Abstract
Angiostrongylus cantonensis was first discovered in 1934 by Professor Chen Xintao and has become an important emerging pathogen causing human angiostrongyliasis. Rats are permissive host, and mice and human are non-permissive host. The adult worms live in the right ventricle and pulmonary arteries of rats. However, worms can’t develop to adult worm and the IV and V stage worm live in brain of mice and human. Human infect this disease by eating raw or undercooked snails or slugs, paratenic host such as prawns or contaminated vegetables, and water that contain the infective larvae. A. cantonensis has spread from its traditional endemic regions of the Pacific islands and Southeast Asia to the American continent including the USA, Caribbean islands, and Brazil. During the past few years, major outbreaks of human angiostrongyliasis have been reported in mainland China, Taiwan, Thailand, Ecuadorian, French, Germany, India, and Jamaica. Additionally, sporadic cases in travelers who have returned from endemic areas have been reported. Thousands of cases of human angiostrongyliasis have been documented worldwide. The main clinical manifestations of human angiostrongyliasis are eosinophilic meningitis and ocular angiostrongyliasis. In adult patients, the common symptoms were headache, neck stiffness, paresthesia, vomiting, and nausea. The treatment of this disease includes supportive treatment, corticosteroid therapy, and combined therapy with corticosteroids and anthelminthics. However, it is pity that some patients have sequela after treated. Therefore, some new drugs like Chinese herbal medicine have been studied for therapy angiostrongyliasis. The basic research is very important for better solution of angiostrongyliasis, so more and more study are in process, which involve mechanism of brain inflammation, new drugs finding, protein and gene sequence and analysis, new diagnosis method exploring, etc. Whatever, persuading people not to eat raw or undercooked intermediate and paratenic hosts is the most effective method for prevention and control angiostrongyliasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Angiostrongylus cantonensis
- China
- Finding history
- Molecular biology
- Clinical aspects
- Geographical distribution
- Population distribution
- Route of infection
- Epidemiological factors
- Diagnosis
- Chemotherapy
- Prevention
- Inflammation mechanism
- Geographical strain
- Development of new drugs
14.1 Introduction
The nematode Angiostrongylus cantonensis was discovered in the pulmonary arteries and hearts of domestic rats in Guangzhou (Canton), China, by Chen Xintiao in 1934 (Chen 1935). A. cantonensis is a metastrongyloid nematode that normally lives in the right ventricle and pulmonary arteries of rats, and rats are definitive (permissive) hosts (Bhaibulaya 1975). While many species of rats can carry patent infections, the Norwegian rat (Rattus norvegicus) and the black rat (Rattus rattus) are considered the most important definitive hosts. In wild populations of rats, A. cantonensis infections induce little disease, as expected for an efficient parasite (Bhaibulaya 1975; Maizels and Yazdanbakhsh 2003; Fernando 2001). Dogs, humans, horses, Australian native mammals (e.g., possums, macropods, macrobats), and birds (e.g., Tawny frogmouths), and various zoo animals are nonpermissive “accidental” hosts that are infected after ingesting third-stage larvae (L3) in intermediate hosts (molluscs) (Bhaibulaya 1975; Kliks and Palumbo 1992) or transport hosts (such as planarians, frogs, fish, and crustaceans). In the past ten years, major outbreaks were reported in endemic regions, especially in mainland China. So far, more than 2,800 people has been reported to infect A. cantonensis (Wang et al. 2008). Therefore, angiostrongyliasis is not only endemic disease but also the severe public health problem and worth paying attention to. Morera and Céspedes (1970) described Angiostrongylus costaricensis causing an abdominal eosinophilic ileocolitis as a new human disease. Several cases are asymptomatic or show light symptoms. However, in cases showing severe symptoms, the disease is often characterized by acute abdominal pain related to lesions in the ileocolic region, with presence of intense eosinophilia, eosinophilic infiltration of the intestinal wall, eggs in the submucosa, and nematodes in the mesenteric arteries. Therefore, the characteristic of angiostrongyliasis induced by A. cantonensis is eosinophil infiltration in CNS.
14.2 Finding History of Angiostrongyliasis in China and Other Countries
Angiostrongylus cantonensis was discovered in the pulmonary arteries and hearts of domestic rats in Guangzhou (Canton), China, by Chen Xintiao in 1934 (Chen 1935). A. cantonensis is a rat lungworm, which occasionally causes human angiostrongyliasis with the main clinical manifestation of eosinophilic meningitis. The first human case of angiostrongyliasis was reported in Taiwan in 1945 (Rosen et al. 1961). In 1984, He et al. reported the first human case of A. cantonensis infection in the mainland of China (He et al. 1984). After that, infection cases were reported continuously. So far, angiostrongyliasis cases were reported in about 10 provinces or cities, involving Guangzhou, Hongkong, Wenzhou, Shanghai, Beijing, Tianjin, Heilongjiang, Liaoning, Hainan, Yunnan, and Fujian (Wang et al. 2010). In past decade, some outbreaks of angiostrongyliasis happened in China. In 2004, China’s Minister of Technology has defined angiostrongyliasis as emerging infectious disease.
Thailand is one of the major source of human angiostrongyliasis. At least 1,337 cases of human angiostrongyliasis have been reported. The high infective rate among Thai population is associated with custom of eating raw or undercooked snails (Plia spp.) with alcohol, which is especially popular among young adult men (Cross and Chen 2007; Schmutzhard et al. 1988). Since two cases of eosinophil meningitis induced by A. cantonensis were reported in Hawaii in 1962 (Rosen et al. 1962, 1967), the parasite has been found in the Pacific islands and southeast Asia. The first case of human angiostrongyliasis in the Caribbean islands was reported in Cuba in 1973 (Pascual et al. 1981). Some surveys reported increasing numbers of human A. cantonensis infection in Costa Rica and Jamaica (Slom et al. 2002; Vazquez et al. 1993; Lindo et al. 2004). There was a group of 23 US travelers and about half of them occurred eosinophilic meningitis after returning from Jamaica in 2000 (Slom et al. 2002).
14.3 Biology of Angiostrongyliasis
14.3.1 Life Cycle
As a zoonotic pathogen, A. cantonensis, a rat lungworm, is endemic in south Asia, the Pacific islands, Australia, and the Caribbean islands. The life cycle of this nematode involves rats as the definitive host, molluscs as intermediate hosts, and crustaceans (prawns and land crabs) (Fig. 14.1), predacious land planarians (flatworms in the genus Platydemus), frogs, and monitor lizards as paratenic (transfer or transport) hosts. Human beings acquire A. cantonensis after eating intermediate or paratenic hosts or vegetables that contain the infective larvae (the third stage) of the worm. Once swallowed, the infective larvae are digested from those vectors and invade intestinal tissue, causing human enteritis, before passing through the liver (Yii 1976). Cough, rhinorrhoea, sore throat, malaise, and fever can develop when the worms move through the lung (Cross 1978). Finally, the larvae reach the central nervous system in about 2 weeks and cause eosinophilic meningitis.
The major pathological changes of human angiostrongyliasis occur in the brain (Eamsobhana and Tungtrongchitr 2005; Chotmongkol et al. 2006; Sonakul 1978). According to autopsy studies, the external surface and spinal cord are generally normal, and gross hemorrhage is not commonly seen. Infiltration of lymphocytes, plasma cells, and eosinophils is commonly reported in the meninges and around intracerebral vessels (Eamsobhana and Tungtrongchitr 2005; Sonakul 1978).
