Abstract
Polymyxins (polymyxin B and colistin) are bactericidal pentacationic cyclic lipodecapeptides that act specifically on Gram-negative bacteria. They were largely abandoned because of their toxicity to kidney proximal tubuli. Now they have been reinstated, in spite of their toxicity, in the therapy of severe infections caused by extremely multiresistant strains. Such strains are now rapidly emerging and spreading. The nephrotoxicity of polymyxins does complicate the therapy, may even require its discontinuation, and must be weighed against the beneficial effects on patient survival. Furthermore, in the recent years it has become increasingly clear that the current dosage regimens are suboptimal in critically ill patients. Clinicians are advised to use larger doses, but this further increases nephrotoxicity. Since there is notably synergy between polymyxins and several other antibiotics, combination therapies may be useful, and clinical evidence for their advantages is currently accumulating. Novel, less nephrotoxic compounds that have strong antibacterial activity would be very welcome. The nephrotoxicity of polymyxins might be related to their very highly cationic nature. In contrast to the old polymyxins, which carry five positive charges, NAB739 carries three positive charges only. The activity of NAB739 against Escherichia coli and Klebsiella pneumoniae is quite close to that of polymyxin B. Pieces of indirect evidence suggest that NAB739 might be less nephrotoxic than the old polymyxins. Ongoing studies compare the efficacy and nephrotoxicity of NAB739 and polymyxin B in animal models. Useful compounds might also include NAB7061 and NAB741, both carrying three positive charges. They lack potent direct action but sensitize Gram-negative bacteria to other antibiotics by facilitating their entry inside the cell.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Escherichia coli and Klebsiella pneumoniae cause almost 40 % of all community-acquired bacteremias and approximately one-third of all healthcare-associated bacteremias. Out of all Gram-negative bacteremias they cause approximately 60–75 %. E. coli and K. pneumoniae strains that elaborate extended-spectrum β-lactamases and especially CTX-M type enzymes have spread at a stunning pace everywhere both in the hospitals and in the community worldwide. In most countries the situation has long been out of control (see Paitan and Ron, this volume).
Now, the largest worry is the carbapenemase-producing strains of E. coli, K. pneumoniae and other Enterobacteriaceae (especially the NDM and KPC strains) that are resistant to almost all antibiotics except polymyxins (polymyxin B, colistin) and tigecycline (see Genilloud and Vicente, this volume). Their ongoing spread might follow the path of the CTX-M strains. This would be disastrous since only extremely few antibiotics with a novel mode of action against Gram-negatives have entered the clinical phases of development.
Most of the extremely resistant Gram-negative “superbacteria” are still susceptible to polymyxins. This antibiotic group was discovered in the mid-1940s, and subsequently used in the intravenous therapy. However, polymyxins were soon largely abandoned because of toxicity, especially toxicity to kidney proximal tubuli. Today they have been reinstated as the last-line intravenous therapy to treat infections caused by strains that are resistant to practically all other agents.
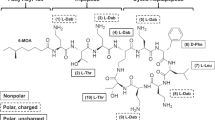
Polymyxin B and colistin (i.e., polymyxin E) (Fig. 8.1) are rapidly bactericidal pentacationic cyclic lipodecapeptides that act on Gram-negative bacteria. Gram-positives, eukaryotic microbes, and mammalian cells are typically resistant. Polymyxins interact with the anionic lipopolysaccharide (LPS) molecules located in the outermost cell structure, the outer membrane (OM) (Vaara 1992, 2010). As shown for polymyxin B nonapeptide (PMBN) (Tsubery et al. 2000), their action is determined by proper three-dimensional conformation, since the enantiomer is completely inactive. Polymyxins permeabilize the OM and then damage the cytoplasmic membrane (Nikaido 2003; Vaara 1992). Several recent general or focused reviews on polymyxins are available (Bergen et al. 2011; Landman et al. 2008; Lim et al. 2010; Michalopoulos and Falagas 2011; Nation and Li 2009; Vaara 2010; Yahav et al. 2011; Zawascki et al. 2007).
Structure of polymyxin B, colistin, CB-182,804, NAB739, NAB7061, and NAB741. Boxed parts indicate locations where the compounds are not identical. Abbreviations for the nontrivial amino acyl residues: Dab diaminobutyryl, Abu aminobutyryl. Other abbreviations: MHA/MOA the mixture of methyl octanoyl and methyl heptanoyl, OA octanoyl, 2-CPAC 2-chloro-phenylaminocarbonyl, cy the cyclic portion indicated with brackets. The positive charge of the free γ-amino group is also shown
Both polymyxin B and colistin are mixtures of related lipodecapeptides. Most of the variation is due to various fatty acyl residues linked to the N-terminus of the peptides. Altogether 39 and 36 different components have been detected in commercial preparations of polymyxin B and colistin, respectively (Van den Bosche et al. 2011). However, as shown for the most predominant components of polymyxin B, their antibacterial activity appears to be identical (Tam et al. 2011). Furthermore, the activities of polymyxin B and colistin are identical (Gales et al. 2001).
Polymyxin B is used as such but colistin is used as its prodrug, colistin methanesulfonate (CMS). In CMS, the free amino groups are blocked by sulfomethylation to yield an uncharged, inactive derivative that in aqueous solutions and in vivo slowly hydrolyzes to free colistin.
Besides the intravenous use of CMS, aerosolized CMS is used in the therapy of Gram-negative lung infections in cystic fibrosis and, to a limited degree, in other patients (Nation and Li 2009; Yahav et al. 2011). Polymyxin B bound to polystyrene fibers is used in hemoperfusion as a means to remove endotoxins (LPS) from the blood of septic patients (Davies and Cohen 2011; Nation and Li 2009; Yahav et al. 2011).
In a few recent years, two pharmaceutical companies, both aiming to develop polymyxin derivatives that are less nephrotoxic, have published data on multiple properties of their derivatives.
