Abstract
In the katydid genus Neoconocephalus, males typically produce continuous calls with an extremely fast pulse rate of about 200/s. Divergence from this ancestral pattern includes alternation of pulse periods resulting in a double-pulse pattern, and the grouping of pulses into chirps. Double-pulse patterns evolved five times independently in the genus. Analysis of the female preferences and call recognition mechanisms revealed that in three species with double-pulse pattern, females have independently evolved new mechanisms for recognizing the derived call pattern. In the remaining two species with double-pulse pattern, females retain the ancestral recognition mechanism and exhibit no preference for the derived temporal pattern. These results suggest that males are leading the evolutionary divergence of call patterns in this genus. We propose a hypothetical scenario in which genetic bottlenecks and founder effects arising from the climatic history of North America contributed to the rapid diversification of calls in this genus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
The diversity of acoustic communication signals has long-fascinated naturalists and biologists of diverse interests. The evolutionary mechanisms generating and maintaining the diversity of signals and signal recognition mechanisms remain a controversial topic, despite a large body of research performed over the last four decades.
The importance of sexual selection and especially female choice for the diversification of communication signals has been widely recognized and well documented in many systems (Andersson and Simmons 2006). For example, in a radiation of Hawaiian crickets (genus Laupala), species differ significantly in the pulse rate of male calls, and female preferences for pulse rate provide an explanation for this diversity (Grace and Shaw 2011; Mendelson and Shaw 2005). Directional female preferences for certain call parameters that potentially encode male quality or condition (e.g., chirp/pulse rate, chirp duration, and carrier frequency) are common (Andersson 2006). However, among closely related species, such directional preferences typically point in the same direction (e.g., toward longer calls or lower carrier frequency) and thus are unlikely to contribute to call divergence. Accordingly, call traits under directional sexual selection typically are not divergent among closely related species.
In contrast, call traits that establish diversity, such as differences in call pattern or rhythm, are typically under stabilizing selection by female preferences (Helversen and Helversen 1994). Such a situation is less obviously explained by sexual selection models of signal evolution. To understand the evolutionary processes that lead to this divergence, comparative studies involving many species are necessary. We summarize here a series of studies on the evolution of a novel call character, the “double-pulse pattern” in Neoconocephalus (Orthoptera, Tettigoniidae). This call character evolved multiple times convergently in this group, and the phenotypes of female call recognition support a rarely considered model of divergence. We contrast the situation in Neoconocephalus with that in a similarly well studied group of Hawaiian crickets.
10.2 The Katydid Genus Neoconocephalus
The natural history and acoustic communication system has been reviewed in detail by Greenfield (1990). In short, the American genus Neoconocephalus is most likely the closest relative of the genus Ruspolia, which occurs throughout the western hemisphere. About 25 Neoconocephalus species exist in the Caribbean, Central and North America; the South American Neoconocephalus fauna has not been systematically revised and it is unclear how many additional species exist in this range (Greenfield 1990).
All Neocononcephalus species are grassland species with typical habitats ranging from marshes to prairies. Most species have wide areas of occurrence with overlapping ranges. Males produce calls by rubbing their forewings (elytra) against each other. Females are silent and, when receptive, walk or fly toward calling males (phonotaxis). It is common to hear up to four species calling at the same time in one habitat. Morphological diversity among the Neoconocephalus species is limited and the male calls are the main diagnostic character, in addition to the shape and coloration of the cone (fastigium) between the antennae (Walker and Greenfield 1983; Walker 2012).
All Neoconocephalus and Ruspolia species have long wings and are strong fliers. Long range dispersal has been observed in several species. For example, Ruspolia swarms have been observed on ships in the Atlantic, several hundreds of km from the African coast, supporting the hypothesis that the American Neoconocephalus fauna originated through colonization from African Ruspolia (reviewed in Greenfield 1990). We found calling N. triops more than 150 km north of their known range following the occurrence of strong low pressure weather systems. The high mobility predisposes Neoconocephalus to rapid colonization of new habitats (e.g., after glaciations) and thus might play an important role in the evolution of the call diversity.
10.3 Diversity of Male Calls in Neoconocephalus
Male calls in Neoconocephalus are unusual for Tettigoniids in two respects. First, the spectral energy is concentrated in a narrow low frequency band with center frequency typically at 10–15 kHz, and with ultrasonic components strongly attenuated (Schul and Patterson 2003). Second, the rate of the pulses produced by the repetitive opening and closing movements of the forewings is typically extremely high at 150–250 Hz (Greenfield 1990).
