Abstract
The chapter specifically deals with the structure–activity relationship studies on various classes of hydroxamates acting as matrix metalloproteinase (MMP) inhibitors. Among all classes of MMP inhibitors, hydroxamates are important in that their zinc-binding group CONHOH makes them a bidentate ligand to act with any metal-containing enzyme. Most of the MMP inhibitors developed by pharmaceutical companies belong to this category of compounds. The position of hydroxamate nitrogen suggests that it is protonated and forms a hydrogen bond with carbonyl oxygen of the enzyme backbone. In addition to zinc-binding affinity, several other properties of the hydroxamic acids depending upon their structures control their MMP inhibition activity. Various categories of hydroxamates such as succinyl, malonic acid, sulfonamide-based, aryl acid-based, sulfone-based, N-benzoyl aminobutyric acids, aminoproline-based, aminopyrrolidine-based, and phosphonamide/phosphinamide-based hydroxamates have been found to act as MMP inhibitors. A detailed structure–activity relationship (SAR) study of all these categories of hydroxamates has been presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hydroxamates
- MMPIs
- Succinyl hydroxamates
- Malonic acid hydroxamates
- Sulfonamide-based hydroxamates
- Aryl acid-based hydroxamates
- Sulfone-based hydroxamates
- Phosphonamide/phosphinamide-based hydroxamates
- Structure–activity relationships
1 Introduction
More than 100 years ago, Lossen discovered the first hydroxamic acid and in the present time it is one of the well-studied compounds having numerous applications. The pharmacological potential of hydroxamic acids in a variety of disease conditions, such as viral diseases (Torres 1995; Szekeres et al. 1997), malaria, Alzheimer’s disease (Parvathy et al. 1998; El Yazal and Pang 2000), allergic diseases (Igeta et al. 2000; Valapour et al. 2002), tuberculosis (Miller 1989; Shingledecker et al. 2000), cancer, cardiovascular diseases (Jeng and Lombaert 1997), and metal poisoning (Domingo 1998; Weisburger and Weisburger 1973) is well reported. This diverse profile of hydroxamic acids can be attributed to their efficiency in blocking a variety of enzymes, viz., ureases (Zhang et al. 1999; Mishra et al. 2002), peroxidases (Tsukamoto et al. 1999), matrix metalloproteinases (MMPs) (Leung et al. 2000; Hidalgo and Eckhardt 2001), hydrolases (Brown et al. 2004a, b), lipoxygenases (Muri et al. 2002), cyclooxygenases (Dooley et al. 2003; Connolly et al. 1999), histone deacetylases (Marks et al. 2000; Johnstone 2002; Jung 2001; Kelly et al. 2002), peptide deformylases (Chen et al. 2004), etc. In the past decades, an extraordinary work has been carried out on their design, synthesis, and structure–activity relationships (SARs) which support their diverse therapeutic properties (Lipczynska-Kochany 1988). Here we focus on the SAR studies of several groups of hydroxamic acids/hydroxamates relevant to their biomedical applications as matrix metalloproteinase inhibitors (MMPIs).
2 Structural Features of MMPIs
MMPs belong to the family of proteolytic enzymes and regulate a plethora of physiological and pathological functions. Their complex role also contributes to unintended side effects during clinical trials. For more than three decades, MMPs have been heralded as promising targets for the treatment of different diseases as discussed before and scientists have been involved in finding potent inhibitors for them. The unique site specificity and selectivity of MMPIs for different MMP targets (Gupta and Patil 2012) have been the focus of recent research. Over activation of MMPs results in an imbalance between the activity of MMPs and tissue inhibitors of metalloproteinases (TIMPs) that can lead to a variety of pathological disorders (Aranapakam et al. 2003a, b; Venkatesan et al. 2004; Brown et al. 2004a). Although the role of each MMP is not known for certain, the study of their inhibition has evoked great interest. A variety of connective tissues and proinflammatory cells including fibroblasts, osteoblasts, endothelial cells, macrophages, neutrophils, and lymphocytes excrete these MMPs, of which most are expressed as inactive zymogens, that are subsequently processed by other proteolytic enzymes, e.g., serine proteases, furin, plasmin, and others, to generate the active forms. Under normal physiological conditions, the proteolytic activity of the MMPs is controlled at any of the following three known stages: transcription, activation of the zymogens, or inhibition by TIMPs. In pathological conditions, this equilibrium is shifted toward increased MMP activity leading to tissue degradation (Cheng et al. 2000; Kontogiorgis et al. 2005).
Since all MMPs belong to the family of zinc-containing enzymes, all contain, in common, a zinc atom (divalent cation Zn2+), and through this metal atom they affect the amide bond hydrolysis. In the amide hydrolysis, this Zn2+ ion is generally tetrahedrally coordinated to three donor groups from the enzyme and a water molecule (Leung et al. 2000; Gupta 2007). Based on the conclusions drawn by various research groups, the basic structural features required for an effective MMPI are: (i) the presence of a functional group, such as a carboxylic group (COOH), hydroxamic group (CONHOH), and sulfhydryl group (SH), that may be able to chelate the active site Zn2+ ion of the enzyme (such a group is referred to as a zinc-binding group, ZBG), (ii) at least one functional group capable of hydrogen bonding with the enzyme backbone, and (iii) one or more side chains that can have effective van der Waals interactions with the enzyme subsites. Based on these requirements, a large number of synthetic MMP inhibitors (MMPIs) have been reported by various research groups from industry and academia (Supuran and Scozzafava 2002).
Many of the MMPIs have been investigated by employing computational methods like substrate-based design (Johnson et al. 1987), structure-based design (Babine and Bender 1997), and combinatorial chemistry (Shuttleworth 1998; Whittakar 1998). In the development of synthetic MMPIs, substrate-based design has been the principal approach and three classes of compounds have been developed, viz, (a) compounds that have amino acid residues on both sides of ZBG, e.g., Pn– ---P2–P1–ZBG–P1′–P2′---- –Pn′; (b) compounds that have amino acids residues on only right-hand side of the ZBG, e.g., ZBG–P1′–P2′ ------ –Pn′ and are called right-hand (RHS) inhibitors; and (c) compounds that have amino acids residues on only left-hand side of the ZBG, e.g., Pn– ---P2–P1–ZBG and are called left-hand side (LHS) inhibitors (Here P’s and P′’s refer to the standard nomenclature of amino acid residues as defined in peptide substrates) (Babine and Bender 1997).
3 Hydroxamates as MMPIs
Among all the classes of MMPIs, the hydroxamates, that contain hydroxamic acid group (CONHOH), have been more extensively studied. The synthetic MMPIs have been categorized in structure-based drug design into three classes, i.e., compounds with amino acid residues on both sides of ZBG, compounds with amino acid residues on only the right-hand side of ZBG, and compounds with amino acid residues on only the left-hand sides of ZBG. Among all these three classes, the right-hand side inhibitors were mostly found to be more potent (Gupta 2007; Whittaker et al. 1999). Further, the hydroxamic acid-based MMPIs have been categorized based on their structural features as:
-
(1)
Succinyl hydroxamates
-
(2)
Malonic acid-based hydroxamates
-
(3)
Sulfonamide-based hydroxamates
-
(4)
Aryl acid-based hydroxamates
-
(5)
Anthranilic acid-based hydroxamates
Hydroxamates are among one of the most explored zinc-binding compounds for the development of MMPIs, and most interestingly the first three MMPIs used to treat cardiovascular diseases are from the hydroxamate category (Whittaker et al. 1999). In 1978, Nishino and Power (1978) first introduced a hydroxamate as a ZBG for designing an inhibitor, thermolysin, which provided an encouragement to develop hydroxamate-containing MMPIs (Moore and Spilburg 1986a, b).
At Pfizer, Robinson et al. (2000) designed some nonpeptidic and sulfonamide hydroxamates with an objective to improve the selectivity and were successful in the development of pyrrolidinone-based hydroxamates, e.g., a (Fig. 1), having good selectivity for MMP-1. Further structural modifications led to novel series of imidazolidine-based MMPIs, such as b (Fig. 1), as strong inhibitors of MMP-13 (Robinson et al. 2001). Simultaneously, compound c (Fig. 1) has been developed for the treatment of osteoarthritis (Aranapakam et al. 2003a, b) and SAR studies concluded a better potency of aromatic sulfonyl compounds than that of aliphatic/heteroaromatic sulfonyl derivatives. Some tetrahydopyran-centered sulfone hydroxamates, such as d (Fig. 1),were developed as selective inhibitors of MMP-13 at Pfizer (Noe et al. 2004) and further structure optimization led to the development of e (Fig. 1) having sub-nanomolar potency against MMP-2 (Salvino et al. 2000).
4 Story of Some Clinical Success
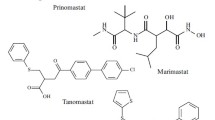
The designing of earlier MMPIs was based on the knowledge of the amino acid sequence of collagen at the site of cleavage by MMP-1 (collagenase-1). Among the very first hydroxamates studied, batimastat (BB-94, 1) and marimastat (BB-2516, 2) that were initially found to be clinically useful were the peptidic inhibitors. It was observed that for this type of inhibitors, the presence of a P1′ residue (α to the hydroxamate moiety) leads to a broad-spectrum activity against a variety of MMPs (Whittaker et al. 1999; Bottomley et al. 1998). However, both were withdrawn after clinical trials for cancer due to poor selectivity and poor bioavailability. These studies concluded that compounds which mimicked the sequences of the right-hand side of the cleavage site (primed sites P1′, P2′, P3′, etc.), with a hydroxamic acid moiety incorporated as the zinc-binding group, exhibited better inhibition (Yao et al. 2001).