2 Antimicrobial Activities of Novel Polymyxins
2.1 CB-182,804
The first and thus far the only novel derivative to enter the clinical phase I was CB-182,804, developed by Cubist Pharmaceuticals, Inc. (Keith et al. 2010). It is otherwise identical to polymyxin B, but the fatty acyl moiety linked to the N-terminus of the polymyxin B peptide is 2-chloro-phenylamino-carbonyl (Fig. 8.1). The MIC90 of CB-182,804 (i.e., the concentration of CB-182,804 that inhibits growth of 90 % of the strains) for E. coli, K. pneumoniae, and Pseudomonas aeruginosa are four-fold, two-fold, and four-fold that of polymyxin B, respectively (Quale et al. 2012).
In the neutropenic mouse thigh infection model, the PD50 value of CB-182,804 (i.e., the dose of CB-182,804 that is required to protect 50 % of the animals) was somewhat lower than that of polymyxin B, when P. aeruginosa was used as the challenge organism (Arya et al. 2010). When Acinetobacter baumannii was used, CB-182,804 was slightly less active than polymyxin B. In the septicemic mouse model with E. coli, the PD50 of CB-182,804 was significantly higher than that of polymyxin B or colistin (Arya et al. 2010).
2.2 NAB739
NAB739 is under development at Northern Antibiotics Ltd. (Vaara 2010). It has its cyclic part identical to that of polymyxin B, but its side chain consists of octanoyl-threonyl-D-serinyl (Fig. 8.1). Accordingly, its linear part lacks the two positively charged diaminobutyryl (Dab) residues present in the linear part of polymyxin B and colistin. Accordingly, NAB739 carries three positive charges only.
In two evaluations (Vaara et al. 2008 and Northern Antibiotics, data on file), the MIC90 values of NAB739 and polymyxin B for E. coli were identical, in the first one 1 μg/ml and in the second case 2 μg/ml. For K. pneumoniae, the MIC90 of NAB739 and polymyxin B were 2 μg/ml and 1 μg/ml, respectively. Unlike that of several other cationic drugs, the activity of NAB739 is not affected by divalent cations (Ca2+, Mg2+).
The MIC range of NAB739 and polymyxin B for the polymyxin-susceptible carbapenemase-producing strains of E. coli and K. pneumoniae (n = 9, including KPC, OXA-48, VIM, and IMP-producing strains) were 1–4 μg/ml and 1–2 μg/ml, respectively (Vaara et al. 2010b).
Against Acinetobacter NAB739 is 4-fold less active than polymyxin B, but low subinhibitory concentrations of NAB739 sensitize A. baumannii to other antibiotics by facilitating their access inside the cell. As low a concentration as 0.5 μg/ml of NAB739 reduces the MIC of rifampin from 4 to 0.05 μg/ml (Vaara et al. 2008). The MIC of clarithromycin is reduced from 8 to 0.5 μg/ml, and the MIC of vancomycin is reduced from 256 to 3 μg/ml (Vaara et al. 2008).
Against P. aeruginosa NAB739 is 8-fold less active than polymyxin B and the MIC90 is 16 μg/ml (Northern Antibiotics, data on file). Another derivative, NAB740, which carries decanoyl as the fatty acyl tail, is more active against P. aeruginosa, but against Enterobacteriaceae it is inferior to NAB739 (Vaara et al. 2008). Furthermore, des-fatty acyl derivatives such as PMBN and many others are quite active against P. aeruginosa but virtually lack activity against Enterobacteriaceae (Katsuma et al. 2009; Sato et al. 2011; Tsubery et al. 2001; Vaara 1992).
The presence of fresh normal human serum (NHS, 20 %) decreases the MIC of NAB739 for P. aeruginosa ATCC 27853 by a factor of 8–16 and yields MIC values as low as 0.25–0.5 μg/ml (Northern Antibiotics, data on file). The MIC for E. coli ATCC25922 decreases from 1–2 to 0.5 μg/ml. The MIC of polymyxin B in the presence of 20 % NHS is <0.06 μg/ml for both strains. Vaara and coworkers have previously shown that PMBN acts synergistically with the complement against Enterobacteriaceae and P. aeruginosa (Vaara and Vaara 1983; Vaara et al. 1984). The combination of PMBN with human, guinea pig, rabbit, and rat serum is strongly bactericidal, but no synergy can be found with mouse serum (Vaara et al. 1984).
Inherently polymyxin-resistant bacterial species as well as strains that have acquired resistance to polymyxin are resistant to NAB739 (Vaara et al. 2008, 2010b).
NAB739 was found to be effective in treating experimental E. coli peritoneal and bacteremic infection in mice at a dose identical to that of polymyxin B (Vingsbo Lundberg et al. 2010). To avoid inactivation of the polymyxins by mucin, the polyanionic polymer commonly used to suppress phagocytosis in the mouse peritonitis model, the K1 capsule-elaborating strain IH3080 (O18:K1:H7) was used. It is virulent to mice in the peritonitis model even in the absence of mucin.
2.3 The Sensitizer Derivatives NAB7061 and NAB741
NAB7061 and NAB741 (Vaara et al. 2008, 2010a, b; Vaara 2010), both under development at Northern Antibiotics, have their cyclic part identical to that of polymyxin B, but their side chain consists of octanoyl-threonyl-aminobutyryl, and acetyl-threonyl-D-serinyl, respectively (Fig. 8.1). Hence they carry only three positive charges, as does NAB739.
Hydrophobic antibiotics (such as macrolides, rifamycins, and many others) as well as large molecules (such as vancomycin) are widely used against Gram-positive bacteria but inactive against most Gram-negative bacteria, because these drugs do not effectively cross the OM (Nikaido 2003; Vaara 1992). NAB741 and NAB7061 lack potent direct antibacterial activity, but, by disrupting the bacterial OM, they facilitate the access of these antibiotics inside the Gram-negative cell. At 4 μg/ml, NAB7061 decreased the MIC of rifampin for E. coli (n = 11), other polymyxin-susceptible Enterobacteriaceae (n = 12), and A. baumannii (n = 3) by factors of 85–750, 10–2,000, and 25–125, respectively (Vaara et al. 2008). With clarithromycin (see Kirst, this volume), the corresponding factors were 90–750, 10–1,000, and 40–100, respectively. The antibacterial properties of NAB741 (Vaara et al. 2010) are similar to those of NAB7061.