We conducted a Bayesian character state analysis of male call traits based on the molecular phylogeny of Neoconocephalus (Snyder et al. 2009). This analysis revealed that the ancestral call state is a continuous ‘buzz’ consisting of pulses repeated with a single, fast repetition rate (Fig. 10.1). Three derived call patterns occur in Neoconocephalus: (1) discontinuous calls, where pulses are grouped in a second order time structure (chirps, or verses), (2) a dramatic reduction of the pulse rate below 50 Hz, and (3) the presence of two alternating pulse periods, which results in pulse pairs or ‘double-pulses.’ Five species in Neoconocephalus have this double-pulse pattern. Quite astonishingly, this novel call trait occurs at five tips in the phylogeny (Fig. 10.1, arrows). Bayesian character state reconstruction confirmed that this novel call trait evolved at least five times independently in this group, providing an excellent opportunity to study the evolutionary processes underlying the diversification of the communication system.
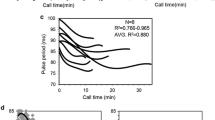
Diversity of male calls in Neoconocephalus katydids. Three lines of call diversification occur in this genus (a–c). a Female N. bivocatus on the ‘Kramer Kugel’. b Most species produce a single-pulse period repeated at extremely fast rates, with loud pulses produced during the closing movements of the tegmina (top trace), while five species have alternating pulse periods, resulting in a double-pulse pattern (bottom trace). c A second line of diversification is a dramatic reduction of pulse rate. Filled arrowheads indicate sounds produced during the closing movements of the tegmina, open triangles those during the opening movement. d Most species produce continuous calls (top), while some have added a second order time structure (verses or chirps) to their calls (bottom). e Phylogenetic relationship among 17 Neoconocephalus species; total evidence tree based on Amplified Fragment Length Polymorphisms (AFLP) and nuclear and mitochondrial DNA sequences (from Snyder et al. 2009). The support values of all in-group nodes are >0.98. Arrows denote the five species with calls with double-pulse pattern. T and M labels indicate the ‘temperate’ and ‘N. maxillosus’ clades referenced in the text
The distribution of call diversity among Neoconocephalus species shows an interesting pattern of rapid evolutionary diversifications as well as phenotypic stability. Seven temperate species form one monophyletic clade (label T in Figs. 10.1, 10.2), which contains the same diversity in call traits as is found in the complete genus (Fig. 10.2). The sibling taxon to the temperate clade is the tropical species N. triops, which has an extremely large range from the southern USA to Argentina (Greenfield 1990). While pulse rate varies by about 20 % across this range, all populations studied so far have the derived double-pulse pattern. However, the genetic diversity among different Caribbean and Central American populations of N. triops is greater than that among the seven species of the temperate clade, which have much higher phenotypic call diversity. Applying standard molecular clocks (Brower 1994) to the ultrametric tree (Fig. 10.2) suggests that the radiation of the temperate species occurred within the last 100,000 years, i.e., within the last glacial cycle. Similarly short divergence times appear in another branch of the phylogeny (the N. maxillosus clade, M in Figs. 10.1, 10.2), which is similar in call diversity. The genus thus includes examples of both stability of call phenotypes despite genetic diversity in N. triops, and call diversification despite little genetic diversity within the temperate and N. maxillosus clades.
Relative time tree of the genus Neoconocephalus. The branch lengths indicate the relative timing of divergence with the numbers indicating the relative age of the nodes. Estimates of the divergence time of the temperate clade (green shading) range from 18,000 to 72,000 years, suggesting that the call divergence in this clade evolved during the last glacial cycle (for methods see below). The species are labeled as in Fig. 10.1. For N. triops (purple shading), four populations are given separately (TT Trinidad, PR Puerto Rico, CR Costa Rica, US continental USA). The columns on the right show the presence of the three derived call traits in each species (DC, discontinuous calls, red; DP double pulses, blue; S slow pulse rate, yellow) with filled squares indicating the derived state and open squares the ancestral state (continuous calls, single-pulse pattern, fast pulse rate, respectively). The labels indicate the first two letters of the species name. T and M indicate the ‘temperate’ and ‘N. maxillosus’ clades referenced in the text (Methods: Tree is based on mitochondrial DNA sequences (CO1, 875 bp), which are included in the total evidence tree in Fig. 10.1. It was built using Bayesian methods in BEAST (v. 1.72; Drummond et al. 2006; Drummond and Rambaut 2007). Divergence times were estimated using BEAST and r8 s (v. 1.71; Sanderson 2003). Mutation rates were assumed in the range from 1.1 to 3.83 %/per million years.)
10.4 Double-Pulse Pattern: A Novel Signal Trait in Neoconocephalus
In species with the ancestral ‘single-pulse pattern’, males call by opening and closing their forewings without interruptions: the wings do not rest in the closed position before the new cycle begins. The derived double-pulse pattern in Neoconocephalus is produced by four movements: a full opening of the forewings, a partial closing, a partial re-opening, and a full closing (Fig. 10.3; Walker 1975). The two sound pulses produced during the closing movements have alternating pulse periods and typically different durations and amplitudes: the pulses produced during the partial closing movement are shorter and of lower amplitude than the ones produced during the full closure (Fig. 10.3; Walker 1975). In all five species with double-pulse pattern, the double-pulses are repeated without a silent interval occurring between them, indicating that the forewings do not rest between the double-pulses. This is markedly different from other Tettigoniid species with double-pulses, where wing movement stops and extended silent intervals separate double-pulses (Fig. 10.3; Heller 1988). The similarity of the motor pattern among the five Neoconocephalus species suggests that the same changes of the motor pattern (and probably similar mutations) underlie the independent evolution of the double-pulse pattern.
