Abstract
The present chapter overviews some aspects of the anatomy, histology and physiology of the female genital tract, focusing especially on the uterus, through which sperm are transported. Moreover, some aspects of sperm interaction with uterinic epithelial cells are referred to in line with recent studies. This chapter also deals with other relevant aspects, such as the functional role of ejaculate volume on sperm transport, and the effects of placing sperm at different parts of the sows’ tract in fertility and prolificacy rates. Finally, it ends with the role of reproductive immunology in response to spermatozoa, seminal plasma and short- and long-term extenders within the intrauterine environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: The Female Reproductive Tract
1.1 A General Overview of the Swine Genital Tract
The sow’s reproductive tract is formed by the following organs, listed here in the reverse direction of the pathway followed by spermatozoa: ovaries, oviducts, uterus, cervix, vagina and external genitalia (Fig. 5.1). All these organs, except the ovaries, form the tubular genitalia.
Each of the two ovaries has a length of approximately 5 cm and presents an irregular shape as a result of numerous follicles and corpora lutea protruding from the surface (Edström 2009). Their main function is to produce follicles, oestrogens (mainly oestradiol), and progesterone (Michael and Schofield 1969).
The oviducts are tubular conduits connecting the ovaries with the uterus. Each oviduct has a length of about 20 cm and can be divided into three parts starting from the ovarian side: infundibulum, ampulla and isthmus. The ampullary-isthmic junction is the site of fertilisation (Hafez 1993).
The uterus is formed by two long uterine horns and a short body. In non-pregnant sows, the length of each uterine horn is about 60–90 cm. Spermatozoa pass through both horns before reaching the oviducts, and both horns are the sites of implantation and foetal development. The other part of the uterus, the uterine body, which is small if compared to other domestic species, is located at the junction of the two uterine horns (Hafez 1993; Thibault et al. 1993).
The cervix is a muscular conduit connecting the vagina and the uterus and the site of semen deposition during natural mating and artificial insemination (AI). Its length is about 25 cm and it has internal interdigitating mucosal prominences. Cervical morphology depends on the stage of the oestrus cycle, since it is dilated when the sow is in heat and constricted during pregnancy and the remaining period of the oestrus cycle (Hafez 1993).
The vagina reaches from the cervix to the urethral orifice. Since the urethra connects the bladder to the vagina, the vagina serves at the same time as a passageway for urine and for the piglets at birth.
Finally, there are the external genitalia, consisting of the vestibulum and the vulva, which is in turn formed by the labia, the clitoris and the vestibular glands. The vestibulum extends from the urethral orifice to the vulva, and the vulva is the external portion of the genital tract of the sow. Its aspect also changes depending on the stage of the oestrus cycle and on sow parity, so that it becomes red and swollen just prior to the oestrus and more swollen in gilts than in sows (Edström 2009).
Although the books covering the female genital tract usually start with the description of the ovaries and end with that of the external genitalia, this chapter will follow the opposite direction starting with the external genitalia. The main purpose is to focus on the transit of a spermatozoon within the female reproductive tract, i.e. from the deposition site towards the ampullary-isthmic junction.
1.2 Histology of the Swine Reproductive Tract: General Pattern
The tubular genitalia follow a pattern that is commonly observed in most tissue sections (Edström 2009).
First, we can distinguish the mucosa, which surrounds the lumen and consists of an epithelium that fulfils different functions depending on the organ (e.g. uterus vs. oviduct). Underlying the epithelium, there is a layer of connective tissue with varying thickness and structure among the reproductive organs. In this connective tissue, we can find other cell types such as the immune cells, which play a relevant role as will be explained below (Finn and Porter 1975; Hafez 1993; Edström 2009; Yáñiz et al. 2006).
Surrounding the mucosa, there is the muscularis, made up of two layers of smooth muscle cells, a circular inner and a longitudinal outer layer.
Finally, there is an outer layer of connective tissue that surrounds the organs. While in the peritoneal cavity the organs are covered by the peritoneum, a serosa consisting of a simple squamous epithelium, in the pelvic cavity there is a tunica adventitia of loose connective tissue. The reproductive organs are contained within the pelvic cavity, except for the most cranial part of the vagina (which has a serosa), and are therefore surrounded by loose connective tissue (Edström 2009).
As observed from histological samples, ovaries, oviducts and the uterus are mainly innervated by autonomous nerves, while the pudendum nerve is the one that innervates the vagina, the vulva and the clitoris by sensorial and parasympathetic fibres (Hafez 1993).
2 Concepts Relating to Swine Reproductive Physiology
2.1 Puberty and Sexual Maturation
In pigs, females reach puberty at the age of 6–7 months. However, this age can be influenced by breed, season, management and/or nutritional factors (see Chap. 4). In fact, the ovaries are controlled by the hypothalamus and the pituitary gland and their activity determines both the onset of puberty and other subsequent events.
Pregnancy lasts approximately 113 days and during lactation, sows are still in anoestrus. The interval between weaning and oestrus is about 4–6 days (Knobil and Neill 1994).
2.2 The Oestrous Cycle
The domestic pig (Sus domesticus) is a poly-oestral species, which means that the female has regular oestrus cycles throughout the year, the cycle being interrupted when the females are pregnant or lactating (the anoestrus period).
In swine, the oestrus cycle averages 21 days, ranging between 18 and 24 days, and is defined as the period of time from the onset of one oestrus to the onset of the next. This cycle can be divided into three different stages: proestrus (1–3 days), oestrus (1–3 days), metoestrus (2–3 days) and dioestrus (13–18 days), and the first day of standing oestrus is generally considered to be the first day of the cycle.
The oestrus corresponds to the ovarian follicular phase, as follicles are the predominant structures in the ovaries. About 3 days after the onset of standing heat, follicular growth is accelerated by the follicle-stimulating hormone (FSH) (Fig. 5.2). Around 20 follicles, between 6 and 10 in each ovary, are ovulated in each oestrous cycle leading to an increase in the corresponding number of corpora lutea (Hafez 1993; Knobil and Neill 1994).
Oestrous cycle in pigs. a Plasma concentrations of 17β-oestradiol and progesterone, b Plasma concentrations of FSH, LH and Inhibin-A, and c development of follicular and corpora lutea (CL). The vertical line in the figures represents time of ovulation (Soede et al. 2011, Reproduced with permission).
There are three different organs involved in the control of the oestrous cycle: the hypothalamus, the hypophysis and the ovaries. The hypothalamus secretes the gonadotropin-releasing hormone (GnRH), which stimulates the hypophysis to secrete FSH and luteinising (LH) hormones (Blödow et al. 1990). FSH stimulates follicles to produce oestradiol (Fig. 5.2); each follicle contains a maturing oocyte and the granulosa cells responsible for its secretion. This oestradiol stimulates, in turn, the follicular growth and acts on the external genitalia, on the cervix and on the uterus, thereby preparing the female both for mating and for implanting the fertilised egg in the endometrium (Eiler and Nalbandov 1977). Oestradiol produces the typical signs of the oestrus, i.e. swollen and hyperaemic vulva, restlessness and riding behaviour, increase in secretory activity and hypertrophy and oedema of the genital tract (Mburu et al. 1998). Finally, the increase in oestradiol levels stimulates the hypophysis to release LH, a peak of this hormone leading to ovulation (Fig. 5.2) (Eiler and Nalbandov 1977). Ovulation occurs at the beginning of the last third of the oestrus stage, i.e. approximately 40 h after the onset of oestrus.
As far as the different stages of the oestrous cycle are concerned, pro-oestrus lasts, as mentioned, between 1 and 3 days and is characterised by follicular growth and regression of the corpus luteum of the previous cycle (Espey and Lipner 1994). Granulose cells inside the developing follicles produce oestrogen, responsible for the typical outer signs of an approaching oestrus.
During oestrus (1–3 days), the sow or gilt is sexually receptive and thus accepts mating by showing a standing reflex (stiffness) when the loin is firmly pressed in the presence of a boar. The increase in oestradiol levels provokes oedema of the oviducts, endometrium, cervix and vulva, this effect being more pronounced in gilts than in sows, an increase in the production of vaginal mucus, and, finally, ovulation itself. There are secondary signs that the female exhibits during the oestrus: increased nervous activity, desire to seek the boar, loss of appetite, changes in vocalisation and a male-like sexual behaviour (pursuing, nosing and mounting other females) (Eiler and Nalbandov 1977; Espey and Lipner 1994).
Metoestrus (days 2–3) and dioestrus (days 13–18) are collectively called the luteal phase, with the corpora lutea being the main functional ovarian structures during this phase. After ovulation, ruptured follicles evolve into luteinised cells, which form the corpora lutea and produce progesterone, about 1–2 days after mating (Shille et al. 1979). Then, there is a decrease in the oestradiol levels and an increase in progesterone (Noguchi et al. 2010; Soede et al. 2011).
Finally, at dioestrus the corpora lutea continue producing progesterone, which results in stimulation of secreting activity in the uterine glands. During this stage, the uterus is prepared for foetal membrane attachment and placentation. If the sow becomes pregnant, the corpora lutea continue to release progesterone together with relaxin until parturition is approaching, so that progesterone is responsible for maintaining pregnancy. On the other hand, the high levels of progesterone block the onset of a new oestrus cycle because they inhibit GnRH secretion in the hypothalamus, which in turn impedes complete maturation and ovulation of new follicles (Hafez 1993; Mburu et al. 1998; Mwanza et al. 2000; Razdan et al. 2001).
If there is no conception, luteolysis occurs when the end of dioestrus is approaching in the presence of prostaglandin F2α (PGF2α), a hormone secreted by the non-pregnant uterus that diffuses from the uterine vein and the ovarian artery to the ovaries (Shille et al. 1979). Luteolysis leads to a decrease in progesterone levels by day 17 of the cycle, which also involves return of hypothalamic stimuli. Release of GnRH is restored in this way, and a new cycle starts with the development of new follicles in the ovary (Knobil and Neill 1994; Mirando et al. 1995).
Lactation is also capable of inhibiting the oestrous cycle, which will be restored 4–7 days after piglet weaning; this period of time depending on several factors, such as the length of lactation, parity, nutrition or season (Knobil and Neill 1994). In fact, shortening lactation and performing weaning as soon as possible is very important in economic terms for AI centres and pig breeders.
The length of oestrus is variable and may last from only 12 h in gilts to up to 60 h or more in sows. Since the real time of its onset is rarely known, it is recommended that a female receives at least two matings or two inseminations in oestrus. This practice ensures that spermatozoa will be present in the oviduct when fertilisation occurs, with the corresponding optimisation of farrowing rates and litter size (Thibault et al. 1993). Here, the role of the sperm reservoir is very important, as will be discussed in the Chap. 6. Other aspects of reproductive physiology relating to AI and mating will be taken up in the (Chap. 12) of this book.
3 External Genitalia
The external genitalia comprise the vestibulum, the major and minor labia, the clitoris and the vestibular glands (Hafez 1993).