Both agents also sensitize target bacteria to the bactericidal activity of the complement system present in fresh serum (Vaara 2010; Vaara et al. 2010a).
The sensitizer activity of NAB7061 has been demonstrated also in vivo in the therapy of experimental E. coli peritoneal infection in mice (Vingsbo Lundberg et al. 2010). In contrast to NAB7061 or erythromycin alone, the combination of NAB7061 and erythromycin was effective in the therapy.
Accordingly, NAB7061 and NAB741 may find value when used as a combination with a suitable partner antibiotic.
3 Determining MIC Values of Polymyxins
Determining the MIC values of polymyxin B and colistin can be performed either by broth microdilution or by agar dilution method, both according to the CLSI standard protocol, the most recent version labeled as M07-A9 (CLSI 2012). For broth microdilution testing, CLSI recommends cation-adjusted Mueller–Hinton broth (CAMHB) that contains 20–25 mg of Ca2+ per liter and 10–12.5 mg of Mg2+ per liter. For agar dilution testing, CLSI recommends Mueller–Hinton agar (MHA) without addition of cation supplements (CLSI 2012). CLSI has also given quality control ranges for polymyxin B and colistin MIC determinations.
Excellent correlation (r = 0.96–0.98) has been reported between broth microdilution and agar dilution tests for both polymyxin B and colistin, as tested by using a representative set of clinical isolates (Gales et al. 2001). A trend toward higher MIC results with the agar method was observed but 94.3 % of the MIC results were ±1 log2 dilution between the methods used for both compounds. Another study with a larger collection of clinical isolates and determining MICs for colistin only did not find such a trend; 85.4 % of the MIC results were identical and 96.8 % were ±1 log2 dilution between the methods (Lo-Ten-Foe et al. 2007).
It should however be remembered that polymyxins are adsorbed to glass, plastic, and sterilization filters. The adsorption of radiolabelled mono-acetyl polymyxin B is in relative terms very intense in dilute solutions and especially in water and in media that have low ionic strength (Vaara et al. 1979 and Vaara, unpublished). In a recent study (Karvanen et al. 2011), altogether 74 % of colistin was lost during dilution steps from stock to 0.125 μg/ml and during subsequent incubation in uninoculated CAMHB in polystyrene wells at 37 °C for 24 h. At higher concentrations of colistin, the loss gradually decreased but was still 22 % at the intended colistin concentration of 8 μg/ml. The adsorption was less intense in polypropylene than in polystyrene.
Also the novel lipoglycopeptides (see Marcone and Marinelli, this volume) are quite sticky. Approximately 90 % of radiolabelled oritavancin was adsorbed to polystyrene in 1 h, when the initial concentration was 1 μg/ml (Arhin et al. 2008). The nonionic detergent polysorbate 80 (Tween 80) at 0.002 % almost completely inhibited the adsorption and reduced the MIC of oritavancin for Staphylococcus aureus by a factor of 30 (Arhin et al. 2008). Polysorbate 80 was also used in the dalbavancin susceptibility assays (Rennie et al. 2007). However, the use of polysorbate to inhibit the adsorption of polymyxins in bacterial susceptibility studies will cause problems. Both polysorbate and polymyxins are membrane-active agents and may act synergistically against the Gram-negative OM and cytoplasmic membrane. As low a concentration as 0.001 % polysorbate 80 has been shown to be synergistic with polymyxin B against P. aeruginosa, and at higher concentrations of polysorbate 80, the synergy was even more pronounced (Brown and Winsley 1971).
Less polymyxin is probably adsorbed to plastic materials in the agar dilution tests than in microbroth dilution tests, since polymyxins diffuse rather poorly in agar. On the other hand, polymyxins can be expected to bind to the anionic sulfated carbohydrate polymers of agar.
4 Acquired Resistance to Polymyxin and to NAB Compounds
Acquired polymyxin resistance in clinical isolates is rare. In the worldwide SENTRY program, susceptibility of 40,625 Gram-negative isolates, collected in 2006–2009, to colistin and polymyxin B isolates was studied (Gales et al. 2010). Susceptibility was interpreted as the MIC ≤ 2 μg/ml. Susceptibility rates of colistin for E. coli, K. pneumoniae, Acinetobacter spp., and P. aeruginosa were 99.9, 98.6, 99.2, and 99.8 %, respectively. For strains isolated in 2009, the corresponding rates were 99.8, 98.2, 97.9, and 99.5 %, respectively. Polymyxin B displayed almost identical susceptibility rates.
In strains isolated in the CANWARD program from Canadian hospitals during 2007–2009, the colistin susceptibility rates (susceptibility interpreted as above) for E. coli, K. pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, A. baumannii, and P. aeruginosa were 99.4, 97.1, 95.4, 83.6, 93.5, and 91.6 %, respectively (Walkty et al. 2009). In a continuation study that included also the strains isolated in 2009, the susceptibility rates for ESBL-producing E. coli, ampC-producing E. coli, Acinetobacter spp., and P. aeruginosa were 98.7, 100, 94.3, and 90.9 %, respectively (Simner et al. 2011; Zhanel et al. 2011).
In the material collected in 2009 from 16 hospitals in Brooklyn, NY and Staten Island, NY and consisting of 5,489 strains of E. coli, K. pneumoniae, Enterobacter spp., A. baumannii, and P. aeruginosa, the susceptibility rates for polymyxin B (susceptibility interpreted as the MIC ≤ 2 μg/ml) were 99.8, 96, 76, 97, and 99.5 %, respectively (Landman et al. 2010; Quale et al. 2012).
Amongst imipenem nonsusceptible isolates of K. pneumoniae in the SENTRY collection, 12 % were resistant to colistin (Sader et al. 2011). Colistin-resistant K. pneumoniae strains may develop during therapy (Lee et al. 2009). They may also cause hospital outbreaks. Colistin-resistant KPC-producing K. pneumoniae strains belonging to the international epidemic clone ST258 have been encountered and reported to cause hospital infections (Bogdanovich et al. 2011).