Forewing movements (top trace) and sound pulses (bottom trace) during call production of a N. robustus, a species with single-pulse pattern, and three species with double-pulse pattern: b N. bivocatus, c Tettigonia viridissima, d Metrioptera oblongicollis. In N. bivocatus the wings do not stop between double-pulses, while in other Tettigoniid species the wings rest between double-pulses. Note the differences in time scale between a, b and c, d. From Walker (1975) (a, b) and Heller (1988) (c, d)
Anecdotal findings of hybrid individuals give additional clues regarding the evolution of the double-pulse pattern. Hybrid individuals between N. bivocatus (a double-pulse species) and N. nebrascensis (a single-pulse species with discontinuous calls) produce a double-pulse pattern within the verse structure of N. nebrascensis (Büttner 2002, and unpublished). Thus, the double-pulse pattern does not mix with the verse pattern of N. nebrascensis, indicating that double-pulses and verse structure are not homologous traits. Thus, double-pulses are a modification of the single-pulse pattern, rather than an added second order time structure.
In addition to the five occurrences in Neoconocephalus, double-pulsed calls also evolved in many other Tettigoniid genera, e.g., Tettigonia, Platycleis, Metrioptera, Decorana, Sepiana, Tessellana (Heller 1988), Atlanticus (Walker 1975), and Orchelimum (Thomas and Alexander 1962). Double-pulse rhythms are also found in other signal modalities, e.g., in the vibratory signals of the katydid genus Meconem (Heller 1988), or in the blinking patterns of courting fireflies (Coleoptera: Lampyridae; Lloyd 1984). The propensity of Neoconocephalus species to evolve the double-pulse pattern, and the prevalence of such double-pulse rhythms across communication modalities and phylogenetic groups, suggests a predisposition of basic neuronal rhythm generators to switch from a single-pulse rhythm to this derived pattern. If such a predisposition exists, it is plausible that only a small number of mutations—potentially even a single mutation—might be required for this novel pattern to arise.
10.5 Female Call Recognition and Preferences
To the human ear, Neoconocephalus calls with fast pulse rates and single-pulse patterns sound continuous without amplitude modulation. Due to nonlinearities in our auditory signal transduction, we perceive a fast pulse rate of about 200 Hz as the pitch of a pure tone underlying the high pitched carrier signal (McDermott and Oxenham 2008).
In species with a fast single-pulse pattern, female preferences reflect human perception: Females respond to continuous signals without amplitude modulation (Fig. 10.4a), but are highly selective against signals that contain silent intervals longer than a few ms (Deily and Schul 2004, 2009). Females are not selective for any particular temporal pattern (e.g., pulse rate or pulse duration), as long as the signal does not contain longer silent intervals. While this call recognition mechanism does not select for a specific temporal pattern, it forces males to maintain a fast pulse rate that is effectively perceived as a continuous signal. It is worth noting that the female call recognition mechanism accepts a much wider range of the signal parameter space (defined by pulse and interval duration) than is occupied by the conspecific male calls (Fig. 10.4b). This does not mean that females are unselective, as they are highly selective for the interval duration. However, the large accepted parameter space provides the potential for diversification of the temporal call pattern, without losing attractiveness (Deily and Schul 2004).
Call recognition in female N. robustus, a species with fast single-pulse calls. a Phonotaxis scores (mean ± s.e.m., n = 10) in response to a call model consisting of pulses (p) and an unmodulated sine wave (s). b Response field of N. robustus females to combinations of pulse interval and duration. The gray area denotes the range of stimulus parameters that elicit a significant phonotactic response. After Deily and Schul (2004)
This mechanism in which females respond to calls that lack silent gaps has been described in five species (Greenfield 1993; Deily and Schul 2004, 2009; Bush and Schul 2010) and occurs in at least two more (N. exciliscanorus, N. palustris, unpublished data). The distribution of this recognition mechanism in the phylogeny of Neoconocephalus makes it very likely that it represents the ancestral state. In species with a verse or chirp pattern (N. nebrascensis, N. exciliscanorus) an additional mechanism is involved in recognizing the second order time structure (Deily and Schul 2009, unpublished results).