3.1 Vestibulum
The union between vestibulum and the vagina is characterised by the presence of the urethral orifice, and sometimes by the vestigial hymen. During the development of sexual organs, two ducts, the Müllerian and the Wolffian, coexist until the sex of the embryo is defined. In females, the Wolffian duct regresses to a remnant called Gartner’s duct that leads to the vestibulum. Particularly, the vestibulum has subepithelial lymphatic nodules in the connective tissue stroma. Bartholin ducts are the conduit between vestibulum and Bartholin glands, which are located on the external lateral wall of the vaginal vestibulum (Knobil and Neil 1994). These glands secrete a viscous liquid into the vestibulum that is more viscous during oestrus, and presents a tubo-alveolar structure similar to that of the boar bulbourethral glands (see Chap. 3).
3.2 Major and Minor Labia
The major labia contain fat deposits, elastic tissue and a thin muscular layer, and the external surface presents the same structure as the epidermis. The integument of major labia contains numerous sebaceous and tubular glands.
Minor labia have a nucleus made of spongeous connective tissue, their surface containing numerous, large sebaceous glands (Hafez 1993).
3.3 The Clitoris
The ventral commissure of the vestibulum hides the clitoris, which has the same embryonic origin as the boar penis. It is formed by erectile tissue covered by a squamous and stratified epithelium and presents numerous sensorial nerve terminations. It is long and sinuous and ends in a small cone or tip (Hafez 1993).
4 The Vagina
4.1 Anatomy and Histology
The vagina extends from the cervix to the urethral orifice and is the receptacle for the boar penis during copulation (Fig. 5.1). The height of its epithelium also depends on the stage of the oestrus cycle, so that the maximum thickness is observed in the late proestrus (Knobil and Neill 1994).
The vaginal wall consists of a superficial epithelium, a muscularis and a serosa.
The muscularis is not as developed as in the external parts of the uterus, and consists of two thinner smooth muscle layers. The inner is circular and the outer is longitudinal and continuous with the uterus. The muscularis contains a large number of blood vessels, nervous bundles and connective tissue both dense and lax (Hafez 1993).
The superficial epithelium is formed by squamous stratified cells and does not contain secretory glands. The surface of epithelial vaginal cells contains microborders ordered longitudinally or in circles. In this pluristratified epithelium, cells form a firm and consistent structure because the microborders of one cell interact with those of another.
The reproductive cycle influences the morphology and disposition of microborders, so that they exhibit a regular pattern during pregnancy but present inner pores throughout the oestral cycle (Hafez 1993).
4.2 Physiological Responses: Contractions and Vaginal Fluid
Vaginal contractility plays a main function in psychosexual responses and seems to play an indirect role in sperm transport (Langendijk et al. 2005; Levin 2011; Suarez and Pacey 2006). It is stimulated by the vaginal fluid during stimulation previous to coitus.
This vaginal fluid consists of transuded secretions through the vaginal wall and also contains vulvar secretions coming from sebaceous and sudoriparous glands. Moreover, this fluid contains traces of endometrial fluid and cervical mucus, as well as exfoliated cells from the vaginal epithelium (Hafez 1993).
4.3 Functions of the Vagina
The vagina is the copulatory organ in the female. After coitus and ejaculation, the seminal plasma is not transported into the uterus but it is either expelled or absorbed through the vaginal wall. When absorbed, the components of seminal plasma trigger physiological responses in other parts of the female reproductive tract, such as the endometrium and the ovary, that affect sow reproductive physiology and improve the chances of conception and pregnancy success (Robertson 2007; O’Leary et al. 2011). Accordingly, within these physiological responses triggered by seminal plasma components, we can find inflammatory responses, including altered patterns of cytokine secretion that facilitate embryo development and implantation (Robertson 2007), and regulation of ovulation timing, corpus luteum development and steroid production in the ovary (O’Leary et al. 2002). Specifically, the cytokines and prostaglandins that seminal plasma contains bind to receptors on target cells in the cervix and uterus, thereby activating changes in gene expression that lead to modifications in structure and function of the female tissues (Kaczmarek et al. 2010) (see also Sect. 5.8). In the case of endometrium, seminal plasma induces pro-inflammatory cytokines and cyclooxygenase-2 and causes recruitment of macrophages and dendritic cells. Apart from seminal plasma components, spermatozoa also contribute in male–female signalling because interaction with seminal plasma factors modulates neutrophil influx into the uterine luminal cavity. Recently, transforming growth factor-β (TGF-β), a potent immune-modulating cytokine present in the seminal plasma of boars, mice and humans, has been suggested to be involved in the immune changes of female reproductive tract elicited by seminal fluid (O’Leary et al. 2011).
On the other hand, vaginal secretions present a low pH which is unfavourable for spermatozoa. A complex interaction between cervical mucus, vaginal secretion and seminal plasma form a buffer system that protects sperm until it is transported towards the micelles of cervical mucus.
Finally, we must mention that the vagina acts as an excretory duct for secretions of the uterine body, endometrium and oviducts, and it also functions as parturition canal. Different physiological features are involved in all these functions: contraction, extension, involution, secretion and absorption (Hafez 1993).
5 The Cervix
5.1 Anatomy and Histology
The cervix is located in the pelvic cavity, where semen is deposited after mating or after conventional AI (Fig. 5.1). As stated, its length is about 25 cm and has internal interdigitating mucosal prominences (pulvini cervicales). It can be divided into two different regions: the shorter is the uterine region, with a length of 6–7 cm, while the larger is the vaginal region, which is about 12–14 cm long. The cervix has two different ends, the so-called utero-cervical end being more pronounced than the cervico-vaginal end (Edström 2009).
In terms of histological structure, the cervical epithelium undergoes cyclic variations and is formed by stratified squamous cells and columnar cells that can cover more than 90 % of the mucosa. The mucosa presents folds and the underlying stroma is made up of connective tissue containing capillaries and small blood vessels.
The muscularis is formed by two layers, the inner circular layer and the outer longitudinal one, that are arranged in bundles and do not extend into the mucosal prominences. The muscularis is surrounded by a serosa of loose connective tissue towards the abdominal cavity (Hafez 1993).
5.2 Cervical Changes During the Oestrous Cycle
The cervix becomes increasingly firm and projects horizontally into the abdominal cavity when the oestrus is coming, and it progressively softens to hang limply over the pubic border by 7 of the oestrous cycle (Rigby 1967; Meredith 1977; Edström 2009).
The literature has reported inconsistent results regarding the histological properties of the cervix during oestrus. Thus, while Steinbach and Smidt (1970) did not report any significant cyclic variation in the height of the epithelium at the uterine part of the cervix, Smith and Nalbandov (1958) observed that the cervix was mostly constricted during oestrus. Indeed, and according to these authors:
-
1.
The constriction/firmness reaches its maximum by days 1 and 2 of the oestrous cycle.
-
2.
After this, the cervix progressively relaxes and softens up to day 9 of the cycle.
-
3.
From days 13–14, the cervix gradually increases its constriction/firmness (Edström 2009).
Although the oestrous cycle does not affect the size of cervical lumen at the uterine portion, the changes in cervical consistency seem to be caused by fluctuations in the rigidity of the tissue rather than by modulations of the muscular contractility. In fact, cervical consistency depends on oestrogen levels. Thus, the increase in firmness of the cervix is related to the rise in oestradiol levels that precedes the oestrus (Kunavongkrit et al. 1983; Edström 2009), while softening of the cervix during the post-oestrus phase occurs as a consequence of the absence of oestradiol in plasma. In this respect, there are contradictory reports on the effects of the presence/absence of sexual hormones during post-oestrus (Smith and Nalbandov 1958; Kunavongkrit et al. 1983), but it seems that it is the absence of oestrogen rather than the presence of progesterone which is responsible for cervical softening (Edström 2009).
5.3 Cervical Changes Related to Pregnancy and Parturition
The cervix also changes during pregnancy and parturition and its physiological status differs from that described during the oestrous cycle.
When sows are pregnant, the main function of the cervix is to protect the uterus (Eldridge-White et al. 1989). This explains why the extensibility and the lumen diameter of the uterine part is lower than those of the vaginal portion during the first 80 days and suggests that, at least during this period of time, the uterine part is more involved in the protection of the uterus than the vaginal region. By day 80 of pregnancy, the uterine cervix increases its softness and extensibility, with no differences when compared to the consistency of the vaginal portion. Here, it is important to keep in mind that the cervix has to be extensible during parturition to allow passage of the foetuses. The increase in firmness is related to progesterone levels, which are high throughout the entire pregnancy, while the softness of the uterine portion appears to be related to relaxin (O’Day et al. 1989) and oestradiol levels, increasing from day 80 until the end of pregnancy (Eldridge-White et al. 1989). It must be also taken into account that progesterone and relaxin are produced by the corpora lutea, and oestrogen is produced by the placenta (Edström 2009).
Finally, it is noteworthy that relaxin influences the cervical connective tissue, reducing the collagen concentration and increasing water content, dry weight and the glycosaminoglycan to collagen ratio (O’Day-Bowman et al. 1991). During late pregnancy, relaxin appears to cause histological changes in the cervix, thereby reducing collagen density and influencing the organisation of muscle fibres and collagen fibre bundles (Winn et al. 1993).
6 The Uterus
6.1 Anatomy and Histology
In the domestic pig, the uterus is formed by a short (3–4 cm) body (corpus) and two long uterine horns (cornuae uteri) (Fig. 5.1). The length of each uterine horn depends on age and whether the female is pregnant or not. Thus, it measures about 60 cm in gilts, 100 cm in non-pregnant sows and may reach 200 cm in the pregnant sow. Notwithstanding, the uterus acquires its full morphological traits by the second oestrus after puberty (Schnurrbusch and Erices 1979; Norrby 2010).
Like the oviducts and ovaries, the uterus is located in the abdominal cavity and is suspended from the abdominal wall by the broad ligaments, including the mesometrium and continuing as mesosalpinx and mesovarium (Finn and Porter 1975).
In the uterine wall, we can distinguish three different layers: the endometrium, surrounding the uterine lumen, the myometrium, and the perimetrium, which is the most outer layer in the vicinity of the abdominal cavity (Edström 2009; Norrby 2010).
The endometrium is the uterine mucosa and consists of a simple to pseudostratified, columnar epithelium (Eslaminejad et al. 2007), depending on the phase of the oestrous cycle, and a loose connective tissue stroma with capillaries and small blood vessels with ciliated and secretory cells, which produce and secrete hormones and nutrients for embryo development (Walter and Bavdek 1997). The subepithelial layer is highly vascularised and infiltrated by several types of cells of the immune system (Romek and Karasinksi 2011).