Probably, the highest rates of polymyxin resistance are encountered in P. aeruginosa strains isolated from cystic fibrosis (Lim et al. 2010).
In A. baumannii, K. pneumoniae, and P. aeruginosa, heteroresistance to polymyxins has been reported (Lim et al. 2010; Yahav et al. 2011). A small subpopulation of cells survive the antibiotic treatment. In broth cultures this is seen as an initially slow but exponential turbidity increase over time, due to the growth of these persisters. While the clinical significance remains unclear, it might still be advantageous to use polymyxin compounds in combination with another antibiotic that suppresses the development of persister cells, as suggested by many authors (Bergen et al. 2011; Garonzik et al. 2011; Landman et al. 2008; Nation and Li 2009; Yahav et al. 2011; Zawascki et al. 2007). Perhaps some of the novel compounds currently developed by the pharmaceutical industry and active against Gram-positives may find use as a suitable adjunct or “partner” antibiotic to polymyxins. Such compounds could include novel oxazolidinones (see Zappia, this volume), ketolides (see Kirst, this volume), lipopeptides (see Baltz, this volume), peptide deformylase inhibitors (see East, this volume), pleuromutilins (see Kirst, this volume), and inhibitors of FabI (see Genilloud and Vicente, this volume). Furthermore, partners could also include agents that inhibit targets such as LpxC enzymes, present in Gram-negatives only, but do not effectively penetrate their OM.
The mechanism of polymyxin resistance was elucidated by Vaara and his coworkers already more than 30 years ago. They reported, that the polymyxin-resistant pmrA mutants of Salmonella typhimurium have an altered lipopolysaccharide in their OM (Vaara et al. 1981). It is less anionic, due to increased decoration by 4-aminoarabinose and phosphoryl ethanolamine (Vaara et al. 1981). This resistance mechanism was then found universal amongst Enterobacteriaceae and P. aeruginosa (Gunn 2008; Landman et al. 2008; Lim et al. 2010; Raetz et al. 2007; Vaara 2010). Furthermore, similar changes in lipid A confer the resistance in several species that are intrinsically resistant to polymyxins, such as Proteus mirabilis, Burkholderia cepacia, and Chromobacterium violaceum, as reviewed by Vaara (1992).
The pmrAB regulon is best characterized in Salmonella typhimurium, where it regulates at least 20 genes including those involved in the decoration of the lipid A by 4-aminoarabinose and phosphoryl ethanolamine (Gunn 2008; Raetz et al. 2007). Conditions that activate pmrAB have recently been thoroughly reviewed (Gunn 2008; Raetz et al. 2007).
The mechanisms that mediate polymyxin resistance have profound effects on the structure and function of the bacterial OM. The OM permeability barrier of the polymyxin-resistant enterobacterial mutants is compromised, as evidenced by their increased susceptibility to other agents such as certain antibiotics and bile acids (Froelich et al. 2006; Murata et al. 2007; Vaara 1981; Vaara and Vaara 1994). This might explain why for instance polymyxin-resistant strains of E. coli are still extremely scarce (see above).
In A. baumannii, polymyxin resistance is mediated by at least two alternative mechanism. One is analogical to the mechanism described above for other bacteria and involves decoration of lipid A with phosphoryl ethanolamine (Beceiro et al. 2011) while the other is a result of complete loss of lipopolysaccharide (Moffatt et al. 2010) Polymyxin-resistant strains of A. baumannii are susceptible to many antibiotics normally active on Gram-positives only and they have reduced in vivo fitness and decreased virulence (Fernández-Reyes et al. 2009; Li et al. 2007; Lôpez-Rojas et al. 2011; Rolain et al. 2011).
Regarding novel polymyxins, the polymyxin-resistant strains of E. coli, K. pneumoniae, Acinetobacter spp,. and P. aeruginosa as well as the polymyxin-resistant variant strain CL5762B of KPC-producing K. pneumoniae are resistant to NAB739 (Vaara et al. 2008, 2010b, and Northern Antibiotics, data on file). On the other hand, the sensitizer compound NAB7061 sensitizes CL5762B to rifampin and clarithromycin by factors of 24 and 12, respectively (Vaara et al. 2010b).
Some of the polymyxin derivatives recently synthesized by Li et al. (2010) have activity against polymyxin-resistant strains of A. baumannii and P. aeruginosa but the polymyxin-resistant K. pneumoniae strains remain resistant. These compounds are more hydrophobic than the old polymyxins and might have detergent-like activity. For instance, in Compound 2, the hydrophobicity of polymyxin is increased by substituting 2-aminodecanoyl (Ada) for leucyl in the heptapeptide ring portion, to yield octyl instead of isobutyl as the R group in this position (position 7 according to the standard numbering of the amino acid residues in the polymyxin decapeptide). The fatty acyl tail consists of an octanoyl residue. For polymyxin-susceptible reference strains P. aeruginosa ATCC27853, A. baumannii ATCC19606, and K. pneumoniae ATCC13883, the MICs of Compound 2 for are 4 μg/ml, 4 μg/ml, and 2 μg/ml, respectively. Unfortunately, the MIC for E. coli has not been disclosed. Compounds 3–5 are analogical to Compound 2 in having, besides the fatty acyl tail, larger hydrophobic moieties (hexyl, bisphenyl) than isobutyl at the position 7. All these four compounds carry five positive charges.
Compound 1 resembles NAB739 and carries only three positive charges. It lacks Dab at the position 1, has Thr at the position 3 and nonanoyl as the fatty acid tail, but, in contrast to NAB739, aminodecanoic acid (Ada) at position 7.