Among the non-peptide MMPIs, the sulfonamide hydroxamate derivatives CGS27023A (3a) and CGS25966 (3b) have entered clinical trials (Heath and Grochow 2000). In this case, NMR spectroscopy and the three-dimensional solution structure were used to define the mode of binding with MMP-3 (Zhang et al. 2000). The isopropyl and pyridylmethyl substituents were found to accommodate the hydrophobic S1 and S2′ subsites. Compound 4 from the similar chemical category was found an MMP-13 inhibitor at subnanomolar range (Kimura et al. 2001).

Some sulfone MMPIs (5, 6) having hydroxamic acid moiety were found to have bidentate interactions with MMP-13 in the reported X-ray crystal structures. The selectivity of these compounds for MMP-13 was attributed to their affinity for the S1′ pocket. This class of inhibitors mimics a carbonyl interaction in peptide-based inhibitors and one of the oxygen of sulfone group serves as the hydrogen bond acceptor from the amide group of Leu185. However, of these two, 6 was withdrawn from Phase II clinical trials for osteoarthritis due to musculoskeletal side effects (Tu et al. 2008).

Simultaneously, a series of reverse hydroxamate peptides (7–9) were reported as broad-spectrum inhibitors of tumor necrosis factor-α (TNF-α) converting enzyme (TACE) and MMPs (Andrews et al. 2000). Compound 7 had IC50 values of 19, 20, 16, and 42 nM for MMP-1, -3, -9, and TACE inhibition, respectively, but failed to show any specificity for TACE against MMPs and this may be the reason behind their side effects such as tendonitis and musculoskeletal effects. Compound 9, prepared in a combinatorial fashion, was found to inhibit MMP-3 and -13 (IC50 > 100 nM) as well as TACE and TNF-α (IC50 < 100 nM).

5 SAR Studies
5.1 Succinyl Hydroxamic Acid Derivatives
Succinyl hydroxamates have been found to be much stronger MMPIs than those belonging to other groups (Johnson et al. 1987) and thus they have been the most widely studied MMPIs until recently. Compounds 1 (batimastat) and 2 (marimastat) are a few of the potent MMPIs that belong to this category (Whittaker et al. 1999; Supuran and Scozzafava 2002; Levin et al. 2004). Both the compounds showed very good activities in several disease models and another compound (10) was found to be orally bioavailable. The bioavailability of 10 was attributed to the presence of a hydrophilic OH moiety at α-carbon, which could probably increase the water solubility of the compound (Levin et al. 2004). Some compounds were obtained by incorporating cis-(1S, 2R)-amino-2-indanol scaffold and optimized as potent, selective, and orally bioavailable inhibitor of  aggrecanase. A series of 13- and 14-membered macrocyclic amines, such as 11 and 12, were developed by linking P1 and P2′ groups of succinic acid-based inhibitors. The selectivity profile of compound 12 against MMP-8 and -9 was attributed to the macrocyclic template and the long phenylpropyl P1 group accommodating in the deep S1 pocket.
aggrecanase. A series of 13- and 14-membered macrocyclic amines, such as 11 and 12, were developed by linking P1 and P2′ groups of succinic acid-based inhibitors. The selectivity profile of compound 12 against MMP-8 and -9 was attributed to the macrocyclic template and the long phenylpropyl P1 group accommodating in the deep S1 pocket.

A large series of succinyl hydroxamates (13) having variations in P1′ and P3′ groups and P2′ as t-butyl group had been evaluated against MMP-2 and -3 (Fray et al. 2001; Fray and Dickinson 2001). The SAR conclusions drawn in the studies were that at P1′ position, the phenylpropyl substituent was conducive to MMP-2 and -3 inhibition and that the o-F and o-Me at phenyl ring showed remarkable improvement in MMP-3 selectivity as compared to larger groups like Et, OMe, and CF3 which caused significant loss of activities. These authors concluded that the size of R1- and R2-substituents contributes toward MMP-2 selectivity. At R2 position, the Me and t-Bu groups were well tolerated in comparison to the cycloalkyl group. It was also noted that chirality of R2 plays an important role, i.e., R-enantiomer retains potency similar to Me analog against MMP-3 but leads to a loss of potency against MMP-2. Compound 14 was identified as a potent and selective MMP-3 inhibitor having 303 times selectivity against MMP-2 (Fray and Dickinson 2001).

5.2 Malonic Acid-Based Hydroxamates

The malonic acid-based hydroxamates were observed to exhibit nonsubstrate-like binding, e.g., compound 15 binds with the Zn2+ of MMPs in the same manner as normal hydroxamates do, but its secondary binding is quite different. The C-terminal Ala-Gly-NH2 moiety adopts a bent conformation that is inserted into the S1′ pocket. Thus, it exhibited nonsubstrate-like binding to the active site and consequently represented a new interesting lead for obtaining malonic acid-based MMP inhibitors (Roedern et al. 1998; Krumme et al. 1998). The SAR studies have shown hydrophobic interactions at S1 subsite of the substituents like isobutyl, (CH2)2Ph, CH2Ph, or Ph. For S1′ subsite the OEt and N-morpholide were not favored while the C-terminal aromatic groups were found to improve inhibitory potency. There is further improvement in activity with NH-n-octyl substituent.
5.3 Sulfonamide-Based Hydroxamates
Sulfonamide-based hydroxamates, as represented by 16, contain a sulfonamide moiety and involve hydrogen bonding as well as direct hydrophobic interaction with S1′ pocket to improve the enzyme-inhibitor binding. Some of these inhibitors (3, 4) were reported to act as efficient MMPIs (Shuttleworth 1998; Whittaker et al. 1999; Supuran and Scozzafava 2002) and further change in their structural features could lead to better inhibitors (Jeng et al. 1998; Whittaker et al. 1999; Hannessian et al. 1999).

