Abstract
What accounts for the beauty and singularity of arthropods is the cuticle that enables them to compete in their small world. What we see is the surface but what does it look like inside? In the past two centuries, starting with the discovery of chitin as a major component of the arthropod cuticle by Odier (1823), a vast number of publications contributed to the understanding of cuticle architecture and composition (reviewed in Locke 2001; Moussian 2010). The arthropod cuticle is a multifunctional coat that defines and stabilises the shape of the body, appendages and internal organs including the hindgut, the foregut and, in insects, the tracheae, preventing dehydration and infection, and protecting against predators of the same scale. As an exoskeleton, additionally, it allows locomotion and flight. Witnessing the ecological success and relevance of arthropods, the cuticle is a highly versatile device facilitating formation of many different body shapes that reflect habitat adaptation, and indeed, arthropods populate a broad range of ecological habitats ranging from oceans to deserts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
What accounts for the beauty and singularity of arthropods is the cuticle that enables them to compete in their small world. What we see is the surface but what does it look like inside? In the past two centuries, starting with the discovery of chitin as a major component of the arthropod cuticle by Odier in 1823, a vast number of publications contributed to the understanding of cuticle architecture and composition (reviewed in Locke 2001; Moussian 2010). The arthropod cuticle is a multifunctional coat that defines and stabilises the shape of the body, appendages and internal organs including the hindgut, the foregut and, in insects, the tracheae, preventing dehydration and infection, and protecting against predators of the same scale. As an exoskeleton, additionally, it allows locomotion and flight. Witnessing the ecological success and relevance of arthropods, the cuticle is a highly versatile device facilitating formation of many different body shapes that reflect habitat adaptation, and indeed, arthropods populate a broad range of ecological habitats ranging from oceans to deserts.
In a given species, environmental constraints may also dictate stage- and tissue-specific differences in the physical properties of the cuticle. Usually, for instance, in caterpillars and other insect larvae, the body cuticle is soft and elastic serving as a hydrostatic jacket withstanding the internal pressure of the haemolymph, thereby allowing locomotion (Fig. 8.1). In the same animal, the head skeleton consists of hard cuticle required for mastication and probably to shield the brain. Hard body cuticle, by contrast, is the prevalent cuticle type of mainly adult animals, especially covering their dorsal side that is usually more exposed to the environment than the ventral side. Sclerites of hard cuticle are joined by soft cuticle rendering the exoskeleton pliable.
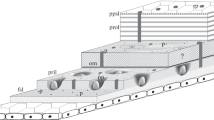
Cuticle architecture. Upper image The typical arthropod cuticle is a layered extracellular structure produced by a monolayer of epithelial cells at their apical side. The polarity of these cells is illustrated by the presence of adherens junctions (AJ) at apicolateral positions of the lateral membrane and the septate junctions (SJ) underneath. The outermost layer is the envelope (env), a relatively new term for this structure. In the literature, it has been described as the lipid-bearing outer epicuticle or the cement layer. The epicuticle (epi), formerly called inner epicuticle, is an ultrastructurally distinct layer beneath the envelope, and also contains lipids and proteins, but is devoid of chitin. The innermost procuticle (pro) is a chitin–protein matrix attached to the surface of the epithelial cell. Lower image Different types of cuticles are present within one animal, for example, in the Panorpa vulgaris (mecoptera) first instar larva with abdominal soft cuticle and a coloured and hard thoracic and head cuticle
Along with its relative advantages, the cuticle makes an arthropod’s life also more complicated: to accommodate possible habitat changes during the life cycle of an organism and to allow growth from one developmental stage to the next, the cuticle has to be detached from the epithelial surface, shed and replaced by a new one (see Chap. 6). This implies stage-specific composition reflecting the required physical properties.
Commonly, cuticles are composed of lipids and waxes, glycosylated and unglycosylated proteins, the polysaccharide chitin and catecholamines. Additionally, especially in crustaceans, minerals such as calcite may be incorporated. Species-, stage- and tissue-specific differences mainly rely on lipid and wax composition, different albeit related proteins, the amounts of chitin and the degree of covalent cross-links by, for example, catecholamines. Analogous to the vertebrate skin, lipids and waxes are implicated in preventing water loss and are mainly coating the surface of the animal. Whereas vertebrates employ sphingolipids such as ceramides (Madison 2003; Harding 2004; Jensen and Proksch 2009), insects apply neutral lipids (n-alkanes and n-alkenes) and wax esters as water repellents. The genomic sequences of many arthropods, mainly insects, have led to the discovery and bioinformatics characterisation of several classes of putative structural cuticle proteins, many of which harbour chitin-binding domains. These classes have been excellently described recently by Willis (2010). Concerning chitin, the second most abundant polysaccharide on earth, molecular biology of arthropod cuticle chitin synthesis has been inspired by advances in research on fungal chitin (Merzendorfer 2006). However, since chitin is highly organised in arthropods, while it seems not to be particularly organised in fungal cell walls, insights from this side are rather limited. Ordered packing of cuticle components involves covalent and non-covalent interactions between them. Major covalent linkages are mediated by catecholamines that eventually also cause cuticle tanning.
Looking at the literature published in the last century or so cuticle organisation is, in spite of the variety of components, basically pretty well conserved between distantly related arthropod species. This observation in turn implies that the molecular mechanisms of cuticle formation are largely conserved as well, permitting the possibility of using model arthropods to answer this fundamental biological problem. In the last few years, molecular and genetic approaches in the insects Drosophila melanogaster and Tribolium castaneum have indeed boosted our understanding about cuticle differentiation. Classic histology paired with recent molecular data together draw an exciting scheme of cuticle differentiation that is summarised in this chapter.
8.2 Architecture and Composition of the Cuticle
The common denominator of virtually all cuticles is, with very few exceptions, their stereotypic organisation in three ultrastructurally distinct horizontal layers (Fig. 8.2). There are numerous terms for the different cuticle layers, and in this chapter, the newest unifying nomenclature proposed by Locke (2001) is used.
Chitin arrangement. The procuticle is the cuticle layer harbouring chitin that associates with proteins, which are necessary both as structural and as functional co-factors. In electron micrographs, chitin microfibrils that consist of around 18 chitin fibres appear as grey parabolic fibres although chitin itself is not contrasted by lead or uranium, suggesting that electron density of these fibres is due to associated proteins (Neville 1975; Neville et al. 1976). In the procuticle, chitin fibres are bundled as microfibrils, which in turn are arranged in parallel to each other forming horizontal chitin sheets, the so-called laminae (a). Often, laminae are stacked helicoidally, probably conferring elasticity and flexibility to the procuticle. Oblique sections of such procuticles give the impression that chitin microfibrils are oriented as parabolic arches (b, compare to a). This architecture of the procuticle was first described by Yves Bouligand in 1965 in crustaceans. In 1969, Neville and Luke ascribed chitin organisation in the insect procuticle to Bouligand’s model (Neville and Luke 1969a, b). Helicoidal arrangement of chitin laminae is not a paradigm. In some hard cuticle, chitin–protein complexes are organised as bricks probably making the cuticle stiff (c, c′ shows a magnification of the framed region in c). Scale bars are 500 nm
8.2.1 The Surface Envelope
The outermost layer composed of neutral lipids, wax esters and proteins is the envelope, which is a composite structure with a thickness of around 25 nm consisting of several alternating electron-dense and electron-lucid sheets. Lipids and waxes predominantly localise to the body surface. Some lipids seem to be free molecules and are easily washed out by organic solvents such as hexane. This has allowed the identification of the molecules in various insects by gas chromatography and mass spectrometry (Nelson et al. 2001, 2002, 2003, 2004; Patel et al. 2001; Nelson and Charlet 2003; Everaerts et al. 2010). The majority of molecules at the insect surface are neutral lipids like long-chain alkanes and alkenes, long-chain alcohol and fatty acid esters. For instance, the most abundant neutral lipids in D. melanogaster imagines are 7-tricosene (male) and 7,11-heptacosadiene (female). The obvious role of lipids and waxes is to protect the animal against dehydration and soaking (Gibbs 1998, 2011). In addition, they are reported to act as pheromones in various insects (Tillman et al. 1999; Howard and Blomquist 2005). In an exciting work using matrix-assisted laser desorption/ionisation (MALDI) imaging, that combines mass spectrometric identification of molecules with their localisation in the tissue, lipids (e.g. heptacosane and nonacosane) were identified on the surface of insect wings (Vrkoslav et al. 2010).
In 1933, Wigglesworth named the major component at the surface of Rhodnius prolixus cuticulin, which he proposes to be composed of lipids and sclerotin, a protein–quinone complex (Wigglesworth 1933, 1990). In his earlier work, Locke termed the outermost layer cuticulin (Locke 1966). Sclerotin can be visualised by silver precipitation after harsh chloroform extraction of masking surface lipids (Wigglesworth 1985). This argentaffin staining method reveals that in addition to a surface localisation, sclerotin is also present between the lamellae of the procuticle and in pore canals, nano-scaled tubes that run through the entire cuticle from the apical surface of the cell to the surface of the cuticle (Wigglesworth 1990). Hence, lipids seem not only to form a sheet-like barrier at the surface of the animal but also to impregnate the entire cuticle either to prevent water loss or to contribute to cuticle architecture (Wigglesworth 1975). The wall of pore canals also displays esterase activity that probably contributes to wax synthesis (Locke 1961). Taken together, production of lipids and waxes is conceivably initiated in the cytoplasm, followed by deposition into the pore canals by an unknown mechanism. Some of these lipids and waxes interact with proteins like sclerotin, others persist as free molecules. Both are subsequently modified and travel through the pore canals to their final site. One should be aware that these conclusions are based on fixed material; hence, for a dynamic view on cuticle, lipid biochemistry, molecular and genetic data are important to confirm or reject the working model presented in Fig. 8.3.
Cuticle production pathways. Cellular and molecular mechanisms of cuticle production can be subsumed in three pathways. Cuticle lipid biology (1). Lipids are either provided by lipid droplets or synthesised in mitochondria and the smooth endoplasmic reticulum (sER) by elongases and desaturases. Lipid deposition and organisation involve transfer by as yet unidentified transporters into extracellular pore canals (pc) that transport lipids to their destination (1a). For lipid organisation, lipid-binding proteins (sclerotin) are secreted through the canonical secretory pathway (1b). Sclerotin, through a yet unidentified reaction, forms a complex with polyphenols and lipids to form a waterproof barrier (1c). Free long-chain alkanes and alkenes are also present at the surface of the animal. Cuticle protein biology (2). Proteins are delivered to the extracellular space via the canonical secretory pathway (2a). Here, during sclerotisation and melanisation, they react with catecholamines (NADA and NBAD, 2b) that are transported to the extracellular space by yet unknown transporters. Synthesis of catecholamines starts in the cytoplasm, where dopamine and L-Dopa are formed by the tyrosine hydroxylase (TH) and Ddc (2c). Protein cross-linking comprises as yet unidentified membrane-bound or extracellular peroxidases (2d) that catalyse dityrosine formation between proteins (2e). Chitin biology (3). The canonical secretory pathway localises the chitin synthase to plaques at the tip of membrane corrugations and proteins assisting chitin synthesis and organisation to the membrane or the extracellular space (3a). Chitin organisation occurs in the extracellular space (3b). Secretory vesicles are depicted as grey circles
The molecular and biochemical pathways of cuticular hydrocarbon synthesis and transport have been studied in some insects, and the enzymes responsible have been identified in a few model species. For instance, biosynthesis of bombykol from Bombyx mori, a twice desaturated C16 alcohol which was the first lipid pheromone isolated by Butenandt (Butenandt et al. 1961a, b) branches out from the canonical fatty acid biosynthesis pathway (Matsumoto 2010). In brief, palmitic acid (C16) is desaturated at C10 and C12 by the specific acyl-CoA desaturase Bmpgdesat1, and the carboxyl group is reduced to an alcohol by the reductase pgFAR. This is a rather simple pathway that also seems to be present in other lepidopterans. Other fatty acid derivatives may require more complex modifications such as chain shortening. Nevertheless, it is possible, in principle, that the biosynthesis of many, if not all, cuticular hydrocarbons may follow this scheme (Fig. 8.3).