Accordingly, the mode of action of the derivatives with increased hydrophobic properties differs from the classic and quite specific mode of action of polymyxins and might merely resemble the rather nonspecific action of cationic detergents. It has long been known that the polymyxin-resistant pmrA mutants of S. typhimurium and their analogues in E. coli with identical alterations in the lipid A are as susceptible as their parents to octapeptin EM49 (Meyers et al. 1974; Vaara 1981). EM49 is structurally very similar to polymyxins but is more hydrophobic, since it lacks two hydrophilic amino acids and carries a fatty acyl tail that is two methylene units longer than the one of polymyxin B. To the cationic detergents benzalkonium chloride and cetyltriammonium chloride, these mutants are even more susceptible than their parents (Vaara 1981; Vaara and Vaara 1994).
5 Pharmacokinetic and Pharmacodynamic Considerations
The pharmacokinetics of CMS and colistin released from CMS has extensively been reviewed elsewhere (Couet et al. 2012; Michalopoulos and Falagas 2011; Nation and Li 2009). In the recent years, it has become increasingly clear that the current dosage regimens of CMS are suboptimal in critically ill patients (Bergen et al. 2011; Couet et al. 2012; Garonzik et al. 2011; Markou et al. 2008; Micalopoulos and Falagas 2011). While clinicians are advised to use larger doses of CMS (Couet et al. 2012), the nephrotoxic potential may cause problems (see below). Another approach is combination therapy (Bergen et al. 2011; Garonzik et al. 2011; Nation and Li 2009; Yahav et al. 2011). The synergy of polymyxins in vitro with many agents, especially with those that are normally excluded by an intact bacterial OM, has thoroughly been reviewed by several authors (Landman et al. 2008; Nation and Li 2009; Yahav et al. 2011; Zawascki et al. 2007). Clinical evidence for the advantages of combination therapies is accumulating (Nation and Li 2009; Yahav et al. 2011).
The serum half-life of polymyxin B in humans with normal renal function and, as determined for one patient with renal insufficiency (serum creatinine, 3.5 mg/dL), has been reported to be 13.6 h and 11.5 h, respectively (Kwa et al. 2008, 2011). Only less than 1 % of the given dose is excreted in urine (Zawascki et al. 2008).
The area under the unbound concentration–time curve to MIC ratio (fAUC/MIC) has been reported as the pharmacokinetic/pharmacodynamic index that best predicts the efficacy of colistin against P. aeruginosa and A. baumannii in animal models (thigh and lung infections in neutropenic mice) and in one in vitro model (Bergen et al. 2011).
6 Nephrotoxicity of Polymyxin Compounds
The nephrotoxicity of polymyxins does complicate the therapy, may even require its discontinuation, and must be weighed against the beneficial effects on patient survival (Falagas and Rafailidis 2009).The nephrotoxicity rate depends on the definitions for toxicity, correlates with the age and cumulative total dose, and increases in patients with abnormal renal function. It varies in the recent literature from 10 to 30 % (Falagas and Kasiakou 2006; Falagas et al. 2010; Landman et al. 2008; Oliveira et al. 2009; Pastewski et al. 2008; Zawascki et al. 2007), and in selected materials may be as high as 43–80 % (Garonzik et al. 2011; Hartzell et al. 2009; Pastewski et al. 2008; Pogue et al. 2011). The nephrotoxic potential of CMS and polymyxin B appear to be similar (Falagas and Kasiakou 2006; Oliveira et al. 2009).
Polymyxins and aminoglycosides (see Kirst and Marinelli, this volume), another group of strongly cationic compounds, are nephrotoxic, because they damage renal proximal tubuli. Both are bound to the megalin macroprotein in the brush-border membrane (BBM) of the epithelial cells of the proximal tubuli and then effectively taken up by these cells (Vaara 2010). This extensive tubular reabsorption results in high concentrations of the drugs inside the cells. This may explain, at least partially, the nephrotoxicity of polymyxins (Zawascki et al. 2008).
The development of two polymyxin derivatives, both carrying five positive charges, has been discontinued. The first of them was PMBN. It lacks the fatty acid tail and the N-terminal amino acyl residue (Dab) of polymyxins but still carries five positive charges. Its direct antibacterial action is weak albeit against P. aeruginosa, but, as first shown by Vaara et al., PMBN has retained the ability to permeabilize the OM (Vaara 1992; Vaara and Vaara 1983). However, in rodent experiments, PMBN was still considered to be too nephrotoxic, and its development for therapeutic purposes was discontinued in 1984 (Vaara 1992; Vaara, unpublished).
CB-182,804 was reported to be less nephrotoxic to cynomolgus monkeys than polymyxin B (Coleman et al. 2010), even though its superiority of CB-182,804 over polymyxin B looked very modest. CB-182,804 entered into a Phase 1 clinical trial in February 2009, and in September 2010 the project was discontinued.
The polymyxin derivatives of the NAB series carry only three positive charges. Their affinity for isolated rat kidney BBM is only approximately one-seventh (NAB739) or one-fifth (NAB7061) of that of polymyxin B and approximately one-third of that of gentamicin (Vaara et al. 2008).
In trivial nonpolarized porcine renal proximal tubular LLC-PK1 cells that express megalin only very poorly (Servais et al. 2006), polymyxin B elicits a marked degree (approx 50 %) of necrosis at 0.5 mM, whereas the NAB compounds are inert even at 1 mM (Mingeot-Leclercq et al. 2012). In artificially permeabilized (electroporated) LLC-PK1 cells, where polymyxins enter freely and unselectively, polymyxin B induces total necrosis at as low a concentration as 0.016 mM, while an approximately 8-fold concentration of NAB739 and NAB7061 and an approximately 32-fold concentration of NAB741 is required for the same effect (Mingeot-Leclercq et al. 2012). Accordingly, the studies showed that NAB739, NAB7061, and NAB741 have a substantially lower necrotic potential toward LLC-PK1 cells than polymyxin B. Polarized cells that express megalin, form BBM and actively bind polymyxins (see above) and internalize them would be more suitable as targets, but the technology is very demanding. In such polarized cells that mimic natural conditions, the fold difference between the cytotoxicity of the NAB compounds and polymyxin B can be expected to be even more larger than that observed with artificially permeabilized cells, since the affinities of the NAB compounds for isolated rat BBM expressing megalin are significantly lower than that of polymyxin B (see above),
The renal clearances of NAB741, NAB739, and NAB7061 after a single intravenous dose in rats are approx. 400-, 50-, and 30-fold higher than that of colistin (Ali et al. 2009; Vaara et al. 2010a). The higher renal clearance indicates a difference in the relative contributions of renal clearance mechanisms (glomerular filtration, tubular secretion, and tubular reabsorption). In contrast to polymyxins, NAB739 and NAB741 do not undergo net tubular uptake (NAB7061 was not included in the study).