The myometrium is the tunica muscularis of the uterus and it is made up of three layers of smooth musculature. The inner layer is thicker and forms two circular patterns that come from the oviducts and extend to the cervix. The middle layer is constituted by fibres randomly organised, which run lengthwise, widthwise and diagonally, and supports the large blood vessels that nourish the myometrium. Finally, the outer layer is thinner and longitudinal (Finn and Porter 1975). On the other hand, the myometrium thickens during pregnancy to allow embryo post-implantation development, and its contractile activity depends on the status of the (pregnant or non-pregnant) female and the stage of foetal development (Michael and Schofield 1969), so that it increases at the moment of parturition.
Finally, there is the perimetrium, which is the outer layer of the uterus and is formed by a layer of connective tissue covered by a serosa, in a similar fashion to the oviducts (Edström 2009). It has the typical composition of loose connective tissue, but it contains a large number of lymphatic vessels.
6.2 Uterine Changes During the Oestrus Cycle
During the oestrous cycle, the morphological properties of the endometrium vary (Walter and Bavdek 1997). Thus, and in accordance with what we stated previously, the nature of the uterine epithelium changes depending on the phase of the oestrous cycle, as follows:
-
1.
At oestrus and early dioestrus, the epithelium is high columnar and pseudostratified (Stroband et al. 1986; Kaeoket et al. 2001).
-
2.
During dioestrus, it is low columnar.
-
3.
At late dioestrus and proestrus it is simple cuboidal or low columnar (Kaeoket et al. 2001).
The mitotic activity in the epithelium is maximal at proestrus and oestrus (Stroband et al. 1986), whereas the secretory activity in the uterine glands is the highest at dioestrus. During late dioestrus, proestrus and oestrus, there is uterine oedema in the subepithelial connective tissue (Walter and Bavdek 1997).
On the other hand, mast cells, macrophages, lymphocytes, plasma cells and eosinophils have also been found in the endometrium (Kaeoket et al. 2001), and the phase of the oestrous cycle also influences the number of lymphocytes, neutrophils, eosinophils and plasma cells. Thus, and regarding the surface epithelium, lymphocytes are mainly found during oestrus and early dioestrus, while macrophages are mainly present at proestrus and oestrus (Kaeoket et al. 2001). As far as the submucosa is concerned, lymphocytes are the dominating cell type during all the stages of the oestrous cycle especially at oestrus and early dioestrus, when they are more numerous. Moreover, there is a massive infiltration of neutrophils in the submucosa during proestrus and oestrus (Fig. 5.3), but these immune cells are not observed during the other stages of the oestrous cycle (Stroband et al. 1986; Kaeoket et al. 2001). More information relating to reproductive immunology will be given in Sect. 5.8.
Transmission electron micrograph showing the entry of polymorphonuclear leucocytes (arrows) from the lamina propria (lp) into the luminal epithelium and towards the lumen (lu) of the uterus in an oestrous sow. (Rodríguez-Martínez et al. 2010, Reproduced with permission)
6.3 Interaction of Spermatozoa with Cells of the Uterine Epithelium
Although billions of spermatozoa arrive at the uterus after copulation or AI, only a few thousand of them ever reach the oviducts (Matthijs et al. 2003) so that the uterine passage involves a large number of sperm cells being lost. This radical decrease may be explained by two different phenomena (Taylor et al. 2008):
-
1.
The backflow within 4 h after AI (Viring and Einarsson 1980, 1981; Steverink et al. 1998), and
-
2.
The possible selection of the spermatozoa before entering the oviduct (Matthijs et al. 2003).
Upon arrival, sperm cells have to face two different cell types: uterine epithelial cells (UEC) and neutrophilic granulocytes [polymorphonuclear neutrophils; see Nathan (2006) for a general review], and both seem to play an active part in a selective-like process by becoming attached to viable spermatozoa (Taylor et al. 2008, 2009a). Indeed, spermatozoa can be attacked and phagocytosed by neutrophilic granulocytes, which migrate in great number after insemination/mating into the uterus lumen (Pursel et al. 1978; Rozeboom et al. 1999; Matthijs et al. 2003). However, the influx of neutrophilic granulocytes takes place within the first 3–4 h post-insemination, and due to the backflow, they are confronted by a reduced number of viable spermatozoa (Taylor et al. 2008).
Although the exact molecular mechanisms by which spermatozoa bind to UEC and neutrophilic granulocytes is unknown, it seems quite clear that only viable spermatozoa are able to bind these two cell types (Taylor et al. 2008). Indeed, using ex vivo and in vitro experiments in pigs, Taylor et al. (2008) observed that many spermatozoa were retained within the uterus strongly enough to resist vigorous flushing. These authors also showed that the number of non-viable spermatozoa that were flushed out was similar to the number of non-viable spermatozoa in the insemination dose. In contrast, the number of viable spermatozoa recovered after flushing was lower than that of the dose. These inseminations were performed with spermatozoa diluted in a long-term extender (Androhep™, Minitüb) or in seminal plasma, the number of viable spermatozoa retained in the uterine segments being higher in the former than in the latter.
As far as the mechanism by which viable sperm cells adhere to UEC is concerned, it seems that specific surface molecules (carbohydrates) could mediate the binding between the two cell types. This possibility is reinforced by observations by Taylor et al. (2008) when comparing the acrosome integrity of spermatozoa diluted in a commercial extender with spermatozoa diluted in seminal plasma. The assessment of the acrosome integrity in this study was performed using the lectin peanut agglutinin (PNA; see also Sect. 9.3.10 for more information about lectins and acrosome integrity), which binds to damaged acrosomes, so that PNA-stained spermatozoa could not be found, in principle, attached to UEC. The PNA modulated binding was more obvious with sperm cells in the commercial extender, whereas the seminal plasma inhibited the binding of PNA. Although PNA-specific sugars may not be the dominant structures involved in the interaction between viable spermatozoa and UEC, this could be a valid hypothesis of why the number of retained spermatozoa decreases when they are suspended in their seminal plasma. Apart from this evidence, and bearing in mind that in vitro cultured UEC retain functional characteristics (Cox and Leese 1997), it is also well-known that viable spermatozoa preferentially attach to oviductal epithelial cells (Töpfer-Petersen et al. 2002; Yeste et al. 2009) and to epididymal epithelial cells (Yeste et al. 2012), this fact being explained by the function of the sperm reservoir in the former case. In addition, sperm binding to oviductal epithelial cells (OEC) is also mediated by carbohydrate interactions (DeMott et al. 1995; Green et al. 2001; Töpfer-Petersen et al. 2002; Wagner et al. 2002), and the ability of sperm cells to adhere to OEC depends on their viability and their functional status. Therefore, as will be seen in the Chap. 6, only viable, morphologically normal and uncapacitated spermatozoa are able to bind oviductal (Fazeli et al. 1999; Green et al. 2001; Töpfer-Petersen et al. 2002; Yeste et al. 2009) and epididymal epithelial cells (Yeste et al. 2012). Furthermore, this preferential binding has not only been seen in reproductive but also in non-reproductive epithelial cells (Yeste et al. 2009, 2012), although it occurs to a lower extent in the latter case. All these data back the hypothesis put forward by Taylor et al. (2008) by which only viable spermatozoa are able to bind UEC, and emphasises the functional role of sperm binding to epithelial cells in different parts of male and female reproductive tracts (epididymal, uterine and oviductal).
Finding an explanation for the reduction in the number of spermatozoa within the uterus is complex, but the most reasonable one is that only viable spermatozoa are able to bind UEC. The other possible explanation for the reduction in viable spermatozoa in the experiment of Taylor et al. (2008) could be that they were damaged during ex vivo incubation and subsequently retained within the female tract. However, this possibility is quite unlikely because if damaged sperm cells increase their binding ability to UEC, the number of non-viable spermatozoa would also decrease after flushing, and Taylor et al. (2008) did not report changes in the number of non-viable spermatozoa.
Regarding the role of sperm binding to UEC, Taylor et al. (2008) have hypothesised that the retention of sperm cells in the uterus could protect the viable spermatozoa from being removed with the backflow, or that it plays some role in sperm maturation. As such, this phenomenon should be considered as a positive selection process. On the other hand, a negative-selection process might also exist that would consist of a certain part of the viable sperm population being actively prevented from reaching the oviduct. Thus, given that the preference of neutrophils to interact with viable spermatozoa has been reported (Taylor et al. 2008), a negative-selection process involving subsets of viable sperm cells unable to attach UEC has been proposed. Although this hypothesis suggests that these spermatozoa would not be considered suitable for fertilisation, we must also say that finding a possible biological meaning for this process is quite difficult and speculative. Another possible explanation for the role of sperm binding to UEC is that spermatozoa might induce signals favouring subsequent inflammatory responses. Intriguingly, Rozeboom et al. (1999), comparing the influx of neutrophils after insemination between extenders with and without spermatozoa, observed an increase in neutrophils in the former but not in the latter.
Finally, seminal plasma significantly inhibits sperm binding ability to UEC and to neutrophilic granulocytes. This indicates its important protective role and also shows its relevance, which has to be considered for AI, especially when using low dosages of spermatozoa.
6.4 Communication from Uterus to Ovarium
Uterus and ovary are in communication (Michael and Schofield 1969), but the exact mechanism is unknown. A possible signal transfer from the uterus to the ovary could involve cells present in the uterus (epithelial and others, including spermatozoa) that would release or lead to release cytokines such as the granulocyte–macrophage colony-stimulating factor (GM-CSF) and the TNF-α (Schuberth et al. 2008). Local mediators would then reach the ovarian stroma and pre-ovulatory follicles and would bind to receptors expressed on the surface of ovarian cells. The physiological route for these cytokines would be the lymphatic ducts and the transfer from the uterine vein to the utero-ovarian artery (Schuberth et al. 2008).
This hypothesis is supported by previous observations. Thus, for example, boar seminal plasma stimulates uterine epithelial cells to secrete GM-CSF (O’Leary et al. 2004), and can induce the advancement of ovulation when contacting with the epithelium at the utero-tubal junction (Waberski et al. 1995, 1996, 1999, 2006) (Fig. 5.4).