Lipid production occurs predominantly in sub-epidermal oenocytes, which are considered to be hepatocyte-like cells involved in lipid homoeostasis (Gutierrez et al. 2007). In D. melanogaster, these cells are organised as paired clusters of five cells each in every abdominal segment (Wigglesworth 1970; Gutierrez et al. 2007). Their activity as secretory organs correlates with cuticle moulting (Wigglesworth 1970). In the frame of their systemic function as lipid relays, they supply epidermal cells with cuticle lipid precursors. How are lipids that are produced and stored in oenocytes delivered to epidermal cells? In a simple yet unproven scenario depicted in Fig. 8.4, cuticle lipid precursors are produced and stored in oenocytes (and the fat body), as lipid droplets and crystal-like inclusions, and released into the haemolymph as lipophorin complexes, that are taken up by epidermal cells at their basal side by lipophorin receptors (LpR), through an endocytosis-independent pathway, as described for D. melanogaster nurse cells (Parra-Peralbo and Culi 2011). The lipid precursors are modified and processed accordingly within the epidermis and transported to the differentiating cuticle by unknown transporters. Pore canals that connect the epidermal apical plasma membrane with the cuticle surface are involved in further lipid modification and delivery, mainly to the cuticle surface. Hence, oenocytes systemically participate at cuticle differentiation.
Lipids and cuticle formation. Many cuticle lipid precursors are produced in sub-epidermal clusters of oenocytes. Lipids are bound to lipophorin and transported through the haemolymph to the basal side of cuticle producing epithelial cells. Here, they are internalised via the lipophorin receptor (LpR). In these cells, they may be modified (lipids*) and transported to the cuticle by a yet unidentified mechanism involving pore canals (pc) to form the protein–lipid complex cuticulin
8.2.2 The Epicuticle
Underneath the envelope, there is the epicuticle, which is mainly composed of largely unidentified proteins and lipids, probably covalently cross-linked. The interaction between the components of this layer does not result in a conspicuous texture, as indicated by electron micrographs of the epicuticle, which rather displays an amorphous ultrastructure. Deposition of the epicuticle in D. melanogaster depends on the activity of the steroid hormone ecdysone, since ecdysone-deficient larvae do not produce an epicuticle (Gangishetti et al. 2012). Obviously, expression of epicuticle producing enzymes and structural proteins is triggered by the ecdysone-signalling pathway. Candidate proteins are those that do not contain a chitin-binding domain directing them to the procuticle (see below). For instance, there are glycine-rich proteins with GGYGG or GGxGG repeats, called “cuticle protein glycine-rich” (CPG) in lepidopterans (Futahashi et al. 2008a), alanine-rich cuticle proteins (CPLCA) confined to dipterans, and apidermins in hymenopterans (Kucharski et al. 2007), that do not have obvious chitin-binding domains. Remarkably, many of these proteins are specific to single arthropod orders. Despite this specificity, they share several common features. First, they are relatively small proteins of around 10 kDa. Second, despite some conserved sequences, they are characterised by low structural complexity, that is, they probably do not adopt a complex tertiary structure (Andersen 2011). To verify whether these proteins are indeed components of the epicuticle, immunodetection on thin sections should be performed, along with genetic and RNA interference (RNAi) analyses to elucidate their role.
8.2.3 The Procuticle
The procuticle is the innermost cuticle layer and harbours the N-acetylglucosamine (GlcNAc) polymer chitin in association with proteins. Usually, chitin orientation in the procuticle is not random as in fungi, but crystalline (Neville 1965a; Neville et al. 1976). The core molecule of chitin crystals is a bundle of on average 20 chitin fibres arranged antiparallel to each other (Vincent and Wegst 2004), an arrangement that is named α-chitin. These nanofibers, which have a diameter of around 30 Å, associate with proteins to form microfibrils with a diameter of around 100 nm. These microfibrils are arranged in parallel, to form two-dimensional sheets called laminae, which are stacked, with each lamina twisted by a small angle with respect to the lamina below. This helicoid pattern (Fig. 8.2) was described for the first time by Bouligand in 1965 through extensive ultrastructural analyses of crustacean cuticles (Bouligand 1965). Subsequently, Luke and Neville found that chitin in insect cuticles adopts the Bouligand arrangement as well (Neville and Luke 1969a, b). An interesting tissue with a specialised procuticle is the eye lens of insects. It consists of twisted chitin laminae that are arranged as a spherical extracellular matrix (Yoon et al. 1997). The lens cuticle serves as a protective structure, especially for digging insects, but may also be a light collector. Alternatively, in some cases, laminae may also be arranged like plywood (Neville and Luke 1969a, b; Neville et al. 1976; Cheng et al. 2009). For instance, the elytral cuticle of the red flour beetle T. castaneum is characterised by tightly packed protein–chitin brick-like units that do not display a helicoidal organisation (Fig. 8.2). In cockroaches and water bugs, Neville has found that chitin orientation changes from lamellate to non-lamellate following a circadian rhythm of light and dark (Neville 1965b). In crustaceans, nanofibrils do not run straight but meander, creating a honeycomb-like structure when viewed from above (Raabe et al. 2005). This structure is thought to prevent crack progression. Whether this pattern is present in other arthropods, including insects, remains to be investigated. Occasionally, pore canals, which are probably useful for cuticle repair, interrupt the crystalline organisation of the chitin–protein matrix.
What are the cellular and molecular requirements of chitin assembly? Ordered chitin synthesis at the apical plasma membrane of epithelia certainly has an important impact on chitin organisation. Indeed, the chitin synthase complex, visible as electron-dense plaques in electron micrographs, resides at the crest of repetitive plasma membrane corrugations. In D. melanogaster, these corrugations, called apical undulae, are longitudinal structures that are stabilised by microtubules (Moussian et al. 2006). In other arthropods, these structures have not been described, and rather microvilli-like units are regarded as the sites of chitin synthesis (Locke 2001, 2003). Elimination or reduction of chitin synthase activity are lethal in D. melanogaster and T. castaneum and cause cuticle disorganisation and collapse (Arakane et al. 2004, 2005b, 2008; Moussian et al. 2005a, b; Tonning et al. 2006). Cuticular proteins coagulate and are unable to ensure the formation of a uniform cuticle. Hence, the presence of chitin is essential for uniform thickness of the cuticle.
Arthropods possess more than one chitin synthase, but these enzymes do not have redundant functions (Merzendorfer 2006, 2011). The epidermal and tracheal chitin synthase 1 or A (CS-1 or CHS-A) is required for cuticle production, whereas the midgut chitin synthase 2 or B (CS-2 or CHS-B) contributes to the formation of the midgut peritrophic matrix that protects the midgut epithelial cells from pathogens and digestive enzymes (Lehane and Billingsley 1996). It should also be pointed out that partner proteins of chitin synthase itself, that is, other constituents of the plaques have not been identified to date.
Fusion of vesicles carrying cuticle proteins occurs in the valleys between microvilli-like membrane corrugations. Thus, chitin synthesis and secretion of chitin-binding proteins are spatially separated processes, and probably, some extracellular self-assembly mechanisms are needed for a stereotypic association of chitin with its partners. Recent genetic data underline that along with structural proteins, secreted enzymes and membrane-inserted factors are required for chitin organisation. Four chitin organising proteins, Knickkopf (Knk), Retroactive (Rtv), Serpentine (Serp) and Vermiform (Verm) have been identified and to some extent characterised in the last few years in D. melanogaster and T. castaneum (Moussian et al. 2005a, b, 2006a, b; Luschnig et al. 2006; Tonning et al. 2006; Arakane et al. 2009; Chaudhari et al. 2011). Collectively, respective mutations in the genes coding for these factors provoke an unordered mass of chitin in the procuticle. Serp and Verm contain a chitin-binding domain and have significant similarity with chitin deacetylases from bacteria, suggesting that deacetylation of chitin to chitosan is a central trimming reaction modifying chitin. However, biochemical proof for this function of Serp and Verm is lacking. Assuming that they may truly deacetylate chitin, their enzymatic activity argues that deacetylated chitin is essential for chitin organisation. Indeed, partially deacetylated chitin has been proposed to raise accessibility of chitin to proteins (Neville 1975). Serp and Verm have another domain that may be crucial for their function in organising the procuticle. N-terminal to the chitin deacetylase signature is a low-density lipoprotein (LDL) domain that presumably enables these enzymes to bind to lipids, including cholesterol. Does this point to a connection between Wigglesworth’s cuticulin and chitin organisation? In summary, it is obvious that the (pro)cuticle has a certain capacity for self-assembly, that is, it is not a simple structure deposited by the epithelial cell as a finished and ready to function product.
Knk and Rtv are membrane-associated factors of unknown biochemical function. Knk is inserted into the apical plasma membrane via a C-terminal Glycosylphosphatidylinositol (GPI) anchor, while Rtv has a C-terminal transmembrane domain (Moussian et al. 2005a, b, 2006a, b). Knk has three conserved domains: at the N-terminus, just after the signal peptide, there is a tandem of DM13 motifs, followed by a middle DOMON domain (Aravind 2001; Iyer et al. 2007). These domains have been proposed to transport electrons to a yet unidentified substrate. Since chitin organisation is severely disrupted in knk mutant D. melanogaster larvae, it is possible that chitin may be the substrate of Knk. However, a requirement for chitin as an electron receiver has not been reported. Experimental evidence for these hypotheses is still missing. In T. castaneum, besides its implication in chitin organisation, Knk has a second function which is to protect chitin from degradation by chitinases (Chaudhari et al. 2011). This finding as illustrated in Fig. 8.5 indicates that organisation of chitin may be the result of balanced chitin production, degradation, that is, shortening and modification by deacetylases, while structural proteins eventually stabilise and conserve an optimal status. Rtv is a small protein (151 aa), which is conserved in arthropods (Moussian et al. 2005b; Havemann et al. 2008). It belongs to the Ly6-type protein family and is characterised by three loops exposing highly conserved aromatic amino acids that are hypothesised to bind to partners. Other Ly6-type proteins appear to be important for sorting events during secretion of proteins of the lateral plasma membrane (Hijazi et al. 2009; Nilton et al. 2010). Based on these findings, one may speculate that Rtv is needed for trafficking of chitin organising factors to the apical plasma membrane. Indeed, this has recently been demonstrated to be the case in T. castaneum (Chaudhari et al. 2013).