Altogether the results indicate that the nephrotoxicity of the NAB compounds might be lower than that of the old polymyxins.
At least in some experimental setups, such as in those using murine and human macrophage cells, colistin appears to be less cytotoxic than polymyxin B (Das et al. 2011). Even though the relevance of these findings in clinical settings remains unclear, it is still quite possible that an analogue of NAB739 where the side chain is octanoyl-threonyl-D-serinyl and the cyclic peptide part is identical to that of colistin might offer some advantages over NAB739.
7 Neurotoxicity of polymyxin compounds
Neurotoxic side effects of intravenously given polymyxin B and CMS in contemporary clinical settings are considered as rare, mild, and reversible (Falagas and Kasiakou 2006; Lim et al. 2010; Nation and Li 2009; Yahav et al. 2011; Zawascki et al. 2007). Severe neurotoxicity, including neuromuscular blockade and apnea, are extremely rare or nonexistent (Falagas and Kasiakou 2006), as are also the other acute reactions such as those mediated by histamine release. Polymyxin B is administered as an infusion over 1 h, whereas CMS, which liberates free colistin slowly, can be given as a shorter infusion.
PMBN lacks the fatty acid tail of polymyxins and is 150 times less active than polymyxin in causing neuromuscular blockade, 15 times less toxic in an acute toxicity assay in mice, and 25 times less active in releasing histamine from rat mast cells (Vaara 1992), and. also other des-fatty acyl derivatives of polymyxins are better tolerated than polymyxin B and colistin in acute toxicity assays (Katsuma et al. 2009; Sato et al. 2011). It could be anticipated that NAB741, the des-fatty acyl derivative of NAB739, resembles PMBN and the other des-fatty acyl derivatives in being better tolerated than fatty acyl-carrying polymyxins in acute toxicity assays.
8 Conclusions
Polymyxins are desperately needed in the therapy of severe infections caused by extremely multiresistant strains of Gram-negative bacteria. One can predict that the exponential spread of carbapenemase-producing strains of E. coli and K. pneumoniae in hospitals and in the community will mimic the triumph already seen in the case of the extended-spectrum betalactamase CTX-M –producing strains.
The nephrotoxicity of the old polymyxins shadows their use. In addition, in the recent years it has become increasingly clear that the current dosage regimens are suboptimal in critically ill patients. Clinicians are advised to use larger doses, but this further increases nephrotoxicity. Since there is notably synergy between polymyxins and several other antibiotics, combination therapies may be useful, and clinical evidence for their advantages is currently accumulating.
Novel, less nephrotoxic compounds that have strong antibacterial activity would be very welcome. Unfortunately, the development of CB-182,804 was discontinued after the clinical Phase I.
The nephrotoxicity of polymyxins might be related to their very highly cationic nature. In contrast to the old polymyxins and CB-182,804, which carry five positive charges, NAB739 carries three positive charges only. Pieces of indirect evidence suggest that it might be less nephrotoxic than the old polymyxins. Ongoing studies compare the efficacy and nephrotoxicity of NAB739 and polymyxin B in animal models.
Useful compounds might also include NAB7061 and NAB741, both carrying three positive charges. They lack potent direct action but sensitize bacteria to other antibiotics.
References
Ali FE, Cao G, Poydal A, Vaara T, Nation RL, Vaara M, Li J (2009) Pharmacokinetics of novel antimicrobial cationic peptides NAB7061 and NAB739 in rats following intravenous administration. J Antimicrob Chemother 64:1067–1070
Arhin FF, Sarmiento I, Belley A, McKay GA, Draghi DC, Grover P, Sahm DF, Parr TR Jr, Moeck G (2008) Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother 52:1597–1603
Arya A, Li T, Zhang S, Chuong L, Kang C, Zhang X, Mortin LI (2010) Efficacy of CB-182,804, a novel polymyxin analog, in rat and mouse models of Gram-negative bacterial infections. Abstr 50th Intersci Conf Antimicrob. Agents Chemother, Boston, abstr F1-1627
Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N (2011) Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379
Bergen PJ, Li J, Nation RL (2011) Dosing of colistin-back to basic PK/PD. Curr Opin Pharmacol 11:464–469
Bogdanovich T, Adams-Haduch JM, Tian GB, Nguyen MH, Kwak EJ, Muto CA, Doi Y (2011) Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin Infect Dis 53:373–376
Brown MRW, Winsley BE (1971) Synergism between polymyxin and Polysorbate 80 against Pseudomonas aeruginosa. J Gen Microbiol 68:367–373
CLSI (2012) M07–A9. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th edn. Clinical and Laboratory Standards Institute, Wayne
Coleman S, Deats T, Pawliuk R, Chavan A, Oleson FB Jr (2010) CB-182,804 is less nephrotoxic as compared to polymyxin B in monkeys following seven days of repeated intravenous dosing. Abstr 50th Intersci Conf Antimicrob Agents Chemother, Boston, abstr F1-1630
Couet W, Grégoire N, Marchand S, Mimoz O (2012) Colistin pharmacokinetics: the fog is lifting. Clin Microbiol Infect 18:30–39
Das D, Buyk J, Van Bambeke F, Lorent J, Mingeot-Leclercq MP, Gobin P, Couet W, Tulkens PM (2011) Abstr 51th Intersci Conf Antimicrob Agents Chemother, abstr F2-170. Comparative assessment of antibacterial and ccytotoxic effects of colistin (CST) colistin methanesulfonate (CMS) and polymyxin B (PMB) using Pseudomonas aeruginosa, macrophages and renal cells, and liposomes
Davies B, Cohen J (2011) Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect Dis 11:65–71
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27
Falagas ME, Rafailidis PI (2009) Nephrotoxicity of colistin: new insight into an old antibiotic. Clin Infect Dis 48:1729–1731
Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A (2010) Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents 35:194–199
Fernández-Reyes M, Rodriguez-Falcón M, Chiva C, Pachón J, Andreu D, Rivas L (2009) The cost of resistance to colistin in Acinetobacter baumannii: a proteomic perspective. Proteonomics 9:1632–1645
Froelich JM, Tran K (2006) Wall D (2006) A pmrA constitutive mutant sensitizes Escherichia coli to deoxycholic acid. J Bacteriol 188:1180–1183
Gales AC, Reis AO, Jones RN (2001) Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol 39:183–190
Gales AC, Jones RN, Sader HS (2010) Contemporary activity of colistin and polymyxin B against a worldwide collection of gram-negative pathogens: results from the SENTRY antimicrobial surveillance program (2006–09). J Antimicrob Chemother 66:2070–2074
Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL (2011) Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294
Gunn JS (2008) The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol 16:284–290
Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintraub A, Wortmann G (2009) Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48:1724–1728
Karvanen M, Malmberg C, Mohamed A, Lagerbäck M, Friberg LE, Cars O (2011) Colistin is extensively lost during normal experimental conditions. Abstr 51th Intersci Conf Antimicrob Agents Chemother, Chicago, abstr D-690.