10.5.1 Recognition of Calls with Double-Pulse Pattern: Three Species with Derived Mechanisms
Male calls of N. bivocatus and N. triops have fast double-pulse rates of 80–110/s, i.e., they maintained the ancestral fast pulse rate of approximately 200 wingstrokes/s. Females of both species recognize the conspecific rate of the double-pulses: they respond with phonotaxis only if the rate of the double-pulses is in the correct range (Deily and Schul 2004; Beckers and Schul 2008). The actual double-pulse structure of the call is not necessary, as double-pulses can be replaced by a single pulse of appropriate length. Females respond to the correct (double-) pulse rate in a wide range of duty cycles (Fig. 10.5a,b). Although preferences were similar in these two species, they appear to differ in the mechanisms generating them. In N. triops, call recognition is most likely generated by a neuronal oscillation: females show significant responses to half the double-pulse rate and to stimuli with rhythmically altered intervals (Fig. 10.5c). In N. bivocatus, in contrast, we could not detect either of these responses (Fig. 10.5c), suggesting that a different neuronal mechanism (e.g., temporal integration) underlies rate recognition (see Bush and Schul 2006 for a detailed discussion of the experimental logic).
Call recognition in female N. bivocatus and N. triops, two species with double-pulse pattern. a Phonotaxis scores in response to call models with the conspecific double-pulse pattern (dp), an unmodulated sine wave (s), and a model with the double-pulses merged into one long pulse (mrg). b Response fields: the gray area denotes the range of stimulus parameters that elicit significant responses. c Phonotaxis scores (mean ± s.e.m, n = 8–10) to stimuli in which every second interval was stretched, either rhythmically (r) so that all pulses fell at times where pulses occur in the standard model (std), or arrhythmically (a-1, a-2), so that half of the pulses occurred during silent intervals in the standard model. Strong responses to the rhythmical stimulus (r) indicate rate recognition based on neuronal resonance. Data for a, b taken from Deily and Schul (2004), and Beckers and Schul (2008)
In both species, females respond to a distinctly different pattern than is generated by the conspecific males: females recognize a single rate (i.e., the repetition rate of the double-pulses) while males produce pulses with two alternating rates. This “nonparallel” evolution of male calls and female recognition (Schul and Bush 2002) is a strong argument against genetic coupling of the sender and receiver subunits of the communication system (Butlin and Ritchie 1989; Bloake 1991), which was recently implicated in the diversification of a cricket communication system (Shaw and Lesnick 2009; Wiley and Shaw 2010). In Neoconocephalus, it is unlikely that the same mutation(s) would alter the call pattern generator toward double-pulses in males and at the same time halve the preferred pulse rate in females. Furthermore, as described below, the change in male call is not always accompanied by a change in female call recognition. The nonparallel changes of sender and receiver indicate that they are mainly linked through the function of the communication system, rather than by shared genetics. This raises the question of how the match between sender and receiver is assured during the evolution of the novel calls and recognition mechanisms. We propose an evolutionary scenario in the final section of this chapter.
Male calls of N. affinis also have a double-pulse pattern, however, the wingstroke rate is much slower with approximately 24 wingstrokes/s or 12 double-pulses/s. Female N. affinis evaluate the double-pulse rate and respond when it is near the conspecific value. In contrast to the previous two species, the double-pulse pattern is necessary and double-pulses cannot be replaced by one long pulse Fig. 10.6. Extensive testing (Bush et al. 2009) revealed that females recognize the amplitude modulation (AM) rate in the frequency domain (Schmidt et al. 2008) and require not only the rate of the double-pulses, but also its first harmonic (i.e., the wingstroke or single-pulse rate). A detailed behavioral analysis of the recognition mechanisms indicated that neuronal resonance underlies the recognition of at least the fundamental AM rate (Bush et al. 2009).
The double-pulse pattern of male calls evolved in these three species independently. Accordingly, the mechanisms of female call recognition differ among the three species, also indicating independent origins of these derived preferences. While the derived male pattern is similar in all three species and thus appears to be caused by similar changes in the neuronal call pattern generator, the three derived call recognition mechanisms differ qualitatively among the three species, suggesting major differences in auditory processing. This makes a common predisposition for the recognition of double-pulses unlikely in Neoconocephalus.
10.5.2 Recognition of Calls with Double-Pulse Pattern: Two Species Retain the Ancestral Mechanisms
Male calls of N. retusus and N. maxillosus both have the double-pulse pattern with wingstroke rates close to the ancestral state of about 200/s. Although the pattern is clearly double-pulsed, the difference between the two alternating pulse periods is less pronounced than in the three species previously described (Bush and Schul 2010). Surprisingly, females of neither species required the double-pulse pattern for call recognition but responded well to calls with single-pulse pattern or to continuous signals without AM Fig. 10.7a. Females responded well when the intervals between pulses were short enough; signals with longer intervals were unattractive, independent of the pulse rate (Bush and Schul 2010). Thus, female call recognition was identical to that of species with single-pulse pattern, i.e., it remains in the ancestral state, while male calls have a derived temporal pattern. Furthermore, females did not show preferences for either their derived conspecific pattern or the ancestral single-pulse pattern, as the intervals between double-pulses are short enough for female call recognition Fig. 10.7b (Bush and Schul 2010).