While analogs of 16 were found to possess nanomolar potencies against MMP-1, -2, -8, and -9 (Supuran and Scozzafava 2002), 17 (a sulfone derivative) was observed to be very strong, highly selective, and orally bioavailable MMP inhibitor (Whittaker et al. 1999).
All the sulfonamide-based hydroxamates studied were derived from α-amino acids, with a single sp 3-hydridized carbon atom separating the sulfonamide nitrogen and the zinc chelating hydroxamic acid moiety. Assuming that an increase in this separation, with connecting atoms held rigidly, may lead to more potent compounds, Levin et al. (2001) attempted to design aryl acid-based sulfonamide hydroxamates as represented by an anthranilic acid-based scaffold (18). They prepared three regioisomeric (ortho, meta, and para) analogs of 18 with R1 = CH2Ph and R2 = OCH3. Of these, the ortho analog 19 was found to be the most potent inhibitor with an IC50 value below 1 μM against MMP-1, -9, and -13.

Similarly, a new class of N-substituted arylsulfonamido-based hydroxamic acid inhibitors was developed by Rossello et al. (2004) having at the sulfonamido nitrogen either an oxyalkyl side chain (20) or simply an alkyl side chain (21), instead of simply hydrogen atom.

Compounds belonging to the series of 20 were found capable of blocking tumor cell invasion by potent and selective inhibition of MMP-2 and -9. Compound (R)-22, an analog of 20 with R-configuration at carbon α to hydroxamic group, showed a very good inhibitory activity profile toward MMP-2, -9, and -14 with IC50 equal to 0.41, 16, and 7.7 nM, respectively (Rossello et al. 2005a).

In continuation to this, Rossello et al. (2005b) further reported some twin hydroxamic acids ((R,R)-23) using some suitable linkers as ‘a’ and ‘b’. Among the series, only compounds having hydroxyl and hydroxylamine substituents at R-position had shown activities against MMP-1, -2, -9, and -14, but were found to be poorly active as compared to the monomeric compound (R)-22. The hydroxamic acid moiety was found to be essential as proven by the loss of activity of carboxylic analog toward MMP-2 and -9 and it was not evaluated against MMP-1 and -14.

In a series of tetrahydroisoquinoline-based sulfonamide hydroxamates (24) studied by Ma et al. (2004), most compounds were found to display potent inhibition activity for some selected MMPs and it was observed that
-
The variation of substituents at the 6- and 7-positions and arylsulfonyl group showed marked differences in potency and selectivity and also imparted some subtle isozyme selectivity. Thus these positions plays some role toward activity as seen by alteration of activity due to 6-hydroxyl/benzoxyl and 7-methoxy substituents.
-
Among the 6-hydroxy analogs, the 4′-Me substituted derivative was found to be more potent than 4′-H or 4′-OMe substituted, however, the latter was found to be the most favored for MMP-15 inhibition.

Yang et al. (2008) reported a few promising series of β-N-biaryl ether sulfonamide hydroxamates (25–30) as novel gelatinase (MMP-2 and -9) inhibitors, in which analogs of 28 were observed to have great selectivity for MMP-9/MMP-2 over MMP-1. Here the group attached to the sulfonamide nitrogen is referred as P1′. Some preliminary SAR conclusions were derived which demonstrated the advantage and potential of a β-N-biaryl ether sulfonamide moiety in the design of MMP inhibitors.
-
The pairing of methanesulfonyl group with biaryl ether type P1′ moiety affords single-digit nanomolar activity against MMP-9.
-
A β-N-biaryl ether sulfonamide with a methyl substituent at P1′ (28; R = H; IC50 = 6.6 nM) was found to be about 5-fold more potent than an α-N-biaryl ether sulfonamide (25; R2 = Me; IC50 = 31 nM) against MMP-9.
-
The introduction of small α-substituents (such as OMe/OH/Me at R-position) in 28, 29, and 30 and the chirality of the α-position (R vs. S) in 26 and 28 had marginal influence on the IC50 and potency.
-
Various substituents on the sulfonamide had the rank order for potency against MMP-2 and -9 as: methyl > ethyl > n-propyl > iso-propyl > NMe2 > phenyl.
-
The potency was restored to double-digit nanomolar range against MMP-9 on replacing the phenyl with a benzyl group at R1-position.
-
The preference shown by analogs having para-substituted phenyl moiety at R1-position in 29 for MMP-2 over MMP-9 enzyme could be attributed to the tunnel-like S1′ subsite of MMP-2, which shows better tolerance of the longer P1′ moiety than the S1′ pocket of MMP-9.
-
In 29, R2 = Cl or CH3 was observed to be more important than any other R2-substituent.
-
Replacement of the phenyl ring with a heteroaryl moiety in 29 (Y = N) was found to reduce potency as the hydrophobic residues surrounding the S1′ pocket typically favor a more lipophilic P1′ moiety.

In a new series of arylsulfonamidic scaffold (31), selective for MMP-13 inhibition (Nuti et al. 2009), the following structure–activity relationships were observed.

The 4-substituted biphenyl group at Ar was found contributing toward the inhibitory profile especially against MMP-2 as compared to the unsubstituted biphenyl for all tested enzymes without affecting the selectivity profile. Among the various substitutions on biphenyl moiety, the 4-methylthio (32) and 4-chlorobenzoxy substituents were found to be most significant for inhibition of MMP-13 (IC50 = 7.2 and 19 nM, respectively). It also exhibited a slight to good selectivity over MMP-1, -2, -3, -14, -16, and TACE. Compound with an isopropyl group as P1 substituent (R-substituent) was identified to be a promising slow-binding inhibitor of MMP-13 at nanomolar concentration, but with very high selectivity for it as compared to MMP-1, -14, and TACE.
In a series of 4-butylphenyl(ethynylthiophene)sulfonamido-based hydroxamates (33) studied by Nuti et al. (2011) for the inhibition of MMP-3, -8, -9, -14, and -25 and for the effective treatment of glioma, a compound having benzophenone substituent (R = –PhCOPh) was identified to have nanomolar potency against MMP-2, -9, and -25 but to be weaker against other members of MMP family. It was also observed that the MMP-2 inhibition activity of 33 was governed by its P1′ group, i.e., 4-butylphenylethynylthiophene, but its enzyme’s selectivity profile by its P1 group (α-substituent). The elongated α-chain contributes toward selectivity. The compound with benzophenone moiety was indentified to have highest selectivity over MMP-1, -3, -8, and -14.

5.4 Sulfone-Based Hydroxamates
Some α- and β-piperidinesulfone hydroxamic acids (34–38) were studied by Becker et al. (2005) as potent inhibitors of MMP-2, -9, and -13. Among them, 35 with R = propargyl (SC-276) was selected for further development as it demonstrated excellent antitumor activity against MX-1 breast tumor in mice when dosed orally as monotherapy or in combination with paclitaxel. This work culminated in the discovery of a thioether sulfone hydroxamate (36, R = propargyl) having excellent efficacy in murine xenograft tumor models and antiangiogenesis assays and exhibited excellent potency for target enzymes and selectivity against MMP-1. The unsubstituted α-sulfone (37) maintained good inhibitory potency against MMP-13 (IC50 = 5 nM) and -2 (IC50 = 2.6 nM) and was selective against MMP-1 (IC50 = 6600 nM). It was, therefore, concluded that the α-sulfone hydroxamates could be developed as potent MMP inhibitors (Becker et al. 2001b; Barta et al. 2003). Consequently, Beckers et al. (2001a) prepared an α,α-dimethyl analog (38) that had nanomolar potency better that β-sulfones against MMP-13 and -2 (IC50 = 0.25 for MMP-13 and 0.1 for MMP-2).