Communication from uterus to ovary, where seminal plasma may contribute to the advancement of ovulation when contacting with the epithelium at the utero-tubal junction. In addition, seminal plasma also initiates the immune cell infiltration (CD4+, Treg cells and a small increase in NK cells) and de novo protein synthesis in the endometrium that may prepare the uterine environment for embryonic development. The biologically active molecules from uterine or seminal plasma origin can reach the ovarian and oviduct tissues directly or via the ovarian and uterine arteries. Abbreviations mean: OA Ovarian artery, UA Uterine artery, UV Uterine vein, T reg T regulatory cells, NK natural killer cells and AA arterio-arterial anastomoses connecting uterine and ovarian arteries (Kaczmarek et al. 2010, Reproduced with permission)
6.5 Distribution of Spermatozoa Within the Intrauterine Environment, UTJ, and Oviduct After Artificial Insemination: CAI, IU and DIU
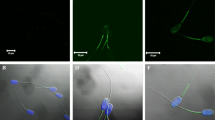
Tummaruk et al. (2007) and Tummaruk and Tienthai (2010) have investigated the number of spermatozoa in the crypts of the utero-tubal junction (UTJ) (Fig. 5.5) and the oviduct of sows, approximately 24 h after intrauterine insemination (IU; Belstra 2002) and deep intrauterine insemination (DIU; Martínez et al. 2001a, b; Vázquez et al. 2005), and compared them with that of (conventional) cervical artificial insemination (CAI). These studies have been the first to show, by using the histological examination technique, the distribution of spermatozoa in the UTJ, caudal isthmus, cranial isthmus and ampulla of the oviduct in sows after low-dose IU and DIU, compared with conventional AI. Accordingly, these authors have observed that the number of spermatozoa in the UTJ and caudal isthmus depend on the insemination technique used (2296 in conventional, 729 in post-cervical/IU, and 22 in DIU). Notwithstanding, they have observed that most of the viable spermatozoa are located in groups in the epithelial crypts within the oviduct in pre- and peri-ovulatory periods during standing oestrus (Mburu et al. 1996 ; Sumransap et al. 2007). These spermatozoa will remain uncapacitated until ovulation takes place (Rodríguez-Martínez et al. 2005).
Distribution of spermatozoa in epithelial crypts of the utero-tubal junction (UTJ) of a sow approximately 24 h after insemination at a 100× magnification, and b 400× magnification. Abbreviations mean: SP spermatozoa, RBC red blood cell, E epithelium and S subepithelium. (Tummaruk and Tienthai 2010, Reproduced with permission)
When using conventional and post-cervical AI, the spermatozoa are found on both sides of the UTJ and caudal isthmus (Tummaruk and Tienthai 2010). In contrast, DIU results in a significantly diminished number of spermatozoa in the sperm reservoir (UTJ and caudal isthmus) after 24 h of insemination compared with AI and IU. This lower number of sperm cells in the sperm reservoir is associated with decreased litter size compared to conventional AI (Martínez et al. 2001a, b, 2006; Vázquez et al. 2005). Moreover, partial and unilateral fertilisation in sows is higher when using DIU than when using CAI (Martínez et al. 2006). According to Tummaruk and Tienthai (2010), this finding may be related to the formation of the sperm reservoir on only one side.
Finally, and still regarding with sperm distribution throughout female reproductive tract, it is worth noting that the formation of the sperm reservoir relies on both spermatozoa and seminal plasma components (Rodríguez-Martínez et al. 2005) as a certain number of them are passively transported throughout the uterine lumen and escape from phagocytosis. In addition, Rodríguez-Martínez et al. (2005) have also shown that within the boar ejaculate, the first 10 ml of the sperm-rich fraction contain a sperm subpopulation that is more effective in terms of sperm reservoir colonising than the rest of the fraction.
7 Sperm Transport Throughout the Uterus
7.1 Introduction
Semen is deposited in the female reproductive tract, the location depending on the species (Suarez and Pacey 2006) and whether natural mating or AI is used. For example, in cattle, semen is deposited in the cranial segment of the vagina in natural mating (First et al. 1968), while spermatozoa are left in the uterine body in AI. In porcine species, semen is deposited in the narrow cervical canal in natural mating and CAI (Rodríguez-Martínez 2007). Alternative techniques of artificial insemination involve other places of semen deposition; thus, intrauterine insemination leaves semen in the uterine body, and deep intrauterine insemination leaves it in the vicinity of the utero-tubal junction (UTJ) (see also Chap. 12).
Three phases can be distinguished in semen transport through the sow tract:
-
1.
A passively transuterine transport immediately after semen deposition.
-
2.
The colonisation of the lower oviduct, forming the semen reservoir.
-
3.
A slow release from the reservoir towards the venue of fertilisation, i.e. the ampullary-isthmic junction, which is a peri-ovulatory event (Barratt and Cooke 1991).
Thus, semen is first deposited in the cervix of the sow after natural mating or CAI (First et al. 1968; Langendijk et al. 2005). From this point, it is flushed into the lumen of the uterine body and the spermatozoa are then transported through the uterine horns up to the oviducts, where fertilisation takes place.
Although the exact mechanism by which male gametes are transported through the mammalian uterus is not completely known (Rousseau and Ménézo 1993), it seems that it consists of a passive process relying on the flow of sperm-containing fluids in the uterine lumen. This process appears to be driven by gravitational force and uterine contractility rather than by the intrinsic motility of spermatozoa (Langendijk et al. 2002a, 2005). Thus, because the contractile activity of the uterus in sperm transport plays such a key role (Suarez and Pacey 2006), the next subsections are focused on myometrial activity and on the factors that modulate this activity.
Another important aspect to be considered in sperm transport throughout the uterus is that this organ provides a hostile environment for spermatozoa due to phagocytosis. However, phagocytosis inhibitors such as caffeine and calcium increase the number of viable spermatozoa when added to insemination doses (Woelders et al. 2000; Woelders and Matthijs 2001). Only when spermatozoa arrive at the end of the uterine horns, i.e. when the sperm cells reach the UTJ and the first part of the oviduct, are they safe, because they can form the sperm reservoir by directly contacting the oviductal epithelial cells (Töpfer-Petersen et al. 2002).
Finally, we must mention that it is not completely known how spermatozoa survive in the uterus despite sperm-UEC interaction, or which role myometrial contractions play in sperm transport throughout the uterine horns. It also remains unclear what happens when the sperm cells stay in the uterine horns for a long time and whether this fact may affect their ability to enter the oviduct and to fertilise.
7.2 Contractile Activity of the Uterus
One important aspect in swine reproductive physiology is uterine activity around oestrus. This activity has been studied using invasive (Brüssow et al. 1988; Claus et al. 1989) and non-invasive methods (Von Döcke and Worch 1963; Langendijk et al. 2002b), both of which have advantages and disadvantages, as Langendijk et al. (2005) have successfully reviewed.
Specifically, by using non-invasive methods for monitoring the intraluminal pressure of the uterus, Von Döcke and Worch (1963) inserted a fluid-filled balloon at the cervical end of the uterus, while Langendijk et al. (2002b) adapted a catheter developed by Hazeleger and Kemp (1994) for non-surgical embryo transfer in sows. From these and other studies, several authors have observed that uterine activity varies during the oestrus cycle, distinguishing the following four different stages:
-
1.
From 2 to 4 days before oestrus, the myometrial activity, in terms of frequency and amplitude of contractions, is low. In a study by Langendijk et al. (2002b), about 50 % of females did not show contractility within this period, whereas the others presented a lower frequency and amplitude of contractions when compared with uterine activity during oestrus.
-
2.
When oestrus is coming, both the number of sows showing myometrial activity and the frequency and the amplitude of myometrial contractions increase.
-
3.
During oestrus, the number of sows showing myometrial activity and the frequency and amplitude of contractions are at their highest. At this time, the amplitude and duration of electrical bursts are more pronounced and the myometrium is more sensitive to electrical inputs (Claus et al. 1989). This leads to an increase of sperm transport.
-
4.
After oestrus, myometrial activity decreases again.
7.3 Uterine Contractions: Cervico-Tubal and Tubo-Cervical
As stated, sperm transport is a passive process. When the spermatozoa are deposited in the cervix, their passage into the uterine body depends on gravity and on the relaxation of the myometrium. The luminal pressure of the uterus consists of baseline pressure in the relaxed state, with periodical increases owing to electrical bursts.
Stimulating uterine contractions can extend the time needed for the uptake of semen during insemination (Langendijk et al. 2002c) and can also increase semen backflow so that this may delay the influx of semen from the AI catheter into the uterus. In this case, it is very important to keep in mind that a high ejaculate backflow may reduce farrowing rates (Steverink et al. 1998; Langendijk et al. 2002c), especially in some unfavourable situations such as using low sperm concentration or low ejaculate volume, or when there is a long period of time between insemination and ovulation.
Uterine contractions are involved in the transport of spermatozoa from the cervix to the UTJ through the uterine horns, and they also take part in the transfer of sperm cells into the oviducts (Baker and Degen 1972). The main role of the uterine contractions is evident when myometrial contractility is reduced as a result of administering a β-adrenergic agonist before insemination. In this case, spermatozoa spend more time in their transport through the uterine horns. This fact provokes, in turn, a decrease in both the number of spermatozoa at the oviduct and fertilisation rates (Langendijk et al. 2002c).
Contraction waves originate at the cervical and tubal ends of the uterine horns, where Taverne (1982) has observed the most pacemaker activity during parturition. Depending on the direction, three types of uterine contractions can be distinguished: tubo-cervical, cervico-tubal and undirected contractions (Brüssow et al. 1988); all three simultaneously observed during oestrus. In all cases, the direction of contractility seems to be a coordinated process, which depends on the phase of oestrus and can be affected by stimuli involved in mating. In the propagation of these contractions, communication between myometrial cells plays a key role. This cell-to-cell communication depends on the number of gap junctions, which increases during the oestrus when oestradiol levels are high (Verhoeff et al. 1986).
The tubo-cervical directed contractions are important to eject the seminal plasma after mating and are also important in the distribution of spermatozoa in the two uterine horns (Woelders and Matthijs 2001), while the cervico-tubal contractions play a key role in sperm transport towards the oviduct (Langendijk et al. 2005).
7.4 Female Factors Influencing Myometrial Activity
Myometrial activity is affected by several factors that are intrinsic to the female. In this respect, it is noteworthy to mention the modulating role of female hormones (oestrogens, progesterone and LH), parity and the individual variation within and among animals (First et al. 1968).
7.4.1 Influence of Female Hormones
Langendijk et al. (2005) have mentioned the modulating role of oestradiol and progesterone on the tissue and on plasma levels in sows. Thus, oestrogens have been reported to increase uterine activity, while progesterone exerts a decreasing effect. In other mammalian species like human, oestrogens increase both the myosin content of myometrial cells (Michael and Schofield 1969) and the pacemaker activity of myometrial cells (Finn and Porter 1975), while in sheep they increase gap junctions among myometrial cells, as mentioned above (Verhoeff et al. 1986).
In swine, oestradiol and oxytocin affect the levels of PGF2α released by the uterus (Edgerton et al. 2000) so that uterine activity is modulated upstream by oestradiol and oxytocin. However, the exact role of both oxytocin and prostaglandins is not exactly known, since, whereas some authors have reported a diurnal fluctuation in the levels of these hormones (Edgerton et al. 2000), others have observed that this variation is quite low (Schille et al. 1979).
Apart from oestradiol, progesterone and oxytocin, LH also seems to be involved in the regulation of uterine activity (First et al. 1968; Ziecik et al. 1992) since LH/human chorionic gonadotropin (hCG)-receptors are present in the myometrium during oestrus, and (Flowers et al. 1991) have demonstrated that hCG suppresses the myometrial activity of the swine uterus. However, it is still unclear whether the high levels of LH during the pre-ovulatory period exert some effects on the regulation of uterine activity.