Katsuma N, Sato Y, Ohki K, Okimura K, Ohnishi K, Sakura N (2009) Development of des-fatty acyl-polymyxin B decapeptide analogs with Pseudomonas aeruginosa-specific antimicrobial activity. Chem Pharm Bull 57:332–336
Keith DD, Borders D, Curran WV, Leese RA, Mahamoon A, Jarolman H, Francis ND, Zhang Y, Arya A, Cotroneo N, Mortin LI, Silverman J, Brian P (2010) Synthesis and activity of CB-182,804: a novel polymyxin analog active against clinically relevant Gram-negative bacteria. Abstr 50th Intersci Conf Antimicrob Agents Chemother, Boston, abstr F1-1619.
Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, Tam VH (2008) Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis 60:163–167
Kwa AL, Abdelraouf K, Low JG, Tam VH (2011) Pharmacokinetics of polymyxin B in a patient with renal insufficiency: a case report. Clin Infect Dis 52:1280–1281
Landman D, Georgescu C, Martin DA, Quale J (2008) Polymyxins revisited. Clin Microbiol Rev 21:449–465
Landman D, Urban C, Bäcker M, Kelly P, Shah N, Babu E, Bratu S, Quale J (2010) Susceptibility profiles, molecular epidemiology, and detection of KPC-producing Escherichia coli isolates from the New York City vicinity. J Clin Microbiol 48:4604–4607
Lee J, Patel G, Huprikar S, Calfee DP, Jenkins SG (2009) Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. Antimicrob Agents Chemother 47:1611–1612
Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C (2007) Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin Infect Dis 45:594–598
Li J, Velkov T, Nation RL, Thompson PE (2010) Patent application WO2010/130007 A1
Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A, Forrest A, Bulitta J, Tsuji BT (2010) Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacother 30:1279–1291
López-Rojas R, Domínguez-Herrera J, McConnell MJ, Docobo-Peréz F, Smani Y, Fernández-Reyes M, Rivas L, Pachón J (2011) Impaired virulence and in vivo fitness of colistin-resistant Acinetobacter baumannii. J Infect Dis 203:545–548
Lo-Ten-Foe JR, de Smet AM, Diederen BMW, Kluytmans JAJ, van Keulen PHJ (2007) Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother 51:3726–3730
Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G (2008) Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther 30:143–151
Meyers E, Parker WL, Brown WE (1974) EM49: a new polypeptide antibiotic active against cell membranes. Ann NY Acad Sci 234:493–501
Michalopoulos AS, Falagas ME (2011) Colistin: recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann Intensive Care 1:30
Mingeot-Leclercq MP, Tulkens PM, Denamur S, Vaara T, Vaara M (2012) Novel polymyxin derivatives are less cytotoxic than polymyxin B to renal proximal tubular cells. Peptides 35:248–252
Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD (2010) Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977
Murata T, Tseng W, Guina T, Miller SI, Nikaido H (2007) PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica Serovar typhimurium. J Bacteriol 189:7213–7222
Nation RL, Li J (2009) Colistin in the 21st century. Curr Opin Infect Dis 22:535–543
Nikaido H (2003) Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656
Oliveira MS, Prado GVB, Costa SF, Grinbaum RS, Levin AS (2009) Polymyxin B and colistimethate are comparable as to efficacy and renal toxicity. Diagn Microbiol Infect Dis 65:431–434
Pastewski AA, Caruso P, Parris AR, Dizon R, Kopec R, Sharma S, Mayer S, Ghitan M, Chapnick EK (2008) Parenteral polymyxin B use in patients with multidrug-resistant Gram-negative bacteremia and urinary tract infections: a retrospective case series. Ann Pharmacother 42:1177–1187
Pogue JM, Lee J, Marchaim D, Yee V, Zhao JJ, Chopra T, Lephart P, Kaye KS (2011) Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 53:879–884
Quale J, Shah N, Kelly P, Babu E, Bäcker M, Rosas-Garcia G, Salamera J, George A, Bratu S, Landman D (2012) Activity of polymyxin B and the novel polymyxin analogue CB-182,804 against contemporary Gram-negative pathogens in New York City. Microb Drug Resist 13:574–581
Raetz CRH, Reynolds CM, Trent MS, Bishop RE (2007) Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem 76:295–329
Rennie RP, Koeth L, Jones RN, Fritsche TR, Knapp CC, Killian SB, Goldstein BP (2007) Factors influencing broth microdilution antimicrobial susceptibility test results for dalbavancin, a new glycopeptide agent. J Clin Microbiol 45:3151–3154
Rolain JM, Roch A, Castanier M, Papazian L, Raoult D (2011) Acinetobacter baumannii resistant to colistin with impaired virulence: a case report from France. J Infect Dis 204:1146–1147
Sader HS, Farrell DJ, Jones RN (2011) Susceptibility of Klebsiella spp. to colistin and polymyxin B: results from the SENTRY Antimicrobial Surveillance Program (2006–2009). Int J Antimicrob Agents 37:174–175
Sato Y, Shindo M, Sakura N, Uchida Y, Kato I (2011) Novel des-fatty acyl-polymyxin B derivatives with Pseudomonas aeruginosa-specific antimicrobial activity. Chem Pharm Bull 59:597–602