This pattern in which females are in the ancestral state and males are in the derived state means that in these two species, males are leading the evolutionary divergence of the communication system and that female choice is not the driving force. Other factors linked to female choice, such as localizability or the transmission of the preferred pattern through the habitat, do not play a role here: localizability was included in the evaluation of the female preferences, and degradation of the pulse pattern does not interfere with the ancestral call recognition mechanism, as echoes would mask silent intervals (Bush and Schul 2010). Male–male interactions in Neoconocephalus take place at the chirp, but not at the pulse level (Meixner and Shaw 1986; Greenfield 1983, 2005), at time scales an order of magnitude longer than the pulse pattern in these species. Thus, intrasexual competition also fails to explain the novel call pattern here.
Natural selection may also have profound influence on signal traits. Eavesdropping predators/parasitoids and energetic costs may lead to the evolution of novel traits (Zuk and Kolluru 1998). Neoconocephalus katydids are commonly hosts of acoustically orienting tachinid flies. The double-pulse pattern seemingly does not provide an advantage, as species with double-pulse pattern are among the heavily parasitized species; infection rates of 75–90 % occur both in single and double-pulse species (Burk 1982; Talwar 2007, see Chap. 4 by Hedwig and Robert). The double-pulse pattern also has the same number of pulses as an equivalent single-pulse pattern and should have similar energetic cost. If there were selection to save energy during calling, male N. retusus or N. maxillosus could have reduced the pulse rate while maintaining the single-pulse pattern; double-pulses introduce longer silent intervals and thus impose a faster minimum pulse rate (=wing stroke rate) than single-pulses to remain attractive. As discussed in detail by Bush and Schul (2010), neither sexual nor natural selection provide convincing explanations for the evolution of the double-pulse pattern in N. retusus and N. maxillosus. This leaves the hypothesis of neutral evolution, i.e., that the novel call pattern evolved through mutations and genetic drift. It is important to bear in mind that neutral evolution is the evolutionary null hypothesis, which can only be supported by the absence of evidence for alternative explanations. Of course, one can never exclude the possibility that selection acted on this trait in the past but is no longer detectable. In the absence of convincing arguments for selective advantages of the double-pulse pattern, however, neutral evolution is the most parsimonious explanation.
Call recognition in female N. affinis, a species with double-pulse pattern and a much slower pulse rate than typical for Neoconocephalus (approximately 12 double pulses/s). Phonotaxis scores (mean ± s.e.m., n = 8–11) to a recording (1) and model (2) of a conspecific call were high, while a continuous sine wave (3) did not elicit responses. A model with the double-pulse replaced by one long pulse was also unattractive (4); adding a second amplitude modulation during this long pulse (5, 6) restored the attractiveness of the model. Thus, the double-pulse structure is necessary for female responses, which differs from the other two species shown in Fig. 10.5. Data from Bush et al. (2009)
Call recognition and preferences in female N. retusus and N. maxillosus, two species with double-pulse pattern. a Phonotaxis scores (mean ± 95 % confidence interval, n = 6–8) in response to call models with double-pulses (dp) and unmodulated sine waves (s). Strong responses to the sine wave resemble the situation in single-pulse species (Fig. 10.4) rather than that in double-pulse species (Figs. 10.5, 10.6). b Absence of female preference for the double-pulse structure. Phonotaxis scores (mean ± 95 % CI, n = 9–10) of N. retusus females toward calls that vary from single-pulses to extreme double-pulses as measured by the ratio of period 2/period 1 (p2/p1, see inset). The experiment was run in two consecutive years with different females. The arrow indicates the mean ratio found in natural male calls
10.6 A Scenario for the Evolution of Double-Pulse Pattern in Neoconocephalus
We develop here a scenario for the evolution of the novel call traits and recognition mechanisms in Neoconocephalus based on the findings that (1) the double-pulse pattern evolved five times independently, (2) the females of three species have derived call recognition mechanisms, whereas (3) in two species, female call recognition remains in the ancestral state and no convincing adaptive explanation for the double-pulse pattern exists.
We propose that the few mutations required to change the male call pattern to a double-pulse pattern happened as the first step, with female recognition remaining in the ancestral state. As long as this mutant/derived call pattern still falls within the attractive range of female call recognition, i.e., if the intervals between double-pulses are short enough to be tolerated, it will not reduce the attractiveness of the derived calls (Fig. 10.4). Thus, the new call pattern would be selectively neutral and could persist in the population, resulting in populations in which some males produce the ancestral single-pulse pattern and others the new double-pulse pattern.
The likelihood of a neutral trait becoming fixed in a large population is low. The ecological history of North America, however, suggests that founder events, genetic bottlenecks, and population fragmentation have likely been frequent occurrences in the history of this genus, greatly increasing the probability of fixation of a neutral trait through genetic drift. Throughout the Pleistocene, ranges of Neoconocephalus have changed dramatically as a result of repeated glacial—interglacial cycles. During glaciations, habitats suitable for Neoconocephalus in North America were restricted to small stretches near the Gulf of Mexico (Adams 1997). The Neotropics were much drier, such that ranges of tropical Neoconocephalus were certainly very different from the ranges that exist today. During interglacials, suitable habitats spread through the Caribbean and eastern parts of North America. Strong flight muscles and a capacity for long range dispersal provided opportunities for rapid range expansion of Neoconocephalus as habitats became suitable.