Finally, the activity of the myometrium not only depends on the levels of circulating hormones but also on the sensitivity of their receptors (Smith and Toft 1993; Weigel 1996). In this regard, Thilander et al. (1990) and Wathes et al. (1996) have reported that numbers of receptors interacting with oestradiol, progesterone and oxytocin depend on the circulating levels of oestradiol and progesterone.
7.4.2 Influence of Parity: Primiparous Versus Multiparous
Apart from the hormonal modulating role, myometrial activity is also affected by parity. Indeed, the frequency of contractions during oestrus has been reported to be higher in primiparous sows than in multiparous sows, while the amplitude of uterine contractions is lower in the former (59 mm Hg vs. 45–51 mm Hg) (Langendijk et al. 2005).
7.4.3 Inter- and Intra-Individual Variations in Female Uterine Activity
On the other hand, there is high inter-individual variation in uterine activity in swine (Langendijk et al. 2002a, b). This variation is detected in the frequency and amplitude of contractions throughout the entire oestrous cycle, i.e. not only during the standing oestrus but also within the period around oestrus. Thus, sows presenting a relatively high level of uterine activity during the days before oestrus have also been reported to display a relatively high level of uterine activity during oestrus. Furthermore, when sows present a longer heat (from 2 to 3 days), they also maintain the high level of uterine activity during oestrus for a longer period of time. In these sows, the decline of oestradiol levels in peripheral blood occurs later.
Apart from the mentioned inter-individual variation, intra-individual differences also exist when comparing oestrous cycles within the same sow (Langendijk et al. 2002a, b). Thus, and according to the above-mentioned, the inter- and intra-individual variations reasonably depend on individual factors such as circulating hormone levels, sensitivity of their receptors and sow parity.
7.5 Boar Factors Influencing Myometrial Activity of Females
Apart from female factors influencing uterine activity, there are male factors that are related to sexual stimuli before, during and after copulation. Langendijk et al. (2005), reviewing the state-of-the-art of this issue, have distinguished between two boar-dependent stimuli: sensory and seminal plasma-related stimuli.
7.5.1 Sensory Stimuli
Within sensory stimuli, at least four different factors can be distinguished: visual (boar presence), olfactory, tactile and auditory.
First, one of these sensory stimuli is the visual presence of a boar, which increases the levels of oxytocin in the sow and her myometrial activity (Claus and Schams 1990). However, these boar-mediated effects are only observed in those sows that have a below average frequency of uterine contractions (Langendijk et al. 2002a). On the other hand, the increase in myometrial activity due to the presence of a boar is not only related to the magnitude of oxytocin release, although the same effect is observed when sows are treated with oxytocin injected intramuscularly (Langendijk et al. 2002c).
As for the effect of olfactory stimulation on uterine activity, a review of the literature provides inconsistent results. Thus, whereas some papers report that olfactory stimulation with 5-α-androstenone increases the release of oxytocin and uterine activity in a similar fashion to what occurs during mating (Maffeo et al. 1993; Mattioli et al. 1986), others (Langendijk et al. 2002c) observe no effect of olfactory stimulation on oxytocin release and myometrial activity.
As far as tactile stimulation is concerned, touching the back and the flanks of the female in the absence of a boar triggers receptive behaviour but has no effect either on oxytocin levels or on uterine activity (Langendijk et al. 2002c). Moreover, although tactile stimulation of the female cervix, either by using normal and transcervical catheters (Claus and Schams 1990), or by massaging the vulva and the clitoris, does not alter oxytocin release, stimulating the cervix does enhance uterine activity. Furthermore, flushing a significant volume of semen extender or saline solution also stimulates myometrial activity (Claus et al. 1989) but this increase seems to be due to the effect of catheter insertion rather than to the infused volume (Langendijk et al. 2005).
Since this cervical stimulation effect is independent on oxytocin release, modulation of myometrium contractility appears to involve adrenergic and/or cholinergic pathways (Langendijk et al. 2005). Accordingly, the swine myometrium presents adrenergic and cholinergic receptors (Claus and Schams 1990), adrenergic receptors being mainly present in the longitudinal muscle layer and cholinergic receptors being mainly located in the circular muscle layer (Taneike et al. 1990).
The effect of the adrenergic and cholinergic receptors on myometrial contractility depends on the type of receptor. Therefore, contractility is initiated when cholinergic and α-adrenergic receptors are stimulated, while β-adrenergic receptors suppress myometrial contractility (Langendijk et al. 2005).
7.5.2 Seminal Plasma-Related Stimuli
The effect of seminal plasma on uterine activity is clearer than that of sensory stimulation (Langendijk et al. 2005). Seminal plasma stimulates myometrial contraction in vitro, owing to the composition of seminal plasma, which contains oestradiol (Claus 1990; Langendijk et al. 2002c).
Therefore, after copulation and AI, the oestrogens present in the boar ejaculate stimulate the endometrium, inducing an immediate release of PGF2α. Moreover, the effect of oestrogens on LH and follicular PGF2α is likely to contribute to the timing of ovulation in response to mating (Claus 1990; Waberski et al. 2006), and this stimulation of myometrium contractility mediated by the oestrogens that boar ejaculate contains is maintained for a few hours. Although the intrauterine infusion of oestrogens at the same level as the boar ejaculate causes similar effects on uterine contractility, the effect mediated by mating with a boar has a higher extent (Claus 1990; Langendijk et al. 2005).
In short, oestrogens in seminal plasma have a clear effect on endometrium and uterine activity by stimulating PGF2α-release during the standing oestrus, while, from a review of the literature, the effects of sensory stimulation are, in contrast, less clear and sometimes controversial. In fact, although it is quite evident that both cervical stimulation and the presence of a boar increase uterine activity, it is less clear that tactile and olfactory stimuli have any effect (Langendijk et al. 2005).
7.6 Relevance of Ejaculate Volume in Sperm Transport in the Female Reproductive Tract
Another relevant issue concerns the putative functional role of the ejaculate volume. In AI, the infused ejaculate volume depends on the catheter used, thereby distinguishing among conventional or cervical (CAI) and intrauterine insemination (IU), this latter being either post-cervical (post-CAI) or deep intrauterine (DIU) (see Chap. 12).
While CAI requires high semen volume (≥80 mL), less volume is needed when using IU (Casas et al. 2010; Martínez et al. 2001a, b). These data underline the importance of the functional role of the ejaculate volume on fertility and prolificacy rates, because when CAI is performed using low semen volume, fertility rates decrease. This fact indicates that large semen volume is needed in CAI, because it probably plays a role in flushing the spermatozoa from the cervix into the uterine body (Langendijk et al. 2005). This would prevent the retention of spermatozoa in the cervical folds. In contrast, the sperm volume would not play such a key role in the transport of spermatozoa throughout the uterine horns and the oviducts, since good farrowing rates are obtained when much lower volumes of semen (~30 mL in IUI, ≤15 mL in DUI) are deposited in the uterus.
On the other hand, a large amount of semen in the sow’s genital tract may be required to accelerate the transport of male gametes from the uterine body to the tubal end of the uterine horns (Langendijk et al. 2005). Given that phagocytosis of spermatozoa occurs in the uterine horns and this reduces the number of available sperm cells, large semen volume may be needed, therefore, when insemination takes place before ovulation (Woelders and Matthijs 2001).
7.7 Effects of Stimulating Myometrial Contractility on Sperm Transport and Farrowing Rates
Stimulating myometrial contractility can also positively affect farrowing rates, i.e. fertility and prolificacy, and these effects can be assessed through two different approaches. The first consists of infusing seminal plasma before insemination, which stimulates uterine activity, as mentioned above. By using this methodology, Viring and Einarsson (1980) observed that sperm transport increased at the oviduct from 1 to 6 h after insemination, while Waberski (1996) reported an increase in the number of accessory spermatozoa in the zona pellucida (ZP) of 3- to 4-day-old embryos without noting any effect on fertilisation rates.
The other approach for stimulating uterine activity consists of combining low semen volume with an intravenous injection of a high dose of oxytocin after insemination. This approach seems to improve the percentage of fertilised oocytes (from 58 to 72 %) (Stratman et al. 1959).
Despite the positive effects mentioned of stimulating myometrial contractility on sperm transport and fertilisation, this also entails disadvantages such as the increase in backflow. Thus, although Peña et al. (1998, 2000) have demonstrated that the hormonal stimulation of myometrial contractility at the time of insemination increases the farrowing rates during the low-fertility season, stimulating uterine contractility can also decrease sperm transport to the oviducts and fertilisation. Furthermore, Hazeleger and Kemp (1994), in another study, infused a high dose of cloprostenol before insemination, and negative rather than positive effects were observed. Thus, the degree of stimulation is key here and a critical concept.
In short, uterine contractility under physiological conditions plays an important role for rapid transport of sperm cells throughout the uterus and up to the oviducts, because the spermatozoa are safer in the oviducts than in the uterine horns (Langendijk et al. 2005). However, it is important to find the right balance when stimulating myometrial contractility, because it can increase sperm transport and fertilisation rates when used at a suitable level, but it can reduce the uptake of semen by the uterus and increase the risk of backflow at a higher level.
7.8 Effects of Prostaglandins on Reproductive Performance: Myometrial Contractility, Sperm Transport and Quality
Prostaglandins (PGs) are eicosanoids that are widely distributed in vertebrate tissues and play multiple roles in a wide array of physiological processes (Kingsley et al. 2005; Flower 2006). These hormones are produced by the bis-dioxygenation of arachidonic acid (20:4) to form hydroperoxy endoperoxide (PGG2), followed by the reduction of the PGG2 to hydroxyl endoperoxide (PGH2), in a process catalysed by cyclooxygenases. Hydroxyl endoperoxide is then transformed by different enzymes to PGs and thromboxane A2 (Kingsley et al. 2005). Such cyclooxygenases are present in the apical region of the head, the post-acrosomal region and the midpiece of the tail of ejaculated and epididymal bovine spermatozoa, as immunohistochemical studies have shown (Shalev et al. 1994).
Prostaglandins are related to several reproductive processes, being present in seminal fluid (Templeton et al. 1978; Kaczmarek et al. 2010) and in cervical mucus (Charbonnel et al. 1982). Human spermatozoa are even able to synthesise prostaglandins (Roy and Ratnam 1992) and, in bovine, spermatozoa have even been reported to induce prostaglandin synthesis and secretion in oviductal epithelial cells (Kodithuwakku et al. 2007). In vitro, PGs produce different effects on tubal smooth muscle because prostaglandin F2α (PGF2α) increases tubal muscle contractility (Pérez-Martínez et al. 1998), whereas prostaglandin E2 (PGE2) inhibits the contraction of circular muscles (Lindblom et al. 1978). However, both are needed since the transport of the embryo and the communication between the embryo and the oviduct involves prostaglandin action through PGE2 and PGF2α receptors (Mwanza et al. 2002a; Wanggren et al. 2006; Kaczmarek et al. 2010) (Fig. 5.4).