Knickkopf function. Knickkopf (Knk) has two functions during cuticle formation (a). Knk is a GPI-anchored protein that assists in chitin organisation (1). Ordered chitin is protected from degradation through chitinase-5 by free Knk (2). During moulting, Knk is removed from the cuticle allowing chitinases to degrade chitin (b)
The crystalline configuration of chitin suggests a non-random association of chitin with proteins at each level of organisation. In the past, an arsenal of peptide sequences of cuticle proteins was identified biochemically and through intensive efforts of localised genome sequencing (Chihara et al. 1982; Snyder et al. 1982; Silvert et al. 1984; Doctor et al. 1985; Fristrom et al. 1986; Wolfgang et al. 1986; Andersen et al. 1995). Today, using sequence information retrieved by classical biochemical work, sequenced insect genomes are being consulted to identify the whole complement of cuticle proteins. Among these, over 100 chitin-binding proteins are classified in two groups: cuticle proteins with Riddiford and Rebers motif (cuticle protein R&R, CPR) and Tweedle proteins (Tang et al. 2010; Willis 2010). CPRs constitute the group with most members. In addition to an N-terminal signal peptide, which directs their deposition to the extracellular space via the canonical secretory pathway, they contain at least one R&R domain that has been shown to bind chitin in vitro (Rebers and Willis 2001; Togawa et al. 2004; Tang et al. 2010). D. melanogaster has 102, Anopheles gambiae 156 and Aedes aegypti 240 CPR coding genes that are organised in distinct clusters. In general, CPR proteins are small, and besides their R&R domain, their sequences are very diverse. This indicates that they play a structural rather than an enzymatic role in chitin organisation. One may also argue that structural diversity of CPR proteins ensures non-perfect, but flexible chitin organisation. The large number of CPRs may also suggest tissue- and stage-dependent expression of different clusters. The Tweedle group of cuticle proteins is less diverse and comprises 27 members in D. melanogaster, and only 12 and 9 in An. gambiae and Ae. aegypti, respectively. They share a domain of unknown function (DUF243) preceded by a signal peptide underscoring that they are extracellular proteins. In contrast to CPR proteins, and although they may bind to chitin in vitro (Tang et al. 2010), Tweedle proteins lack a known chitin-binding domain. Some insight into their function comes from D. melanogaster genetic approaches. Dominant mutations in some Tweedle genes in D. melanogaster cause a tubby phenotype, indicating that these proteins are involved in cuticle structuring that has an impact on body shape (Guan et al. 2006). Tweedle proteins do not participate in basic cuticle organisation as they are insect specific and seem to be absent in crustaceans such as Daphnia pulex. Tweedle genes are expressed in a tissue and stage-specific manner, suggesting that they are key components in different cuticle types. In a recent bioinformatics approach, Cornman defined two motifs, GYR and YLP with exposed tyrosine residues that are present in members of CPRs and Tweedle proteins (Cornman 2010). These motifs are also present in other cuticle proteins such as CPLCGs and CPF/CPFLs. Corman proposes that these motifs are involved in protein–protein interaction. Extensive interaction between cuticle proteins, via their GYR and YLP motifs, and association with chitin is indeed an attractive model for a chitin-cuticular network where all components are linked together. In Sect. 8.4, we will encounter types of cross-linking of cuticular components that classically has been viewed as a stabilising element of cuticle structure.
8.2.4 Cuticle Irregularities
The naked cuticle is an extracellular matrix with uniform thickness. Cuticular protrusions like scutes, denticles and bristles disrupt cuticle uniformity, affecting the texture or thickness of distinct cuticle layers. Scutes in centipedes, for instance, are ridges in the epicuticle coinciding with the outline of hypodermal cells, suggesting that cells dictate epicuticle irregularity (Fusco et al. 2000). In D. melanogaster, chitin in larval ventral denticles and adult sensory bristles is not textured as in the naked cuticle, but is unorganised (Uv and Moussian 2010; Nagaraj and Adler 2012). Most cuticle irregularities probably arise from different cell identities that were determined during early pattern formation and from cell-immanent planar polarity. Cell- and tissue-position-specific programs may instruct efficiency and duration of chitin synthesis and deposition of specific proteins and lipids in cuticle protrusions. During bristle formation in the thorax of D. melanogaster, for instance, correct localisation of the bristle-specific membrane-bound zona pellucida (ZP) protein Dusky-like (Dyl) depends on the small GTPase Rab11 that functions in all epidermal cells (Nagaraj and Adler 2012). In this work, it was also proposed that Dyl is the effector of Rab11 in chitin synthesis and cuticle deposition. Since Dyl is not expressed in non-bristle cells, these findings imply that another Rab11 effector acts in these cells to mediate Rab11’s role in cuticle formation.
8.3 Cuticle Producing Epithelial Cells
What are the cellular programs that bring about the 3D architecture fundamental for cuticle function as a protective and supporting extracellular tunic? Evidently, molecular pathways of cuticle differentiation are deployed in the cytoplasm and organelles of polarised epithelial cells, producing cuticle components or precursors that travel through the active apical plasma membrane to the extracellular space where they are eventually modified and assembled.
8.3.1 Properties of Cuticle Producing Epithelial Cells
The cuticle is an extracellular matrix produced by most ectodermal tissues, comprising the epidermis, the fore- and hindgut epithelia, and respiratory organs (i.e. tracheae and book lungs) at their apical side. At stage transitions, to accommodate growth, these cells either divide or expand their apical areas in order to enlarge the surface of the respective tissue. Concomitantly, they shed their cuticle (apolysis) and produce a new one underneath that replaces the old one (ecdysis). The regulation of these processes is reviewed in Chap. 6. Besides the persistent ectodermal tissues, the extraembryonic serosal cells also produce a cuticle, called the serosal cuticle during embryogenesis that transiently prevents embryo desiccation and allows survival during periods of drought (Rezende et al. 2008).
Cuticle producing epithelial cells display the standard polar organisation in apical, lateral and basal domains (Fig. 8.1). At their basal side, they are covered by the basal lamina, an extracellular matrix consisting of a network of collagens and laminins, which are contributed by haemocytes. Studies using the fruit fly D. melanogaster have revealed that the integrity of the basal lamina is a prerequisite for an intact cuticle. Interference with basal lamina assembly through mutations in sparc, for instance, coding for a collagen-binding protein that is provided by haemocytes and stabilises collagen IV in the basal lamina, causes fragmentation of the cuticle (Martinek et al. 2008). A functional basal lamina may indirectly support cuticle production by ensuring cell vitality. A functional basal lamina could also directly influence cuticle production by mediating interaction between the basal plasma membrane of cuticle producing cells and free-floating haemocytes which deliver cuticle proteins and enzymes (Sass et al. 1993, 1994).
The lateral membrane of cuticle producing epithelial cells is decorated by three kinds of junctions. The apicalmost adherens junctions contact neighbouring cells and interact with the actin cytoskeleton, stabilising the tissue. The main players of these structures are the membrane-inserted E-cadherin and the cytoplasmic β-catenin. The extracellular domains of clustered E-cadherin molecules in neighbouring cells bind to each other, while their intracellular parts of the protein, via associated factors, serve as anchors to span a panel of stabilising actin cytoskeleton. Mutations in the D. melanogaster genes coding for E-cadherin shotgun (shg) and β-catenin armadillo (arm) do not affect cuticle differentiation. Ruptures in the cuticle are not due to defective cuticle formation but to the failure to renew cell contact after delamination of neuroblasts from the epidermal primordium (Tepass et al. 1996). However, a possible requirement may be masked by maternally provided proteins. Hence, genetic analyses of the function of adherens junctions during cuticle differentiation is difficult and have not been conducted.
Basal to these junctions, there are the septate junctions that seal the paracellular space, thus preventing free diffusion of water and solutes between the two separated milieus. The assembly of septate junctions seems to be modular. At least two complexes—the Gliotactin–Discs large (Dlg) complex and the core complex including Coracle, Neurexin IV and Nervana 2—come together for full establishment of septate junctions (Schulte et al. 2006; Laprise et al. 2009; Oshima and Fehon 2011). At the septate junction domains, the plasma membrane meanders, enlarging the contact zone and enforcing the belt like character of the epithelium. It is, however, not known whether the septate junction proteins themselves are responsible for membrane curvature. Work in D. melanogaster demonstrates that apical secretion may be compromised in cells with disrupted septate junctions. For instance, Knk, an apical membrane-inserted protein fails to be delivered quantitatively to the apical plasma membrane of tracheal cells in embryos mutant for Fas2 and sinuous that code for septate junction components. Secretion control at the septate junctions and their function as paracellular diffusion barriers and cell shape determinants seems to be independent from each other (Laprise et al. 2010).
The septate junctions are occasionally interrupted by gap junctions that are required for cell–cell communication. In invertebrates, innexins are the major constituents of gap junctions (Bauer et al. 2005). The role of innexins and gap junctions in epidermal differentiation during cuticle production has not been investigated.
Epithelial cell polarity is, as expected, a prerequisite for correct deposition of the cuticle at the apical side of the cell. Cells that fail to polarise do not produce cuticle at random sides, but mostly undergo apoptosis. This is best exemplified by the phenotype of crumbs (crb) mutant larvae in D. melanogaster, which is characterised by patches of cuticle produced by surviving epidermal cells (Tepass et al. 1990). Genetic analyses have revealed that cell polarity is not a stable state but needs maintenance during cuticle differentiation. Abrogation of ER-born vesicle formation through mutations in sec23 and sec24 encoding respective COPII components, for instance, results in cuboidal cells that gradually lose their polarity (Norum et al. 2010). Thus, the canonical secretory pathway is necessary for sustaining polarity.
8.3.2 The Plasma Membrane of Cuticle Producing Cells
During differentiation, cuticle material is deposited into the extracellular space across the apical plasma membrane. However, the apical plasma membrane does not serve simply as an interface, but seems to actively contribute to cuticle assembly. In the D. melanogaster embryo, the envelope, which is the first layer to be formed, is produced as fragments at the tips of irregular protrusions of the apical plasma membrane (Moussian et al. 2006a). Deposition of the envelope is effectuated by the canonical secretory pathway (Norum et al. 2010). Envelope fragments eventually fuse together to give rise to a continuous layer consisting of parallel electron-dense and electron-lucid sheets. The parallel course of the envelope sheets suggests an invariant structural coupling of the components.
