 (RpoN) both affect QS-dependent regulation. In P. aeruginosa, σ S participates in mutual regulation with AHL-dependent QS, in which σ S stimulates a moderate induction of lasR and rhlR, and these QS systems subtly induce rpoS (Schuster et al. 2004). Despite this, P. aeruginosa PAO1 rpoS mutants overproduce PYO, suggesting that σ S also acts independently of AHL to modulate phenazine biosynthesis (Suh et al. 1999). In P. chlororaphis 30-84, σ S is positively regulated by the GacS/GacA two component system and activates phenazine inducing protein (Pip). Pip positively regulates the PhzR/I QS system, which ultimately upregulates the P. chlororaphis phenazine operon, making σ S a positive regulator of phenazine production in this strain (Girard et al. 2006a). Although the downstream effects of σ S on phenazine production differ in P. aeruginosa and P. chlororaphis, the regulator PsrA positively controls σ S activity in both strains (Kojic and Venturi 2001; Girard et al. 2006b). Conflicting results have been reported regarding the effects of σ 54 on the Rhl QS system in P. aeruginosa (Heurlier et al. 2003; Thompson et al. 2003). PA14 mutants lacking functional σ 54 produce less PYO (Hendrickson et al. 2001); this may be because the expression of the CrcZ sRNA (discussed below) is σ 54-dependent (Abdou et al. 2011).
One important target of the AHL-controlled regulatory network in P. aeruginosa is the operon pqsABCDE. This locus is required for the production of another class of chemical signaling molecules called quinolones, and together, the AHL and quinolone signaling pathways form the core of the P. aeruginosa QS signaling cascade (Pesci et al. 1999). pqsA-D encode biosynthetic enzymes that are required for production of 2-heptyl-4-quinolone (HHQ). PqsH, encoded elsewhere in the genome, is a monooxygenase that converts HHQ to Pseudomonas Quinolone Signal (PQS) (Deziel et al. 2004). Both HHQ and PQS activate the transcriptional regulator PqsR (also known as MvfR), but PQS does so with greater efficiency (Xiao et al. 2006; Diggle et al. 2007). PqsR itself activates expression of pqsABCDE; therefore, HHQ/PQS and PqsR participate in an autoregulatory positive feedback loop in which the quinolones potentiate their own production (Fig. 2.5).
pqsE encodes a putative metallo-β-hydrolase of unknown function (Yu et al. 2009) that appears to be “caught” in the positive feedback loop controlling HHQ production: it is induced as a result of this mechanism but is not required for HHQ synthesis. Nevertheless, PqsE is required for phenazine production in P. aeruginosa PAO1 and PA14 (Gallagher et al. 2002; Recinos et al. 2012). Constitutive expression of PqsE in a pqsA mutant background is sufficient to promote phenazine production (Farrow et al. 2008), suggesting that, in the context of phz operon expression, the positive feedback loop that promotes HHQ production serves the sole purpose of tangentially upregulating pqsE (Williams and Camara 2009). The mechanism whereby PqsE promotes phz operon expression remains completely undefined, as PqsE itself does not contain a DNA binding domain. PqsE may be involved in the transformation of an unknown signal (Yu et al. 2009). We hypothesize that this signal controls the activity of an unidentified regulator of phz gene expression.
Until recently, studies examining the roles of quinolones in the regulation of phz gene expression focused on aerobically grown, well-mixed planktonic cultures. Under this condition, PQS is required for phz1 expression and phz1 is a major contributor to phenazine biosynthesis. Our group has evaluated the relative contributions of phz1 and phz2 to phenazine production in aerobic liquid cultures and biofilms in P. aeruginosa PA14 (Recinos et al. 2012). We have reported that, although phz1 is expressed at much higher levels than phz2 in P. aeruginosa PAO1 grown in aerobic liquid cultures (Whiteley et al. 1999; Chugani et al. 2001), in strain PA14 both operons make significant contributions to phenazine production when it is grown under the same conditions.
Interestingly, when P. aeruginosa PA14 is grown as a colony biofilm on agar plates, phz2 alone is sufficient for wild-type phenazine production. Furthermore, HHQ is sufficient to fully activate expression of phz2 in this context. The observation that HHQ rather than PQS is the major regulator of phz gene expression in biofilms is intriguing when considered in the context of oxygen availability. The conversion of HHQ to PQS is catalyzed by PqsH and requires molecular oxygen (Schertzer et al. 2010). In biofilms, which become anoxic at depth due to limited diffusion and oxygen consumption by cells closer to the surface (Dietrich et al. 2013), HHQ is likely produced in greater abundance than PQS. phz2 expression is mediated through PqsE and downstream regulators that are apparently specific for this operon. The mechanism whereby PqsE controls expression of phz1 and phz2, and the mechanisms that confer differential, condition-dependent expression of phz1 and phz2 are currently under investigation but likely include AHL-dependent regulation (Farrow et al. 2008).
2.3.3 Post-transcriptional Regulation
Several regulatory mechanisms have been identified that control, or would be expected to control, pseudomonad phenazine production post-transcriptionally (Fig. 2.6). These mechanisms are diverse and include mRNA binding by proteins and mRNA base pairing with 5′-leader RNA sequences, both of which can affect translation (Sonnleitner and Haas 2011). Additional sRNAs can further modulate the binding of such proteins and cis-acting regulatory RNAs to mRNA. Expression of these protein and RNA regulators is often controlled by two component systems or QS. They can indirectly control phz gene expression by modulating earlier steps in the regulatory cascade, or directly control translation of phz mRNAs. Extensive characterization of post-transcriptional regulators has been conducted in P. aeruginosa phenazine-producing strains, but also in P. fluorescens strains that do not contain phz operons. Our discussion includes references to these P. fluorescens strains as their post-transcriptional regulatory mechanisms may be relevant for the regulation of phenazine production in P. fluorescens 2-79.