Cellular infiltration around living worms is not prominent, but dead worms were usually surrounded by a granuloma, an increase in the number of eosinophils, and sometimes Charcot–Leyden crystals (Eamsobhana and Tungtrongchitr 2005). The physical lesions of tracks and micro-cavities caused by movement of the worms can be found in the brain and even in the spinal cord. The larvae can also move to the eyes and cause ocular angiostrongyliasis with visual disturbance such as diplopia or strabismus in many patients (Punyagupta et al. 1975; Sawanyawisuth et al. 2006).
14.3.2 Morphology
A. cantonensis larvae got from lung tissue of P. canaliculata by means of microanatomy usually appeared to be intact. Few refractive granules were observed in very early stage L1. At early-stage L1, refractive granules began to emerge in the larval body, and in later-stage L1 and L2, they had increased and even obscured the expanded gut (Fig. 14.2) (Lv et al. 2009c). A clear line emerged at the esophagus–intestine junction, dividing the larval body into an anterior section with few refractive granules and a posterior section dense with granules. Later, just before the second molting, the line blurred, and the amount of refractive granules decreased (Fig. 14.3) (Lv et al. 2009c). Some big refractive granules could still be seen in the posterior section of early-stage L3. Late-stage L3 were transparent, and the anus, excretory pore, and esophagus–intestine junction could easily be identified (Fig. 14.4) (Lv et al. 2009c).
The image of first-stage larva of A. cantonensis. (a) Very early first-stage larva (L1) of A. cantonensis recovered from fresh rat feces. The larva moves with a coiled tail (CT). (b) Early L1 of A. cantonensis recovered from P. canaliculata on day 3 postinfection. Unexpanded intestine (UI) presents strand-like. (c) Early L1 of A. cantonensis recovered from P. canaliculata on day 5 postinfection. The intestine is subdivided into two segments: expanded intestine (EI) at the anterior part and unexpanded intestine (UI) at the posterior part. (d) Mid-stage L1 of A. cantonensis recovered from P. canaliculata on day 7 postinfection. The expanded intestine (EI) replaces the unexpanded intestine observed at an earlier stage. (e) Late L1 of A. cantonensis recovered from P. canaliculata on day 11 postinfection. The intestine becomes obscure due to refractive granules, while the esophagus bulbus (EB) anterior to the esophagus-intestine (EI) line is clear, with wrinkles (W) appearing at the anterior end. (f) Late L1 of A. cantonensis recovered from P. canaliculata on day 15 postinfection. The body size has increased approximately to that of L2. The esophagus–intestine (EI) line is clear due to heterogeneous distribution of refractive granules
The second-stage of A. cantonensis. (a) Second-stage larva (L2) of A. cantonensis with one sheath. The anus cuticle (AC) of L1 is completely molted and presents on the sheath (S). The esophagus–intestine (EI) line appears clear. (b) L2 of A. cantonensis without sheath. The larval body is not destroyed, and the esophagus–intestine (EI) line still appears clear. (c) L2 of A. cantonensis with expanded knob-like tips (KT) and rod-like structure (RS). The developed larva shows capability of movement and the esophagus–intestine line has disappeared, whereas the anus cuticle (AC) of L1 is well presented on the sheath (S)
The third-stage larva of A. cantonensis. (a) Third-stage larva (L3) of A. cantonensis with two sheaths. The outer one is the first sheath (FS) produced during the first molting, whereas the inner one is the second sheath (SS) produced during the second molting. (b) Typical L3 of A. cantonensis with expanded knob-like tips (KT) and rod-like structure (RS), but no sheath. (c) Mature and transparent L3 of A. cantonensis. Clearly visible structures include excretory pore (EP), esophagus bulbus (EB), intestine (I), and anus (A)
The larvae in the cranial cavity were mainly at the stage of L4 and L5, which were slender, thread-like roundworm, with a circular mouth in the anterior part. The heads of both sexes were similar, but the tails were different. The female was pointed with the vulva in front of the anus, but the male was broad with copulatory bursa and long spicules. The gastrointestinal tracts and genital tubes were clearly seen inside the coelomic cavity through the translucence body (Fig. 14.5) (OuYang et al. 2012). Adult worms were recovered from the cardiopulmonary systems of rats. These worms had features characteristic of A. cantonensis, including size (males measured 14–15 mm in length; females 24–26 mm in length), body shape, and prominent dark intestine (Fig. 14.6a) (Lindo et al. 2002). The long copulatory spicules in the male worms measured approximately 1.2 mm (Fig. 14.6b) (Lindo et al. 2002). The eggs were got from lung of rats after they were infected 8 weeks (Fig. 14.7) (Gu et al. 2008).
The fourth stage and fifth stage of A. cantonensis from rat and mice brain. Images show the intracranial larvae obtained from rats. A. cantonensis larva was a kind of thread-like, semitransparent nematode with a simple circular mouth (arrowhead) in the anterior part and a tail (arrow) with a copulatory bursa (male) or a vulva (female)
14.4 Molecular Biology of A. cantonensis
It is not incompletely known for the molecular characteristics of A. cantonensis. The expressed sequence tags (ESTs) of A. cantonensis were analyzed in order to get more insight to its genomic expression patterns. About 1,277 ESTs of A. cantonensis in NCBI databases. The result showed that there were 60 ESTs had no match to any of the proteins and gene sequences in the published databases, and 695 ESTs score more than 80. According to the function, the identified 695 ESTs could be grouped into 13 categories related to metabolism, cellular development, immune evasion, host–parasite interactions, and so on. Among them, 65 (9.4 %) were proteases and protease inhibitors, represented 19 potential proteases and protease inhibitors genes; 42 (6.0 %) were allergens or antigens, represented 15 potential antigens/allergens genes (Fang et al. 2010). It is also excepted that the whole genome of A. cantonensis is sequencing by Sun Yat-sen University. The information substantially expand the available genetic information about A. cantonensis and should be a significant resource for A. cantonensis gene research.
In addition, a cDNA library of A. cantonensis fourth-stage larvae was constructed, and ~1,200 clones were sequenced. Bioinformatic analyses revealed 378 cDNA clusters, 54.2 % of which matched known genes at a cutoff expectation value of 10−20. Of these 378 unique cDNAs, 168 contained open-reading frames encoding proteins containing an average of 238 amino acids. Characterization of the functions of these encoded proteins by Gene Ontology analysis showed enrichment in proteins with binding and catalytic activity. The observed pattern of enzymes involved in protein metabolism, lipid metabolism, and glycolysis may reflect the central nervous system habitat of this pathogen (He et al. 2009).
There are many genes of A. cantonensis having been cloned and analyzed. They are cysteine protease inhibitor (Liu et al. 2010), matrix metalloproteinase (Sun et al. 2012), cathepsin B-like cysteine proteinase (Cheng et al. 2012), protein disulfide isomerases (Liu et al. 2012), novel gene encoding 16 kDa protein (Li et al. 2012a), cathepsin B (Han et al. 2011), galectin-10 (Liu et al. 2013), etc. These work establish the foundation for researching the vaccine against A. cantonensis and searching drug target of angiostrongylsis.