Even on a much shorter time scale, Neoconocephalus distributions are fluid. Over the past 12 years, we have observed two dramatic changes in population sizes, including one local near-extinction of a N. robustus population and a large-scale regional drop by >90 % of N. nebrascensis. In both cases, population levels returned to previous sizes within a few years. Founder and bottleneck effects are therefore likely to have been common during the diversification of Neoconocephalus.
Given their high mobility, it is conceivable that new populations were frequently founded by few individuals. If the founders of such new populations had the mutation(s) for a novel call trait, a population with the derived call pattern would be established and have the opportunity to diverge at least somewhat in other traits, before secondary contact with populations in the ancestral state occurred. We hypothesize that N. maxillosus and N. retusus originated in this manner and that the communication system has remained in this state with derived male calls and ancestral female call recognition.
Mutations that influence female call recognition inevitably occur, too. While we generally think of female preferences as quantitative traits which evolve gradually, recent advances in computational neuroscience have demonstrated the qualitative effect that small changes in ion channel composition may cause. For example, adding or replacing a single channel may change the response type of a neuron from integrator to resonator (or vice versa) (Izhikevich 2001). Thus, even a single mutation might have a significant impact on call recognition.
In most cases, mutations of the call recognition mechanism that lead to significant changes of selectivity would not respond to the conspecific (ancestral) call pattern and would thus quickly disappear from the population. In a population in which the derived call trait (e.g., double-pulses) is either common or fixed, there are two scenarios by which a mutation in the female recognition system may spread. First, if the new recognition mechanism does respond well enough to a calling male to elicit a female approach, then founder and bottleneck effects could cause fixation (perhaps followed by refinement) of the derived recognition mechanism. Given the dispersal capabilities of Neoconocephalus and the quickly changing habitats at the end of glaciation, even a single female with the derived recognition mechanism could establish a new, isolated population as these insects spread into newly available habitats following the end of an ice age.
An alternative scenario involves selection rather than genetic drift. The mutation of the recognition system may appear in a population in which the double-pulse pattern is already fixed but call recognition remains in the ancestral state (i.e., similar to N. retusus or N. maxillosus). When this population comes into contact with a different species with single-pulse calls, females with the novel call recognition (responding to double-pulses only) would have an advantage over females with ancestral recognition, as they would avoid heterospecific matings. The novel call recognition would thus be selected for and spread through the population. Either of these scenarios would result in a population fixed both in a novel call pattern and in a novel call recognition mechanism that prefers the novel over the ancestral call pattern.
Given the apparent ease with which double-pulse calls arose in this genus, it is possible that double-pulses evolved and disappeared multiple times in both the single- and double-pulse species throughout history. The new call trait would have been stabilized by a novel female preference, while in the absence of a change in female preference, the call trait may be lost again through drift. In this respect, the current situation in N. maxillosus and N. retusus, with males in the derived state and females in the ancestral state, may be temporary in evolutionary time.
While we propose that the double-pulse pattern evolved in Neoconocephalus through genetic drift, sexual selection has clearly influenced other call parameters within the genus. For example, the call of N. robustus has a significantly lower carrier frequency than its congeners, probably as a result of selection favoring females that avoided heterospecific matings with N. bivocatus (Deily and Schul 2004, 2006). In addition, the female preference for leading males in N. spiza (Greenfield and Roizen 1993; Greenfield and Schul 2008) may have arisen as a result of benefits obtained by females who mate with leading males.
10.7 Comparison to Hawaiian Crickets
The co-evolution of male calls and female preferences has been studied in detail in the Hawaiian cricket genus Laupala (Orthoptera, Gryllidae). Here, male calls vary widely in pulse rate among species, and female preferences are tuned to conspecific pulse rates, functioning as premating isolation barriers (Grace and Shaw 2011; Shaw and Herlihy 2000). Many small effect mutations likely underlie this diversity (Ellison et al. 2011). Indirect (Shaw and Lesnick 2009; Wiley and Shaw 2010) and direct (Wiley et al. 2012) evidence indicates that male calls and female preferences are genetically linked through multiple loci, assuring the function of the communication system during diversification. This is in stark contrast to Neoconocephalus, where call production and call recognition seemingly evolved much more independently and may involve few mutations with large effects.