The proteins RsmA and RsmE modulate secondary metabolism, QS-dependent activities and phenazine production in diverse pseudomonads. Both proteins are found in P. fluorescens and P. chlororaphis, while only RsmA is found in P. aeruginosa (Blumer et al. 1999; Reimmann et al. 2005). In P. fluorescens, RsmA and RsmE have been shown to interact with and inhibit the translation of target mRNAs that contain unpaired ANGGA motifs near the ribosomal binding site; by stabilizing a stem loop that contains the ANGGA motif, RsmA/E prevents the ribosome from binding (Lapouge et al. 2007). In P. aeruginosa strains, RsmA expression increases with cell density and regulates the LasR/I and RhlR/I QS circuits in a post-transcriptional manner (Pessi et al. 2001). It is thought that RsmA binds to lasR and rhlR mRNAs, inhibiting their translation. Though RsmA and RsmE have not been studied in the phenazine-producer P. fluorescens 2-79 (containing the LuxR/I homologues PhzR/I), they may have a similar effect on QS in this strain.
As RsmA is a negative regulator of lasR and rhlR mRNAs, one would predict that mutations in rsmA would lead to phenazine overproduction (Reimmann et al. 2005). Burrowes et al. (2006) found, however, that the phenotype of an rsmA mutant was condition-dependent: the mutant showed decreased PYO production in LB but increased PYO production in a defined medium containing glycerol and alanine (Burrowes et al. 2006). Furthermore, differing phenazine production phenotypes in rsmA mutants in strains PAO1 and M18 may indicate that temperature is an additional environmental parameter that affects this regulatory cascade. In M18, which is typically grown at 28 °C, RsmA is a positive regulator of phenazine production (Zhang et al. 2005). Interestingly, RsmA consensus sequences are present near the ribosomal binding sites of the phzA1 and phzA2 promoters, raising the possibility of direct control of the phenazine biosynthetic operons by RsmA. Preliminary evidence suggests that RsmA and RsmE also regulate expression of the phz operon in P. chlororaphis, although whether this occurs via direct interaction with phz operon mRNA, through regulation of the PhzR/I QS system, or both has not been reported (Wang et al. 2012a).
sRNAs containing the ANGGA motif can compete with target mRNAs for binding sites on RsmA and RsmE, thereby controlling the extent to which these proteins repress their targets. In P. aeruginosa, two of these sRNAs, called RsmY (sometimes referred to as RsmB) and RsmZ, have been identified (Heurlier et al. 2004; Burrowes et al. 2005). P. fluorescens strains produce homologues of these plus an additional sRNA called RsmX (Heeb et al. 2002; Valverde et al. 2003; Kay et al. 2005). Evidence suggests that the genes encoding these sRNAs are directly regulated by GacA. When phosphorylated GacA activates transcription of rsmX, rsmY, and rsmZ, the sRNA products bind to and sequester the translational repressors RsmA and RsmE, allowing expression of RsmA/E target genes (Heurlier et al. 2004; Kay et al. 2006). In P. aeruginosa, additional regulators have been reported to control expression of the RsmY and RsmZ sRNAs. HptB indirectly represses RsmY expression through a complex regulatory cascade (Hsu et al. 2008; Bordi et al. 2010). Furthermore, the BfiS/BfiR two component system induces expression of RNaseG, which specifically degrades RsmZ (Petrova and Sauer 2010). Levels of RsmA and RsmE are also regulated by complicated networks involving two component sensors, sRNAs, and QS systems. These mechanisms further contribute to the complexity of regulation of phz gene expression, but their ultimate effects on phenazine production have not been measured.
The two component system CbrA/CbrB controls expression of phzM via a mechanism analogous to the network linking GacS/GacA to phz operon expression. When CbrB is activated, it induces expression of CrcZ, an sRNA that binds to and sequesters the translational repressor Crc. The CrcZ and phzM transcripts both contain an A-rich motif that is recognized by Crc. CrcZ therefore limits the ability of Crc to inhibit phzM translation, and a crc mutant overproduces PYO due to increased PhzM levels (Huang et al. 2012).
In contrast to RsmX/Y/Z and CrcZ, which are controlled at the transcriptional level by two component systems, the sRNAs Lrs1 and Lrs2 are regulated by QS (Wurtzel et al. 2012). Using P. aeruginosa PA14 as a model strain, Wurtzel et al. identified las boxes in the promoter regions of the lrs1 and lrs2 genes and confirmed their regulation by LasR. In addition, they generated an lrs1 deletion mutant and found that it was defective in PYO production. RNA-seq analysis revealed two major differences in transcript levels between this mutant and the wild-type parent: increased abundance of transcript from the antABC operon, and increased abundance of the PrrF1 and PrrF2 sRNAs (discussed further below). The authors hypothesized that the PYO production defect in the lrs1 mutant arose from increased flux through an anthranilate-catechol conversation pathway (mediated by the products of the antABC operon). Anthranilate and phenazines are produced by pathways that branch from chorismate as a common precursor (Mentel et al. 2009). Notably, increased conversion of anthranilate to catechol also diverts it away from the quinolone biosynthetic pathway. Given that quinolones regulate phz operon expression, indirect Lrs1-dependent downregulation of anthranilate degradation may be important for wild-type levels of quinolone, and therefore phenazine production.