MicroRNAs (miRNAs) are endogenous, small, noncoding RNAs that play key roles in gene expression regulation, cellular function and defense, homeostasis, and pathogenesis. A study term determine and characterize miRNAs of female and male adults of A. cantonensis by Solexa deep sequencing. A total of 8,861,260 and 10,957,957 high quality reads with 20 and 23 conserved miRNAs were obtained in females and males, respectively. No new miRNA sequence was found. Nucleotide bias analysis showed that uracil was the prominent nucleotide, particularly at positions of 1, 10, 14, 17, and 22, approximately at the beginning, middle, and the end of the conserved miRNAs (Chen et al. 2011c). MicroRNA of A. cantonensis third- and fourth-stage larvae and the brain microRNA of infected mice have been sequenced by Sun Yat-sen University. Undoubtedly, these study will establish the foundation for the research of A. cantonensis molecular biology and angiostrongyliasis mechanism.
14.5 Epidemiology
14.5.1 Epidemiology of A. cantonensis Worldwide
Human A. cantonensis infection has evidently increased the public attention worldwide due to outbreaks and also because more and more sporadic cases are being reported in Western tourists in recent years. Over 2,800 cases of human angiostrongyliasis had been documented in approximately 30 countries (Wang et al. 2008). However, there are no doubts, many more cases unreported due to lack of awareness of this parasite within the medical community. During 2008–2012, an additional 120 cases were reported (Table 14.1).
14.5.2 Epidemiology of A. cantonensis in China
China has become one of the major countries where cases of human angiostrongyliasis increased significantly in the past decade. Therefore, much more efforts have been made to investigate the prevalence of A. cantonensis in this country. In mainland China, the first case of human angiostrongyliasis with eosinophilic meningitis was reported in 1984 in Guangdong province. Outbreaks of human infection with A. cantonensis have been reported with increasing frequency in mainland China in recent years, possibly caused by the growing popularity of eating exotic foods such as raw and undercooked snails. One outbreak of human A. cantonensis infection with 65 cases of eosinophilic meningitis was observed in Wenzhou, Zhejiang province, in 1997 (Zheng et al. 2001). An outbreak of five human cases occurred in Liaoning province in 1999 (Lin and Wang 2004) and three outbreaks with a total of 30 cases were reported in Fujian province in 2002 (Yang et al. 2004; Ye et al. 2008; Lin et al. 2003). Two outbreaks with 34 human cases occurred in Yunnan province in 2003 and 2005 (Zhou et al. 2009). Unfortunately, these outbreaks do not seem to have drawn sufficient public attention to the threat of this parasite in China, a situation that contributed to a larger outbreak of some 160 cases in Beijing in 2006. The same exposure pattern was also reported in two outbreaks with a total of 17 human infections in Taiwan in 1998 and 2001 (Tsai et al. 2001a, b) (Fig. 14.8). From the epidemiological survey, the west-central region of Guangdong Province in China is the natural focus of A. Cantonensis and there were 180 positive samples of IgG antibody against A. cantonensisin 1,800 serum samples of the residents, with a positive rate of 10 % (Chen et al. 2011a).
The distribution of A. cantonensis and its outbreaks in China. The endemic regions of A. cantonensis are marked in purple and those with outbreaks of human A. cantonensis are marked with green triangles (Wang et al. 2012)
In China, Pomacea canaliculata and Achatina fulica are main vectors for human infection (Wang et al. 2007). P. canaliculata, native to South America, was introduced to Taiwan and the mainland of China in the 1980s. P. canaliculata has replaced the African giant snail, A. fulica, as a major intermediate host and has become the main source of human infection both in Taiwan and mainland China. A retrospective study of published prevalence of A. cantonensis in mainland China revealed that 22 of 32 species of wild mollusk species (69 %) are infected with the parasite (Lv et al. 2008). Achatina fulica has been recorded with the highest rate and intensity of infections, followed by slugs (Vaginulus spp.) and Pomacea canaliculata. The rates and intensities of infections in terrestrial snails and slugs are higher than in freshwater mollusks. This was confirmed by a recent national survey conducted in China (Lv et al. 2009b). P. canaliculata and A. fulica were found in 11 and 6 provinces, respectively. Out of 11,709 P. canaliculata snails examined, 6.8 % were infected with A. cantonensis. Of 3,549 A. fulica snails examined, 13.4 % were infected with A. cantonensis. The infection prevalence among terrestrial snails was 0.3 %. A total of 5,370 terrestrial slugs were dissected, revealing an infection prevalence of 6.5 %. The prevalence among the other fresh water snails was 0.05 %. In Guangdong province, during 2008–2009, specimens from 510 snails (144 P. canaliculata, 306 A. fulica, and 60 Bradybaena despecta) were digested with pepsin for isolation of A. cantonensis larvae. Prevalence rates of A. cantonensis in P. canaliculata, A. fulica, and B. despecta were 8.3 %, 2.0 %, and 5.0 %, respectively (Qu et al. 2011). After that, the followed survey reported that a total of 795 A. fulica snails and 734 P. canaliculata snails were collected and the average infection rates of these two species were 13.96 % (111 of 795) and 1.50 % (11 of 734), respectively, in 2008–2010 (Yang et al. 2012). However, a recent study demonstrated that P. canaliculata had an average infection rate of 21 %, significantly higher than that of A. fulica (10 %) in Shenzhen, Guangdong province (Zhang et al. 2008). P. canaliculata has replaced A. fulica playing an important role in the epidemiology of A. cantonensis in recent outbreaks of human angiostrongyliasis (Lv et al. 2008).
The retrospective study also revealed that 11 of 15 wild rodent species in mainland China are infected with A. cantonensis. Rattus norvegicus is the most frequently identified host with a generally higher prevalence and intensity of infection compared with other rodents. This was consistent with a national survey that found 32 of 711 rats infected with A. cantonensis (31 R. norvegicus and 1 R. flavipectus) (Lv et al. 2008). In Guangdong, China, researchers captured 288 rats of seven species (257 R. norvegicus, 13 R. flavipectus, 7 R. Losea, 6 R. rattus, 3 Bandicota indica, 1 R. rattus alexandrinus, and 1 Mus musculus) and rats were examined for adult A. cantonensis nematodes in pulmonary arteries and right heart cavities. The result showed that among the 288 rats examined, 27 (9.4 %) from three species (25 R. norvegicus, 1 R. losea, and 1 M. Musculus) were infected with A. cantonensis adults in their cardiopulmonary systems during 2008–2009 (Qu et al. 2011). From 2008 through 2010, a total of 430 rats were captured and 23 rats, from two species, were infected, with an average infection rate of 5.35 % and the infection rate was calculated to be 9.09 % (20 of 220) for R. norvegicus and 15.00 % (3 of 20) for R. flavipectus, respectively (Yang et al. 2012). Interestingly, A. cantonensis was also found in nonhuman primate, equine, and canine species. A. cantonensis was discovered in paratenic host frog species (Hylarana guentheri, Rana limnocharis, and Rana plancyi) and toads (Bufo melanostictus), but has not yet been identified in freshwater shrimp, fish, crabs, or planaria in published studies (Lv et al. 2008). A. cantonensis was not found in any of 652 paratenic hosts collected during a national survey that included frogs, shrimps, crabs, toads, and fish (Lv et al. 2009b).
14.6 Clinical Aspects
The term human angiostrongyliasis refers primarily to eosinophilic meningitis/meningoencephalitis and ocular angiostrongyliasis, which are the major clinical features of A. cantonensis infection in human beings. However, a rare and extremely fatal cases were reported (Sawanyawisuth et al. 2009). The incubation of this disease is highly variable, ranging from 1 day to several months, depending on the number of parasites involved (Yii et al. 1976; Chen et al. 2006; Zheng et al. 2001; Punyagupta et al. 1970). In an outbreak in Beijing, China, the incubation period of 128 (80 %) of 160 patients was 7–35 days (He et al. 2007). In an outbreak in Wenzhou, Zhejiang, China, clinical symptoms appeared in 40 (62 %) of 65 patients on days 6–15 after infection (Xue et al. 2000).