Servais H, Jossin Y, Van Bambeke F, Tulkens PM, Mingeot-Leclercq M-P (2006) Gentamicin causes apoptosis at low concentrations in renal LLC-PK1 cells subjected to electroporation. Antimicrob Agents Chemother 50:1213–1221
Simner PJ, Zhanel GG, Pitout J, Tailor F, McCracken M, Mulvey MR, Lagacé-Wiens PR, Adam HJ, Hoban DJ, Canadian Antimicrobial Resistance Alliance (CARA) (2011) Prevalence and characterization of extended-spectrum β-lactamase- and AmpC β-lactamase-producing Escherichia coli: results of the CANWARD 2007-2009 study. Diagn Microbiol Infect Dis 69:326–334
Tam VH, Cao H, Ledesma KR, Hu M (2011) In vitro potency of various polymyxin B components. Antimicrob Agents Chemother 55:4490
Tsubery H, Ofek I, Cohen S, Fridkin M (2000) The functional association of polymyxin B with bacterial lipopolysaccharide is stereospecific: studies on polymyxin B nonapeptide. Biochemistry 39:11838–11844
Tsubery H, Ofek I, Cohen S, Fridkin M (2001) N-terminal modifications of polymyxin nonapeptide and their effect on antibacterial activity. Peptides 22:1675–1681
Vaara M (1981) Increased outer membrane resistance to ethylenediamine tetraacetate and cations in novel lipid a mutants. J Bacteriol 48:426–434
Vaara M (1992) Agents that increase the permeability of the outer membrane. Microbiol Rev 56:395–411
Vaara M (2010) Polymyxins and their novel derivatives. Curr Opin Microbiol 13:574–581
Vaara M, Vaara T (1983) Sensitization of Gram-negative bacteria to antibiotics and complement by a non-toxic oligopeptide. Nature (London) 303:526–528
Vaara M, Vaara T (1994) Ability of cecropin B to penetrate the enterobacterial outer membrane. Antimicrob Agents Chemother 38:2498–2501
Vaara M, Vaara T, Sarvas M (1979) Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J Bacteriol 139:664–667
Vaara M, Vaara T, Jensen M, Helander I, Nurminen M, Rietschel E, Mäkelä P (1981) Characterization of the lipopolysaccharide from the polymyxin-resistant pmrA mutants of Salmonella typhimurium. FEBS Lett 129:145–149
Vaara M, Viljanen P, Vaara T, Mäkelä PH (1984) An outer membrane-disorganizing peptide PMBN sensitizes E. coli strains to serum bactericidal action. J Immunol 132:2582–2589
Vaara M, Fox J, Loidl G, Siikanen O, Apajalahti J, Hansen F, Frimodt-Moeller N, Nagai J, Takano M, Vaara T (2008) Novel polymyxin derivatives carrying only three positive charges are effective antibacterial agents. Antimicrob Agents Chemother 52:3229–3236
Vaara M, Siikanen O, Apajalahti J, Fox J, Frimodt-Moeller J, He H, Poydyal A, Li J, Nation RL, Vaara T (2010a) A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob Agents Chemother 54:3341–3346
Vaara M, Siikanen O, Apajalahti J, Frimodt-Møller N, Vaara T (2010b) Susceptibility of carbapenemase-producing strains of Klebsiella pneumoniae and Escherichia coli to the direct antibacterial activity of NAB739 and to the synergistic activity of NAB7061 with rifampin and clarithromycin. J Antimicrob Chemother 65:942–994
Van den Bosshe L, Van Schedael A, Chopra S, Hoogmartens J, Adams E (2011) Identification of impurities in polymyxin B and colistin bulk sample using liquid chromatography coupled to mass spectrometry. Talanta 83:1521–1529
Vingsbo Lundberg C, Vaara T, Frimodt-Møller N, Vaara M (2010) Novel polymyxin derivatives are effective in treating experimental E. coli peritoneal infection in mice. J Antimicrob Chemother 65:981–985
Walkty A, DeCorby M, Nichol K, Karlowsky JA, Hoban DJ, Zhanel GG (2009) In vitro activity of colistin (polymyxin E) against 3,480 isolates of Gram-negative bacilli obtained from patients in Canadian hospitals in the CANWARD study, 2007–2008. Antimicrob Agents Chemother 53:4924–4926
Yahav D, Farbman L, Leibovici L, Paul M (2011) Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 18:18–29
Zawascki AP, Goldani LZ, Li J, Nation RL (2007) Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antibiot Chemother 60:1206–1215
Zawascki AP, Goldani LZ, Cao G, Superti SC, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J (2008) Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 47:1298–1304
Zhanel GG, Adam HJ, Low DE, Blondeau J, Decorby M, Karlowsky JA, Weshnoweski B, Vashisht R, Wierzbowski A, Hoban DJ, Canadian Antimicrobial Resistance Alliance (CARA) (2011) Antimicrobial susceptibility of 15,644 pathogens from Canadian hospitals: results of the CANWARD 2007-2009 study. Diagn Microbiol Infect Dis 69:291–306
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Vaara, M. (2014). Old and Novel Polymyxins Against Serious Gram-Negative Infections. In: Marinelli, F., Genilloud, O. (eds) Antimicrobials. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39968-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-39968-8_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39967-1
Online ISBN: 978-3-642-39968-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)