5.5 Sulfone N-Formylhydroxylamines (Retrohydroxamates)
An N-formylhydroxylamine () 39(ABT-770) was investigated by Curtin et al. (2001) to be a potent inhibitor with selectivity for inhibition of MMP-2 over MMP-1. It was moderately active against MMM-9. But in the next communication, the same group of authors (Wada et al. 2002) reported that the replacement of the ether group of 39 by sulfone group led to a compound (40) which had substantially increased MMP-9 inhibition activity but with a loss of selectivity for inhibition of MMP-2 and -9 over MMP-1 and diminished oral exposure. Further, replacement of the biphenyl P1′ substituent in sulfone retrohydroxamates with a phenoxyphenyl group provided compounds (41) that were highly selective for inhibition of MMP-2 and -9 over MMP-1. In this series, optimization of the substituent R adjacent to the retrohydroxamate center in this series led to a clinical candidate (42, ABT-518) which was found to be a highly potent, selective, and orally bioavailable MMP inhibitor that could significantly inhibit tumor growth in animal cancer models.

A small library of N-aryl piperazine α-sulfone hydroxamic acid derivatives was designed to explore the effect of substituent on the distal aryl rings of 43a and 43b (Kolodziej et al. 2010a). Compounds having N-aryl piperazine α-sulfone moiety failed to show any measurable potency for MMP-1 (IC50 > 1000 nM) but had 1000 times MMP-13 selectivity over MMP-1. N-aryl piperazines (44a and 44b) had nanomolar potency for MMP-13 and -2 but moderate selectivity for the former over the latter. Some of their derivatives were found equipotent. As compared to ortho and meta substituted derivatives of 46, the para-substituted derivatives maintained high potency and selectivity for MMP-13.

Kolodzeij et al. (2010b) derived another series of inhibitors (45) by varying the substituent at the aryl ring of 43b. In order to boost the MMP-2 and -13 selectivity, they studied para-substituted analogs and found that the MMP-2 selectivity depended on the size of the substituent, with methoxy being optimal: H < Cl, OH < CH3, CF3 < OMe, OEt, and 4-F-C6H4. The decrease in affinity for MMP-13 was attributed to steric effects. The additional substituents like 2,3-(CH=CH)naphthyl, methyl, and methoxy showed increased MMP-2 potency and the para-substituted N-aryl piperazines showed superior MMP-13 potency.

Some orally active and MMP-1 sparing α-tetrahydropyranyl (α-THP) sulfone hydroxamates, such as 46, and several α-piperidine sulfone hydroxamates (47) were synthesized and found to be potent inhibitors of MMP-2, -9, and -13 by Becker et al. (2010) with oral efficacy in inhibiting tumor growth in mice and left-ventricular hypertrophy in rats and in the bovine cartilage degradation explant system. In most cases, the α-piperidines exhibited greater exposure than the α-THP analogs. An analog of 47 (R = methoxyethyl; SC-78080/SD-2590) was selected for development toward the initial indication of cancer, while its another analog (R = cyclopropyl; SC-77964) and 46 (SC-77774) were identified as backup compounds.

In N-arylsulfonyl-based MMPIs, the poor bioavailability is the major drawback for the development of this family of molecules. To enhance the water solubility, Attolino et al. (2010) applied a structure-based approach and performed structural analysis of 48 (NNGH) with MMP-12 to find that the sec-butyl residue was not directly involved in the binding with MMP. A new series of compounds (49) was then studied, where sec-butyl residue was replaced with hydroxamic acid moiety, to get water soluble potent inhibitors.

All the newly prepared analogs of 49 were evaluated, using NNGH as reference, against MMP-1, -7, -8, -9 -12, and -13 to find that all of them had low nanomolar K i values for the MMPs tested except for MMP-1 and -7.
Analogs of 49 prepared with fluorine and biphenyl substituents instead of methoxy group to compliment the characteristic shape of the S1′ binding pocket were not found beneficial. The lack of favorable interactions of Arg214 with fluorine contributed toward the low affinity of the inhibitors and micromolar Ki value for MMP-1. A decrease in hydrophilicity of the compounds was found and thus the necessity of at least one hydroxyl group was felt.
5.6 N-Benzoyl Aminobutyric Acid Hydroxamates
Nakatani et al. (2006) studied a series of N-benzoyl 4-aminobutyric acid hydroxamate analogs (50–54) as inhibitors of MMP-1, -2, -3, and -9. Most of the compounds, like N-[4-(benzofuran-2-yl)benzoyl]-4-amino-4S-hydroxymethyl butyric acid hydroxamates, were found to be highly potent inhibitors of the gelatinases (MMP-2 and -9) as compared to the corresponding 2S- or 3S-hydroxy analogs.

Ikura et al. (2006) performed chemical modification of the N-benzoyl residue of N-benzoyl γ-aminobutyric hydroxamic acids (55, 56) by introducing electron-rich para-substituents and found it to be effective to increase the inhibitory activity of this class of MMPIs. Analogs having relatively more planar N-acyl residues demonstrated more potency. The three-dimensional arrangement of the two pharmacophores, hydroxamic acid and N-acyl residues, was optimized by chemical modifications of the γ-aminobutyric hydroxamic acid moiety (50) and this moiety was found as best spacer. All the compounds were evaluated for their inhibitory activity against MMP-1, -2, -3, and -9 and the N-benzoyl γ-aminobutyric hydroxamic acid was identified as a new chemical lead for MMP-2 and -9 inhibitions. Further chemical modification was focused on the 4-N-(4-methyl)benzoyl moiety, and a series of N-benzoyl γ-aminobutyric hydroxamic acids was prepared. As compared to 4-(para-alkylphenyl)benzoyl analogs, the 4-(para-substituted phenyl)benzoyl analogs were found to be stronger inhibitors. Introduction of electron-rich substituents (benzofuran-2-yl and para-chlorocinnamyl) at para-position of the N-benzoyl moiety was assumed to be essential for strong activity. A secondary amide was found as a superior linkage compared to the ether or N-methyl amide with respect to the formation of hydrogen bonds with amino acid residues Pro238 and Leu181 in the S1′ pocket.

5.7 Aminoproline-Based Hydroxamates
A few series of hydroxamates (57–60) were reported as MMP inhibitors from an aminoproline scaffold by Natchus et al. (2000) where analogs of 57 were identified to have broad-spectrum activity with sub-nanomolar potency for some enzymes. Further modifications at the P1′ portion of this molecule with longer-chain aliphatic and aromatic substituents were found to affect both potency and selectivity within the MMP family. All compounds were assayed for the inhibition of MMP-1, -2, -3, -7, and -13.