In adult patients, the common symptoms were headache (95 %), neck stiffness (46 %), paresthesia (44 %), vomiting (38 %), and nausea (28 %). Headache, mainly caused by increased intracranial pressure or the direct damage of the larvae, was intermittent, frequent, and severe at first; after repeated lumbar puncture, it became less frequent and less severe and eventually resolved (Yii 1976; Punyagupta et al. 1975). Neck stiffness was usually mild and lasted for a short period. Nuchal rigidity was less common but has been reported in severe cases (Slom et al. 2002; Chau et al. 2003). Paresthesia, which usually persisted for less than 2 weeks and occurred in a variety of anatomical locations (usually in the extremities) was expressed as pain, numbness, itching, or a sensation of worms crawling under the skin (Yii 1976). Vomiting and nausea were probably related to increased intracranial pressure and usually disappeared after the first lumbar puncture. Although few adult patients with visual disturbance or diplopia were reported in China, this symptom was noted in 184 (38 %) of 484 patients in Thailand and 11 (92 %) of 12 patients in the USA (Slom et al. 2002; Punyagupta et al. 1975). Thirty-two percent of adult patients had fever, which was mostly low grade; however, approximately 10 % of these had high-grade fever ranging from 38 °C to 39 °C (Yii 1976; Punyagupta et al. 1975). In addition, the clinical features of human ocular angiostrongyliasis in 35 patients involve visual loss (94.3 %), fundus change (34.3 %), eye redness and pain (17.1 %), eye floater (8.6 %), and blindness (5.7 %) (Diao et al. 2011).
In children, stiff neck and paresthesia were less common than in adult patients, whereas reports of nausea and vomiting were higher. Of 94 (82 %) pediatric patients with nausea and vomiting, 56 % had projectile vomiting, which usually disappeared within 1 week (Yii 1976). Additionally, rates of fever, somnolence, constipation (76 %), and abdominal pain were higher in children than in adults. In adults or children with heavy infections, coma and death can occur (Yii 1976; Chotmongkol and Sawanyawisuth 2002). Especially, children have lower immunity than human, the rate of death increased, and the autopsy found adult worm in pulmonary artery (Li et al. 2001).
14.7 Diagnosis
14.7.1 Parasite Diagnosis
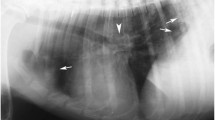
Human angiostrongyliasis is confirmed by detection of A. cantonensis in patients. However, the detection rate is frequently low (Punyagupta et al. 1975; Yii 1976). The diagnosis is, therefore, primarily based on clinical symptoms and medical history. The typical clinical manifestation of human angiostrongyliasis is eosinophilic meningitis. However, other causes for this clinical presentation must be considered (Lo and Gluckman 2003). Medical history of eating intermediate or paratenic hosts of A. cantonensis critical for diagnosis. The detection of eosinophils and brain lesions are also helpful for diagnosis. Eosinophils account for a large portion of white cell counts in blood and CSF in A. cantonensis infections (Yii 1976; Tsai et al. 2001a). MRI and CT have been used to detect damage in brain for differential diagnosis of A. cantonensis from other parasites. After administration of Gd-DTPA, multiple round or oval contrast-enhanced nodules, diameter ranging from 3 to 10 mm, were seen on T1WI, and the resolution of lesions in the pia mater was faster than that in parenchyma of the brain (Fig. 14.9) (Jin et al. 2005; Ogawa et al. 1998; Hasbun et al. 2001).
Brain MR images obtained at 7 weeks (a–e) and 11 weeks (f) after ingestion of snails. Some lesions presented as (a) hypointense on a unenhanced axial T1WI (TR 500 ms; TE ms) and (b) hyperintense on a transverse T2WI (TR 4,000 ms; TE 100 ms). (c) More lesions were revealed on a corresponding transverse FLAIR image (TR 5,000 ms; TE 110 ms; TI 2,000 ms). Diffuse contrast-enhanced round or oval nodules of different sizes were shown on (d) a transverse T1WI (TR 500 ms; TE 15 ms) and (e) a sagittal T1WI (TR 500 ms; TE 15 ms) after gadolinium administration. The nodular lesions mentioned above disappeared or diminished on a 5-week (f) follow-up transverse T1WI (TR 500 ms; TE 15 ms) after gadolinium compared with (d). Note that pia enhancement (arrow) shown on (d) completely resolved in (f)
14.7.2 Immunological Detection
To effectively diagnose and manage A. cantonensis infection, serological tests such as enzyme-linked immunosorbent assay (ELISA) have been developed to detect the antigens of or antibodies against A. cantonensis in serum or cerebrospinal fluid. The detection of circulating antigens in serum or CSF provides a rapid confirmation of infection. Monoclonal antibodies (mAbs) against parasite-specific antigens detect circulating antigen with relatively high specificity and reasonably good sensitivity (Eamsobhana and Yong 2009). Recently, several mAbs against the excretory/secretory (ES) proteins have been developed (Chen et al. 2010). The mAbs against an ES protein of 55 kDa have the highest specificity and sensitivity. The detection rate of antigen in the sera of angiostrongyliasis patients was 100 % and cross-reactions to normal sera or the sera of patient s with other parasitic infection, such as clonochiasis, fasiolopsiasis, ancylostomiasis, anisakiasis, or schistosomiasis were not found (Huang et al. 2010). In addition, antigens from A. cantonensis can also be detected in sera by immuno-PCR (Chye et al. 2004). Human antibodies to A. cantonensis may be generated after infection. Several specific A. cantonensis antigens such as 29 kD, 31 kD, 32 kD, and 66 kD have been identified for immunodiagnosis of the presence of such antibodies (Maleewong et al. 2001; Nuamtanong 1996; Bessarab and Joshua 1997).
14.8 Control
14.8.1 Chemotherapy
Human angiostrongyliasis displays two main forms of clinical presentation: eosinophilic meningitis and ocular angiostrongyliasis. For eosinophilic meningitis, effective supportive treatments are repeated by lumbar puncture and analgesics (Punyagupta et al. 1975; Yii 1976). Corticosteroid therapy has been effective in human angiostrongyliasis. Patients were given a 2-week course of prednisolone (treatment group), 60 mg/day, and compared with those given placebo (control group). The results indicated that a 2-week course of prednisolone was beneficial in relieving headache in patients with eosinophilic meningitis (Chotmongkol et al. 2000). Anthelminthics, such as albendazole and mebendazole, have been used to treat this disease at 15 mg/kg/day or identical placebo for 2 weeks in attempts to more effectively relieve symptoms and reduce their duration. The mean duration of headache was reduced significantly by using albendazole alone (Jitpimolmard et al. 2007). The combination of corticosteroids and anthelminthics has been commonly used for treatment of human angiostrongyliasis. Patients were given a 2-week course of prednisolone, 60 mg/day, and mebendazole, 10 mg/kg/day. Treatment for 2 weeks with the combination regimen of prednisolone and mebendazole is safe and beneficial in relieving headaches in patients with eosinophilic meningitis (Chotmongkol et al. 2006; Wang et al. 2008). Currently, some Chinese herbal medicines display efficacy for treating angiostrongyliasis in animal studies but have not been used in humans (He et al. 2011; Wan and Weng 2004; Shih et al. 2007; Lai et al. 2008; Lai 2006). Surgery is required to remove worms from the eyes of patients with ocular angiostrongyliasis.