Classically, later during pro- and epicuticle production, regular microvilli-like protrusions of the plasma membrane are formed during deposition of the pro- and epicuticle (Fig. 8.6). These structures are somewhat different from midgut cell microvilli. A midgut cell microvillus in arthropods is a cylindrical membraneous structure which is stabilised by a core of actin and associated proteins that are homologous to actin-binding proteins in vertebrates. Indeed, the major microvillar actin-binding and actin-organising proteins such as Espin, Fascin, Villin, Myosin 1A and calmodulin are present in D. melanogaster (Bartles 2000; Tilney et al. 2004; Hegan et al. 2007). Epidermal microvilli are stunted and carry electron-dense plaques at their tips that harbour the chitin synthesis and probably organisation factors. Microvilli formation has been studied especially at the site of bristle formation. The D. melanogaster Espin Forked, for instance, determines the number of microvilli during bristle formation in the pupa by regulating the thickness of actin bundles (Tilney et al. 1998, 2000, 2004). In forked mutant animals, actin bundle number at the plasma membrane is increased and more microvilli are formed, although actin bundle diameter is reduced. Interestingly, mutations in forked and the other microvillus factors are not lethal indicating either functional redundancy between them or involvement of yet unidentified factors.
The plasma membrane of cuticle producing epithelial cells. Classical microvilli (mv) are cylindrical extensions of the apical plasma membrane that have an inner skeleton consisting of actin bundles (a). Organisation of actin involves several actin-binding proteins. At the tip of microvilli-like structures that are protruded during cuticle deposition in various arthropods, an electron-dense plaque harbours the chitin synthesis apparatus. Apical undulae have been demonstrated to form during cuticle deposition in the D. melanogaster embryo (b). These longitudinal protrusions are traced by microtubule filaments at their basis. Actin has not been observed within the undulae, and their supporting inner cytoskeleton is unknown, but does not seem to contain high amounts of Fodrin also known as Spectrin, which accumulates rather at the lateral membrane (Thomas and Kiehart 1994; Das et al. 2008). The taenidia (tae) of insect tracheae follow the spiral extrusion of the apical plasma membrane of tracheal cells (c). The plasma membrane (mem) is stabilised by actin cables that run in parallel to the taenidia. They prevent collapse of the tracheal tube lumen (lum). Scale bar 500 nm
At the cytoplasmic side the microvillar plaques are nourished by chitin monomers GlcNAc that are synthesised from glucose through the Leloir pathway comprising six cytoplasmic enzymes (Moussian 2008). One may speculate that extensive chitin synthesis may necessitate concentration of GlcNAc production at the membrane protrusions. An isoform of the last enzyme of the Leloir pathway in D. melanogaster, UDP-N-acetylglucosamine pyrophosphorylase, has a 37 amino acid N-terminal domain that could mediate its localisation to the apical plasma membrane (Tonning et al. 2006). Mutations in the gene coding for this enzyme are lethal and cause a chitin-less and collapsed cuticle.
Secretion of cuticle proteins occurs at the depression between microvilli. The physical separation of chitin synthesis and protein secretion implies firstly that cuticle assembly takes place in the extracellular space. Secondly, coordination of chitin synthesis and protein secretion is not simply effectuated by direct physical contact between the effectors, but that communication is required at some other as yet unknown hub.
At sites of denticles of the D. melanogaster embryo, the apical plasma membrane forms large protrusions at the posterior half of the cell (Fig. 8.6). The formation of these protrusions obeys cues from planar polarity defined by the asymmetric distribution of Strabismus, Dishevelled, Diego, Prickle and Frizzled at cell junctions (Goodrich and Strutt 2011). The denticle forming protrusion is stabilised by an actin core that involves several factors important for microvilli formation, as well. The apical plasma membrane of denticle forming protrusions is decorated by several membrane-inserted zona pellucida (ZP) proteins that specify distinct domains of the future denticle (Chanut-Delalande et al. 2006; Fernandes et al. 2010). Zye (Zye) and Trynity (Tyn) mark the basis of a denticle, and Miniature (M) separates the apical part of the denticle occupied by Dusky-like (Dyl).
At the end of larval cuticle production during embryogenesis, the apical plasma membrane smoothens. This process is a critical step in differentiation. Mutations in wollknäuel (wol) that codes for the D. melanogaster ALG5 (UDP-glucose:dolichyl-phosphate glucosyltransferase) cause uncontrolled protrusions of the apical plasma membrane at the end of cuticle differentiation (Shaik et al. 2011). This phenotype is associated with persistent apical localisation of Crb, that is, a determinant of the apical plasma membrane identity (Assemat et al. 2008). Thus, removal of Crb from its apical position may be a prerequisite for plasma membrane smoothening.
8.3.3 Secretion of Cuticle Material
The cuticle is an extracellular matrix and is naturally the product of secretion and deposition of its components. Some components are secreted directly via the canonical secretory pathway from the ER tubules, via the Golgi apparatus and secretory vesicles to the plasma membrane, where they are released to the extracellular space. Most if not all proteins such as Verm, Serp and cuticle proteins follow this route. Some other components are produced at or transferred across the apical plasma membrane through membrane-localised enzymes, such as the chitin synthase, that links GlcNAc monomers together and extrudes the polymer chitin probably through a pore formed by the enzyme itself. The apical plasma membrane also harbours those factors required for chitin organisation such as Knk and Rtv (Moussian et al. 2005a, b, 2006b). Like bona fide secreted proteins, the membrane-inserted factors are also positioned within the membrane by the secretory pathway. Hence, many different components and enzymes travel through the secretory pathway at the same time with the same destination, the apical plasma membrane. The topology of the apical plasma membrane argues that at some point, these factors have to be sorted. Where does this take place? In D. melanogaster, we are beginning to understand this process. The Maclura pomifera agglutinin (MPA) recognises some epitopes at the envelope and some within the procuticle of the D. melanogaster larva (Moussian et al. 2007). In larvae mutant for the plasma membrane t-SNARE Syntaxin 1A (Syx1A), some secretory vesicles that erroneously accumulate beneath the apical plasma membrane are MPA positive, but others are not. This indicates that some factors are separated at the exit of the Golgi apparatus.
One enzyme that may define a class of secretory vesicles is the chitin synthase. In fungi, chitin synthases localise to specialised intracellular vesicles of 40–70 nm diameter, the chitosomes that deliver the chitin synthesis complex to the site where this is required during cell division (e.g. Bartnicki-Garcia 2006). Several specific factors are associated with chitosomes and are required for ordered positioning of the chitin synthesis complex. Fungal chitin synthases are, however, not active within chitosomes, suggesting that activation has to be triggered. It seems that localisation to the plasma membrane is mandatory for chitin synthase activity. In yeast, the CaaX protease Ste24 facilitates the localisation of Chs3, the major chitin synthase in yeast, to the plasma membrane through trimming of the Chs3 partner Chs4, which is absent in arthropods (Meissner et al. 2010).
Do Arthropods have chitosomes? In the moth Manduca sexta, it was found that a chitin synthase–specific antibody recognises an epitope at the apical surface but also within the midgut cell (Zimoch and Merzendorfer 2002). Obviously, as membrane-inserted enzymes, chitin synthases travel through the secretory pathway to reach the plasma membrane. Therefore, sorting at some level is imperative. The central question is whether plasma membrane plaques of chitin synthesis are preformed within vesicles, or whether they travel to the plasma membrane where they are assembled. In the D. melanogaster embryo, the plasma membrane t-SNARE Syx1A is dispensable for delivery of chitin synthases and chitin organising factors, such as Knk, to the plasma membrane (Moussian et al. 2007). This finding suggests that another t-SNARE may mediate localisation of plaques to the plasma membrane, in turn arguing (and confirming the MPA data) that different Golgi-born vesicles deliver distinct factors to decorate the apical plasma membrane and to produce the cuticle. In other words, the Golgi apparatus is the main compartment where sorting of cuticle structural and production components takes place.
8.4 Cross-Linking of Cuticle Components
8.4.1 A Dityrosine Transcellular Barrier
Soft body cuticle of caterpillars and larvae has to withstand the internal hydrostatic pressure in order to serve as an exoskeleton. Indeed, in D. melanogaster, mutations in the chitin synthase gene or in knk and rtv that are needed for chitin organisation result in loss of body shape and inability to move, suggesting that chitin is an essential element of the soft exoskeleton (Moussian et al. 2005a, b, 2006a, b). In view of the elaborate interaction between chitin and chitin-binding proteins, one may assume that chitin on its own is not the barrier component opposing water pressure at the cuticle. Indeed, it seems that a network of proteins at the basal side of the procuticle covalently bind to each other via tyrosine residues, probably constructing a network adjacent to the plasma membrane that confers elasticity and stiffness to resist the internal hydrostatic pressure (Shaik et al. 2012). The establishment of this dityrosine network depends on a haem-dependent enzyme, which is yet unknown. Likewise, the sequence of the linked proteins is unknown, as well. Candidate cuticle proteins may be those low complex proteins with GYR- and YLP-like motifs that are characterised by invariant tyrosine (Y) residues (Cornman 2010). The membrane-inserted dual oxidase Duox could be considered as a good candidate for being the haem-dependent enzyme involved in dityrosine formation. It has an intracellular flavoprotein domain that accepts electrons from NADPH, which are transferred across the membrane to the extracellular peroxidase domain that catalyses H2O2 production (Donko et al. 2005). Tyrosines are oxidised and spontaneously react with each other to link neighbouring proteins. It is difficult to assume that this last reaction of dityrosine formation is ordered and specific. Rather, within the range of H2O2 production, tyrosines from all proteins present are potential targets. Indeed, Duox stimulates the production of a dityrosine-based barrier that protects the midgut epithelium in mosquitoes against pathogen entry (Kumar et al. 2010). Consistently, reduction of Duox activity by RNAi in the D. melanogaster wing results in pale wings, suggesting that melanisation and probably sclerotisation are impaired in this tissue (Anh et al. 2011). Arguing against an involvement of Duox in barrier formation, however, reduced Duox activity does not give rise to a respective haem-deficient phenotype. In fungi, a cytochrome 56 protein has been shown to drive extracellular dityrosine formation required to render the cell wall impermeable against loss of proteins. Hence, one cannot exclude that functional redundant enzymes may catalyse tyrosine oxidation in arthropods.