5.8 Aminopyrrolidine-Based Hydroxamates
A diverse family of aminopyrrolidine-based hydroxamate inhibitors were found to act as very potent inhibitors of MMP family with the exception of MMP-1 and -7 by Natchus et al. (2000). At the 4-position of the aromatic sulfonamides, long-chain aliphatic groups were incorporated to enhance the selectivity against the shallow S1′ pocket of enzymes. The X-ray crystallography data of stromelysin-complex was used to explain the binding of aromatic sulfonamide structure into the S1′ pocket and the selectivity profile.
5.9 Phosphinamide/Phosphonamide-Based Hydroxamates
Pikul et al. (1999) studied some phosphinamide-based hydroxamates (61) where compounds having R-configuration at phosphorous were found to be potent inhibitors of MMP-1 and -3. The S-configuration was found inactive. A compound with R1 = CH2CHMe2, R2 = CH2Ph, R3 = Me, and R4 = Ph was found to be active against MMP-1 (IC50 = 20.5 nM) and MMP-3 (IC50 = 24.4 nM) where R4-substituent could play key role in binding with S1′ pocket of the enzymes. The analysis of binding interactions indicated the involvement of Leu164, Ala165, Glu202, and Val163 residues of the enzymes.  Some cyclophosphinamide- and cyclophosphonamide-based hydroxamates (62) were designed by altering the phosphorous substituent interacting with S1′ pocket and evaluated as potent MMPIs (Sorensen et al. 2003). The SAR conclusions were in accordance with those of Pikul et al. (1999) confirming the essential requirement of R-configuration of phosphorous atom and α-carbon. The 7-membered cyclophosphonamide and unsaturated 6-membered cyclophosphinamide hydroxamates were among the most potent inhibitors of MMP-1, -3, and -9. The proposed binding mode at MMP-3 has suggested interactions with Ala165, Leu164, Asn162, and Val163 as well as affinity of R2-substituent toward S1′ pocket.
Some cyclophosphinamide- and cyclophosphonamide-based hydroxamates (62) were designed by altering the phosphorous substituent interacting with S1′ pocket and evaluated as potent MMPIs (Sorensen et al. 2003). The SAR conclusions were in accordance with those of Pikul et al. (1999) confirming the essential requirement of R-configuration of phosphorous atom and α-carbon. The 7-membered cyclophosphonamide and unsaturated 6-membered cyclophosphinamide hydroxamates were among the most potent inhibitors of MMP-1, -3, and -9. The proposed binding mode at MMP-3 has suggested interactions with Ala165, Leu164, Asn162, and Val163 as well as affinity of R2-substituent toward S1′ pocket.

5.10 Non-Peptidyl Hydroxamates
A novel class of non-peptidyl hydroxamates, i.e., derivatives of N-aryl-iminodiacetic acid (IDA) (63–65) has been recently reported by Marques et al. (2006). To further improve the potency and selectivity versus MMP-2 and -13, some structural modifications were carried out to get new MMP-1/MMP-14-sparing hydroxamates inhibitors (Santos et al. 2006). As the specificity of MMP inhibition is correlated with the interactions at the S1′ pocket of the enzyme, the alkylaryl/sulfonylaryl substituents were added at the nitrogen and the carboxylic moiety was replaced by an amide chain.

Among the sulfonamide analogs (64), the para-methoxybenzene, biphenyl, and para-phenoxybenzene showed good inhibitory potency against MMP-2, -7, -8, -9, -13, and -14. The para-phenoxybenzene analog was observed as most potent (IC50 = 1–30 nM) and the order of activity was as: MMP-2 > MMP-13 > MMP-9 > MMP-8 > MMP-16 > MMP-14. The authors concluded that the lipophilic, electrostatic, and steric properties are liable toward MMPI potency and selectivity. Also the sulfonyl group at the nitrogen is important which permits its oxygen to form H–bonds with Leu164 and Ala165 (MMP-2) and directs the lipophilic groups in the S1′ pocket.

