Abstract
Collagen fibers from cattle skin were enzymatically extracted and analyzed. The results of texture analyzer determination and microscopic observation of collagen fibers showed that extract efficiency was affected by pH, enzyme concentration, and combined ratio. The optimal conditions were obtained as follows: adding 0.5 ml 6 ‰ complex enzyme solution (alkaline proteinase: neutral protease = 1:1, w/w) into 1 g leather, ultrasonic shocking for 20 min, incubating in the shaking box at 30 °C for 24 h, and soaking in the pH = 2 hydrochloric acid solution for 7 h. Extracted collagen fibers are potentially used in edible film production and casing making.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
When thought about the packaging innovations for the twenty-first century, the most interesting thing to review was the technologies that are enabling these changes. As packaging innovations were researched across the food industry, there are numerous examples of breakthrough innovations in meat, snack, and beverage industry [1], such as high-density polyethylene coffee containers and polyethylene plastic bags. However, these new materials were not so friendly to the environment in some way. Many polyethylene plastic bags were found to be flying all around in the street at one time. So interest in edible films was rooted in attempts to develop easily degradable packaging, nonaggressive to the environment, thus improving the quality of food products and providing new markets for the materials used in the manufacture of these films [2]. The new food packaging technologies are also developing as a response to consumer demands or industrial production trends toward mildly preserved, fresh, tasty, and convenient food products with prolonged shelf life and controlled quality [3].
Edible films are defined as a thin layer of material which can be consumed and provides a barrier to moisture, oxygen, and solute movement for the food. The material can manufacture a complete food coating or can be disposed as a continuous layer between food components [4]. Edible films can be formed as food coatings and free-standing films, and have the potential to be used with food as gas aroma barrier [5].
Edible films can be produced from materials with film forming ability. During manufacturing, film materials must be dispersed and dissolved in a solvent such as water, alcohol, or mixture of water and alcohol or a mixture of other solvents. Plasticizers, antimicrobial agents, colors, or flavors can be added in this process. Adjusting the pH and heating the solutions may be done for the specific polymer to facilitate dispersion. Film solution is then casted and dried at a desired temperature and relative humidity to obtain free-standing films. In food applications, film solutions could be applied to food by several methods such as dipping, spraying, brushing, and panning followed by drying. Components used for the preparation of edible films can be classified into three categories: hydrocolloids (such as proteins, polysaccharides, and alginate), lipids (such as fatty acids, acyl glycerol, and waxes), and composites [6].
In their native states, proteins generally exist as either fibrous proteins or globular proteins [7]. Fibrous proteins are fully extended and associated closely with each other in parallel structures, generally through hydrogen bonding, to form fibers [8]. At the same time, proteins can assured their biodegradability and environmental compatibility to form films [9], and films made from protein can supplement the nutritional value of the food [10].
Collagen is widely found in nature as the major constituent of skin, bones and connective tissue, which has a high value whether in the field of food or medicine, and cosmetics. So the extraction of collagen from cattle skin will have a high cost-effectiveness and practical value. Plasticizers, antimicrobial agents, colors, or flavors have been added to improve its characteristics, such as mechanical properties and water vapor permeability. Chambi and Grosso [11] produced edible films with gelatin and casein cross-linked with transglutaminase. Vanin et al. [12] studied the effects of plasticizers and their concentrations on the thermal and functional properties of gelatin-based films.
Found by the preceding work, collagen fibers were mainly extracted by chemical methods, such as acid, alkali, which caused serious environment pollution and potential toxicity. Few researches were reported by enzymatic methods, although enzymatic hydrolysis has already used in gelatin production, collagen hydrolyte extraction, which showed enzymatic extraction is an efficient, environment-friendly way for collagen treatment.
The objective of this work was to study the enzymatic extraction conditions for collagen fibers from cattle skin, and to characterize their mechanical properties, providing a basic for produce potentially used in edible film production and casing making.
2 Materials and Methods
2.1 Materials
Cattle skins were obtained from Longbao Collagen Co., Ltd, and the initial pH was 12. Enzymes were purchased from Tianjin Nuoao Technology Co., Ltd. For simplicity in this article, trypsin and high alkali alkaline proteinase were abbreviated as Try and Haap. Alkaline proteinase and neutral protease in food and industry grade were abbreviated as Apf, Api, Npf, and Npi, respectively.