The sRNAs PrrF1 and PrrF2 have been characterized in further detail in P. aeruginosa PAO1. PrrF1 and PrrF2 are encoded by adjacent loci and repressed by the iron-dependent regulator Fur when iron is abundant (Wilderman et al. 2004; Oglesby et al. 2008). They are expressed during iron limitation and base-pair with target mRNAs, preventing their translation. One such target is the transcript of sodB, which encodes superoxide dismutase and is, for unknown reasons, required for PYO production in P. aeruginosa PAO1 (Hassett et al. 1995). Also in this strain, a prrF1/prrF2 mutant shows increased expression of the antABC operon, an effect that seems to contradict the simultaneous upregulation of antABC and prrF1/prrF2 transcripts in the lrs1 mutant of strain PA14 (Wurtzel et al. 2012). This may represent a strain-dependent difference in this branch of the P. aeruginosa sRNA-dependent regulatory network.
Expression of PhrS, an sRNA that positively controls translation of pqsR mRNA, is also controlled by a regulator that responds to an environmental cue: the oxygen-sensitive transcription factor ANR. In the absence of PhrS, pqsR mRNA adopts an intramolecular secondary structure in which an upstream open reading frame base-pairs with the pqsR transcript and inhibits translation. Under oxygen-limited conditions, ANR is activated and induces expression of PhrS. PhrS competes with the pqsR transcript for binding of its 5′ untranslated region, and via this anti-antisense mechanism, exposes the pqsR mRNA to allow for ribosome binding and translation. This regulatory cascade was elucidated in P. aeruginosa PAO1, where a PhrS-overexpressing strain shows increased PYO production due to elevated PqsR levels and quinolone production (Sonnleitner et al. 2011).
Hfq, an abundant mRNA-binding protein found in diverse bacteria, also affects phenazine production through post-transcriptional mechanisms. In P. aeruginosa M18, Hfq binds qscR and phzM mRNA transcripts via AU-rich sequences present in their 5′-leader sequences and inhibits their translation (Wang et al. 2012b). As qscR is a negative regulator of the phz operons, and phzM is required for the conversion of PCA to PYO, Hfq would be expected to enhance phenazine production overall but limit PYO production. In mutants lacking functional Hfq, Wang et al. observed increased PYO production and decreased PCA production, consistent with decreased production of the PhzA-G biosynthetic enzymes, but increased translation of phzM. Formation of the active, hexameric form of Hfq is promoted by the RelA enzyme, a critical regulator of the stringent response to amino acid starvation (Argaman et al. 2012). P. aeruginosa PAO1 relA mutants also overproduce PYO, suggesting that Hfq may regulate translation of the phzM transcript in this strain according to a mechanism similar to the one described for M18 (Erickson et al. 2004).
2.3.4 Environmental Signals and Conditions Affecting phz Gene Expression
Many studies characterizing the conditional dependence of phenazine production have revealed environmental cues that affect the regulation of this process and, in some cases, mechanisms linking the condition to the response. These studies have evaluated the effects of environmental parameters such as temperature, pH, salinity, and oxygen availability. They have also examined how phenazine production is influenced by the availability of carbon and nitrogen sources, phosphate, sulfate, iron, and magnesium. These environmental variables can affect phenazine production by indirectly or directly altering expression of Phz proteins (for example, through their effects on the production of signals upstream in the regulatory cascade (van Rij et al. 2004; Farrow and Pesci 2007)), or they can alter the availability of substrates and thus, flux through the relevant metabolic pathways that support phenazine biosynthesis.
The effect of temperature on phenazine production has been investigated in P. chlororaphis PCL1391, P. fluorescens 2-79, and multiple strains of P. aeruginosa. P. chlororaphis PCL1391 produces the PCA derivative phenazine-1-carboxamide (PCN) at comparable levels when grown at temperatures ranging from 21 to 31 °C, but production is almost undetectable when it is grown at 16 °C (van Rij et al. 2004). In P. fluorescens 2-79, PCA production was found to inversely correlate with temperature in a survey of temperatures ranging from 25 to 37 °C (Slininger and Shea-Wilbur 1995). In P. aeruginosa M18, transcription of phz1 and phz2 is elevated at 28 °C compared to 37 °C, and this correlates with a large increase in PCA production (Huang et al. 2009). In P. aeruginosa PA14, PYO production increases modestly when this strain is grown at 37 °C compared to 28 °C. Using RNA-seq, Wurtzel et al. (2012) found that the transcript abundances of both phz1 and phz2 are elevated at the higher temperature, with a larger effect on phz1 than phz2. These results also indicated the presence of a temperature-dependent transcriptional start site upstream of phzB1. The differential regulation of phzA1 and phzB1 is interesting because these two genes encode highly similar proteins that form heterodimers required for in vivo formation of the phenazine core. Temperature-dependent differences in expression may have consequences for PhzA/B dimerization (Ahuja et al. 2008).
Ambient oxygen levels also influence the production of different phenazine derivatives. In P. aeruginosa, PCA can be biosynthesized anaerobically (Dietrich et al. 2006; Mentel et al. 2009; Recinos et al. 2012). However, oxygen is required for the conversion of 5-methylphenazine-1-carboxylic acid (the product of PhzM, 5-MCA) to PYO by the PhzS monooxygenase. Therefore, PYO production is inhibited in the absence of oxygen. Interestingly, Holliman (1969) reported increased production of the red phenazines aeruginosin A and B in low oxygen conditions; inefficient conversion of 5-MCA to PYO may shunt the biosynthetic pathway toward the production of these alternative phenazines when oxygen is limited. An effect of oxygen limitation on phenazine biosynthesis has also been observed in P. chlororaphis PCL1391, where growth in low oxygen conditions leads to PCN overproduction (van Rij et al. 2004).