6 Conclusion
In conclusion, these SAR studies have indicated the following:
-
(1)
The greatest potency with the MMP inhibitors can be associated with their ability to interact with S1, S1′, and S2′ subsites.
-
(2)
S1′ differs most among the MMPs and a certain degree of specificity can be achieved by varying the P1′ residue of the inhibitors.
-
(3)
Introduction of larger P1′ substituents generally gives greater specificity for MMP-2 and MMP-9.
-
(4)
The P1′ substituent should preferably be a long side chain for the binding with MMP-2, MMP-3, and MMP-9.
In a recent article, Gupta and Patil (2012) have pointed out that the main subsites in MMPs for substrate recognition are the specificity pocket S1′ and, to a lesser extent, S2. The specificity pocket S1′ originates immediately to the right of catalytic Zn2+ ion and considerably differs in size and shape among the various MMPs. Due to this variation in size and shape, S1′ pocket offers selective inhibition of MMPs. The rough classification of S1′ specificity pockets according to the shape and size and the flexibility can aid in the development of selective MMP inhibitors. Because of the variation in the structure of S1′ pocket, modification of the P1′ can be used to introduce substrate specificity. The P1′–S1′ interaction is the main determinant for the affinity of inhibitors and the cleavage position of peptide substrates.
Several quantitative SARs (QSARs) on MMP inhibitors have been carried out. In a recent comprehensive review on QSAR studies on zinc-containing metalloproteinase inhibitors, Gupta (2007) concluded that in addition to binding with the catalytic Zn2+, the MMP inhibitors may also have hydrophobic, steric, and electrostatic interactions with the enzymes that may provide them better potency. Verma and Hansch (2007) also came to the same conclusion from their QSAR studies on some series of hydroxamic acids acting as MMP inhibitors. Some molecular modeling studies have visualized these interactions (Matter et al.1999; Matter and Schwab 1999; Tsai and Lin 2004). The SAR studies presented here also indicated these types of interactions between the inhibitors and various MMPs.
Abbreviations
- MMP:
-
Matrix metalloproteinase
- MMPI:
-
Matrix metalloproteinase inhibitor
- QSAR:
-
Quantitative structure–activity relationship
- SAR:
-
Structure-activity relationship
- TACE:
-
TNF-α converting enzyme
- TIMP:
-
Tissue inhibitors of metalloproteinases
- TNF:
-
Tumor necrosis factor-α
- ZBG:
-
Zinc-binding group
References
Andrews KL, Betsuyaku T, Rogers S, Shipley JM, Senior RM, Miner JH (2000) Gelatinase B (MMP-9) is not essential in the normal kidney and does not influence progression of renal disease in a mouse model of Alport syndrome. Am J Pathol 157:303–311
Aranapakam V, Davis JM, Grosu GT, Baker JL, Ellingboe J, Zask A, Levin JI, Sandanayaka VP, Du M, Skotnicki JS, Di Joseph JF, Sung A, Zhao W, McDevitt J, Xu ZB (2003a) Synthesis and structure-activity relationship of N-substituted 4-arylsulfonylpiperidine-4-hydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem 46:2376–2396
Aranapakam V, Grosu GT, Davis JM, Hu B, Ellingboe J, Baker JL, Skotnicki JS, Zask A, Di Joseph JF, Sung A, Sharr MA, Killar LM, Walter T, Jin G, Cowling R (2003b) Synthesis and structure-activity relationship of a-sulfonylhydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem 46:2361–2375
Attolino E, Calderone V, Dragoni E, Fragai M, Richichi B, Luchinat C, Nativi C (2010) Structure-based approach to nanomolar, water soluble matrix metalloproteinases inhibitors (MMPIs). Eur J Med Chem 45:5919–5925
Babine RE, Bender SL (1997) Molecular recognition of protein-ligand complexes: applications to drug design. Chem Rev 97:1359–1472
Barta TE, Becker DP, Boehm TL, DeCrescenzo GA, Villamil CI, McDonald JJ, Freskos JN, Getman DP (2003) Preparation of arylsulfonyl heterocyclyl hydroxamic acids and related compounds as matrix metalloproteases inhibitors. U.S. Patent 6, 541, 489, 2003. PCT Int. Appl. WO 9925687 A1 990527 CAN 131:18929; AN 1999:350651
Becker DP, Barta TE, Bedell L, DeCrescenzo G, Freskos J, Getman DP, Hockerman SL, Li M, Mehta P, Mischke B, Munie GE, Swearingen C, Villamil CI (2001a) α-amino-β-sulphone hydroxamates as potent MMP-13 inhibitors that spare MMP-1. Bioorg Med Chem Lett 11:2719–2722
Becker DP, Barta TE, Bedell LJ, Boehm TL, Bond BR, Carroll J, Carron CP, DeCrescenzo GA, Easton AM, Freskos JN, Funckes-Shippy CL, Heron M, Hockerman S, Howard CP, Kiefer JR, Li MH, Mathis KJ, McDonald JJ, Mehta PP, Munie GE, Sunyer T, Swearingen CA, Villamil CI, Welsch D, Williams JM, Yu Y, Yao J (2010) Orally active MMP-1 sparing α-tetrahydropyranyl and α–piperidinyl sulfone matrix metalloproteinase (MMP) inhibitors with efficacy in cancer, arthritis and cardiovascular disease. J Med Chem 53:6653–6680
Becker DP, DeCrescenzo G, Freskos J, Getman DP, Hockerman SL, Li M, Mehta P, Munie GE, Swearingen C (2001b) α-alkyl-α-amino-β-sulphone hydroxamates as potent MMP inhibitors that spare MMP-1. Bioorg Med Chem Lett 11:2723–2725
Becker DP, Villamil CI, Barta TE, Bedell LJ, Boehm TL, DeCrescenzo GA, Freskos JN, Getman DP, Hockerman S, Heintz R, Howard SC, Li MH, McDonald JJ, Carron CP, Funckes-Shippy CL, Mehta PP, Munie GE, Swearingen CA (2005) Synthesis and structure-activity relationships of β- and α-piperidine sulfone hydroxamic acid matrix metalloproteinase inhibitors with oral antitumor efficacy. J Med Chem 48:6713–6730
Bottomley KM, Johnson WH, Walter DS (1998) Matrix metalloproteinase inhibitors in arthritis. J Enzyme Inhib 13:79–101
Brown DA, Cuffe LP, Fitzpatrick NJ, Ryan AT (2004a) A DFT study of model complexes of zinc hydrolases and their inhibition by hydroxamic acids. Inorg Chem 43:297–302
Brown DA, Glass WK, Fitzpatrick NJ, Kemp TJ, Errington GJ, Haase W, Karsten F, Mahdy AH (2004b) Structural variations in dinuclear model hydrolases and hydroxamate inhibitor models: synthetic, spectroscopic and structural studies. Inorg Chim Acta 357:1411–1436
Chen D, Hackbarth C, Ni ZJ, Wu C, Wang W, Jain R, He Y, Bracken K, Weidmann B, Patel DV, Trias J, White RJ, Yuan Z (2004) Peptide deformylase inhibitors as antibacterial agents: identification of VRC3375, a proline-3-alkylsuccinyl hydroxamate derivative, by using an integrated combinatorial and medicinal chemistry approach. Antimicrob Agents Chemother 48:250–261
Cheng M, De B, Pikul S, Almstead NG, Natchus MG, Anastasio MV, McPhail SJ, Snider CE, Taiwo YO, Chen L, Dunaway CM, Gu F, Dowty ME, Mieling GE, Janusz MJ, Wang-Weigand S (2000) Design and synthesis of piperazine-based matrix metalloproteinase inhibitors. J Med Chem 43:369–380
Connolly PJ, Wetter SK, Beers KN, Hamel SC, Chen RH, Wachter MP, Ansell J, Singer MM, Steber M, Ritchie DM, Argentieri DC (1999) N-hydroxyurea and hydroxamic acid inhibitors of cyclooxygenase and 5-lipoxygenase. Bioorg Med Chem 9:979–984
Curtin ML, Florjancic AS, Heyman HR, Michaelides MR, Garland RB, Holms JH, Steinman DH, Dellaria JF, Gong J, Wada CK, Guo Y, Elmore IB, Tapang P, Albert DH, Magoc TJ, Marcotte PA, Bouska JJ, Goodfellow CL, Bauch JL, Marsh KC, Margon DW, Davidsen SK (2001) Discovery and characterization of the potent, selective and orally bioavailable MMP inhibitor ABT-770. Bioorg Med Chem Lett 11:1557–1560
Domingo JL (1998) Developmental toxicity of metal chelating agents. Reprod Toxicol 12:499–510
Dooley CM, Devocelle M, Mcloughlin B, Nolan KB, Fitzgerald DJ, Sharkey CT (2003) A novel family of hydroxamate-based acylating inhibitors of cyclooxygenase. Mol Pharmacol 63:450–455