2.2 Collagen Fibers Production
Initial cattle skins were washed to the neutral pH, dried in the air, and then cut into pieces, vibrated 20 min in the ultrasonic oscillator, and cultivated with different enzyme solutions at 30 °C for 24 h. After washed up the residual enzyme, skins were put in hydrochloric acid in order to swell the collagen fibers, and neutralize the acid finally. In control group, cattle skins were treated with distilled water [13, 14]. Each sample was examined in triplicate.
2.3 Mechanical Properties
Firmness and shear force were determined according to the NY/T 1180-2006 using a texture analyzer (TA-XT Plus, Stable Micro Systems, SMS, UK), and the software Texture Expert V 5.1.2.0. The trigger force was 40 g. The speed before and after used initially was 1.50 and 10.00 mm/s, respectively [15]. Mechanical measurements were done in triplicate.
2.4 Histological Study
Cattle skins were cut into small pieces, embedded in the sample care using the Tissue Freezing Medium, sectioned at 4–6 μm thickness using a Freezing splicer (Leica CM1950). After fixed by the stationary liquid [16], the sections were stained with hematoxylin and eosion for microscopic examination using confocal laser scanning microscopy [17, 18].
3 Results and Discussion
3.1 Mechanical Properties
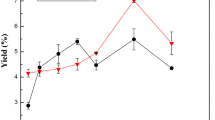
The firmness and shear force of cattle skins were determined using a Texture Analyzer. The data can react rough understanding of the enzymatic degree. The firmness and shear force of cattle skins were studied using six enzymes at 2, 4, 6, and 8 ‰ (w/w) concentrations. Figure 153.1 showed the changes of firmness and shear force under six different enzymes. The initial cattle skin firmness and shear force were 500.424 N and 639.7 N/s, respectively. The firmness and shear force of control group were 258.58 N and 201 N/s, respectively.
In Fig. 153.1, it was obvious that the firmness of skins treated with different enzymes had similar trend with the shear force. Compared with the control group, cattle skins dealt with Npf and Npi were obviously tougher than other groups. However, the Apf, Api, and Haap groups were almost twice softer than first three groups. Try was similar with animal proteinase in the way to degrade collagen, and it also acted on 780–781 sector of the amino acid sequence which is close to the sectors animal proteinase acted [19]. The optimum pH of Npf and Npi is 6.0–7.5, while APf, Api, and Haap is 9–11. Figure 153.1 showed that it was better to choose Apf, Api, or Haap to degrade the collagen.
With enzyme concentration increasing, firmness and shear force decreased quickly under 6 ‰. When the concentration was higher, firmness and shear force increased immediately. In this case, the best concentration of enzyme was 6 ‰.
In Fig. 153.2, firmness and shear force of different enzymes with different ratios (1:1, 1:2, 2:1) were researched. The concentration of different complex enzyme was fixed to 6 ‰ according to Fig. 153.1. It was obvious that the effect was best when Npf/Apf was 1:1. Other groups were similar each other, but they were all too tough compared to the best group. So the best condition was to add 0.5 ml 6 ‰ complex enzyme solution (alkaline proteinase: neutral protease = 1:1, w/w) into 1 g leather.
After cattle skins were broken by enzymes, the isolated protein and many other components were cleaned up together. So the content of fibers was increased relatively. Then it was swelled by acid. Fibers became rougher, and space between fibers was not so tight. As is well-known, protein is hydrophilic, so its size becomes bigger when it absorbs water. However, fibers will be used to manufacture collagen casings by drying it, so the casings contain fibers most, and they have strong tensile strength. The better the fibers are swelled, the stronger casings are made. Figure 153.3 showed that when pH was 2, firmness and shear force of the fibers were both better than pH was 5.
3.2 Microscope Imaging
Figure 153.4 showed four micrographs of the cattle skin in different enzyme conditions: the control group, Api (2 ‰), Api (6 ‰), and the complex enzyme (6 ‰). Enzyme can get rid of the dissociate protein. It was obvious in the micrographs that the majority of materials in cattle skin were collagen fibers. Fibers rank tidily and regularly according to certain directions in the casings [20]. When cattle skins were dealt with alkali proteinase, extracted fibers were obviously thicker than the control ones, which add nothing to the cattle skins. Moreover, with the increasing concentration of enzyme, the fibers were thicker, and the gap between fibers was broader. In addition, it also showed that it was better when enzymes were mixed and the most suitable result was found when the complex enzymes (Alkaline proteinase/Neutral protease) ratio was 1:1.