14.8.2 Prevention
Because of its worldwide distribution, it is impossible to eliminate A. cantonensis from the environment. However, it is possible to avoid or reduce human infection by blocking the transmission pathway of this parasite. The simple method is to persuade people not to eat raw or undercooked intermediate and paratenic hosts in endemic regions. Epidemiological surveys indicate that most cases of human angiostrongyliasis would be avoided in this way. Also some rare cases caused by eating contaminated vegetables can be avoided by effective washing. However, the difficulty for prevention is that most people have no or limited know ledge of the worm and are totally unaware of the danger of consuming it. Therefore, one of the most effective measures would be the spread of knowledge regarding A. cantonensis and its potential for damage to the health of the general population, especially in remote and poor areas of endemic regions. Another approach is persuading people to abandon their habit of eating raw snails and paratenic hosts. Travelers heading to endemic regions must know the dangers of eating raw mollusks and raw vegetables with unknown sources and should avoid these foods. For physicians in both nonendemic and endemic regions, it is necessary to be aware of the existence of these worms, their symptoms, and modes of transmission to suspect and diagnose A. cantonensis infection in humans promptly (Wang et al. 2012).
14.9 Basic Research
In China, there are some research groups from universities or institutes involving in basic reaearches about A. cantonensis, such as Sun Yat-sen University and National institute of parasitic diseases Chinese Center For Disease Control and Prevention(NIPD). The research topics involves genetics of differential isolated strains, mechanism of pathogenesis and inflammation induced by the worm, development of new drugs, molecular biological study (showed in the part of molecular biology of A. cantonensis), etc.
14.9.1 Different Geographic Strain Study
He Han-jiang et al. study the biology, genetics, and virulence of A. cantonensis isolated from Guangdong, Fuzhou, Haikou, Hekou, and Wenzhou in China. Phylogenetic analysis revealed that the combined CO1 and ND4 mtRNA sequences were able to distinguish A. cantonensis isolates from these geographical regions. According to CO1 and ND4 sequences and phylogenetic analysis, there are two geographical origins probably. One geographical origin includes Guangzhou, Haikou, and Fuzhou strains, another geographical origin consist of strains from Wenzhou and Hekou. To compare virulence of A. cantonensis isolates from Guangzhou, Haikou, and Fuzhou, the rate of death, change of weight, worm recovery, neurological function points, and leukocyte counts are detected in infected BALB/c mice. The result showed that the mice infected by L3 from Guangzhou were not different from Haikou. However, these indexes were lower in the mice infected with the worm from Fuzhou region. Therefore, the difference of virulence in A. cantonensis matched up to genes variation. (This part is not published.)
14.9.2 Immunity Reaction of Angiostrongyliasis
The study of immunity against Angiostrongylus cantonensis infection is attempted in animal model. Researches suggest that systemic and local Th2 cytokine responses, especially those involving IL-5, are predominant in A. cantonensis-nfected mice and that IL-5 is an important cytokine underlying the innate resistance of the mouse against A. cantonensis (Sugaya et al. 1997). The levels of interleukin 5 (IL5), IL10, and IL13 in the cerebrospinal fluid (CSF) were markedly higher in 30 patients with eosinophilic meningitis associated with angiostrongyliasis (EOMA) than in the controls (Intapan et al. 2008).CCR3 is a major chemokine receptor that is abundant on the surface of eosinophils and is responsible for their activation and chemotaxis. CCR3 recognizes many chemokines, including CCL11, CCL5 (RANTES), and CCL3 (MIP-1α). Mice received an intraperitoneal injection of anti-CCR3 monoclonal antibody (mAb) (50 μg) at 10 days postinfection (dpi); the levels of CCL11 (eotaxin) in the peripheral circulation and the expression of the Th2-type cytokine interleukin-5 and eosinophil count in the brains were significantly reduced (Chuang et al. 2010). Another important cytokine is IL-33. IL-33 protein and ST2L messenger RNA (mRNA) transcripts in the brains were upregulated during A. cantonensis infection and that both splenocytes and brain mononuclear cells became IL-33 responsive and produced interleukin 5 and interleukin 13. Furthermore, administration of IL-33 to A.cantonensis-infected mice enhanced ST2L expression and cytokine production, which indicated that IL-33 produced in the brain may function as an inflammatory mediator in eosinophilic meningitis induced by A. Cantonensis (Peng et al. 2013).
The types of cells involved in the BBB include astrocyte, microglia, and endothelial cells. When the worm invade into the brain, BBB is damaged. A. cantonensis larvae extracts can induce apoptosis of brain astrocytic cells and brain microvascular endothelial cells and increase the permeability of the BBB in vitro (Hu et al. 2012). Microglia is considered to be the key immune cell in the central nervous system like macrophage. Soluble antigen of the fourth larva of A. cantonensis can induce mice microglia activation and produce IL-5, IL-13, and eotaxin which are related to eosinophil (Wei et al. 2013).
14.9.3 Development of New Drugs
For improving the effect of therapy for angiostrongyliasis, many new drugs have been researched. The results showed that the combination of albendazole and baicalein increased the survival time, decreased body weight loss, neurological dysfunction, leucocyte response, eotaxin concentration, and MMP-9 activity, so the combination of albendazole and baicalein was more effective than either drug administered singly (He et al. 2011). In addition, albendazole combined with a marine fungal extract (m2-9) increased body weight, reduced worm burden, improved learning ability, memory and action, decreased neurological dysfunction and leucocyte response in these mice, and m2-9 is a natural product with potentially significant therapeutic value for angiostrongyliasis and is worthy of further study (Li et al. 2012b). Tribendimidine, a broad-spectrum anti-helmintic drug developed in China, is a derivative of amidantel. The study showed that a strong efficacy of tribendimidine against A. cantonensis and provided suitable alternative treatments to further explore its potential use in treatment of human angiostrongyliasis. These drugs also can induce worm surface damage (Fig. 14.10) (Wang et al. 2013).