8.4.2 Resilin
In 1960, Weis-Fogh published his discovery that the long-range elastic behaviour of parts of the thoracic cuticle in the locust and dragonfly depends on the presence of a glycine-rich rubber-like protein he named resilin (Weis-Fogh 1960). Other cuticles with extreme extensibility like the cuticle covering the alloscutum of ticks, such as Ixodes ricinus, also contain large amounts of resilin. Resilin visualisation is comparably simple as resilin fluoresces upon excitation with UV light by conventional fluorescence or confocal laser scanning microscopy (Michels and Gorb 2012). This characteristic of resilin is due to the presence of di- and trityrosines (Andersen 1964, 1966; Malencik and Anderson 2003). In the following decades, the in vivo physical properties of resilin were investigated in detail mainly by Weis-Fogh himself, Andersen, Edwards, Bennet-Clark, Neville and others (Bennet-Clark 2007). Finally, in the genomic era of insect biology, the full sequence of resilin was uncovered in different insect species (Andersen 2010b; Lyons et al. 2011). Presence of resilin has been reported in crustaceans, as well (Burrows 2009). It was Andersen who first identified the sequence of the resilin monomer, proresilin, encoded by the gene CG15920 in the D. melanogaster genome, by using the amino acid sequence of three tryptic peptides he had obtained from the elastic cuticle of the wing hinges and prealar arms from the desert locust Schistocerca gregaria (Ardell and Andersen 2001). D. melanogaster proresilin has an N-terminal signal peptide that directs it to the apical extracellular space (Fig. 8.7). In addition, the protein is composed of 18 repeats of a 15 residue motif (type A repeat) and 11 repeats of a 13 residue motif (type B repeat), which flank a type 2 Riddiford and Rebers chitin-binding domain (R&R-2) of 62 amino acids. Prolines and glycines occupy distinct positions within both repeats, probably forming β-turns separated by irregular loops, overall yielding a β-spiral conformation, which accounts for protein elasticity. Proresilin’s type A repeats from D. melanogaster and An. gambiae in synthetic polypeptides have elastic and resilient properties comparable to those of recombinant full-length proresilin, underscoring the significance of sequences with low complexity for protein elasticity and resilience (Lyons et al. 2009). Proresilin sequence information allows us to model resilin function within the cuticle: proresilin monomers are polymerised to resilin via di- and trityrosine bridges that were discovered some decades ago, tyrosine residues being present, especially in the N-terminal type A repeats, and associate with chitin via their chitin-binding domain of the R&R-2 type, which in D. melanogaster is indeed able to bind to chitin in vitro (Qin et al. 2009). D. melanogaster possesses one gene coding for two alternatively spliced proresilin isoforms (620 and 575 amino acids). The implication of two isoforms is unclear, as it is not known whether they are expressed in different tissues or stages. The proresilin isoform from the shorter mRNA lacks the R&R-2 domain, suggesting that it may be present in cuticle types with low, or no chitin (Andersen 2010b). Association and non-association with chitin may confer specific elastic properties to the respective cuticle. Low complexity of the proresilin sequence suggests that other low complex cuticle proteins may have similar physical properties. Indeed, another protein encoded by the CG9036 locus in the D. melanogaster genome may constitute a proresilin paralog (Ardell and Andersen 2001).
Resilin. Proresilin has an N-terminal signal peptide (SP) that allows the protein to be secreted to the extracellular space through the canonical secretory pathway. It harbours two types of repeats, the type A and the type B repeat with the consensus sequences GGRPSDSYGAPGGGN and GYSGGRPGGQDLG, respectively, that flank an R&R chitin-binding motif. Tyrosine residues in the repeat sequences may covalently link to tyrosines of neighbouring proteins. Resilin is a polymer of proresilins that are linked to each other via dityrosine bridges. Upon illumination with ultraviolet light (maximum 315 nm), dityrosines emit blue light (409 nm maximum)
Resilin as a biomaterial has been extensively investigated since the identification of its sequence. Similarly, resilin’s function in whole organisms and tissues is well understood. By contrast, resilin cell biology including regulation of its cell-specific expression and extracellular polymerisation is less well analysed. To advance in these problems, a genetic approach in D. melanogaster would be helpful. During D. melanogaster embryogenesis, proresilin is expressed in segmental clusters of epidermal cells and in stretch receptors (Wong et al. 2012). Later, as expected, proresilin expression is detectable in cells at the base of the wing. Mutations in D. melanogaster proresilin should reveal the importance of resilin function in these cells.
8.4.3 Transglutaminase
Another type of protein covalent cross-links in the cuticle, the ε-(γ-glutamyl) lysine bonds, is catalysed by extracellular transglutaminases. Generally, expression and activity of transglutaminases are known to be induced upon injury in both vertebrates and invertebrates. In D. melanogaster, transglutaminase is also robustly expressed during late developmental stages, that is, in L3 larvae, in pupae and the adult flies (Shibata et al. 2010). Abrogation of transglutaminase translation by RNAi causes deformation and tanning failure of the adult abdominal cuticle and wrinkling of the wing. In contrast to the dityrosine network, the transglutaminase cross-links do not constitute a water barrier. Several cuticle proteins have been identified as targets of transglutaminase activity. Fondue, for example, an unknown extracellular protein is deposited into the cuticle during clot formation after wounding (Lindgren et al. 2008). Moreover, extractability of the cuticle proteins Cpr47Ef, Cpr64Ac, Cpr76Bd and Cpr97Eb depends on transglutaminase activity, suggesting that these proteins are normally fixed within the cuticle by ε-(γ-glutamyl) lysine bonds (Shibata et al. 2010). Interestingly, the wing phenotype of transglutaminase depleted animals can at least partially be attributed to the function of Cpr97Eb, as knock-down of this factor causes a similar phenotype. Taken together, transglutaminase-based cross-linking of cuticle components seems to be needed for cuticle pigmentation and stiffness.
8.4.4 Sclerotisation and Melanisation
Since Pryor’s work in the 1940s, hardening (sclerotisation) of the cuticle is known to depend on cross-linking of proteins with phenolic substances (Pryor 1940; Pryor et al. 1946, 1947). Recent advances in the field are excellently summarised by Andersen (2010a, 2012). The basic feature of the sclerotised cuticle is the covalent linkage of cuticle components by the acyldopamines N-acetyldopamine (NADA) and N-β-alanyldopamine (NBAD). Production of phenolic substances starts in the cytoplasm where tyrosine is converted to Dopa by tyrosine dehydroxylase (Pale in D. melanogaster), which is subsequently used to produce dopamine by Dopa-decarboxylase (Ddc). Dopamine is the substrate of the dopamine N-acetyl-transferase and NBAD synthase (Ebony in D. melanogaster) that catalyse the formation of NADA and NBAD, respectively. The catecholamines NADA and NBAD are transported to the extracellular space through as yet unknown transporters. In the differentiating extracellular matrix, they are oxidised to their ortho-quinones that may react with free amino groups of proteins and possibly also deacetylated chitin. The incorporation of these ortho-quinones results in brown cuticle, whereas usage of the oxidation intermediate dehydro-NADA as preferred by insects only lightly colours the cuticle. The extracellular sclerotisation reactions are catalysed by extracellular laccases and tyrosinases (Suderman et al. 2006). Multicopper-containing Laccase 2 in the beetles T. castaneum and Monochamus alternatus has been reported to affect cuticle integrity, and this can be attributed to sclerotisation defects underlining the importance of these modifications (Arakane et al. 2005a; Niu et al. 2008). Only few cuticle proteins have been experimentally shown to be cross-linked via catecholamines. Pioneering work has been performed in Manduca sexta: lysyl groups of the cuticle protein MsCP36 contribute to protein oligomerisation (Suderman et al. 2010). Interestingly, dityrosine bounds were also found to be involved in MsCP36 cross-linking.
Animals suffering Laccase 2 reduction are also pale compared to siblings with normal laccase 2 activity. This nicely underlines that sclerotisation (hardening) and melanisation (pigmentation, tanning) share extracellular enzymes. However, there are also melanisation-specific enzymes (Sugumaran 2009). The extracellular Yellow protein represents a prominent class of melanisation enzymes. Mutations in the D. melanogaster yellow gene are not lethal, but provoke a yellow body colour. The two members of Yellow protein family Yellow-f1 and Yellow-f2 have been shown to be dopachrome-conversion enzymes (Han et al. 2002). Dopachrome, the intramolecular cyclisation product of dopa, is converted to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) that is subsequently used for polymerisation of melanin. An interesting mechanism of melanisation control has been analysed in the D. melanogaster wing (Riedel et al. 2011). Melanin is usually produced at the distal region of the wing procuticle. Confinement of melanisation reactions to this region necessitates timed removal of Yellow from the cuticle by endocytosis regulated by Rab5 and Megalin. Failure to clear the procuticle from Yellow results in extension of melanisation to proximal regions of the procuticle.
Taken together, defects in the melanisation pathway, if they do not impair sclerotisation, are not lethal but cause body colour changes. Indeed, the differential activity of melanising enzymes can be used by nature to create colour patterns. The antagonistic functioning of Yellow and Ebony, for example, generates the striped pattern in the abdomen of adult fruit flies (Wittkopp et al. 2002). Not only in D. melanogaster but also in other insects, Yellow and Ebony are involved in pigmentation patterns (Futahashi et al. 2008b; Arakane et al. 2010).
8.4.5 Calcite Deposition in Crustacean Cuticle
The cuticle of crustaceans is stiffened through internal deposition of calcite. The molecular mechanisms of calcification are being intensively studied in terrestrial isopods by Ziegler and his group. Storage of calcite in the extracellular space and its resorption are highly regulated during moulting in Porcellio saber (Ziegler et al. 2004). A central enzyme of this process is the smooth endoplasmic reticulum Ca2+-ATPase (SERCA), the expression of which is up-regulated at late pre-moult and intermoult stages (Ziegler et al. 2002; Hagedorn et al. 2003). Before the synthesis of the new cuticle, Ca2+ and HCO3 − ions are reabsorbed from the posterior half of the cuticle, that moults first, and are transported through the haemolymph and across the epithelium to the apical extracellular space of anterior sternites, where storage calcite is formed. Concomitantly, protons produced during CaCO3 formation are pumped into the haemolymph through the V-type H+-ATPase (VHA) that localises to the basolateral plasma membrane of these cells at this stage. Abundance of the enzyme is regulated at the transcriptional level. To mobilise Ca2+ and HCO3 − for cuticle remineralisation, the extracellular space is acidified by the VHA that now localises to the apical plasma membrane. Hence, induction of vha and serca transcription and sorting of VHA are the major mechanisms for calcite storage and recycling and cuticle calcification in these animals.
8.5 Tracheal Cuticle
The epidermal cuticle is an even structure and produces protrusions only at distinct sites where trichomes are formed. By contrast, the tracheal cuticle in insects and myriapods, that consists of an envelope, a thin epicuticle and a conspicuous procuticle, is uneven, following regular protrusions of the apical plasma membrane of tracheal epithelial cells (Fig. 8.6) (Lewis 1981; Uv and Moussian 2010). These protrusions, the taenidia, are supported by actin cables that, as spirals, run perpendicularly to the length of the tracheal tube. The formin dDAAM, that organises the polymerisation of actin bundles, has been shown to be required for this pattern in Drosophila (Matusek et al. 2006). In ddaam mutant embryos, the taenidia are disordered but present, suggesting that dDAAM is not directing taenidia formation per se but their orientation. Likewise, mutations in polished rice (pri), a polycistronic gene coding for several short peptides, dramatically impair actin organisation in epidermal and tracheal cells (Kondo et al. 2007). In consequence, epidermal denticles and taenidia are not formed. Thus, the Pri peptides can be considered as master regulators of actin-based membrane protrusions in cuticle producing epithelial cells.
These genes required for epidermal cuticle formation are evidently also acting during tracheal cuticle formation. What then makes the difference between these two types of cuticles? Possibly, chitin organisation dictates the shape of the cuticle. In the epidermal procuticle, chitin preferably adopts the Bouligand pattern, whereas in the taenidial procuticle chitin is rather amorphous. This difference may be due to cuticle-specific chitin-binding proteins that pack chitin in distinct cuticle-typical ways. It is also imaginable that chitin fibre length has an influence on cuticle shape. Consistently, the length of chitin fibres varies in the epidermis and the trachea. In the epidermal cuticle, chitin fibres are rather long, whereas in the taenidia, they are comparably short and therefore indiscernible. Length differences in turn may reflect tissue-specific differences in chitin synthase processivity, which implies tissue-specific co-factors of chitin synthesis.