After enzymatic extraction, collagen fibers were soaked in the hydrochloric acid. When the pH was too low, as well as the concentration of hydrochloric acid was too high, acid can break protein [21], and make it degeneration, so fibers were broken, casings can not be made. Figure 153.5 showed that when pH = 2, fibers were thicker than pH = 7. What is more, the gap between fibers became broader.
4 Conclusions
The collagen fibers from cattle skins were extracted by using the combined enzymolysis method. Effects of enzyme addition, solid-liquor ratio on the extraction, as well as pH efficiency of collagen fibers were investigated in this paper. The optimal processing conditions were obtained as follows: adding 0.6 % compound enzyme solution into 1 g leather, ultrasonic shocking for 20 min, then incubation at 30 °C in shaking box for 24 h, soaked in the pH = 2 hydrochloric acid solution. The results of texture analyzers determination and frozen section microscope observation of collagen fibers showed that in all conditions, the condition with alkaline proteinase/neutral protease = 1:1 was best.
References
Eilert SJ (2005) New packaging technologies for the 21st century. Meat Sci 71:122–127
Chen H (1995) Functional properties and applications of edible films made of milk proteins. J Dairy Sci 78:2563–2583
Dainelli D, Gontard N, Spyropoulos D, Beuken EZ, Tobback P (2008) Active and intelligent food packaging: legal aspects and safety concerns. Trends Food Sci Tech 19:S103–S112
Guilbert S (1986) Technology and application of edible protective films. Food package preserv 1986:371–394
Kester JJ, Fennema OR (1986) Edible films and coatings: a review. Food Tech 40(12):47–59
Donhowe IG, Fennema OR (1993) The effects of plasticizers on crystallinity, permeability, and mechanical properties of methylcellulose films. J Food Process Preserv 17:247–257
Scope RK (1993) Separation by precipitation. In: Protein purification, principles and practice. Springer, New York, pp 71–101
Bourtoom T (2008) Review article edible films and coatings: characteristics and properties. Int Food Res J 15(3):237–248
Krochta JM, Johnston C (1997) Edible and biodegradable polymer films: challenges and opportunities. Food Tech 51:61–64
Gennadios A, Weller L (1990) Edible films and coatings from wheat and corn proteins. Food Tech 44:438–443
Chambi H, Grosso C (2006) Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res Int 39:446–458
Vanin FM, Sobral PJA, Menegalli FC et al (2005) Effects of plasticizers and their concentrations on the thermal and functional properties of gelatin-based films. Food Hydrocolloids 19:899–907
Ya H (2007) Edible collagen casing and application in meat industry. Meat Ind 10:17–19
Li P (2010) Collagen casing and film production. Sci Technol Gelatin 30(2):80–82
NY/T 1180-2006 (2006) Determination of meat tenderness and shear force method. Ministry of Agricultre of the PRC, Beijing
Yao HS, Yang J, Jin XL et al (1998) The fixed method improvement of frozen section. Shanhai Med 21(5):309–310
Adedeji AA, Liu L, Ngadi MO (2011) Microstructural evaluation of deep-fat fried chicken nugget batter coating using confocal laser scanning microscopy. J Food Eng 102:49–57
Achir N, Vitrac O, Trystram G (2010) Direct observation of the surface structure of French fries by UV-VIS confocal laser scanning microscopy. Food Res Int 43:307–314
Zhao SN (1998) Research of collagen extracted from fresh pig skin. Sci Tech Food Ind 5:16–17
Ye Y, Wang KY, Wang B (2003) Characteristics of collagen-chitosan-poval complex film. China Leather 32(23):1–4
Li X, Li YT, Wei SZ (2004) Study on production technology of acid-hydrolysis amino acid seasoning. China Brewing 3:11–15
Acknowledgements
The present work was supported by grants from the Foundation of National 863 Plan (No.2013AA102204), National Natural Science Foundation of China (No. 31000755), and Key Project of Chinese Ministry of Education (No. 211008).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Wang, W., Zhao, K., Ren, W., Liu, T., Zhang, Y. (2014). Enzymatic Extraction and Characteristics of Collagen Fibers from Cattle Skin. In: Zhang, TC., Ouyang, P., Kaplan, S., Skarnes, B. (eds) Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012). Lecture Notes in Electrical Engineering, vol 251. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37925-3_153
Download citation
DOI: https://doi.org/10.1007/978-3-642-37925-3_153
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37924-6
Online ISBN: 978-3-642-37925-3
eBook Packages: EngineeringEngineering (R0)