The effects of pH and salinity on phenazine production have been tested in biocontrol strains, where optimization of soil conditions could facilitate the application of such strains for crop growth promotion. P. chlororaphis PCL1391 produces PCN when grown at pH 7 or pH 8, but not at pH 6 (van Rij et al. 2004). For P. fluorescens 2-79, however, PCA production was maximized at pH 7, partially decreased but still substantial at pH 6, and abolished at pH 8 (Slininger and Shea-Wilbur 1995). Increasing concentrations of salts decreased PCN production in P. chlororaphis PCL1391, but this effect was specific to ionic solutes as xylose did not affect PCN production when introduced at isoosmotic levels, and osmoprotectants did not restore PCN production in a high-salt medium.
Variations in the availability of minerals and the compounds that provide the major elements for biomass can have dramatic effects on phenazine biosynthesis. In a survey of carbon sources for growth of P. chlororaphis PCL1391, van Rij et al. (2004) found that glucose, glycerol, and L-pyroglutamic acid gave rise to the highest levels of PCN production. The amount of PCN produced did not correlate with growth rate, and the most stimulatory carbon sources were not the most abundant organic compounds in the rhizosphere, where the organism is commonly found. Glucose and glycerol have also been found to stimulate PCA production in P. fluorescens 2-79. That glucose and glycerol promote the highest levels of phenazine production is surprising because they are not preferred carbon sources for pseudomonads; unlike E. coli, Pseudomonas species typically utilize organic acids such as succinate before utilizing sugars (Behrends et al. 2009; Rojo 2010; Valentini and Lapouge 2012).
Given that phenazine structures, and particularly that of PCN, contain multiple nitrogen atoms, one would predict that the type of nitrogen source provided would affect phenazine production. Generally, supplementation with amino acids stimulates phenazine production, but the effects of individual amino acids and inorganic nitrogen sources on phenazine production vary widely between species and conditions. Increasing levels of nitrogen provided as ammonium sulfate stimulated PCN production in P. chlororaphis PCL1391, but did not stimulate PCA production in P. fluorescens. Although glutamine is used to form the carboxamide functional group in PCN, the addition of this amino acid to the medium did not stimulate PCN production any more than other individual amino acids such as leucine. All aromatic amino acids stimulate PCN production in P. chlororaphis PCL1391, whereas the effects of phenylalanine, tryptophan, and tyrosine on PYO production in P. aeruginosa appear to be strain- and condition-dependent (Burton et al. 1947, Palmer et al. 2007). The effect of tryptophan in particular is at least partially related to its ability to serve as a precursor for quinolone biosynthesis (Farrow and Pesci 2007).
In both P. aeruginosa and P. chlororaphis PCL1391, PYO and PCN production, respectively, are maximized when the medium contains an intermediate level of phosphate; this is apparently not an artifact of effects on growth (Burton et al. 1947; van Rij et al. 2004). Iron and magnesium supplementation at micromolar levels is required and optimal for growth and phenazine production by P. aeruginosa and P. chlororaphis PCL1391. Because iron and magnesium are often provided as sulfate salts, it can be difficult to decouple their effects from that of varying the sulfur source. The importance of sulfate has been thoroughly evaluated in P. chlororaphis PCL1391, however, where millimolar concentrations are required for maximum production of PCN (van Rij et al. 2004).
2.4 Regulation of Phenazine Biosynthesis in Other Genera
In addition to the Pseudomonas species we have discussed, many diverse species belonging to other genera also produce phenazines with highly derivatized chemical structures (Table 2.1). Relatively little is known about the regulation of phenazine biosynthesis in these species, but recent studies have identified regulators that affect the process in strains of Burkholderia and Streptomyces (Ramos et al. 2010; Saleh et al. 2012). In Burkholderia cenocepacia K56-2, wild-type phenazine production requires a regulator called phenazine biosynthesis regulator (Pbr), which is encoded by a gene that lies near phzF and phzD homologues on the chromosome (Ramos et al. 2010). Pbr binds to the promoter region of the phzF-phzD operon and is required for wild-type expression. In Streptomyces anulatus 9663, regulators of phz gene expression have been identified through characterization of a large gene cluster that includes all of the genes required for PCA biosynthesis and genes required for transformation of PCA to the prenylated phenazines endophenazines A and E (Saleh et al. 2012). One of these regulators, encoded by the gene ppzV, is similar to a putative TetR-family regulator called EpzV found in S. cinnamonensis, another phenazine-producer. Inactivating the ppzV gene in a strain expressing the large phenazine biosynthetic cluster led to loss of the ability to produce prenylated phenazines but an increase in the amount of unprenylated phenazines, suggesting that the ppzV product regulates PCA derivatization. The second regulator, encoded by ppzY, is similar to transcriptional regulators of the LysR family. Inactivation of ppzY led to a nearly complete defect in all phenazine production, suggesting that the ppzY product is required for expression of PCA biosynthetic genes in S. anulatus 9663.
2.5 Conclusion
Characterization of the regulation of phenazine biosynthesis in diverse Pseudomonas isolates has revealed common mechanisms and hierarchies. As more of the mechanisms regulating phenazine biosynthesis in other genera are uncovered, it will be interesting to compare them to the Pseudomonas paradigm and evaluate their physiological relevance in these new contexts. The intricacy of the networks controlling phenazine production in Pseudomonas is becoming clear at a time when phenazines themselves are gaining recognition for their roles in bacterial physiology, which include intercellular signaling and redox balancing. The multilayered cascades that modulate phenazine biosynthesis are consistent with their new status as primary players in cellular metabolism and communication. Indications that not just the core genes for PCA synthesis, but also the genes for PCA modification, are regulated at multiple levels may suggest that different phenazines perform different physiological roles, consistent with their unique chemistries.