Scanning electron microscopy images showing results after treatment with edible oil (a), tribendimidine early treatment (b), tribendimidine late treatment (c), and albendazole treatment (d). Images show larvae, taken from the control group, with clear and regular epidermal folds (a). The epicuticule of the larvae from tribendimidine early treatment group was damaged and incomplete (TBD7) (b). The epidermal fold is fuzzy and its structure is not clear on the larvae from the tribendimidine late treatment group (TBD14) (c). The epidermal fold is clear and regular on larvae from albendazole-treated mice (d)
References
Bessarab IN, Joshua GW (1997) Stage-specific gene expression in Angiostrongylus cantonensis: characterisation and expression of an adult-specific gene. Mol Biochem Parasitol 88:73–84
Bhaibulaya M (1975) Comparative studies on the life history of Angiostrongylus mackerrasae Bhaibulaya, 1968 and Angiostrongylus cantonensis (Chen, 1935). Int J Parasitol 5:7–20
Chau TT, Thwaites GE, Chuong LV, Sinh DX, Farrar JJ (2003) Headache and confusion: the dangers of a raw snail supper. Lancet 361:1866
Chen HT (1935) Un nouveau nematoda pulmonaire: Pulmonema cantonensis, n.g.n. sp. des rats de Canton. Ann Parasitol Hum Comp 13:312–317
Chen WL, Zhong JM, Chen H, Wu SY, Ding L (2006) Eosinophilic meningitis: 31 cases report. Chin J Misdiagnostics 6:4668–4669
Chen MX, Zhang RL, Chen JX, Chen SH, Li XH, Gao ST, Geng YJ, Huang DN, Ai L, Xu MJ, Zhu XQ (2010) Monoclonal antibodies against excretory/secretory antigens of Angiostrongylus cantonensis. Hybridoma 29:447–452
Chen D, Zhang Y, Shen H, Wei Y, Huang D, Tan Q, Lan X, Li Q, Chen Z, Li Z, Ou L, Suen H, Ding X, Luo X, Li X, Zhan X (2011a) Epidemiological survey of Angiostrongylus cantonensis in the west-central region of Guangdong Province, China. Parasitol Res 109:305–314
Chen F, Chen SR, Li KR, Li TH, Fang W, Luo JJ (2011b) Investigation on outbreak of Angiostrongyliasis cantonensis due to consumption of snail food in Dali City. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 23:687–690 (Article in Chinese)
Chen MX, Ai L, Xu MJ, Zhang RL, Chen SH, Zhang YN, Guo J, Cai YC, Tian LG, Zhang LL, Zhu XQ, Chen JX (2011c) Angiostrongylus cantonensis: identification and characterization of microRNAs in male and female adults. Exp Parasitol 128:116–120
Cheng M, Yang X, Li Z, He H, Qu Z, He A, Wu Z, Zhan X (2012) Cloning and characterization of a novel cathepsin B-like cysteine proteinase from Angiostrongylus cantonensis. Parasitol Res 110:2413–2422
Chotmongkol V, Sawanyawisuth K (2002) Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health 33:231–234
Chotmongkol V, Sawanyawisuth K, Thavornpitak Y (2000) Corticosteroid treatment of eosinophilic meningitis. Clin Infect Dis 31:660–662
Chotmongkol V, Sawadpanitch K, Sawanyawisuth K, Louhawilai S, Limpawattana P (2006) Treatment of eosinophilic meningitis with a combination of prednisolone and mebendazole. Am J Trop Med Hyg 74:1122–1124
Chuang CC, Su KE, Chen CW, Fan CK, Lin FK, Chen YS, Du WY (2010) Anti-CCR3 monoclonal antibody inhibits eosinophil infiltration in Angiostrongylus cantonensis-infected ICR mice. Acta Trop 113:209–213
Chye SM, Lin SR, Chen YL, Chung LY, Yen CM (2004) Immuno-PCR for detection of antigen to Angiostrongylus cantonensis circulating fifth-stage worms. Clin Chem 50:51–57
Cross JH (1978) Clinical manifestations and laboratory diagnosis of eosinophilic meningitis syndrome associated with angiostrongyliasis. Southeast Asian J Trop Med Public Health 9:161–170
Cross JH, Chen ER (2007) Angiostrongyliasis. In: Murrell KD, Fried B (eds) Food-borne parasitic zoonoses. Springer, New York, NY, pp 263–290
Deng ZH, Lv S, Lin JY, Lin RX, Pei FQ (2011) An outbreak of angiostrongyliasis in Guanging, People’s Republic of China: migrants vulnerable to an emerging disease. Southeast Asian J Trop Med Public Health 42:1047–1053
Diao Z, Wang J, Qi H, Li X, Zheng X, Yin C (2011) Human ocular angiostrongyliasis: a literature review. Trop Doct 41:76–78
Dorta-Contreras AJ, Padilla-Docal B, Moreira JM, Robles LM, Aroca JM, Alarcón F, Bu-Coifiu-Fanego R (2011) Neuroimmunological findings of Angiostrongylus cantonensis meningitis in Ecuadorian patients. Arq Neuropsiquiatr 69:466–469
Eamsobhana P, Tungtrongchitr A (2005) Angiostrongyliasis in Thailand. In: Arizono N, Chai JY, Nawa Y, Takahashi Y (eds) Food-borne helminthiasis in Asia, vol 1, Asian parasitology. The Federation of Asian Parasitologists, Chiba, Japan, pp 183–197
Eamsobhana P, Yong HS (2009) Immunological diagnosis of human angiostrongyliasis due to Angiostrongylus cantonensis (Nematoda:Angiostrongylidae). Int J Infect Dis 13:425–430
Fang W, Xu S, Wang Y, Ni F, Zhang S, Liu J, Chen X, Luo D (2010) ES proteins analysis of Angiostrongylus cantonensis: products of the potential parasitism genes? Parasitol Res 106:1027–1032
Fernando RL (2001) Angiostronglyosis. In: Fernando RL (ed) Tropical infectious diseases. Greenwich Medical Media, London, pp 107–110
Gu JB, Liu M, Li H, Luo YL, Li XX, Chen XG, Zhan XM (2008) Construction of the life cycle of Angiostrongylus cantonensis in laboratory. Nan Fang Yi Ke Da Xue Xue Bao 28:551–554 (Article in Chinese)
Han YP, Li ZY, Li BC, Sun X, Zhu CC, Ling XT, Zheng HQ, Wu ZD, Lv ZY (2011) Molecular cloning and characterization of a cathepsin B from Angiostrongylus cantonensis. Parasitol Res 109:369–378
Hasbun R, Abrahams J, Jekel J, Quagliarello VJ (2001) Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 345:1727–1733
He JZ, Zhu SH, Yang SQ, Yu BW, Chen YS, Hu GX, Wang SB, Wang L (1984) First discover and evidence of Angiostrongylus cantonensis in the cerebrospina fluid from the case of the population of the mainland of China. Acad J Guangzhou Med Coll 12:56–61 (Article in Chinese)
He ZY, Jia L, Huang F, Liu GR, Li J, Dou XF, Wang QY, He X, Gao ZY, Yang P, Wu J (2007) Survey on the outbreak of human angiostrongyliasis in Beijing. Chin J Public Health 23:1241–1242 (Article in Chinese)
He H, Cheng M, Yang X, Meng J, He A, Zheng X, Li Z, Guo P, Pan Z, Zhan X (2009) Preliminary molecular characterization of the human pathogen Angiostrongylus cantonensis. BMC Mol Biol 10:97
He HJ, Lv ZY, Li ZY, Zhang LY, Liao Q, Zheng HQ, Su WY, Rao SQ, Yu XB, Wu ZD (2011) Efficacy of combined treatment with albendazole and baicalein against eosinophilic meningitis induced by Angiostrongylus cantonensis in mice. J Helminthol 85:92–99
Hu X, Li JH, Lan L, Wu FF, Zhang EP, Song ZM, Huang HC, Luo FJ, Pan CW, Tan F (2012) In vitro study of the effects of Angiostrongylus cantonensis larvae extracts on apoptosis and dysfunction in the blood-brain barrier (BBB). PLoS One 7:e32161