On the other hand, some prostaglandins affect sperm function (PGE1, PGE2, 19-OH-PGE, 19-OH-PGF and PGF1α), while others do not, or just to a lower extent (PGF2α) (Gottlieb et al. 1988; Maes et al. 2003; Yeste et al. 2008). Indeed, prostaglandins E1 and E2 (PGE1 and PGE2) increase the velocity and the penetrating ability of human spermatozoa (Aitken and Kelly 1985), thereby changing their functional competence. Moreover, seminal plasma and follicular fluid contain PGE1 and PGE2 that promote a Ca2+-influx in human spermatozoa (Blackmore et al. 1990; Baldi et al. 1991; Joyce et al. 1987; Margalioth et al. 1988; Thomas and Meizel 1988; Shimizu et al. 1998), and in the case of PGE1 acts as well as an in vitro capacitating factor for mouse spermatozoa (Herrero et al. 1997). Prostaglandin F1α reduces sperm motility, while 19-OH-PGE increases sperm motility and penetration ability, and 19-OH-PGF diminishes ATP concentration in human spermatozoa (Bendvold et al. 1984).
As far as the hormone PGF2α is concerned, it has been used in swine operations for the synchronisation and induction of farrowing and to increase the libido of boars (Hawk 1983; Estienne and Harper 2004; Mwanza et al. 2002b; Szurop et al. 1986). This hormone contributes like other components of seminal plasma (Waberski et al. 1996, 1999, 2006; Kaczmarek et al. 2010) to the timing of ovulation in response to mating in sows (Claus 1990) and, as stated before, it is an important smooth muscle contractile agent that exerts a significant uterotonic effect via the specific PGF2α-receptor that has been identified in the myometrium of humans, swine, sheep and rats (Friel et al. 2005). Prostaglandin F2α binds to the PGF2α-receptor and a signal transduction pathway, which leads to the mobilisation of intracellular Ca2+, is then activated (Olson et al. 2003). The role of this mechanism is so important that failure in parturition occurs when the PGF2α-receptor gene is knocked out (Sugimoto et al. 1997). Furthermore, significant changes in the plasma concentration of PGF2α are observed within 15–21 min after starting stimulation and AI, reaching a plateau after 30 min (Madej et al. 2005). The effects of oxytocin are partially mediated by PGF2α, which also augments the expression of an oxytocin receptor (Mirando et al. 1995).
All this background finds its practical application in AI procedures. Indeed, one strategy for increasing fertility outcomes consists of adding different substances to cooled or frozen seminal doses in order to improve their storage, maintain their function and survival and/or increase farrowing rates (Yeste 2008). Accordingly, the addition of PGF2α to extended semen used in AI increases farrowing rates (Gustaffson et al. 1975; Gamcik et al. 1980; Hawk 1983; Kos and Bilkei 2004) because it enhances myometrial contractility (Gil et al. 1998; Cheng et al. 2001; Kos and Bilkei 2004; Friel et al. 2005). However, some concentrations of PGF2α can be cytotoxic (Maes et al. 2003; Yeste et al. 2008) and this is for boar spermatozoa when added to extended seminal doses at concentrations higher than 12.5 mg·100 mL−1. Sperm viability drops dramatically above this threshold and the reduction in general sperm motility, in specific kinematic parameters (VSL, VCL and VAP) and in the osmotic resistance of spermatozoa is very significant. In contrast, PGF2α concentrations of 2.5, 5 and 10 mg·100 mL−1 are not harmful to spermatozoa and the addition of 5 mg of PGF2α·100 mL−1 has still been reported to have a positive effect on maintaining sperm viability after 6 and 10 days of storage in a short-term extender at 15 °C (Yeste et al. 2008).
8 Reproductive Immunology in the Female Tract
8.1 Introduction
Spermatozoa within the uterus are not only able to attach to UEC but they can also interact and be phagocytosed by resident leucocytes. This may explain the reduction in the number of spermatozoa in the sperm population flushed out from the sow. However, the number of these leucocytes appears to be too low to explain solely the loss of so many spermatozoa in such a short time period. Schuberth et al. (2008) have reviewed the state-of-the-art of reproductive immunology in sows and gilts. In this regard, some crucial aspects have to be kept in mind.
First, insemination is followed in many species by attraction and activation of leucocytes, with subsequent biological consequences (Robertson 2007). Nonetheless, relevant advances have been made in this field in recent years, but more research is still required to unveil the exact molecular mechanisms that regulate the post-mating inflammatory reaction.
Second, the immune response to copulation depends on the species, amount and composition of seminal plasma, semen extenders and number of spermatozoa (Schuberth et al. 2008). Thus, there are differences among species in the volume of ejaculate reaching the uterine lumen directly or loosely after passage through the cervix. As an example, the immune response induced by spermatozoa triggers a neutrophil influx similar to the one induced by bacteria in equine species (Gorgens et al. 2005a).
The interaction of spermatozoa with neutrophilic granulocytes has been described in several species, including pigs (Matthijs et al. 2000, 2003; Rozeboom et al. 2001), horses (Troedsson et al. 2005), ruminants (Strzemienski 1989) and humans (Blanco et al. 1992). However, in this direct neutrophil-spermatozoa interaction, neutrophilic granulocytes preferentially target aged, non-viable or capacitated spermatozoa, as described for porcine and other mammalian species like humans (Vogelpoel and Verhoef 1985; Eisenbach 2003; Matthijs et al. 2003).
On the other hand, as previously stated, some aspects of this interaction are known, whilst others remain unclear. Despite complementary factors, natural anti–sperm antibodies or carbohydrate–protein interactions have been suggested in this regard (Matthijs et al. 2000; Rozeboom et al. 2001; Troedsson et al. 2005). It remains unknown whether the interaction of spermatozoa with neutrophilic granulocytes is due to random attachment or involves sperm-specific molecules that are recognised by the leucocytes. From these three possible interactions, neither complementary factors (Matthijs et al. 2000; Rozeboom et al. 2001) nor natural anti-sperm antibodies (Kalaydjiev et al. 2002; Troedsson et al. 2005) seem to be involved in pigs, even though more research is needed on this point. The other speculated possibility would involve carbohydrate-mediated interactions of spermatozoa with neutrophilic granulocytes (Ofek and Sharon 1988), since the former exhibit lectins on their surface, which mediate, in turn, interaction with other cells like OEC (Green et al. 2001; Ekhlasi-Hundrieser et al. 2005; Töpfer-Petersen et al. 2002, 2008; Wagner et al. 2002). However, more recently Taylor et al. (2008) have shown that lectins do not seem to mediate binding between neutrophilic granulocytes and spermatozoa. In short, viable spermatozoa can bind neutrophilic granulocytes, while non-viable male gametes cannot, so that this interaction seems to be specific rather than random and involves membrane surface molecules, although their exact nature has remained hitherto unveiled. This topic will be taken up again in a specific subsection of the present chapter (Sect. 5.8.4).
Insemination is the starting point of communication with the female organism, which allows optimal pregnancy success. Immune reactions in response to mating/AI have an influence on the ovulation process, sperm selection, induction and maintenance of immunological tolerance regarding paternally derived antigens, restructuration of endometrial tissue for implantation and placentation and immunological support of foetal tissues during pregnancy (Robertson 2005; 2007) (Fig. 5.6). As an example of this phenomenon, boar semen may specifically accelerate ovulation in sows, as Waberski et al. (1995, 1997) showed by using a surgical model that consisted of gilts with a clamped uterine horn. In this experiment, these authors observed that semen accelerated ovulation only on the infused uterine side, while it did not affect ovulation timing on the other one.
Actions of seminal plasma in the female reproductive tract. Active moieties in seminal plasma and associated with spermatozoa interact with cervical and uterinic epithelial cells at mating to induce synthesis of pro-inflammatory cytokines. These cytokines cause the recruitment and activation of inflammatory cells in the uterine endometrium, including macrophages, dendritic cells and granulocytes. The macrophages and dendritic cells have roles in remodelling of the endometrial tissue and in activating maternal immune tolerance of pregnancy. Neutrophils traversing the endometrial epithelium into the lumen act to clear debris and maintain uterine sterility. Epithelial cytokines activated by seminal plasma are also secreted into the luminal fluid, where they exert trophic actions on the developing pre-implantation embryo (Robertson 2005, Reproduced with permission)
Specifically, semen constituents induce a series of immunological reactions when contacting with cervical and uterine tissues (Figs. 5.4 and 5.6). This response seems to be logical, since semen is a foreign material for the female reproductive tract organism and the aim of the sow’s immune system is to eliminate it (Schuberth et al. 2008). In fact, the mucosal immune system in the female reproductive tract has to maintain a balance between the presence of commensal bacteria, sexually transmitted bacterial and viral pathogens, allogeneic spermatozoa and an immunologically distinct foetus (Wira et al. 2005; Ochiel et al. 2008). In this regard, it is worth noting that seminal plasma induces changes in cell populations of the uterine mucosa by increasing the amount of MHC class II-positive cells, which means an immediate and local cellular response against seminal plasma, especially at the UTJ (Waberski et al. 2006).
Epithelial cells that line the cervix, the uterus and the oviducts provide a first line of defence that confers continuous protection by providing a physical barrier as well as secretions that contain bactericidal and virucidal agents. These epithelial cells of the female reproductive tract are also able to respond to pathogens, in part through Toll-like receptors. Toll-like receptors (TLRs) are a broad family of innate immunity receptors that play critical roles in detecting and responding to invading pathogens. Thus, epithelial cells, macrophages, natural killer cells and neutrophils in the oviducts, uterus and cervix act via TLRs, which confer protection through the production of chemokines and cytokines. Chemokines and cytokines recruit and activate immune cells, as well as bactericidal and virucidal agents, which provide protection at times when adaptive immunity is down-regulated by steroid hormones to meet the constraints of procreation. Thus, in the female reproductive tract, TLRs enhance innate immune protection and, when necessary, contribute to the initiation of an adaptive immune response (Ochiel et al. 2008).
Finally, we must mention that some members of the TLR family (Tlr1–Tlr9) and some TLR-adapter proteins, such as TLR adaptor molecule 1 and NFKBIA, have also been indentified in some organs of the rat male reproductive tract (testis, epididymis and vas deferens) (Palladino et al. 2007). These TLRs have also been detected on epididymal rat spermatozoa (Palladino et al. 2008), while TLR2 and TLR4 have also been found in the membranes of human and mouse ejaculated spermatozoa (Fujita et al. 2011). In addition, bacterial endotoxins have been reported to negatively affect sperm function and survival by activating TLR-dependent pathways that lead to cell death. Therefore, TLRs appear to play important roles in innate immunity not only in female but also in male reproductive tract (Wira et al. 2005; Palladino et al. 2007, 2008; Fujita et al. 2011).