Although our understanding of the complicated networks controlling phenazine production is still developing, a hint at this complexity has long been evident in the variability of phenazine production that is apparent among species, isolates, and even repeat cultivations of the same strain. Differences in phenazine production among strains of the same species likely arise in part from subtle discrepancies in regulatory networks and sensing mechanisms. On the other hand, differences between repeat experiments imply that, although many of the conditions and regulators that affect phenazine production have been identified, unrecognized variables can still alter phenazine production in unpredictable ways. Elucidating the parameters and mechanisms that affect this process has the potential to facilitate the use of beneficial phenazine-producing pseudomonads in agriculture, support the development of therapeutics for patients suffering from P. aeruginosa infections, and allow us to learn new techniques for controlling antibiotic production in diverse species.
References
Abdou L, Chou HT, Haas D et al (2011) Promoter recognition and activation by the global response regulator CbrB in Pseudomonas aeruginosa. J Bacteriol 193(11):2784–2792
Ahuja EG, Janning P, Mentel M et al (2008) PhzA/B catalyzes the formation of the tricycle in phenazine biosynthesis. J Am Chem Soc 130(50):17053–17061
Argaman L, Elgrably-Weiss M, Hershko T et al (2012) RelA protein stimulates the activity of RyhB small RNA by acting on RNA-binding protein Hfq. Proc Natl Acad Sci USA 109(12):4621–4626
Balasubramanian D, Schneper L, Kumari H et al (2013) A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucl Acids Res 41(1):1–20
Ballard RW, Palleroni NJ, Doudoroff M et al (1970) Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol 60(2):199–214
Beatson SA, Whitchurch CB, Sargent JL et al (2002) Differential regulation of twitching motility and elastase production by Vfr in Pseudomonas aeruginosa. J Bacteriol 184(13):3605–3613
Behrends V, Ebbels TM, Williams HD et al (2009) Time-resolved metabolic footprinting for nonlinear modeling of bacterial substrate utilization. Appl Environ Microbiol 75(8):2453–2463
Birkofer L (1947) Das Verhalten der Diazoketone bei der katalytischen Hydrierung; eine neue Synthese des Threonins. Chem Berichte 80(1):83–94
Blumer C, Heeb S, Pessi G et al (1999) Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci USA 96(24):14073–14078
Bordi C, Lamy MC, Ventre I et al (2010) Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol Microbiol 76(6):1427–1443
Bourret RB, Silversmith RE (2010) Two-component signal transduction. Curr Opin Microbiol 13(2):113–115
Burrowes E, Abbas A, O’Neill A et al (2005) Characterisation of the regulatory RNA RsmB from Pseudomonas aeruginosa PAO1. Res Microbiol 156(1):7–16
Burrowes E, Baysse C, Adams C et al (2006) Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152(Pt 2):405–418
Burton MO, Eagles BA, Campbell JJ (1947) The amino acid requirements for pyocyanin production. Can J Res 25(4):121–128
Byng GS, Eustice DC, Jensen RA (1979) Biosynthesis of phenazine pigments in mutant and wild-type cultures of Pseudomonas aeruginosa. J Bacteriol 138(3):846–852
Caldwell CC, Chen Y, Goetzmann HS et al (2009) Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol 175(6):2473–2488
Chancey ST, Wood DW, Pierson LS 3rd (1999) Two-component transcriptional regulation of N-acyl-homoserine lactone production in Pseudomonas aureofaciens. Appl Environ Microbiol 65(6):2294–2299
Chin-A-Woeng TFC, van den Broek D, de Voer G et al (2001) Phenazine-1-carboxamide production in the biocontrol strain Pseudomonas chlororaphis PCL1391 is regulated by multiple factors secreted into the growth medium. Mol Plant Microbe Interact 14(8):969–979
Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ (2003) Phenzines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157:503–523
Choi EJ, Kwon HC, Ham J et al (2009) 6-Hydroxymethyl-1-phenazine-carboxamide and 1,6-phenazinedimethanol from a marine bacterium, Brevibacterium sp. KMD 003, associated with marine purple vase sponge. J Antibiot (Tokyo) 62(11):621–624
Chugani SA, Whiteley M, Lee KM et al (2001) QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 98(5):2752–2757
Clemo GR, Daglish AF (1948) Structure of the pigment of Chromobacterium iodinum. Nature 162(4124):776
De Maeyer K, D’Aes J, Hua GK et al (2011) N-Acylhomoserine lactone quorum-sensing signalling in antagonistic phenazine-producing Pseudomonas isolates from the red cocoyam rhizosphere. Microbiology 157(Pt 2):459–472
Deziel E, Lepine F, Milot S et al (2004) Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci USA 101(5):1339–1344
Dieppois G, Ducret V, Caille O et al (2012) The transcriptional regulator CzcR modulates antibiotic resistance and quorum sensing in Pseudomonas aeruginosa. PLoS ONE 7(5):e38148
Dietrich LE, Price-Whelan A, Petersen A et al (2006) The phenazine pyocyanin is a terminal signaling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61(5):1308–1321
Dietrich LE, Okegbe C, Price-Whelan A et al (2013) Bacterial community morphogenesis is intimately linked to the intracellular redox state. J Bacteriol. doi:10.1128/JB.02273-12
Diggle SP, Matthijs S, Wright VJ et al (2007) The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14(1):87–96