Huang DN, Chen MX, Geng YJ, Li XH, Gao ST, Zhang RL (2010) Detection of circulating antigen of Angiostrongylus cantonensis by 12D5 and 21B7 monoclonal antibodies. Chin J Epidemiol 31:79–82
Intapan PM, Kittimongkolma S, Niwattayakul K, Sawanyawisuth K, Maleewong WJ (2008) Cerebrospinal fluid cytokine responses in human eosinophilic meningitis associated with angiostrongyliasis. Neurol Sci 267:17–21
Jin E, Ma D, Liang Y, Ji A, Gan S (2005) MRI findings of eosinophilic myelomeningoencephalitis due to Angiostrongylus cantonensis. Clin Radiol 60:242–250
Jitpimolmard S, Sawanyawisuth K, Morakote N, Vejjajiva A, Puntumetakul M, Sanchaisuriya K, Tassaneeyakul W, Tassaneeyakul W, Korwanich N (2007) Albendazole therapy for eosinophilic meningitis caused by Angiostrongylus cantonensis. Parasitol Res 100:1293–1296
Kliks MM, Palumbo NE (1992) Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med 34:199–212
Lai SC (2006) Chinese herbal medicine Yin-Chen-Extract as an adjunct to anthelmintic albendazole used against Angiostrongylus cantonensis-induced eosinophilic meningitis or meningoencephalitis. Am J Trop Med Hyg 75:556–562
Lai SC, Chen KM, Chang YH, Lee HH (2008) Comparative efficacies of albendazole and the Chinese herbal medicine long-dan-xie-gan-tan, used alone or in combination, in the treatment of experimental eosinophilic meningitis induced by Angiostrongylus cantonensis. Ann Trop Med Parasitol 102:143–150
Li DN, He A, Wang Y, Liang Y, Li ZY, Men JX, Zhan XM (2001) Three lethal cases of Angiostrongylus cantonensis infected children. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 19:310–311 (Article in Chinese)
Li ZY, Lv ZY, Wei J, Liao Q, Zheng HQ, Wu ZD (2012a) Cloning and characterization of a novel gene encoding 16 kDa protein (Ac16) from Angiostrongylus cantonensis. Parasitol Res 110:2145–2153
Li ZY, Sun R, Li J, Song YX, Lin YC, Zeng X, He HJ, Wei J, Yang F, Zheng HQ, Lv ZY, Wu ZD (2012b) Efficacy of albendazole combined with a marine fungal extract (m2-9) against Angiostrongylus cantonensis-induced meningitis in mice. J Helminthol 86:410–417
Lin W, Wang XT (2004) Epidemiology of Angiostrongylus cantonensis in mainland. Chin J Zoonoses 20:1004–1007 (Article in Chinese)
Lin JX, Li YS, Zhu K, Chen BJ, Cheng YZ, Lin JC, Cao Y, Chen RZ (2003) Epidemiological study on group infection of Angiostrongylus cantonensis in Changle City. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 21:110–112 (Article in Chinese)
Lindo JF, Waugh C, Hall J, Cunningham-Myrie C, Ashley D, Eberhard ML, Sullivan JJ, Bishop HS, Robinson DG, Holtz T, Robinson RD (2002) Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis 8:324–326
Lindo JF, Escoffery CT, Reid B, Codrington G, Cunningham-Myrie C, Eberhard ML (2004) Fatal autochthonous eosinophilic meningitis in a Jamaican child caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 70:425–428
Liu YH, Han YP, Li ZY, Wei J, He HJ, Xu CZ, Zheng HQ, Zhan XM, Wu ZD, Lv ZY (2010) Molecular cloning and characterization of cystatin, a cysteine protease inhibitor, from Angiostrongylus cantonensis. Parasitol Res 107:915–922
Liu Q, Yang X, Zhang M, Wang L, Liu J, Chen J, He A, Li Z, Wu Z, Zhan X (2012) Molecular characterization and immunolocalization of a protein disulfide isomerase from Angiostrongylus cantonensis. Parasitol Res 110:2501–2507
Liu LH, He HJ, Lv ZY, Wei J, Zeng X, Liang JY, Zheng HQ, Yu XB, Sun X, Wu ZD (2013) The mRNA level of the galectin-10 of Angiostrongylus cantonensis induced by reactive oxygen stress. Parasitol Res 112:933–943
Lo RV III, Gluckman SJ (2003) Eosinophilic meningitis. Am J Med 114:217–223
Luessi F, Sollors J, Torzewski M, Muller HD, Siegel E, Blum J, Sommer C, Vogt T, Thomke F (2009) Eosinophilic meningitis due to Angiostrongylus cantonensis in Germany. J Travel Med 16:292–294
Lv S, Zhang Y, Steinmann P, Zhou XN (2008) Emerging angiostrongyliasis in mainland China. Emerg Infect Dis 14:161–164
Lv S, Zhang Y, Chen SR, Wang LB, Fang W, Chen F, Jiang JY, Li YL, Du ZW, Zhou XN (2009a) Human angiostrongyliasis outbreak in Dali, China. PLoS Negl Trop Dis 3:e520
Lv S, Zhang Y, Liu HX, Hu L, Yang K, Steinmann P, Chen Z, Wang LY, Utzinger J, Zhou XN (2009b) Invasive snails and an emerging infectious disease: results from the first national survey on Angiostrongylus cantonensis in China. PLoS Negl Trop Dis 3:e368
Lv S, Zhang Y, Liu HX, Zhang CW, Steinmann P, Zhou XN, Utzinger J (2009c) Angiostrongylus cantonensis: morphological and behavioral investigation within the freshwater snail Pomacea canaliculata. Parasitol Res 104:1351–1359
Maizels RM, Yazdanbakhsh M (2003) Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol 3:733–744
Maleewong W, Sombatsawat P, Intapan PM, Wongkham C, Chotmongkol V (2001) Immunoblot evaluation of the specificity of the 29-kDa antigen from young adult female worms Angiostrongylus cantonensis for immunodiagnosis of human angiostrongyliasis. Asian Pac J Allergy Immunol 19:267–273
Malvy D, Ezzedine K, Receveur MC, Pistone T, Crevon L, Lemardeley P, Josse R (2008) Cluster of eosinophilic meningitis attributable to Angiostrongylus cantonensis infection in French policemen troop returning from the Pacific Islands. Travel Med Infect Dis 6:301–304
Mattis A, Mowatt L, Lue A, Lindo J, Vaughan H (2009) Ocular angiostrongyliasis-first case report from Jamaica. West Indian Med J 58:383–385
Morera P, Céspedes R (1970) Angiostrongylus costaricensis n.sp. (Nematoda: Metastrongyloidea), a new lungworm occurring in man in CostaRica. Rev Biol Trop 18:173–185
Nuamtanong S (1996) The evaluation of the 29 and 31 kDa antigens in female Angiostrongylus cantonensis for serodiagnosis of human angiostrongyliasis. Southeast Asian J Trop Med Public Health 27:291–296
Ogawa K, Kishi M, Ogawa T, Wakata N, Kinoshita M (1998) A case of eosinophilic meningoencephalitis caused by Angiostrongylus cantonensis with unique brain MRI findings. Rinsho Shinkeigaku 38:22–26 (Article in Japanese)
OuYang L, Wei J, Wu Z, Zeng X, Li Y, Jia Y, Ma Y, Zhan M, Lei W (2012) Differences of larval development and pathological changes in permissive and nonpermissive rodent hosts for Angiostrongylus cantonensis infection. Parasitol Res 111:1547–1557
Pascual Gispert JE, Aguilar Prieto PH, Galvez Oviedo MD (1981) Finding of Angiostrongylus cantonensis in the cerebrospinal fluid of a boy with eosinophilic meningoencephalitis. Rev Cubana Med Trop 33:92–95
Paul A, Pammal AT (2008) Ocular parasitosis: a rare cause of hypertensive uveitis. Indian J Ophthalmol 56:501–502