Differences in ecology between Neoconocephalus and Laupala provide a potential explanation for the contrasting evolutionary scenarios. Laupala divergence occurred across the Hawaiian islands over the last 4–6 million years in a relatively stable tropical environment, with newly appearing islands providing space for diversification. In contrast, repeated cycles of ice-ages and interglacials dramatically altered the climate and habitats where Neoconocephalus evolved and occurs today. These different climatic conditions might favor different evolutionary processes resulting in contrasting phenotypic and genetic patterns. We acknowledge that we have not yet identified the genetic basis underlying the diversity of the communication system in Neoconocephalus, but rather infer our hypothetical scenario from a large collection of phenotypic, phylogenetic and ecological data. Additional studies including the genetic as well as neuronal basis of Neoconocephalus communication are ultimately needed to provide a complete and conclusive picture. At the current stage, Neoconocephalus serves as a reminder that evolutionary patterns are complex and might lead to surprising explanations.
10.8 Conclusion
Among Neoconocephalus katydids, diversity in the communication system consists largely of qualitative changes in both male calls and female recognition mechanisms. In some species, changes in male calls have preceded changes in female recognition, suggesting that males rather than females may lead the divergence of the communication system, and that sender and receiver may evolve more independently of each other than is commonly assumed. The rate of diversification since the last glacial cycle suggests that genetic drift (e.g., founder effects) may have contributed to the radiation within this genus. Further research is needed to identify the mechanisms of divergence, including potential differences in patterns of divergence between organisms living in tropical versus temperate ecological zones.
References
Adams J (1997) Preliminary vegetation maps of the world since the last glacial maximum: an aid to archaeological understanding. J of Archaeol Sci 24:623–647
Andersson M (2006) Condition-dependent indicators in sexual selection: development of theory and tests. In: Lucas JR, Simmons LW (eds) Essays in animal behaviour: celebrating 50 years of animal behaviour. Elsevier, Amsterdam, pp 253–267
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol (Amst) 21:296–302. doi:10.1016/j.tree.2006.03.015
Beckers OM, Schul J (2008) Developmental plasticity of mating calls enables acoustic communication in diverse environments. Proc R Soc Lond B 275:1243–1248
Bloake CRB (1991) Coevolution of sender and receiver of sexual signals: genetic coupling and genetic correlations. Trends Ecol Evol 6:225–227. doi:10.1016/0169-5347(91)90027-U
Brower AVZ (1994) Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc Natl Acad Sci USA 91:6491–6495
Burk TE (1982) Evolutionary significance of predation on sexually signaling males. Fla Entomol 65:90–104
Bush SL, Schul J (2006) Pulse-rate recognition in an insect: evidence of a role for oscillatory neurons. J Comp Physiol A 192:113–121. doi:10.1007/s00359-005-0053-x
Bush SL, Schul J (2010) Evolution of novel signal traits in the absence of female preferences in Neoconocephalus katydids (Orthoptera, Tettigoniidae). PLoS ONE 5(8):e12457
Bush SL, Beckers OM, Schul J (2009) A complex mechanism of call recognition in the katydid Neoconocephalus affinis (Orthoptera: Tettigoniidae). J Exp Biol 212:648–655. doi:10.1242/jeb.024786
Butlin R, Ritchie MG (1989) Genetic coupling in mate recognition systems: what is the evidence. Biol J Linn Soc 37:237–246. doi:10.1111/j.1095-8312.1989.tb01902.x
Büttner UK (2002) Charakterisierung der Gesänge von fünf in Missouri (USA) heimischen Neoconocephalus-Arten (Orthoptera, Tettigoniidae). Diplom Thesis, Friedrich Alexander University, Erlangen
Deily JA, Schul J (2004) Recognition of calls with exceptionally fast pulse rates: Female phonotaxis in the genus Neoconocephalus (Orthoptera: Tettigoniidae). J Exp Biol 207:3523–3529
Deily JA, Schul J (2006) Spectral selectivity during phonotaxis: a comparative study in Neoconocephalus (Orthoptera, Tettigoniidae). J Exp Biol 209:1757–1764
Deily JA, Schul J (2009) Selective phonotaxis in Neoconocephalus nebrascensis (Orthoptera: Tettigoniidae): call recognition at two temporal scales. J Comp Physiol A 195:31–37
Drummond AJ, Rambaut A (2007) BEAST: bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88
Ellison CK, Wiley C, Shaw KL (2011) The genetics of speciation: genes of small effect underlie sexual isolation in the Hawaiian cricket Laupala. J Evolution Biol 24:1110–1119. doi:10.1111/j.1420-9101.2011.02244.x
Grace JL, Shaw KL (2011) Coevolution of male mating signal and female preference during early lineage divergence ofthe Hawaiian cricket, Laupala cerasina. Evolution 65:2184–2196. doi:10.1111/j.1558-5646.2011.01278.x
Greenfield MD (1983) Unsynchronized chorusing in the coneheaded katydid Neoconocephalus affinis (Beauvois). Anim Behav 31:102–112