Egland KA, Greenberg EP (1999) Quorum sensing in Vibrio fischeri: elements of the luxl promoter. Mol Microbiol 31(4):1197–1204
Erickson DL, Lines JL, Pesci EC et al (2004) Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect Immun 72(10):5638–5645
Farrow JM 3rd, Pesci EC (2007) Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J Bacteriol 189(9):3425–3433
Farrow JM 3rd, Sund ZM, Ellison ML et al (2008) PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol 190(21):7043–7051
Fordos MJ (1859) Recherches sur la matière colorante des suppurations bleues: pyocyanine. Rec Trav Soc d’Émul Sci Pharm 3:30
Gallagher LA, McKnight SL, Kuznetsova MS et al (2002) Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol 184(23):6472–6480
Gebhardt K, Schimana J, Krastel P et al (2002) Endophenazines A-D, new phenazine antibiotics from the arthropod associated endosymbiont Streptomyces anulatus. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot (Tokyo) 55(9):794–800
Gerber NN (1967) Phenazines, phenoxazinones, and dioxopiperazines from Streptomyces thioluteus. J Org Chem 32(12):4055–4057
Gerber NN, Lechevalier MP (1964) Phenazines and phenoxazinones from Waksmania aerata sp. nov. and Pseudomonas iodina. Biochemistry 3:598–602
Gerber NN, Lechevalier MP (1965) 1,6-Phenazinediol-5-oxide from microorganisms. Biochemistry 4:176–180
Gessard C (1894) On the blue and green coloration that appears on bandages. Rev Inf Dis 6:S775–S776
Giddens SR, Feng Y, Mahanty HK (2002) Characterization of a novel phenazine antibiotic gene cluster in Erwinia herbicola Eh1087. Mol Microbiol 45(3):769–783
Girard G, Barends S, Rigali S et al (2006a) Pip, a novel activator of phenazine biosynthesis in Pseudomonas chlororaphis PCL1391. J Bacteriol 188(23):8283–8293
Girard G, van Rij ET, Lugtenberg BJJ et al (2006b) Regulatory roles of psrA and rpoS in phenazine-1-carboxamide synthesis by Pseudomonas chlororaphis PCL1391. Microbiology 152(Pt 1):43–58
Goodman AL, Kulasekara B, Rietsch A et al (2004) A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev Cell 7(5):745–754
Goodman AL, Merighi M, Hyodo M et al (2009) Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes and Dev 23(2):249–259
Haagen Y, Gluck K, Fay K et al (2006) A gene cluster for prenylated naphthoquinone and prenylated phenazine biosynthesis in Streptomyces cinnamonensis DSM 1042. ChemBioChem 7(12):2016–2027
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3(4):307–319
Hansford GS, Holliman FG, Herbert RB (1972) Pigments of Pseudomonas species. IV. In vitro and in vivo conversion of 5-methylphenazinium-1-carboxylate into aeruginosin A. J Chem Soc Perkin Trans I 1:103–105
Hassett DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177(22):6330–6337
Heeb S, Haas D (2001) Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact 14(12):1351–1363
Heeb S, Blumer C, Haas D (2002) Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J Bacteriol 184(4):1046–1056
Hendrickson EL, Plotnikova J, Mahajan-Miklos S (2001) Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. J Bacteriol 183(24):7126–7134
Herbert RB, Holliman FG (1969) Pigments of Pseudomonas species. II. Structure of aeruginosin B. J Chem Soc Perkin 1(18):2517–2520
Heurlier K, Denervaud V, Pessi G et al (2003) Negative control of quorum sensing by RpoN (sigma54) in Pseudomonas aeruginosa PAO1. J Bacteriol 185(7):2227–2235
Heurlier K, Williams F, Heeb S et al (2004) Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J Bacteriol 186(10):2936–2945
Holliman FG (1969) Pigments of Pseudomonas species. I. Structure and synthesis of aeruginosin A. J Chem Soc Perkin 1(18):2514–2516
Hsu JL, Chen HC, Peng HL et al (2008) Characterization of the histidine-containing phosphotransfer protein B-mediated multistep phosphorelay system in Pseudomonas aeruginosa PAO1. J Biol Chem 283(15):9933–9944
Huang J, Xu Y, Zhang H, Li Y, Huang X, Ren B, Zhang X (2009) Temperature-dependent expression of phzM and its regulatory genes lasI and ptsP in rhizosphere isolate Pseudomonas sp. strain M18. Appl Environ Microbiol 75(20):6568–6580
Huang J, Sonnleitner E, Ren B et al (2012) Catabolite repression control of pyocyanin biosynthesis at an intersection of primary and secondary metabolism in Pseudomonas aeruginosa. Appl Environ Microbiol 78(14):5016–5020
Hunter RC, Klepac-Ceraj V, Lorenzi MM et al (2012) Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol 47(6):738–745
Johnson LE, Dietz A (1969) Lomofungin, a new antibiotic produced by Streptomyces lomondensis sp. n. Appl Microbiol 17(5):755–759
Juhas M, Wiehlmann L, Huber B et al (2004) Global regulation of quorum sensing and virulence by VqsR in Pseudomonas aeruginosa. Microbiology 150(Pt 4):831–841
Kay E, Dubuis C, Haas D (2005) Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc Natl Acad Sci USA 102(47):17136–17141
Kay E, Humair B, Denervaud V et al (2006) Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol 188(16):6026–6033
Khan SR, Mavrodi DV, Jog GJ et al (2005) Activation of the phz operon of Pseudomonas fluorescens 2-79 requires the LuxR homolog PhzR, N-(3-OH-hexanoyl)-L-homoserine lactone produced by the LuxI homolog PhzI, and a cis-acting phz box. J Bacteriol 187(18):6517–6527