Peng H, Sun R, Zhang Q, Zhao J, Wei J, Zeng X, Zheng H, Wu Z (2013) Interleukin 33 mediates type 2 immunity and inflammation in the central nervous system of mice infected with Angiostrongylus cantonensis. J Infect Dis 207:860–869
Punyagupta S, Bunnag T, Juttijudata P, Rosen L (1970) Eosinophilic meningitis in Thailand. Epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. Am J Trop Med Hyg 19:950–958
Punyagupta S, Juttijudata P, Bunnag T (1975) Eosinophilic meningitis in Thailand. Clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 24:921–931
Qu ZY, Yang X, Cheng M, Lin YF, Liu XM, He A, Wu ZD, Zhan XM (2011) Enzootic angiostrongyliasis, Guangdong, China, 2008–2009. Emerg Infect Dis 17:1335–1336
Rosen L, Laigret J, Boils PL (1961) Observation on an outbreak of eosinophilic meningitis on Tahiti, French Polynesia. Am J Hyg 74:26–42
Rosen L, Chappell R, Laqueur GL et al (1962) Eosinophilic meningoencephalitis caused by a metastrongylid lung-worm of rats. JAMA 179:620–624
Rosen L, Loison G, Laigret J et al (1967) Studies on eosinophilic meningitis: 3, epidemiologic and clinical observations on Pacific islands and the possible etiologic role of Angiostrongylus cantonensis. Am J Epidemiol 85:17–44
Sawanyawisuth K, Kitthaweesin K (2008) Optic neuritis caused by intraocular angiostrongyliasis. Southeast Asian J Trop Med Public Health 39:1005–1007
Sawanyawisuth K, Kitthaweesin K, Limpawattana P, Intapan PM, Tiamkao S, Jitpimolmard S, Chotmongkol V (2006) Intraocular angiostrongyliasis: clinical findings, treatments and outcomes. Trans R Soc Trop Med Hyg 101:497–501
Sawanyawisuth K, Takahashi K, Hoshuyama T, Sawanyawisuth K, Senthong V, Limpawattana P, Intapan PM, Wilson D, Tiamkao S, Jitpimolmard S, Chotmongkol V (2009) Clinical factors predictive of encephalitis caused by Angiostrongylus cantonensis. Am J Trop Med Hyg 81:698–701
Schmutzhard E, Boongird P, Vejjajiva A (1988) Eosinophilic meningitis and radiculomyelitis in Thailand, caused by CNS invasion of Gnathostoma spinigerum and Angiostrongylus cantonensis. J Neurol Neurosurg Psychiatry 51:80–87
Shih PC, Lee HH, Lai SC, Chen KM, Jiang ST, Chen YF, Shiow SJ (2007) Efficacy of curcumin therapy against Angiostrongylus cantonensis-induced eosinophilic meningitis. J Helminthol 81:1–5
Sinawat S, Sanguansak T, Angkawinijwong T, Ratanapakorn T, Intapan PM, Yospaiboon Y (2008) Ocular angiostrongyliasis: clinical study of three cases. Eye (Lond) 22:1446–1448
Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S (2002) An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 346:668–675
Sonakul D (1978) Pathological findings in four cases of human angiostrongyliasis. Southeast Asian J Trop Med Public Health 9:220–227
Sugaya H, Aoki M, Abe T, Ishida K, Yoshimura K (1997) Cytokine responses in mice infected with Angiostrongylus cantonensis. Parasitol Res 83:10–15
Sun R, Li ZY, He HJ, Wei J, Wang J, Zhang QX, Zhao J, Zhan XM, Wu ZD (2012) Molecular cloning and characterization of a matrix metalloproteinase, from Caenorhabditis elegans: employed to identify homologous protein from Angiostrongylus cantonensis. Parasitol Res 110:2001–2012
Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, Tsai TH, Lin WR, Huang CK, Yen MY, Yen CM (2001a) Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med 111:109–114
Tsai TH, Liu YC, Wann SR, Lin WR, Lee SJ, Lin HH, Chen YS, Yen MY, Yen CM (2001b) An outbreak of meningitis caused by Angiostrongylus cantonensis in Kaohsiung. J Microbiol Immunol Infect 34:50–56
Vazquez JJ, Boils PL, Sola JJ, Carbonell F, de Juan Burgueño M, Giner V, Berenguer-Lapuerta J (1993) Angiostrongyliasis in a European patient: a rare cause of gangrenous ischemic enterocolitis. Gastroenterology 105:1544–1549
Wan KS, Weng WC (2004) Eosinophilic meningitis in a child raising snails as pets. Acta Trop 90:51–53
Wang QP, Chen XG, Lun ZR (2007) Invasive freshwater snail, China. Emerg Infect Dis 13:1119–1120
Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR (2008) Human angiostrongyliasis. Lancet Infect Dis 8:621–630
Wang H, Qiu H, Qiu JB (2010) The research progress of angiostrongyliasis spread and prevalence. Exp Lab Med 28:377–378
Wang QP, Wu ZD, Wei J, Owen RL, Lun ZR (2012) Human Angiostrongylus cantonensis: an update. Eur J Clin Microbiol Infect Dis 31:389–395
Wang J, Wei J, Zeng X, Liang JY, Wu F, Li ZY, Zheng HQ, He HJ, Wu ZD (2013) Efficacy of tribendimidine against Angiostrongylus cantonensis infection in the mice. Parasitol Res 112:1039–1046
Wei J, Wu F, Sun X, Zeng X, Liang JY, Zheng HQ, Yu XB, Zhang KX, Wu ZD (2013) Differences in microglia activation between rats-derived cell and mice-derived cell after stimulating by soluble antigen of IV larva from Angiostrongylus cantonensis in vitro. Parasitol Res 112:207–214
Xue DY, Ruan YZ, Lin BC, Zheng RY, Fang JQ, Zhao QX, Li MF, Pan CW (2000) Epidemiological investigation on an outbreak of Angiostrongyliasis cantonensis in Wenzhou. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 18:176–178 (Article in Chinese)
Yang FZ, Zhang YZ, Tu ZP, Xu LS (2004) Survey on the outbreak of human angiostrongyliasis caused by eating snails. Strait J Prev Med 10:44–45 (Article in Chinese)
Yang X, Qu Z, He H, Zheng X, He A, Wu Y, Liu Q, Zhang D, Wu Z, Li Z, Zhan X (2012) Enzootic angiostrongyliasis in Guangzhou, China, 2008–2010. Am J Trop Med Hyg 86:846–849
Ye DG, Luo B, Liu BD, Zheng P (2008) Epidemiological study of Angiostronglus cantonensis infection in Fuzhou City. J Trop Med 8:938–940
Yii CY (1976) Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg 25:233–249
Zhang RL, Chen MX, Gao ST, Geng YJ, Huang DN, Liu JP, Wu YL, Zhu XQ (2008) Enzootic angiostrongyliasis in Shenzhen, China. Emerg Infect Dis 14:1995–1996
Zheng RY, Jin R, Lin BC, Pan CW, Xue DY (2001) Probing and demonstrating etiological factors for outbreak of Angiostrongyliasis cantonensis in Wenzhou. Shanghai J Prev Med 13:105–107
Zhou Z, Barennes H, Zhou N, Ding L, Zhu YH, Strobel M (2009) Two outbreaks of eosinophilic meningitis in Yunann (China) clinical, epidemiological and therapeutical issues. Bull Soc Pathol Exot 102(2):75–80 [Article in French]
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Wei, J., Wu, Z. (2014). Angiostrongylus cantonensis in China. In: Mehlhorn, H., Wu, Z., Ye, B. (eds) Treatment of Human Parasitosis in Traditional Chinese Medicine. Parasitology Research Monographs, vol 6. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39824-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-39824-7_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39823-0
Online ISBN: 978-3-642-39824-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)