Tracheael chitin synthesis is subdivided into two phases. First, a luminal chitin rod is produced that is needed for tube diameter adjustment (Devine et al. 2005; Tonning et al. 2005; Moussian et al. 2006a, b). Abrogation of chitin synthesis or organisation results in irregular and cystic tracheal lumen. The cellular mechanisms, that is, the localisation of the chitin synthesis machinery during this process has not been investigated. Second, ordered activity of chitin synthase complexes at the crests of spiral corrugations of the apical plasma membrane produces the tracheal cuticle chitin.
Less is known about the thin cuticle lining book lungs in arachnids and the epithelial cells that produce these organs. Like insect and myriapod tracheae, book lungs are ectodermal and have a lumen interspersed with lamellae that are partially stabilised by cellular structures (pillars) and cuticular trabeculae (Kamenz et al. 2005; Scholtz and Kamenz 2006). A thorough molecular and histological description of book lung development in different arachnids would teach us about the importance of the cuticle for the stability and function of internal structures.
8.6 Control of Cuticle Differentiation
Cuticle differentiation during embryogenesis is the last process to occur before hatching. Construction of the layers presupposes controlled expression of the components. Expression analyses of 6003 genes in D. melanogaster (44 % of all genes) by in situ hybridisation and microarray time-course data revealed that epidermal differentiation at the end of embryogenesis, that is, cuticle formation does not require maternal input, but is largely accomplished by zygotic factors (Tomancak et al. 2007). Several transcription factors cooperate in this mission as shown in Fig. 8.8. The evolutionary conserved CP2-type transcription factor Grainyhead (Grh), for instance, transcribes a subset of cuticle genes including knk in D. melanogaster (Gangishetti et al. 2012). The expression of another subset of cuticle genes including serp and verm is regulated by ecdysone-induced transcription factors such as βFtzF1 (Gangishetti et al. 2012). These two transcription factors cooperate in regulating the expression of a third subset of genes like dsc73. The relatively mild mutant phenotype of βFtzF1 mutant larvae compared to the rather strong mutant phenotype of ecdysone-deficient larvae indicates that other ecdysone-induced transcription regulators act together with βFtzF1 to drive cuticle differentiation. Indeed, βFtzF1 and DHR3 have overlapping functions during late D. melanogaster embryonic development (Ruaud et al. 2010). Expression of serp and verm also depends on Ribbon (Rib), a BTB/POZ domain nuclear factor with a broad range of functions during embryogenesis (Luschnig et al. 2006). Interestingly, Rib also controls the expression of crb, which codes the membrane-inserted determinant of apical plasma membrane, thereby indirectly influencing cuticle formation (Kerman et al. 2008). Tramtrack (Ttk), a zinc-finger transcription factor, is essential for correct cuticle architecture, as demonstrated in a microarray experiment by its regulatory activity on several cuticle genes, including cpr78Cb, which codes for an R&R protein (Araujo et al. 2007; Rotstein et al. 2012). It is clear from this short summary that a master regulator of cuticle differentiation does not seem to exist.
Transcriptional regulation of cuticle differentiation during D. melanogaster embryogenesis. Five transcription factors—Ribbon, the Ecdysone receptor EcR, βFtzF1, Grainyhead and Tramtrack—have been reported to date to drive expression of cuticle genes in the embryo of D. melanogaster. EcR, Ribbon and Tramtrack are regulators of major morphogenetic and differentiation events, whereas Grainyhead is rather specific to epidermal differentiation. The relatively mild mutant phenotype of βFtzF1 mutant larvae suggests that it may act redundantly to other ecdysone-induced transcription factors (see text)
Post-embryonic cuticle formation during moulting is more complicated. Generally, ecdysone is a central regulator of cuticle renewal (Charles 2010). In fruit flies, the ecdysone inducible transcription factor DHR38 is essential for adult cuticle formation, by regulating the transcription of cuticle genes, such as Acp65A, which is also regulated by the Broad complex transcription factor isoform BR–C Z1, and Ddc, which is essential for cuticle sclerotisation and melanisation (Cui et al. 2009; Kozlova et al. 2009). Interestingly, DHR38 also regulates the formation of glycogen (Ruaud et al. 2011), which may be the source of the chitin monomer GlcNAc. It remains to be shown whether chitin synthesis relies on DHR38 activity. In imaginal discs of B. mori, βFtzF1 and the ecdysone-induced Broad complex transcription factor isoform BR–C Z4 together regulate the expression of BMWCP5, which codes for a cuticle protein (Wang et al. 2009). Another Broad complex transcription factor isoform, BR–C Z2, has been proposed to regulate the expression of another cuticle gene, BmorCPG11 in B. mori that is probably not under the control of βFtzF1 (Ali et al. 2012). In summary, ecdysone-signalling induces the expression of cuticle genes in a locus-specific manner. Moreover, impact of ecdysone on cuticle differentiation is stage specific. For example, mutations in dhr38 do not affect larval cuticle formation, but impair adult cuticle integrity. Moreover, mutations in the Broad complex genes in D. melanogaster are not larval lethal, and therefore, the respective transcription factors are not essential for cuticle formation in the larvae. Overall, regulation of cuticle differentiation is obviously a stage-specific phenomenon.
References
Ali MS, Wang HB, Iwanaga M, Kawasaki H (2012) Expression of cuticular protein genes, BmorCPG11 and BMWCP5 is differently regulated at the pre-pupal stage in wing discs of Bombyx mori. Comp Biochem Physiol B 162:44–50
Andersen SO (1964) The cross-links in resilin identified as dityrosine and trityrosine. Biochim Biophys Acta 93:213–215
Andersen SO (1966) Covalent cross-links in a structural protein, resilin. Acta Physiol Scand Suppl 263:1–81
Andersen SO (2010a) Insect cuticular sclerotization: a review. Insect Biochem Mol Biol 40:166–178
Andersen SO (2010b) Studies on resilin-like gene products in insects. Insect Biochem Mol Biol 40:541–551
Andersen SO (2011) Are structural proteins in insect cuticles dominated by intrinsically disordered regions? Insect Biochem Mol Biol 41:620–627
Andersen SO (2012) Cuticular sclerotization and tanning. In: Gilbert LI (ed) Insect molecular biology and biochemistry. Elsevier, Amsterdam, pp 167–192
Andersen SO, Hojrup P, Roepstorff P (1995) Insect cuticular proteins. Insect Biochem Mol Biol 25:153–176
Anh NT, Nishitani M, Harada S, Yamaguchi M, Kamei K (2011) Essential role of Duox in stabilization of Drosophila wing. J Biol Chem 286:33244–33251
Arakane Y, Hogenkamp DG, Zhu YC, Kramer KJ, Specht CA, Beeman RW, Kanost MR, Muthukrishnan S (2004) Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem Mol Biol 34:291–304
Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ (2005a) Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA 102:11337–11342
Arakane Y, Muthukrishnan S, Kramer KJ, Specht CA, Tomoyasu Y, Lorenzen MD, Kanost M, Beeman RW (2005b) The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol Biol 14:453–463
Arakane Y, Specht CA, Kramer KJ, Muthukrishnan S, Beeman RW (2008) Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol 38:959–962
Arakane Y, Dixit R, Begum K, Park Y, Specht CA, Merzendorfer H, Kramer KJ, Muthukrishnan S, Beeman RW (2009) Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem Mol Biol 39:355–365
Arakane Y, Dittmer NT, Tomoyasu Y, Kramer KJ, Muthukrishnan S, Beeman RW, Kanost MR (2010) Identification, mRNA expression and functional analysis of several yellow family genes in Tribolium castaneum. Insect Biochem Mol Biol 40:259–266
Araujo SJ, Cela C, Llimargas M (2007) Tramtrack regulates different morphogenetic events during Drosophila tracheal development. Development 134:3665–3676
Aravind L (2001) DOMON: an ancient extracellular domain in dopamine beta-monooxygenase and other proteins. Trends Biochem Sci 26:524–526
Ardell DH, Andersen SO (2001) Tentative identification of a resilin gene in Drosophila melanogaster. Insect Biochem Mol Biol 31:965–970
Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D (2008) Polarity complex proteins. Biochim Biophys Acta 1778:614–630
Bartles JR (2000) Parallel actin bundles and their multiple actin-bundling proteins. Curr Opin Cell Biol 12:72–78
Bartnicki-Garcia S (2006) Chitosomes: Past, present and future. FEMS Yeast Res 6:957–965
Bauer R, Loer B, Ostrowski K, Martini J, Weimbs A, Lechner H, Hoch M (2005) Intercellular communication: the Drosophila innexin multiprotein family of gap junction proteins. Chem Biol 12:515–526
Bennet-Clark H (2007) The first description of resilin. J Exp Biol 210:3879–3881
Bouligand Y (1965) Sur une architecture torsadée répandue dans de nombreuses cuticules d’Arthropodes. C R Acad Sci (Paris) D 261:4864–4867
Burrows M (2009) A single muscle moves a crustacean limb joint rhythmically by acting against a spring containing resilin. BMC Biol 7:27
Butenandt A, Beckmann R, Hecker E (1961a) Über den Sexuallockstoff des Seidenspinners. I. Der biologische Test und die Isolierung des reinen Sexual-Lockstoffes Bombykol. Hoppe Seylers Z Physiol Chem 324:71–83
Butenandt A, Beckmann R, Stamm D (1961b) Über den SexuaIIockstoff des Seidenspinners. II. Konstitution und Konfiguration des Bombykols. Hoppe Seylers Z Physiol Chem 324:84–87
Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) Shavenbaby couples patterning to epidermal cell shape control. PLoS Biol 4(9):e290. doi:10.1371/journal.pbio.0040290
Charles JP (2010) The regulation of expression of insect cuticle protein genes. Insect Biochem Mol Biol 40:205–213
Chaudhari SS, Arakane Y, Specht CA, Moussian B, Boyle DL, Park Y, Kramer KJ, Beeman RW, Muthukrishnan S (2011) Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc Natl Acad Sci USA 108:17028–17033
Chaudhari SS, Arakane Y, Specht CA, Moussian B, Kramer KJ, Muthukrishnan S, Beeman RW (2013) Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of Tribolium castaneum. PLoS Genet 9(1):e1003268
Cheng L, Wang L, Karlsson AM (2009) Mechanics-based analysis of selected features of the exoskeletal microstructure of Popillia japonica. J Mater Res 24:3253–3267
Chihara CJ, Silvert DJ, Fristrom JW (1982) The cuticle proteins of Drosophila melanogaster: stage specificity. Dev Biol 89:379–388