Khan SR, Herman J, Krank J et al (2007) N-(3-hydroxyhexanoyl)-l-homoserine lactone is the biologically relevant quormone that regulates the phz operon of Pseudomonas chlororaphis strain 30-84. Appl Environ Microbiol 73(22):7443–7455
Kojic M, Venturi V (2001) Regulation of rpoS gene expression in Pseudomonas: involvement of a TetR family regulator. J Bacteriol 183(12):3712–3720
Lapouge K, Sineva E, Lindell M et al (2007) Mechanism of hcnA mRNA recognition in the Gac/Rsm signal transduction pathway of Pseudomonas fluorescens. Mol Microbiol 66(2):341–356
Lasseur A (1911) These de Faculte des Sciences de l’Universite de Nancy
Lau GW, Ran H, Kong F et al (2004) Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun 72(7):4275–4278
Laursen JB, Nielsen J (2004) Phenazine natural products: biosynthesis, synthetic analogues, and biological activity. Chem Rev 104(3):1663–1686
Lechevalier H, Lechevalier MP (1965) Classification of aerobic actinomycetes based on their morphology and their chemical composition. Ann Inst Pasteur (Paris) 108(5):662–673
Ledgham F, Ventre I, Soscia C et al (2003) Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol Microbiol 48(1):199–210
Lee JH, Lequette Y, Greenberg EP (2006) Activity of purified QscR, a Pseudomonas aeruginosa orphan quorum-sensing transcription factor. Mol Microbiol 59(2):602–609
Liang H, Li L, Kong W et al (2009) Identification of a novel regulator of the quorum-sensing systems in Pseudomonas aeruginosa. FEMS Microbiol Lett 293(2):196–204
Mavrodi DV, Blankenfeldt W, Thomashow LS (2006) Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Annu Rev Phytopathol 44:417–445
Mavrodi DV, Peever TL, Mavrodi OV et al (2010) Diversity and evolution of the phenazine biosynthesis pathway. Appl Environ Microbiol 76:866–879
Mavrodi DV, Parejko JA, Mavrodi OV et al (2012) Recent insights into the diversity, frequency and ecological roles of phenazines in fluorescent Pseudomonas spp. Environ Microbiol:doi. doi:10.1111/j.1462-2920.2012.02846.x
McLaughlin HP, Caly DL, McCarthy Y et al (2012) An orphan chemotaxis sensor regulates virulence and antibiotic tolerance in the human pathogen Pseudomonas aeruginosa. PLoS ONE 7(8):e42205
Meighen EA (1991) Molecular biology of bacterial bioluminescence. Microbiol Rev 55(1):123–142
Mentel M, Ahuja EG, Mavrodi DV et al (2009) Of two make one: the biosynthesis of phenazines. ChemBioChem 10:2295–2304
Oglesby AG, Farrow JM 3rd, Lee JH et al (2008) The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J Biol Chem 283(23):15558–15567
Palmer KL, Aye LM, Whiteley M (2007) Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189(22):8079–8087
Pearson JP, Gray KM, Passador L et al (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 91(1):197–201
Pearson JP, Passador L, Iglewski BH et al (1995) A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92(5):1490–1494
Pesci EC, Milbank JB, Pearson JP et al (1999) Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 96(20):11229–11234
Pessi G, Williams F, Hindle Z, Heurlier K et al (2001) The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J Bacteriol 183(22):6676–6683
Petrova OE, Sauer K (2010) The novel two-component regulatory system BfiSR regulates biofilm development by controlling the small RNA rsmZ through CafA. J Bacteriol 192(20):5275–5288
Pierson LS 3rd, Pierson EA (2010) Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 86:1659–1670
Pierson LS 3rd, Keppenne VD, Wood DW (1994) Phenazine antibiotic biosynthesis in Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J Bacteriol 176(13):3966–3974
Ramos CG, Sousa SA, Grilo AM et al (2010) The Burkholderia cenocepacia K56-2 pleiotropic regulator Pbr is required for stress resistance and virulence. Microb Pathog 48(5):168–177
Rampioni G, Schuster M, Greenberg EP et al (2009) Contribution of the RsaL global regulator to Pseudomonas aeruginosa virulence and biofilm formation. FEMS Microbiol Lett 301(2):210–217
Recinos DA, Sekedat MD, Hernandez A et al (2012) Redundant phenazine operons in Pseudomonas aeruginosa exhibit environment-dependent expression and differential roles in pathogenicity. Proc Natl Acad Sci USA 109:19420–19425
Reimmann C, Beyeler M, Latifi A et al (1997) The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol 24(2):309–319
Reimmann C, Valverde C, Kay E et al (2005) Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J Bacteriol 187(1):276–285
Rojo F (2010) Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol Rev 34(5):658–684
Rusman Y, Oppegard LM, Hiasa H et al (2013) Solphenazines A-F, glycosylated phenazines from Streptomyces sp. strain DL-93. J Nat Prod 76(1):91–96
Saleh O, Flinspach K, Westrich L et al (2012) Mutational analysis of a phenazine biosynthetic gene cluster in Streptomyces anulatus 9663. Beilstein J Org Chem 8:501–513
Schertzer JW, Brown SA, Whiteley M (2010) Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol Microbiol 77(6):1527–1538
Schoental R (1941) The nature of the antibacterial agents present in Pseudomonas pyocyanea cultures. Brit J Exp Pathol 22(3):37–147
Schroeter J (1872) Ueber einige durch Bacterien gebildete Pigmente. Beiträge zur Biologie der Pflanzen Band 1(Zweites Heft):109–126
Schuster M, Hawkins AC, Harwood CS et al (2004) The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol 51(4):973–985
Seeger K, Flinspach K, Haug-Schifferdecker E et al (2011) The biosynthetic genes for prenylated phenazines are located at two different chromosomal loci of Streptomyces cinnamonensis DSM 1042. Microb Biotechnol 4(2):252–262