Greenfield MD (1990) Evolution of acoustic communication in the genus Neoconocephalus: discontinuous songs, synchrony, and interspecific interaction. In: Bailey W, Rentz D (eds) The Tettigoniidae: biology, systematics, and evolution. Springer, New York, pp 72–97
Greenfield MD (1993) Inhibition of male calling by heterospecific signals: artifact of chorusing or abstinence during suppression of female phonotaxis? Naturwissenschaften 80:570–573
Greenfield MD (2005) Mechanisms and evolution of communal sexual displays in arthropods and anurans. Adv Study Behav 35:1–62
Greenfield MD, Roizen I (1993) Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364:618–620
Greenfield MD, Schul J (2008) Mechanisms and evolution of synchronous chorusing: emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: Tettigoniidae). J Comp Psychology 122:289–297
Heller KG (1988) Bioakustik der Europäischen Laubheuschrecken. Margraf, Weikersheim
Ov Helversen, Dv Helversen (1994) Forces driving coevolution of song and song recognition in grasshoppers. In: Schildberger K, Elsner N (eds) Neural basis of behavioural adaptations. Fischer, Stuttgart, pp 253–284
Izhikevich EM (2001) Resonate-and-fire neurons. Neural Netw 14:883–894
Lloyd JE (1984) Evolution of a firefly flash code. Florida Entomol 67:228–239
McDermott JH, Oxenham AJ (2008) Music perception, pitch, and the auditory system. Curr Opin Neurobiol 18:452–463. doi:10.1016/j.conb.2008.09.005
Mendelson T, Shaw K (2005) Rapid speciation in an arthropod. Nature 433:375–376. doi:10.1038/433375a
Meixner AJ, Shaw KC (1986) Acoustic and associated behavior of the coneheaded katydid Neoconocephalus nebrascensis (Orthoptera: Tettigoniidae). Annals Entomol Soc Am 79:554–565
Sanderson MJ (2003) r8 s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19:301–302. doi:10.1093/bioinformatics/19.2.301
Schmidt A, Ronacher B, Hennig RM (2008) The role of frequency, phase and time for processing of amplitude modulated signals by grasshoppers. J Comp Physiol A 194:221–233. doi:10.1007/s00359-007-0295-x
Schul J, Bush SL (2002) Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc Lond B 269:1847–1852
Schul J, Patterson AC (2003) What determines the tuning of hearing organs and the frequency of calls? a comparative study in the katydid genus Neoconocephalus (Orthoptera, Tettigoniidae). J Exp Biol 206:141–152
Shaw KL, Herlihy DP (2000) Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proc Biol Sci 267:577–584. doi:10.1098/rspb.2000.1040
Shaw KL, Lesnick SC (2009) Genomic linkage of male song and female acoustic preference QTL underlying a rapid species radiation. P Natl Acad Sci Usa 106:9737–9742. doi:10.1073/pnas.0900229106
Snyder RL, Frederick-Hudson KH, Schul J (2009) Molecular phylogenetics of the genus Neoconocephalus (Orthoptera, Tettigoniidae) and the evolution of temperate life histories. PLoS ONE 4:e7203. doi:10.1371/journal.pone.0007203.t001
Talwar M (2007) Function and evolution of the call spectrum in the katydid genus Neoconocephalus. Dissertation, University of Missouri, Columbia
Thomas ES, Alexander RD (1962) Systematics and behavioral studies on the meadow grasshoppers of the Orchelimum concinnum group (Orthoptera: Tettigoniidae). Occas Pap Mus Zool Univ Mich 626:1–31
Walker TJ (1975) Stridulatory movements of eight species of Neoconocephalus (Tettigoniidae). J Insect Physiol 21:595–603
Walker TJ (2012) The singing insects of North America—Katydids. http://buzz.ifas.ufl.edu/crickets.htm. Cited 1 June 2012
Walker T, Greenfield M (1983) Songs and systematics of Caribbean Neoconocephalus (Tettigoniidae, Orthoptera). Trans Amer Ent Soc 109:357–389
Wiley C, Ellison CK, Shaw KL (2012) Widespread genetic linkage of mating signals and preferences in the Hawaiian cricket Laupala. P Roy Soc B-Biol Sci 279:1203–1209. doi:10.1098/rspb.2011.1740
Wiley C, Shaw KL (2010) Multiple genetic linkages between female preference and male signal in rapidly speciating Hawaiian crickets. Evolution 64:2238–2245. doi:10.1111/j.1558-5646.2010.01007.x
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by predators and parasitoids. Q Rev Biol 73:415–438
Acknowledgments
We thank the numerous members of our lab for their contributions and support. Oli Beckers, Josh Deily, and Rob Snyder each contributed significantly to this research. Tom Walker and Michael Greenfield introduced JS and SLB to many aspects of Neoconocephalus natural history and helped locate many species for these studies. We would like to thank Peter Heinecke for his help in assembling and maintaining our walking compensator (“Kramer Kugel”), which has been instrumental for this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Schul, J., Bush, S.L., Frederick, K.H. (2014). Evolution of Call Patterns and Pattern Recognition Mechanisms in Neoconocephalus Katydids. In: Hedwig, B. (eds) Insect Hearing and Acoustic Communication. Animal Signals and Communication, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40462-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-40462-7_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40461-0
Online ISBN: 978-3-642-40462-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)