8.2 Variation of Local Resident Leucocytes During the Oestrous Cycle
The uterus has features of a mucosa-associated lymphoid tissue similar to other tracts, like the digestive tract. However, as mentioned above, the uterus undergoes cyclic changes, which affect not only the endometrium but also the leucocyte populations within the endometrium and the uterine lumen (Bischof et al. 1994; Kaeoket et al. 2001).
Regarding the surface epithelium of endometrium, lymphocytes are mainly found during oestrus and early dioestrus, while macrophages are mainly found at proestrus and oestrus (Kaeoket et al. 2001). As far as the submucosa is concerned, lymphocytes are the dominating cell type during all stages of the oestral cycle, especially at oestrus and early dioestrus, when they are more numerous. Moreover, there is a massive infiltration of neutrophils in the submucosa during proestrus and oestrus, but these immune cells are not observed during the other stages of the oestrus cycle (Stroband et al. 1986; Rodríguez-Martínez et al. 1990; Bischof et al. 1994; Kaeoket et al. 2001) (Fig. 5.3). These neutrophilic granulocytes form a resident population in the uterine lumen just before ovulation (Matthijs et al. 2003; Rozeboom et al. 1998, 1999).
Furthermore, a considerable variation in terms of this cell population has been observed among individuals. Thus, Schuberth et al. (2008) have reported that a leucocyte population within uteri of gilts varies before ovulation from 0 to 2.7 × 109 leucocytes, with variable fractions of monocytes and granulocytes. In fact, it still remains unclear whether these luminal leucocytes are significantly involved in the insemination-induced signalling cascade, even though this seems a reasonable assumption.
8.3 The Immunological Response After Mating/Insemination Within the Intrauterine Environment
8.3.1 The Influx of Neutrophilic Granulocytes
As stated, an inflammatory response in the female reproductive tract of several mammalian species including porcine (Lovell and Getty 1968; Matthijs et al. 2003; Rozeboom et al. 1999), mice (Robertson et al. 1996; Robertson 2007) and equine (Gorgens et al. 2005a, b) occurs after copulation/insemination. This response is characterised by an influx of neutrophils in the uterine lumen, which is usually the highest from 1 to 12 h after mating or AI. The duration of this peak also depends on the species, as Katila (1995) reported an elevated number of uterine leucocytes in mares up to 48 h after insemination. In fact, this potent neutrophil influx in the equine species may be related to the mentioned intensity of immune response, which is similar to the one observed in response to bacteria (Gorgens et al. 2005a).
Spermatozoa are chemotactic as previous studies conducted in mice, pigs and humans have shown (Yoshida and Yoshida 2011; Zuccarello et al. 2011; Li et al. 2012). Indeed, chemotaxis of spermatozoa towards ovum is a widespread phenomenon that occurs in most forms of life from plants to mammals and plays important roles in ensuring fertilisation (Yoshida and Yoshida 2011). In fact, the fundamental mechanisms underlying sperm chemotaxis seem to be common among all mammalian species and the intracellular calcium concentration is an important factor for the regulation of chemotactic behaviour in spermatozoa (Yoshida and Yoshida 2011). Related to this, ion channels, such as CatSper (a pH-regulated calcium-selective ion channel), KSper (Slo3), voltage-gated proton channel Hv1 or P2X2, have been found in human and/or mouse sperm tails and are also involved in sperm chemotaxis and sperm hyperactivation (Lishko et al. 2012). Recently, Zuccarello et al. (2011) have investigated chemotaxis in human spermatozoa and they have observed that SDF-1 (stromal cell-derived factor-1), a chemokine expressed in the oocytes, endometrium and follicular fluid, is involved in sperm chemotaxis, since its specific receptor CXCR-4 (chemokine CXC motif receptor 4) is present on the sperm head. When SDF-1 interacts with CXCR-4, sperm hyperactivation is induced and there is an increase of the intracellular calcium levels, without inducing the acrosome exocytosis (Zuccarello et al. 2011).
Schuberth et al. (2008) have proposed that spermatozoa may induce chemotaxis of neutrophils by binding to endometrial cells and/or present leucocytes. As mentioned, the interaction between spermatozoa and neutrophilic granulocytes has been reported in porcine, human and other mammalian species such as ruminants and horses (Strzemienski 1989; Blanco et al. 1992; Matthijs et al. 2000, 2003; Troedsson et al. 2005) (Fig. 5.6). However, it still remains unknown whether the interaction between both cell types occurs randomly or a specific binding exists. In this regard, Taylor et al. (2008) have reported that those sperm cells that interact with neutrophilic granulocytes have their membrane intact. Although membrane integrity does not seem the only functional parameter to be taken into account when assessing sperm-neutrophil interaction, these authors have observed that membrane-damaged spermatozoa do not bind polymorphonuclear neutrophils. In this context, sperm motility also seems to be involved in this interaction.
Finally, the interaction between spermatozoa and neutrophils also seems to be modulated by some seminal plasma components. In horses (Alghamdi et al. 2004) and in boars (Taylor et al. 2008; Li et al. 2012), seminal plasma reduces the chemotactic and phagocytotic activities of polymorphonuclear neutrophils when co-incubated with spermatozoa, while sperm diluted in semen extender does not. Here, it is also interesting to highlight the modulating role of seminal plasma constituents in sperm interactions since, as mentioned, they also inhibit the interaction of spermatozoa with uterine epithelial cells (see Sect. 5.6.3). All these aspects are discussed below.
8.3.2 Immune Mediators in Response to Spermatozoa and Seminal Plasma
After insemination, uterine and cervical cells synthesise and release cytokines, chemokines and other local mediators that are involved in the subsequent cellular response (Schuberth et al. 2008). These local mediators include cytokines, as granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-6 (IL-6), and chemokines (Robertson 2007; Schuberth et al. 2008). Accordingly, Pandya and Cohen (1985) in human, and Robertson et al. (2000) in mice have reported that pro-inflammatory factors stimulate the infiltration of uterine and cervical tissues by macrophages, dendritic cells and granulocytes. In humans, Jeremias et al. (1998, 1999) found that semen induced the expression of IL-10 (anti-inflammatory TH2-related cytokine) and HSP1A1 (HSP70) in peripheral blood mononuclear cells from women in co-culture, but did inhibit the expression of interferon-gamma (IFN-γ) when phytohaemagglutinin, a potent IFN-γ-inducing T cell mitogen, was added to co-culture conditions. Hence, these authors suggested that human semen is both an inducer of an anti-inflammatory (TH2) immune response and an inhibitor of pro-inflammatory (TH1) cell-mediated immunity. In pigs, O’Leary et al. (2004) also observed a response to mating/insemination, since seminal plasma induces the uterine expression of GM-CSF, IL-6 and monocyte chemotactic protein-1 (MCP-1), leading to monocyte and dendritic cell recruitment in the endometrial stroma.
Cytokines expressed by endometrial cells are considered as a part of the inflammatory response to insemination and are involved in the recruitment of leucocytes (Fig. 5.6). In swine, Rozeboom et al. (1998, 1999) and Matthijs et al. (2003) have proposed that cytokines are involved in the neutrophilic response to insemination. This hypothesis is in agreement with the findings obtained by Taylor et al. (2009b) who, in a study assessing the levels of mRNA of the five following transcripts: TNF-α, TGF-β, IL-10, CXCL8 and COX-2, demonstrated that only the concentrations of CXCL8-encoding mRNA in the presence of a commercial extender were correlated with the recruitment of neutrophilic granulocytes, while the other cytokines did not appear to be involved. As previously mentioned, seminal components elicit inflammatory responses in the female reproductive tract, including altered patterns of cytokine secretion, which have consequences for early embryo development and implantation (O’Leary et al. 2002). Indeed, boar seminal plasma appears to synergise in activating an inflammatory response and downstream changes in the female tract after insemination. In this regard, it is worth remembering that seminal plasma elicits endometrial changes, with induction of pro-inflammatory cytokines and cyclooxygenase-2, causing recruitment of macrophages and dendritic cells. Spermatozoa contribute by interacting with seminal plasma factors to modulate neutrophil influx into the luminal cavity. The cascade of changes in local leucocyte populations and cytokine synthesis persists throughout the preimplantation period (Robertson 2007).
As we have stated, the humoral response can be triggered by seminal plasma, spermatozoa, or by both. In humans and mice, it seems that it is the seminal plasma rather than sperm that is mainly responsible for triggering the immunological response (Robertson et al. 1996; Robertson 2007). Moreover, the components of the extenders (both short- and long-term) infused at the time of insemination may affect the immunological events, i.e. cytokine and chemokine release as well as cellular infiltration, and their nature and amount can vary, thereby triggering a different humoral response. Indeed, comparing the differential effects of seminal plasma, semen extender and sperm preparations on the endometrial expression of cytokines, Schuberth et al. (2008) observed that spermatozoa modulated the expression of interleukin-8, GM-CSF and TGF-β after 3 h post-insemination. However, the modulation of this cytokine expression did not appear to be exactly the same when spermatozoa were infused with seminal plasma or with semen extender. Related to this observation, Taylor et al. (2009b) found that the presence or absence of seminal plasma or extender also influenced the immune response in sows slaughtered after insemination. Accordingly, spermatozoa in semen extender (Taylor et al. 2009c) appear to stimulate the neutrophil influx in the uterine lumen, while seminal plasma suppresses it (Rozeboom et al. 1999; Taylor et al. 2009c). Specifically, seminal plasma components rather than sperm cells are thought to decrease the female immune response after insemination, but the down-regulated cytokines include pro- as well as anti-inflammatory mediators. This raises reasonable doubts about the real meaning of decreasing cytokine expression in response to insemination, since both pro- and anti-inflammatory mediators are down-regulated in response to insemination.
Seminal plasma attenuates neutrophil immigration and function both in vivo (Rozeboom et al. 1998, 1999) and in vitro (Bischof et al. 1994; Gorgens et al. 2005a). This effect has also been reported in humans (Binks and Pockley 1999), rats (Galdiero et al. 1989) and cattle (Gilbert and Fales 1996). In vivo, the effects of seminal plasma (with or without spermatozoa) are partially reflected by a lower but still significant up-regulation of interleukin-8 expression compared to semen extender alone (Taylor et al. 2008). Schuberth et al. (2008) have reported that seminal plasma effectively blocks the interleukin-8-induced neutrophil chemotaxis. However, in this study it appeared that the anti-chemotactic effect of seminal plasma was more related to the strongly induced agglutination of neutrophils, which hinders in vitro migration.