Siehnel R, Traxler B, An DD et al (2010) A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 107(17):7916–7921
Slininger PJ, Shea-Wilbur MA (1995) Liquid-culture pH, temperature, and carbon (not nitrogen) source regulate phenazine productivity of the take-all biocontrol agent Pseudomonas fluorescens 2-79. Appl Microbiol Biotechnol 43(5):794–800
Sonnleitner E, Haas D (2011) Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Appl Microbiol Biotechnol 91(1):63–79
Sonnleitner E, Abdou L, Haas D (2009) Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 106(51):21866–21871
Sonnleitner E, Gonzalez N, Sorger-Domenigg T et al (2011) The small RNA PhrS stimulates synthesis of the Pseudomonas aeruginosa quinolone signal. Mol Microbiol 80(4):868–885
Suh SJ, Silo-Suh L, Woods DE et al (1999) Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol 181(13):3890–3897
Tanabe I, Obayashi A (1971) Cultivation of Brevibacterium stationis var. iodiniofaciens and its iodinin production. Memoirs of the Faculty of Agriculture, Kagoshima University 8(1):373–389
Thompson LS, Webb JS, Rice SA et al (2003) The alternative sigma factor RpoN regulates the quorum sensing gene rhlI in Pseudomonas aeruginosa. FEMS Microbiol Lett 220(2):187–195
Tipton CD, Rinehart KL Jr (1970) Lomofungin. I. Degradative studies of a new phenazine antibiotic. J Am Chem Soc 92(5):1425–1426
Turner JM, Messenger AJ (1986) Occurrence, biochemistry and physiology of phenazine pigment production. Adv Microb Physiol 27:211–275
Valentini M, Lapouge K (2012) Catabolite repression in Pseudomonas aeruginosa PAO1 regulates the uptake of C(4) -dicarboxylates depending on succinate concentration. Environ Microbiol. doi:10.1111/1462-2920.12056
Valverde C, Heeb S, Keel C et al (2003) RsmY, a small regulatory RNA, is required in concert with RsmZ for GacA-dependent expression of biocontrol traits in Pseudomonas fluorescens CHA0. Mol Microbiol 50(4):1361–1379
van den Broek D, Chin-A-Woeng TFC, Eijkemans K et al (2003) Biocontrol traits of Pseudomonas spp. are regulated by phase variation. Mol Plant Microbe Interact 16(11):1003–1012
van Rij ET, Wesselink M, Chin-A-Woeng TFC et al (2004) Influence of environmental conditions on the production of phenazine-1-carboxamide by Pseudomonas chlororaphis PCL1391. Mol Plant Microbe Interact 17(5):557–566
Ventre I, Goodman AL, Vallet-Gely I et al (2006) Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci USA 103(1):171–176
Vincent F, Round A, Reynaud A et al (2010) Distinct oligomeric forms of the Pseudomonas aeruginosa RetS sensor domain modulate accessibility to the ligand binding site. Environ Microbiol 12(6):1775–1786
Von Saltza MH, Last JA, Stapleton PG et al (1969) Cyanomycin, its identity with pyocyanine. J Antibiot (Tokyo) 22(2):49–54
Wagner VE, Bushnell D, Passador L et al (2003) Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185(7):2080–2095
Wang D, Yu JM, Pierson LS 3rd et al (2012a) Differential regulation of phenazine biosynthesis by RpeA and RpeB in Pseudomonas chlororaphis 30-84. Microbiology 158(Pt 7):1745–1757
Wang G, Huang X, Li S et al (2012b) The RNA chaperone Hfq regulates antibiotic biosynthesis in the rhizobacterium Pseudomonas aeruginosa M18. J Bacteriol 194(10):2443–2457
Whiteley M, Lee KM, Greenberg EP (1999) Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 96(24):13904–13909
Wilderman PJ, Sowa NA, FitzGerald DJ et al (2004) Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci USA 101(26):9792–9797
Williams P, Camara M (2009) Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12(2):182–191
Wood DW, Pierson LS 3rd (1996) The phzI gene of Pseudomonas aureofaciens 30–84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168(1):49–53
Workentine ML, Chang L, Ceri H et al (2009) The GacS-GacA two-component regulatory system of Pseudomonas fluorescens: a bacterial two-hybrid analysis. FEMS Microbiol Lett 292(1):50–56
Wurtzel O, Yoder-Himes DR, Han K et al (2012) The single-nucleotide resolution transcriptome of Pseudomonas aeruginosa grown in body temperature. PLoS Pathog 8(9):e1002945
Xiao G, Deziel E, He J et al (2006) MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol Microbiol 62(6):1689–1699
Xu H, Lin W, Xia H et al (2005) Influence of ptsP gene on pyocyanin production in Pseudomonas aeruginosa. FEMS Microbiol Lett 253(1):103–109
Yu S, Jensen V, Seeliger J et al (2009) Structure elucidation and preliminary assessment of hydrolase activity of PqsE, the Pseudomonas quinolone signal (PQS) response protein. Biochemistry 48(43):10298–10307
Zhang X, Wang S, Geng H et al (2005) Differential regulation of rsmA gene on biosynthesis of pyoluteorin and phenazine-1-carboxylic acid in Pseudomonas sp. M18. World J Microbiol Biotechnol 21(6–7):883–889
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sakhtah, H., Price-Whelan, A., Dietrich, L.E.P. (2013). Regulation of Phenazine Biosynthesis. In: Chincholkar, S., Thomashow, L. (eds) Microbial Phenazines. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40573-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-40573-0_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40572-3
Online ISBN: 978-3-642-40573-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)