El Yazal J, Pang Y-P (2000) Proton dissociation energies of zinc-coordinated hydroxamic acids and their relative affinities for zinc: insight into design of inhibitors of zinc-containing proteinases. J Phys Chem B 104:6499–6504
Fray MJ, Burslem MF, Dickinson RP (2001) Selectivity of inhibitors of matrix metalloproteinases MMP-3 and MMP-2 by succinyl hydroxamates and their carboxylic acid analogues is dependent on P3′ group chirality. Bioorg Med Chem Lett 11:567–570
Fray MJ, Dickinson RP (2001) Discovery of potent and selective succinyl hydroxamates inhibitors of matrix metalloproteinase-3 (stromelysin-1). Biorg Med Chem Lett 11:571–574
Gupta SP (2007) Quantitative structure-activity relationship studies on zinc-containing metalloproteinase inhibitors. Chem Rev 107:3042–3087
Gupta SP, Patil VM (2012) Specificity of binding with matrix metalloproteinases. In: Gupta SP (ed) Matrix metalloproteinase inhibitors: specificity of binding and structure-activity relationships. Springer, Basel, pp 35–56
Hannessian S, Bouzbouz S, Boudon A, Tucker GC, Peyroulan D (1999) Picking the S1, S1′ and S2′ pockets of matrix metalloproteinases: a niche for potent acyclic sulfonamide inhibitors. Bioorg Med Chem Lett 9:1691–1696
Heath EI, Grochow LB (2000) Clinical potential of matrix metalloproteinase inhibitors in cancer therapy. Drugs 59:1043–1055
Hidalgo M, Eckhardt SG (2001) Matrix metalloproteinase inhibitors: how can we optimize their development? J Natl Cancer Inst 93:178–193
Igeta K, Tobetto K, Saiki I, Odake S, Fujisawa T, Matsuo T, Oku T (2000) PCT Int Appl, WO 00 03,703
Ikura M, Nakatani S, Yamamoto S, Habashita H, Sugiura T, Takahashi K, Ogawa K, Ohno H, Nakai H, Toda M (2006) Discovery of a new chemical lead for a matrix metalloproteinase inhibitor. Bioorg Med Chem 14:4241–4252
Jeng AY, Chou M, Parker DT (1998) Sulfonamide-based hydroxamic acids as potent inhibitors of mouse macrophage metalloelastase. Bioorg Med Chem Lett 8:897–902
Jeng AY, De Lombaert S (1997) Endothelin converting enzyme inhibitors. Curr Pharm Des 3:597–614
Johnson WH, Roberts NA, Borkakoti N (1987) Collagenase inhibitors: their design and potential therapeutic use. J Enzyme Inhib 2:1–22
Johnstone RW (2002) Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1:287–299
Jung M (2001) Inhibitors of histone deacetylase as new anticancer agents. Curr Med Chem 8:1505–1511
Kelly WK, O’Connor OA, Marks PA (2002) Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs 11:1695–1713
Kimura T, Tamakl K, Miyazaki S, Kurakata S, Fujiwara K (2001) PCT Int. Appl. WO 0123363, 5th Apr, 2001
Kolodziej SA, Hockerman SL, Boehm TL, Carroll JN, DeCrescenzo GA, McDonald JJ, Mischke DA, Munie GE, Fletcher TR, Rico JG, Stehle NW, Swearingen C, Becker DP (2010a) Orally bioavailable dual MMP-1/MMP-14 sparing, MMP-13 selective α-sulfone hydroxamates. Bioorg Med Chem Lett 20:3557–3560
Kolodziej SA, Hockerman SL, DeCrescenzo GA, McDonald JJ, Mischke DA, Munie GE, Fletcher TR, Stehle N, Swearingen C, Becker DP (2010b) MMP-13 selective isonipecotamide α-sulfone hydroxamates. Bioorg Med Chem Lett 20:3561–3564
Kontogiorgis CA, Papaioannou P, Hadjipavlou-Litina DJ (2005) Matrix metalloproteinase inhibitors: a review on pharmacophore mapping and (Q)SARs results. Curr Med Chem 12:339–355
Krumme D, Wenzel H, Tschesche H (1998) Hydroxamate derivatives of substrate-analogous peptides containing aminomalonic acid are potent inhibitors of matrix metalloproteinases. FEBS Lett 436:209–212
Leung D, Abbenante G, Fairlie DP (2000) Protease inhibitors: current status and future prospects. J Med Chem 43:305–341
Levin JI, Du MT, Di Joseph JF, Killar LM, Sung A, Walter T, Sharr MA, Roth CE, Moy FJ, Powers R, Jin G, Cowling R, Skotnicki JS (2001) The discovery of anthranilic acid-based MMP inhibitors. Part 1: SAR of the 3-position. Bioorg Med Chem Lett 11:235–238
Levin JI, Nelson FC, Delos SE, Du MT, MacEwan G, Chen JM, Ayral-Kaloustian S, Xu J, Jin G, Cummons T, Barone D (2004) Benzodiazeine inhibitors of the MMPs and TACE. Part 2. Bioorg Med Chem Lett 14:4147–4151
Lipczynska-Kochany E (1988) In some new aspects of hydroxamic acid chemistry. Pr Nauk-Politech Warsz Chem 46:3–98
Ma D, Wu W, Yang G, Li J, Ye Q (2004) Tetrahydroisoquinoline based sulfonamide hydroxamates as potent matrix metalloproteinase inhibitors. Bioorg Med Chem Lett 14:47–50
Marks PA, Richon VM, Rifkind RA (2000) Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92:1210–1216
Marques SM, Chaves S, Rossello A, Tuccinardi T, Santos MA (2006) Metal ions in biology and medicine, vol 9. John Libbey Eurotext, Paris, pp 117–121
Matter H, Schwab W (1999) Affinity and selectivity of matrix metalloproteinase inhibitors: a chemometrical study from the perspective of ligands and proteins. J Med Chem 42:4506–4523
Matter H, Schwab W, Barbier D et al (1999) Quantitative structure-activity relationship of human neutrophil collagenase (MMP-8) inhibitors using comparative molecular field analysis and X-ray structure analysis. J Med Chem 42:1908–1920
Miller MJ (1989) Syntheses and therapeutic potential of hydroxamic acid based siderophores and analogues. Chem Rev 89:1563–1579
Mishra H, Parrill AL, Williamsom JS (2002) Three-dimensional quantitative structure-activity relationship and comparative molecular field analysis of dipeptide hydroxamic acid Helicobacter pylori urease inhibitors. Antimicrob Agents Chemother 46:2613–2618
Moore WM, Spilburg CA (1986a) Peptide hydroxamic acids inhibit skin collagenase. Biochem Biophys Res Commun 136:390–399
Moore WM, Spilburg CA (1986b) Purification of human collagenase with a hydroxamic acid affinity column. Biochemistry 25:5189–5195
Muri EM, Nieto MJ, Sindelar RD, Williamson JS (2002) Hydroxamic acids as pharmacological agents. Curr Med Chem 9:1631–1653
Nakatani S, Ikura M, Yamamoto S, Nishita Y, Itadani S, Habashita H, Sugiura T, Ogawa K, Ohno H, Takahashi K, Nakai H, Toda M (2006) Design and synthesis of novel metalloproteinase inhibitors. Bioorg Med Chem 14:5402–5422
Natchus MG, Bookland RG, De B, Almstead NG, Pikul S, Janusz MJ, Heitmeyer SA, Hookfin EB, Hsies LC, Dowty ME, Dietsch CR, Patel VS, Garver SM, Gu F, Pokross ME, Mieling GE, Baker TR, Foltz DJ, Peng SX, Bornes DM, Strojnowski MJ, Taiwo YO (2000) Development of new hydroxamates matrix metalloproteinase inhibitors derived from functionalized 4-aminoprolines. J Med Chem 43:4948–4963
Nishino N, Powers JC (1978) Peptide hydroxamic acids as inhibitors of thermolysin. Biochemistry 17:2846–2850
Noe MC, Snow SL, Wolf-Gouveia LA, Mitchell PG, Lopresti-Morrow L, Reeves LM, Yocum SA, Liras JL, Vaughn M (2004) 3-hydroxy-4-arylsulfonyltetra hydrohydropyranyl-3-hydroxamic acids are novel inhibitors of MMP-13 and aggrecanase. Bioorg Med Chem Lett 14:4727–4730
Nuti E, Casalini F, Avramova SI, Santamaria S, Cercignani G, Marinelli L, Pietra VL, Novellino E, Orlandini E, Nencetti S, Tuccinardi T, Martinelli A, Lim NH, Visse R, Nagase H, Rossello A (2009) N-O-isopropyl sulfonamide-based hydroxamates: design, synthesis and biological evaluation of selective matrix metalloproteinase-13 inhibitors as potential therapeutic agents for osteoarthritis. J Med Chem 52:4757–4773
Nuti E, Casalini F, Santamaria S, Gabelloni P, Bendinelli S, Pozzo ED, Costa B, Marinelli L, Pietra VL, Novellino E, Bernardo MM, Fridman R, Settimo FD, Martini C, Rossello A (2011) Synthesis and biological evaluation of U87MG glioma cells of (ethynylthiophene) sulfonamide-based hydroxamates as matrix metalloproteinase inhibitors. Eur J Med Chem 46:2617–2629
Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM (1998) Alzheimer’s amyloid precursor protein alpha-secretase is inhibited by hydroxamic acid-based zinc metalloprotease inhibitors: similarities to the angiotensin converting enzyme secretase. Biochemistry 37:1680–1685
Pikul S, McDow Dunham KL, Almstead NG, De B, Natchus MG, Anastasio MV, McPhail SJ, Snider CE, Taiwo YO, Chen L, Dunaway CM, Gu F, Mieling CE (1999) Desgin and synthesis of phosphinamide-based hydroxamic acids as inhibitors of matrix metalloproteinases. J Med Chem 42:87–94
Robinson RP, Laird ER, Blake JF, Bordner J, Donahue KM, Lopresti-Morrow LL, Mitchell PG, Reese MR, Reeve LM, Stam EJ, Yocum SA (2000) Structure-based design and synthesis of a potent matrix metalloproteinase-13 inhibitor based on a pyrrolidinone scaffold. J Med Chem 43:2293–2296
Robinson RP, Laird ER, Donahue KM, Lopresti-Morrow LL, Mitchell PG, Reese MR, Reeves LM, Rouch AI, Stam EJ, Yocum SA (2001) Design and synthesis of 2-ooxo-imidazolidine-4-carboxylic acid hydroxamides as potent matrix metalloproteinase-13 inhibitors. J Med Chem 119:1211–1213
Roedern GE, Brandstetter H, Engh RA, Bode W, Grams F, Moroder L (1998) Bis-substituted malonic acid hydroxamates derivatives as inhibitors of human neutrophil collagenase. J Med Chem 41:3041–3047
Rossello A, Nuti E, Carelli P, Orlandini E, Macchia M, Nencetti S, Zandomeneghi M, Balzano F, Uccello BG, Albini A, Benelli R, Cercignani G, Murphy G, Balsamo A (2005a) N-i-propoxy-N-biphenylsulfonyl aminobutylhydroxamic acids as potent and selective inhibitors of MMP-2 and MT1-MMP. Bioorg Med Chem Lett 15:1321–1326
Rossello A, Nuti E, Catalani MP, Carelli P, Orlandini E, Rapposelli S, Tuccinardi T, Atkinson SJ, Murphyb G, Balsamoa A (2005b) A new development of matrix metalloproteinase inhibitors: twin hydroxamic acids as potent inhibitors of MMPs. Bioorg Med Chem Lett 15:2311–2314
Rossello A, Nuti E, Orlandini E, Carelli P, Rapposelli S, Macchia M, Minutolo F, Carbonaro L, Albini A, Benelli R, Cercignani G, Murphy G, Balsamo A (2004) New N-arylsulfonyl-N-alkoxyaminoacetohydroxamic acids as selective inhibitors of gelatinase A (MMP-2). Bioorg Med Chem 12:2441–2450
Salvino JM, Mathew R, Kiesow T, Narensingh R, Mason HJ, Dodd A, Groneberg R, Burns CJ, McGeehan G, Kline J, Orton E, Tang SY, Morrisette M, Labaudininiere R (2000) Solid-phase synthesis of an arylsulfone hydroxamate library. Bioorg Med Chem Lett 10:1637–1640
Santos MA, Marques SM, Tuccinardi T, Carelli P, Panelli L, Rossello A (2006) Design, synthesis and molecular modeling study of iminodiacetyl monohydroxamic acid derivatives as MMP inhibitors. Bioorg Med Chem 14:7539–7550
Shingledecker K, Jiang S, Paulus H (2000) Reactivity of the cysteine residues in the protein splicing active center of the Mycobacterium tuberculosis RecA intein. Arch Biochem Biophys 375:138–144
Shuttleworth S (1998) An overview of combinatorial chemistry and its applications to the identification of matrix metalloproteinase inhibitors (MMPIs). In: Harvey AL (ed.) Advances in drug discovery techniques. Wiley, New York, p 115
Sorensen MD, Blaehr LKA, Christensen MK, Hoyer T, Latini S, Hjaranaa PJV, Bjoekling F (2003) Cyclic phosphinamides and phosphonamides, novel series of potent matrix metalloproteinase inhibitors with antitumor activity. Bioorg Med Chem 11:5461–5484
Supuran CT, Scozzafava A (2002) Matrix Metalloproteinase (MMPs). In: Smith HJ, Simons C (eds.) Proteinase and peptidase inhibition: recent potential targets for drug development. Taylor and Francis, London, pp 35-61
Szekeres T, Fritzer-Szekeres M, Elford HL (1997) The enzyme ribonucleotide reductase: target for antitumor and anti-HIV therapy. Critical Rev Clin Lab Sci 34:503–528
Torres G (1995) Hydroxyurea, a potential new anti-HIV agent. GMHC Treat Issues 9:7–9
Tsai KC, Lin TH (2004) A ligand-based molecular modeling study on some matrix metalloproteinase-1 inhibitors using several 3D QSAR techniques. J Chem Inf Comput Sci 44:1857–1871
Tsukamoto K, Itakura H, Sato K, Fukuyama K, Miura S, Takahashi S, Ikezawa H, Hosoya T (1999) Binding of salicylhydroxamic acid and several aromatic donor molecules to Arthromyces ramosus peroxidase, investigated by X-ray crystallography, optical difference spectroscopy, NMR relaxation, molecular dynamics, and kinetics. Biochemistry 38:12558–12568
Tu G, Xu W, Huang H, Li S (2008) Progress in the development of matrix metalloproteinase inhibitors. Curr Med Chem 15:1388–1395
Valapour M, Gou J, Schroeder JT, Keen J, Cianferoni A, Casolaro V, Georas SN (2002) Histone deacetylation nhibits IL4 gene expression in T cells. J Allergy Clin Immunol 109:238–245
Venkatesan AM, Davis JM, Grosu GT, Baker J, Zask A, Levin JI, Ellingboe J, Skotnicki JS, Di Joseph JF, Sung A, Jin G, Xu W, McCarthy DJ, Barone D (2004) Synthesis and structure-activity relationships of 4-alkynyloxy phenyl sulfanyl sulfinyl and sulfonyl alkyl hydroxamates as tumor necrosis factor-α converting enzyme and matrix metalloproteinase inhibitors. J Med Chem 47:6255–6269
Verma RP, Hansch C (2007) Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Biorg Med Chem 15:2223–2268
Wada CK, Holms JH, Curtin ML, Dai Y, Florjancic AS, Garland RB, Guo Y, Heyman HR, Stacey JR, Steinman DH, Albert DH, Bouska JJ, Elmore IN, Goodfellow CL, Marcotte PA, Tapang P, Morgan DW, Michaelides MR, Davidsen SK (2002) Phenoxyphenyl sulfone N-formylhydroxylamines (retrohydroxamates) as potent, selective, orally bioavailable matrix metalloproteinase inhibitors. J Med Chem 45:219–232
Weisburger JH, Weisburger EK (1973) Formation and pharmacological, toxicological, and pathological properties of hydroxylamines and hydroxamic acids. Pharmacol Rev 25:1–66
Whittakar M (1998) Discovery of protease inhibitors using targeted libraries. Curr Opin Chem Biol 2:386–396
Whittaker M, Floyd CD, Brown P, Gearing JH (1999) Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev 99:2735–2776
Yang SM, Scannevin RH, Wang B, Burke SL, Wilson LJ, Karnachi P, Rhodes KJ, Lagu B, Murray WV (2008) β-N-biaryl ether sulfonamide hydroxamates as potent gelatinase inhibitors: Part 1. Design, synthesis, and lead identification. Bioorg Med Chem Lett 18:1135–1139
Yao W, Wasserman ZR, Chao M, Reddy G, Shi E, Liu RQ, Covington MB, Arner EC, Pratta MA, Tortorella M, Magolda RL, Newton R, Qian M, Ribadeneira MD, Christ D, Wexler RR, Decicco CP (2001) Design and synthesis of a series of (2R)-N(4)-hydroxy-2-(3-hydroxybenzyl)-N(1)-[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl] butanediamide derivatives as potent, selective, and orally bioavailable aggrecanase inhibitors. J Med Chem 44:3347–3350
Zhang X, Gonnella NC, Koehn J, Pathak N, Ganu V, Melton R, Parker D, Hu SI, Nam KY (2000) Solution structure of the catalytic domain of human collagenase-3 (MMP-13) complexed to a potent non-peptidic sulfonamide inhibitor: binding comparison with stromelysin-1 and collagenase-1. J Mol Biol 301:513–524
Zhang Y, Li D, Houtman JC, Witiak DT, Seltzer J, Bertics PJ, Lauhon CT (1999) Design, combinatorial chemical synthesis and in vitro characterization of novel urea based gelatinase inhibitors. Bioorg Med Chem Lett 9:2823–2826
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Patil, V.M., Gupta, S.P. (2013). Structure–Activity Relationship Studies of Hydroxamic Acids as Matrix Metalloproteinase Inhibitors. In: Gupta, S. (eds) Hydroxamic Acids. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38111-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-38111-9_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38110-2
Online ISBN: 978-3-642-38111-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)