Cornman RS (2010) The distribution of GYR- and YLP-like motifs in Drosophila suggests a general role in cuticle assembly and other protein–protein interactions. PLoS ONE 5(9):e12536. doi:10.1371/journal.pone.0012536
Cui HY, Lestradet M, Bruey-Sedano N, Charles JP, Riddiford LM (2009) Elucidation of the regulation of an adult cuticle gene Acp65A by the transcription factor broad. Insect Mol Biol 18:421–429
Das A, Base C, Manna D, Cho W, Dubreuil RR (2008) Unexpected complexity in the mechanisms that target assembly of the spectrin cytoskeleton. J Biol Chem 283:12643–12653
Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA (2005) Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc Natl Acad Sci USA 102:17014–17019
Doctor J, Fristrom D, Fristrom JW (1985) The pupal cuticle of Drosophila: biphasic synthesis of pupal cuticle proteins in vivo and in vitro in response to 20-hydroxyecdysone. J Cell Biol 101:189–200
Donko A, Peterfi Z, Sum A, Leto T, Geiszt M (2005) Dual oxidases. Phil Trans R Soc B 360:2301–2308
Everaerts C, Farine JP, Cobb M, Ferveur JF (2010) Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5(3):e9607. doi:10.1371/journal.pone.0009607
Fernandes I, Chanut-Delalande H, Ferrer P, Latapie Y, Waltzer L, Affolter M, Payre F, Plaza S (2010) Zona pellucida domain proteins remodel the apical compartment for localized cell shape changes. Dev Cell 18:64–76
Fristrom D, Doctor J, Fristrom JW (1986) Procuticle proteins and chitin-like material in the inner epicuticle of the Drosophila pupal cuticle. Tissue Cell 18:531–543
Fusco G, Brena C, Minelli A (2000) Cellular processes in the growth of lithobiomorph centipedes (Chilopoda: Lithobiomorpha). A cuticular view. Zool Anz 239:91–102
Futahashi R, Okamoto S, Kawasaki H, Zhong YS, Iwanaga M, Mita K, Fujiwara H (2008a) Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem Mol Biol 38:1138–1146
Futahashi R, Sato J, Meng Y, Okamoto S, Daimon T, Yamamoto K, Suetsugu Y, Narukawa J, Takahashi H, Banno Y, Katsuma S, Shimada T, Mita K, Fujiwara H (2008b) yellow and ebony are the responsible genes for the larval color mutants of the silkworm Bombyx mori. Genetics 180:1995–2005
Gangishetti U, Veerkamp J, Bezdan D, Schwarz H, Lohmann I, Moussian B (2012) Grainyhead and ecdysone cooperate during differentiation of the Drosophila skin. Insect Mol Biol 21:283–295
Gibbs A (1998) Water-proofing properties of cuticular lipids. Amer Zool 38:471–482
Gibbs AG (2011) Thermodynamics of cuticular transpiration. J Insect Physiol 57:1066–1069
Goodrich LV, Strutt D (2011) Principles of planar polarity in animal development. Development 138:1877–1892
Guan X, Middlebrooks BW, Alexander S, Wasserman SA (2006) Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc Natl Acad Sci USA 103:16794–16799
Gutierrez E, Wiggins D, Fielding B, Gould AP (2007) Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445:275–280
Hagedorn M, Weihrauch D, Towle DW, Ziegler A (2003) Molecular characterisation of the smooth endoplasmic reticulum Ca(2+)-ATPase of Porcellio scaber and its expression in sternal epithelia during the moult cycle. J Exp Biol 206:2167–2175
Han Q, Fang J, Ding H, Johnson JK, Christensen BM, Li J (2002) Identification of Drosophila melanogaster yellow-f and yellow-f2 proteins as dopachrome-conversion enzymes. Biochem J 368:333–340
Harding CR (2004) The stratum corneum: structure and function in health and disease. Dermatol Ther 17(Suppl 1):6–15
Havemann J, Muller U, Berger J, Schwarz H, Gerberding M, Moussian B (2008) Cuticle differentiation in the embryo of the amphipod crustacean Parhyale hawaiensis. Cell Tissue Res 332:359–370
Hegan PS, Mermall V, Tilney LG, Mooseker MS (2007) Roles for Drosophila melanogaster myosin IB in maintenance of enterocyte brush-border structure and resistance to the bacterial pathogen Pseudomonas entomophila. Mol Biol Cell 18:4625–4636
Hijazi A, Masson W, Auge B, Waltzer L, Haenlin M, Roch F (2009) Boudin is required for septate junction organisation in Drosophila and codes for a diffusible protein of the Ly6 superfamily. Development 136:2199–2209
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Iyer LM, Anantharaman V, Aravind L (2007) The DOMON domains are involved in heme and sugar recognition. Bioinformatics 23:2660–2664
Jensen JM, Proksch E (2009) The skin’s barrier. G Ital Dermatol Venereol 144:689–700
Kamenz C, Dunlop JA, Scholtz G (2005) Characters in the book lungs of Scorpiones (Chelicerata, Arachnida) revealed by scanning electron microscopy. Zoomorphology 124:101–109
Kerman BE, Cheshire AM, Myat MM, Andrew DJ (2008) Ribbon modulates apical membrane during tube elongation through Crumbs and Moesin. Dev Biol 320:278–288
Kondo T, Hashimoto Y, Kato K, Inagaki S, Hayashi S, Kageyama Y (2007) Small peptide regulators of actin-based cell morphogenesis encoded by a polycistronic mRNA. Nat Cell Biol 9:660–665
Kozlova T, Lam G, Thummel CS (2009) Drosophila DHR38 nuclear receptor is required for adult cuticle integrity at eclosion. Dev Dyn 238:701–707
Kucharski R, Maleszka J, Maleszka R (2007) Novel cuticular proteins revealed by the honey bee genome. Insect Biochem Mol Biol 37:128–134
Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C (2010) A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327:1644–1648
Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U (2009) Yurt, Coracle, Neurexin IV and the Na(+), K(+)-ATPase form a novel group of epithelial polarity proteins. Nature 459:1141–1145
Laprise P, Paul SM, Boulanger J, Robbins RM, Beitel GJ, Tepass U (2010) Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr Biol 20:55–61
Lehane MJ, Billingsley PF (1996) Biology of the insect midgut. Chapman & Hall, London
Lewis JGE (1981) The biology of centipedes. Cambridge University Press, Cambridge
Lindgren M, Riazi R, Lesch C, Wilhelmsson C, Theopold U, Dushay MS (2008) Fondue and transglutaminase in the Drosophila larval clot. J Insect Physiol 54:586–592
Locke M (1961) Pore canals and related structures in insect cuticle. J Biophys Biochem Cytol 10:589–618
Locke M (1966) The structure and formation of the cuticulin layer in the epicuticle of an insect, Calpodes ethlius (Lepidoptera, Hesperiidae). J Morphol 118:461–494
Locke M (2001) The Wigglesworth Lecture: Insects for studying fundamental problems in biology. J Insect Physiol 47:495–507
Locke M (2003) Surface membranes, Golgi complexes, and vacuolar systems. Annu Rev Entomol 48:1–27
Luschnig S, Batz T, Armbruster K, Krasnow MA (2006) Serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol 16:186–194
Lyons RE, Nairn KM, Huson MG, Kim M, Dumsday G, Elvin CM (2009) Comparisons of recombinant resilin-like proteins: repetitive domains are sufficient to confer resilin-like properties. Biomacromolecules 10:3009–3014
Lyons RE, Wong DC, Kim M, Lekieffre N, Huson MG, Vuocolo T, Merritt DJ, Nairn KM, Dudek DM, Colgrave ML, Elvin CM (2011) Molecular and functional characterisation of resilin across three insect orders. Insect Biochem Mol Biol 41:881–890
Madison KC (2003) Barrier function of the skin: La raison d’etre of the epidermis. J Invest Dermatol 121:231–241
Malencik DA, Anderson SR (2003) Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 25:233–247
Martinek N, Shahab J, Saathoff M, Ringuette M (2008) Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci 121:1671–1680
Matsumoto S (2010) Molecular mechanisms underlying sex pheromone production in moths. Biosci Biotechnol Biochem 74:223–231
Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J (2006) The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development 133:957–966
Meissner D, Odman-Naresh J, Vogelpohl I, Merzendorfer H (2010) A novel role of the yeast CaaX protease Ste24 in chitin synthesis. Mol Biol Cell 21:2425–2433
Merzendorfer H (2006) Insect chitin synthases: a review. J Comp Physiol B 176:1–15
Merzendorfer H (2011) The cellular basis of chitin synthesis in fungi and insects: common principles and differences. Eur J Cell Biol 90:759–769
Michels J, Gorb SN (2012) Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J Microsc 245:1–16
Moussian B (2008) The role of GlcNAc in formation and function of extracellular matrices. Comp Biochem Physiol B 149:215–226
Moussian B (2010) Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol 40:363–375
Moussian B, Schwarz H, Bartoszewski S, Nusslein-Volhard C (2005a) Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol 264:117–130
Moussian B, Soding J, Schwarz H, Nusslein-Volhard C (2005b) Retroactive, a membrane-anchored extracellular protein related to vertebrate snake neurotoxin-like proteins, is required for cuticle organization in the larva of Drosophila melanogaster. Dev Dyn 233:1056–1063
Moussian B, Seifarth C, Muller U, Berger J, Schwarz H (2006a) Cuticle differentiation during Drosophila embryogenesis. Arthropod Struct Dev 35:137–152
Moussian B, Tang E, Tonning A, Helms S, Schwarz H, Nusslein-Volhard C, Uv AE (2006b) Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 133:163–171
Moussian B, Veerkamp J, Muller U, Schwarz H (2007) Assembly of the Drosophila larval exoskeleton requires controlled secretion and shaping of the apical plasma membrane. Matrix Biol 26:337–347
Nagaraj R, Adler PN (2012) Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development 139:906–916
Nelson DR, Charlet LD (2003) Cuticular hydrocarbons of the sunflower beetle, Zygogramma exclamationis. Comp Biochem Physiol B 135:273–284
Nelson DR, Tissot M, Nelson LJ, Fatland CL, Gordon DM (2001) Novel wax esters and hydrocarbons in the cuticular surface lipids of the red harvester ant, Pogonomyrmex barbatus. Comp Biochem Physiol B 128:575–595
Nelson DR, Olson DL, Fatland CL (2002) Cuticular hydrocarbons of the flea beetles, Aphthona lacertosa and Aphthona nigriscutis, biocontrol agents for leafy spurge (Euphorbia esula). Comp Biochem Physiol B 133:337–350
Nelson DR, Adams TS, Fatland CL (2003) Hydrocarbons in the surface wax of eggs and adults of the Colorado potato beetle, Leptinotarsa decemlineata. Comp Biochem Physiol B: Biochem Mol Biol 134:447–466
Nelson DR, Hines H, Stay B (2004) Methyl-branched hydrocarbons, major components of the waxy material coating the embryos of the viviparous cockroach Diploptera punctata. Comp Biochem Physiol B 138:265–276
Neville AC (1965a) Chitin lamellogenesis in locust cuticle. Q J Microsc Sci 106:269–286
Neville AC (1965b) Circadian organization of chitin in some insect skeletons. Q J Microsc Sci 106:315–325
Neville AC (1975) Biology of the arthropod cuticle. Springer, Berlin
Neville AC, Luke BM (1969a) A two-system model for chitin-protein complexes in insect cuticles. Tissue Cell 1:689–707
Neville AC, Luke BM (1969b) Molecular architecture of adult locust cuticle at the electron microscope level. Tissue Cell 1:355–366
Neville AC, Parry DA, Woodhead-Galloway J (1976) The chitin crystallite in arthropod cuticle. J Cell Sci 21:73–82
Nilton A, Oshima K, Zare F, Byri S, Nannmark U, Nyberg KG, Fehon RG, Uv AE (2010) Crooked, coiled and crimpled are three Ly6-like proteins required for proper localization of septate junction components. Development 137:2427–2437
Niu BL, Shen WF, Liu Y, Weng HB, He LH, Mu JJ, Wu ZL, Jiang P, Tao YZ, Meng ZQ (2008) Cloning and RNAi-mediated functional characterization of MaLac2 of the pine sawyer, Monochamus alternatus. Insect Mol Biol 17:303–312
Norum M, Tang E, Chavoshi T, Schwarz H, Linke D, Uv A, Moussian B (2010) Trafficking through COPII stabilises cell polarity and drives secretion during Drosophila epidermal differentiation. PLoS ONE 5(5):e10802
Odier A (1823) Mémoires sur la composition chimique des parties cornées des insectes. Mém Soc Hist Nat Paris 1:29–42
Oshima K, Fehon RG (2011) Analysis of protein dynamics within the septate junction reveals a highly stable core protein complex that does not include the basolateral polarity protein Discs large. J Cell Sci 124:2861–2871
Parra-Peralbo E, Culi J (2011) Drosophila lipophorin receptors mediate the uptake of neutral lipids in oocytes and imaginal disc cells by an endocytosis-independent mechanism. PLoS Genet 7(2):e1001297
Patel S, Nelson DR, Gibbs AG (2001) Chemical and physical analyses of wax ester properties. J Insect Sci 1:4
Pryor MG (1940) On the hardening of cuticle of insects. Proc R Soc B 128:393–407
Pryor MG, Russell PB, Todd AR (1946) Protocatechuic acid, the substance responsible for the hardening of the cockroach ootheca. Biochem J 40:627–628
Pryor MG, Russell PB, Todd AR (1947) Phenolic substances concerned in hardening the insect cuticle. Nature 159:399
Qin G, Lapidot S, Numata K, Hu X, Meirovitch S, Dekel M, Podoler I, Shoseyov O, Kaplan DL (2009) Expression, cross-linking, and characterization of recombinant chitin binding resilin. Biomacromolecules 10:3227–3234
Raabe D, Romano P, Sachs C, Al-Sawalmih A, Brokmeier H-G, Yi S-B, Servos G, Hartwig HG (2005) Discovery of a honeycomb structure in the twisted plywood patterns of fibrous biological nanocomposite tissue. J Cryst Growth 283:1–7
Rebers JE, Willis JH (2001) A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem Mol Biol 31:1083–1093
Rezende GL, Martins AJ, Gentile C, Farnesi LC, Pelajo-Machado M, Peixoto AA, Valle D (2008) Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Dev Biol 8:82
Riedel F, Vorkel D, Eaton S (2011) Megalin-dependent yellow endocytosis restricts melanization in the Drosophila cuticle. Development 138:149–158
Rotstein B, Molnar D, Adryan B, Llimargas M (2012) Tramtrack is genetically upstream of genes controlling tracheal tube size in Drosophila. PLoS ONE 6(12):e28985
Ruaud AF, Lam G, Thummel CS (2010) The Drosophila nuclear receptors DHR3 and beta FTZ-F1 control overlapping developmental responses in late embryos. Development 137:123–131
Ruaud AF, Lam G, Thummel CS (2011) The Drosophila NR4A nuclear receptor DHR38 regulates carbohydrate metabolism and gycogen storage. Mol Endocrinol 25:83–91
Sass M, Kiss A, Locke M (1993) Classes of integument peptides. Insect Biochem Mol Biol 23:845–857
Sass M, Kiss A, Locke M (1994) Integument and hemocyte peptides. J Insect Physiol 40:407–421
Scholtz G, Kamenz C (2006) The book lungs of Scorpiones and Tetrapulmonata (Chelicerata, Arachnida): evidence for homology and a single terrestrialisation event of a common arachnid ancestor. Zoology (Jena) 109:2–13
Schulte J, Charish K, Que J, Ravn S, MacKinnon C, Auld VJ (2006) Gliotactin and Discs large form a protein complex at the tricellular junction of polarized epithelial cells in Drosophila. J Cell Sci 119:4391–4401
Shaik KS, Pabst M, Schwarz H, Altmann F, Moussian B (2011) The Alg5 ortholog Wollknauel is essential for correct epidermal differentiation during Drosophila late embryogenesis. Glycobiology 21:743–756
Shaik KS, Meyer F, Vazquez AV, Flotenmeyer M, Cerdan ME, Moussian B (2012) delta-Aminolevulinate synthase is required for apical transcellular barrier formation in the skin of the Drosophila larva. Eur J Cell Biol 91:204–215
Shibata T, Ariki S, Shinzawa N, Miyaji R, Suyama H, Sako M, Inomata N, Koshiba T, Kanuka H, Kawabata S (2010) Protein crosslinking by transglutaminase controls cuticle morphogenesis in Drosophila. PLoS ONE 5(10):e13477. doi:10.1371/journal.pone.0013477
Silvert DJ, Doctor J, Quesada L, Fristrom JW (1984) Pupal and larval cuticle proteins of Drosophila melanogaster. Biochemistry 23:5767–5774
Snyder M, Hunkapiller M, Yuen D, Silvert D, Fristrom J, Davidson N (1982) Cuticle protein genes of Drosophila: Structure, organization and evolution of four clustered genes. Cell 29:1027–1040
Suderman RJ, Dittmer NT, Kanost MR, Kramer KJ (2006) Model reactions for insect cuticle sclerotization: cross-linking of recombinant cuticular proteins upon their laccase–catalyzed oxidative conjugation with catechols. Insect Biochem Mol Biol 36:353–365
Suderman RJ, Dittmer NT, Kramer KJ, Kanost MR (2010) Model reactions for insect cuticle sclerotization: participation of amino groups in the cross-linking of Manduca sexta cuticle protein MsCP36. Insect Biochem Mol Biol 40:252–258
Sugumaran M (2009) Complexities of cuticular pigmentation in insects. Pigment Cell Melanoma Res 22:523–525
Tang L, Liang J, Zhan Z, Xiang Z, He N (2010) Identification of the chitin-binding proteins from the larval proteins of silkworm, Bombyx mori. Insect Biochem Mol Biol 40:228–234
Tepass U, Theres C, Knust E (1990) Crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61:787–799
Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Torok T, Hartenstein V (1996) Shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev 10:672–685
Thomas GH, Kiehart DP (1994) Beta heavy-spectrin has a restricted tissue and subcellular distribution during Drosophila embryogenesis. Development 120:2039–2050
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones–an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 29:481–514
Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM (1998) Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J Cell Biol 143:121–133
Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM (2000) Regulation of actin filament cross-linking and bundle shape in Drosophila bristles. J Cell Biol 148:87–100
Tilney LG, Connelly PS, Guild GM (2004) Microvilli appear to represent the first step in actin bundle formation in Drosophila bristles. J Cell Sci 117:3531–3538
Togawa T, Nakato H, Izumi S (2004) Analysis of the chitin recognition mechanism of cuticle proteins from the soft cuticle of the silkworm, Bombyx mori. Insect Biochem Mol Biol 34:1059–1067
Tomancak P, Berman BP, Beaton A, Weiszmann R, Kwan E, Hartenstein V, Celniker SE, Rubin GM (2007) Global analysis of patterns of gene expression during Drosophila embryogenesis. Genome Biol 8:R145
Tonning A, Hemphala J, Tang E, Nannmark U, Samakovlis C, Uv A (2005) A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev Cell 9:423–430
Tonning A, Helms S, Schwarz H, Uv AE, Moussian B (2006) Hormonal regulation of mummy is needed for apical extracellular matrix formation and epithelial morphogenesis in Drosophila. Development 133:331–341
Uv A, Moussian B (2010) The apical plasma membrane of Drosophila embryonic epithelia. Eur J Cell Biol 89:208–211
Vincent JF, Wegst UG (2004) Design and mechanical properties of insect cuticle. Arthropod Struct Dev 33:187–199
Vrkoslav V, Muck A, Cvacka J, Svatos A (2010) MALDI imaging of neutral cuticular lipids in insects and plants. J Am Soc Mass Spectrom 21:220–231
Wang HB, Nita M, Iwanaga M, Kawasaki H (2009) betaFTZ-F1 and Broad-Complex positively regulate the transcription of the wing cuticle protein gene, BMWCP5, in wing discs of Bombyx mori. Insect Biochem Mol Biol 39:624–633
Weis-Fogh T (1960) A rubber-like protein in insect cuticle. J Exp Biol 37:889–907
Wigglesworth VB (1933) The physiology of the cuticle and of ecdysis in Rhodnius prolixus (Triatomidae, Hemiptera); with special reference to the oenocytes and function of the dermal glands. Quart J Microscop Soc 76:270–318
Wigglesworth VB (1970) Structural lipids in the insect cuticle and the function of the oenocytes. Tissue Cell 2:155–179
Wigglesworth VB (1975) Distribution of lipid in the lamellate endocuticle of Rhodnius prolixus (Hemiptera). J Cell Sci 19:439–457
Wigglesworth VB (1985) Sclerotin and lipid in the waterproofing of the insect cuticle. Tissue Cell 17:227–248
Wigglesworth VB (1990) The distribution, function and nature of cuticulin in the insect cuticle. J Insect Physiol 36:307–313
Willis JH (2010) Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem Mol Biol 40:189–204
Wittkopp PJ, True JR, Carroll SB (2002) Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 129:1849–1858
Wolfgang WJ, Fristrom D, Fristrom JW (1986) The pupal cuticle of Drosophila: differential ultrastructural immunolocalization of cuticle proteins. J Cell Biol 102:306–311
Wong DC, Pearson RD, Elvin CM, Merritt DJ (2012) Expression of the rubber-like protein, resilin, in developing and functional insect cuticle determined using a Drosophila anti-Rec 1 resilin antibody. Dev Dyn 241:333–339
Yoon CS, Hirosawa K, Suzuki E (1997) Corneal lens secretion in newly emerged Drosophila melanogaster examined by electron microscope autoradiography. J Electron Microsc (Tokyo) 46:243–246
Ziegler A, Weihrauch D, Towle DW, Hagedorn M (2002) Expression of Ca2+-ATPase and Na+/Ca2+-exchanger is upregulated during epithelial Ca2+ transport in hypodermal cells of the isopod Porcellio scaber. Cell Calcium 32:131–141
Ziegler A, Weihrauch D, Hagedorn M, Towle DW, Bleher R (2004) Expression and polarity reversal of V-type H+-ATPase during the mineralization-demineralization cycle in Porcellio scaber sternal epithelial cells. J Exp Biol 207:1749–1756
Zimoch L, Merzendorfer H (2002) Immunolocalization of chitin synthase in the tobacco hornworm. Cell Tissue Res 308:287–297
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Moussian, B. (2013). The Arthropod Cuticle. In: Minelli, A., Boxshall, G., Fusco, G. (eds) Arthropod Biology and Evolution. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36160-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-36160-9_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36159-3
Online ISBN: 978-3-642-36160-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)