In their study, Taylor et al. (2009b) found an inhibition effect on cytokine expression due to spermatozoa, since the presence of sperm cells abrogated the extender- or seminal plasma-induced up-regulation of interleukin-10 (IL-10), transforming growth factor-β (TGF-β), TNF-α, CXCL-8 (IL-8), COX-2 and ALOX-5 (Fig. 5.7). Moreover, Jiwakanon and colleagues (2011) have recently compared how inseminations with or without spermatozoa in extended seminal plasma affected the expression of pro-inflammatory (interleukin-1β, interleukin-6 and GM-CSF) and suppressive (interleukin-10 and TGF- β) cytokines (Fig. 5.8). Both Jiwakanon et al. (2011) and Taylor et al. (2009b) report no differences in the use of seminal plasma with or without spermatozoa on the expression of the three pro-inflammatory (IL-1β, IL-6 and GM-CSF) and one suppressive (TGF-β) cytokines. In the case of Taylor et al. (2009b) the study was performed 3 h after AI, while in that of Jiwakanon et al. (2011), it was carried out between 5 and 6 h after AI. Notwithstanding, the literature has reported the presence of interleukin-6, interleukin-10 and TGF-β in the oviductal endosalpinx epithelial cell layer (Jiwakanon et al. 2010) and the presence of TGF-β in the endometrial cells (Moussad et al. 2002).
Expression of cytokines, the chemokine CXCL8 (interleukin-8), ALOX-5 and COX-2 in the endometrial tissue 3 h after artificial insemination in the absence (grey bars) or presence (black bars) of spermatozoa. Abbreviations means nil not inseminated, AH inseminated with 98 % (v/v) Androhep™ and 2 % (v/v) seminal plasma, SP inseminated with 98 % (v/v) seminal plasma and 2 % (v/v) Androhep™ (Taylor et al. 2009b, Reproduced with permission)
Immunohistochemical labelling of IL-6, IL-10 and TGF-β1 in the surface epithelium and sub-epithelial connective tissue of the porcine endometrium collected 5–6 h and 35–40 h after treatment. Arrows
 ) indicate endothelial cells and arrow heads (
) indicate endothelial cells and arrow heads ( ) indicate neutrophils (Jiwakanon et al. 2011, Reproduced with permission)
) indicate neutrophils (Jiwakanon et al. 2011, Reproduced with permission)
Within the seminal plasma molecules involved in the immune response, the cytokine TGF-β seems to be the main factor (O’Leary et al. 2011). This cytokine is present as an inactive form, which is activated in the female reproductive tract by plasmin and other enzymes after insemination (Robertson 2005, 2007). Then, TGF-β acts indirectly by inducing cytokine and chemokine expression in the female genital tract as has been shown in the murine uterus (Tremellen et al. 1998). In addition, mammalian seminal plasma contains eicosanoids, such as prostaglandin E2 (PGE2) and interleukin-8, which are the strongest chemotactic agents for neutrophils and cooperatively interact with TGF-β (Palter et al. 2001; Robertson 2005). Indeed, the effects of TGF-β depend on the endometrial induction of appropriate cytokines. Thus, the presence of interleukin-6 is required for TGF-β to induce the generation of interleukin-17 and the production of pro-inflammatory Th-17 cells, which in turn favours the induction of interleukin-8 (Rubtsov and Rudensky 2007) that plays a neutrophil-chemotactic function.
Comparing seminal plasma vs. semen extender without spermatozoa, the former has been reported to induce the expression of interleukin-10, while the latter up-regulates the expression of CXCL-8, TNF-α and COX-2 (Fig. 5.7). This suggests, as previously mentioned, that the media used for extending semen also affects cytokine expression in the sow tract after insemination. In the case of the suppressive cytokine interleukin-10, Taylor et al. (2009b) observed a higher mRNA expression in the presence of seminal plasma without spermatozoa than in inseminations with both seminal plasma and spermatozoa. Moreover, Taylor et al. (2009b) also reported that the mRNA levels encoding TGF-β were higher when AI with a long-term commercial extender without spermatozoa took place than when insemination was performed with the commercial extender containing sperm cells.
8.3.3 Immune Response to the Short- and Long-Term Extenders
Inseminating with extended sperm doses induces a higher influx of neutrophils than insemination of spermatozoa in seminal plasma, or seminal plasma or extender alone (Matthijs et al. 2003). The composition of the various commercial extenders differs among them (Yeste 2008; see also Sect. 10.2.1). Such heterogeneity entails a variety of immunological responses to insemination. In equine species, skim milk extender with egg yolk is strongly chemotactic for equine neutrophils in vitro (Gorgens et al. 2005a, b) and results in significant neutrophil migration into the uterus in vivo (Kotilainen et al. 1994). In pigs, Schuberth et al. (2008) have mentioned that a long-term extender (Androhep™, manufactured by Minitüb) can inhibit the migration of porcine neutrophils in vitro, but, in contrast, causes a massive and significantly higher influx of neutrophils when compared to seminal plasma, which also appears to be independent of the presence or absence of spermatozoa. According to Taylor et al. (2009b), the commercial extender (Androhep™) seems to induce leucocyte migration into the uterus owing to nonspecific irritation of epithelial cells or resident leucocyte populations rather than direct chemotaxis.
Both Taylor et al. (2009b), working with a long-term extender (Androhep™), and Jiwakanon et al. (2011), working with a short-term extender (BTS), have observed that commercial extenders with or without spermatozoa induce higher neutrophilic granulocyte migration into the uterine lumen than seminal plasma (Fig. 5.9). Indeed, in a study using a short-term extender (BTS) that involved suppressive cytokines (Jiwakanon et al. 2011), spermatozoa in BTS appeared to stimulate immune reactivity at about 35 to 40 h after insemination because of down-regulation in the expression of the suppressive cytokine TGF-β and interleukin-10 in the endometrium. These authors also observed that cervical stimulation alone also affects neutrophil infiltration because the presence of neutrophils in the endometrial subepithelial connective tissue, when stimulating the cervix after insemination, was higher than when inseminating with seminal plasma without spermatozoa. The response to this cervical stimulation is similar to the one derived from inseminating with seminal plasma and spermatozoa. This would be in agreement with Woelders and Matthijs (2001), who suggested that the intromission of a volume of liquid after insemination rather than the composition itself is responsible for recruiting neutrophils in the uterine lumen.
Distribution of neutrophils in the surface epithelium and sub-epithelial connective tissue (Sub-CNT) of the sow endometrium collected at: a 5–6 h (insemination with seminal plasma, spermatozoa in BTS, fresh semen in BTS or BTS) or b 35–40 h after treatment (insemination with seminal plasma, spermatozoa in BTS, BTS or catheter only used). Abbreviations mean BTS Beltsville thawing solution, OML one ocular micrometre length and OMA one ocular micrometre area (Jiwakanon et al. 2011, Reproduced with permission)
Both Taylor et al. (2009b) and Jiwakanon et al. (2011) have shown that insemination and/or inseminated components modulate cytokine expression in the swine endometrium too. Jiwakanon et al. (2011) observed that inseminations with seminal plasma in the absence of sperm cells decreased neutrophilic granulocyte infiltration in the gilt endometrium. In short, active seminal constituents, damaged and viable spermatozoa and semen extender, act in concert to modulate early and mid-term immune responses in the female genital tract, although Jiwakanon et al. (2011) did not find a clear relation between the cytokines studied and the presence of polymorphonuclear neutrophils. Moreover, all the mentioned data also suggest that gene regulation after insemination and, thus, the inflammatory response, also relies on the inseminate composition so that artificial extenders contain immune-stimulating agents, while seminal plasma appears to contain immune-suppressors. Thus, and when comparing the endometrial cytokine expression in presence and absence of sperm cells, the extender up-regulates the expression of four of the eight cytokines while the presence of spermatozoa leads to general inhibition (Taylor et al. 2009b) (Fig. 5.7).
8.3.4 Mechanisms and Modulation of Cytokine Expression
Seminal plasma components and spermatozoa modulate the cytokine expression in the endometrial cells (Fig. 5.6). As aforementioned, this modulating effect can be triggered by a direct binding sperm-UEC, by secretion of humoral factors, or by both. We proceed now to discuss these three speculated possibilities.
Direct contact between cells is one of the means by which sperm and epithelial cells interact, as Taylor et al. (2008) have observed. Their data indicate that an inhibition of cytokine expression is possible via direct contact between spermatozoa and endometrial epithelial cells, since up-regulation of such expression is absent when spermatozoa are infused into the uterus.
The other possible means consists of the secretion of humoral factors, which are involved in the communication between leucocytes. In this regard, Huleihel et al. (1999, 2000) have shown that seminal plasma contains cytokines (IL-1 and IL-6).
The degree and intensity of the immune response to insemination/mating is another question that merits discussion. After insemination, Taylor et al. (2009b) observed a significant but small increase in the expression of an array of cytokines, while the neutrophilic response was very strong when compared to baseline values. In agreement with these results, O’Leary et al. (2004) in swine and Gutsche et al. (2003) in humans also observed a quite low induction of cytokine expression in response to mating/exposure to seminal plasma. These findings have led to suggest that the endometrial cytokine response to insemination is moderate.
On the other hand, the mRNA expression of TGF-β in the oviduct mucosa (endosalpinx) is down-regulated when sows are inseminated in the absence of spermatozoa, while the presence of sperm cells up-regulates it (Jiwakanon et al. 2010). From these observations, it has also been concluded the different immunological response of endometrium and endosalpinx to spermatozoa obeys alternative pathways in different parts of the sow’s reproductive tract. Therefore, up-regulation of the suppressive TGF-β in the oviductal mucosa would be consistent with its function, which consists of protecting early embryos in the oviduct at the pre-implantation stages. In contrast, down-regulation of TGF-β observed in the endometrium would take place to clean the uterus before the embryos enter the uterine lumen (Jiwakanon et al. 2010, 2011).
Apart from the role of semen on the swine’s immunological response, the physiological status of the female is another factor to be considered in the immune response of endometrial tissues in insemination/copulation (Taylor et al. 2008). Taking into account various biological meanings of immune cells and immune mediators, the range of cellular responses to different inseminate preparations indicate specific immune responses and warrant a closer look at the level of regulating factors (Schuberth et al. 2008). In sows, an inflammatory reaction in the endometrium takes place after insemination. This process consists of the infiltration of leucocytes in the epithelium and in the sub-epithelial connective tissue (Kaeoket et al. 2003a) and of a massive influx of polymorphonuclear neutrophilic granulocytes into the uterine lumen within a few hours after insemination (Rozeboom et al. 1998; Kaeoket et al. 2003a). Upon arrival of the embryos at the uterus, i.e. between 2 and 3 days after ovulation, the neutrophils are eliminated (Kaeoket et al. 2003a). The immune response to insemination/mating also depends on the moment of insemination with respect to ovulation, i.e. whether insemination takes place before or after ovulation. This is related to the plasma levels of oestrogen and progesterone and to which of these is predominant (Kaeoket et al. 2002, 2003a, b), and may explain why the phagocytic role of neutrophils in the uterine lumen is not dominant in the early phases after insemination.
8.4 The Physiological Role of Neutrophils
8.4.1 Phagocytosis
Phagocytosis cleans the uterine environment after insemination (Robertson 2005). As previously stated, aged, non-viable or capacitated spermatozoa are preferentially targeted by neutrophilic granulocytes (Matthijs et al. 2003; Eisenbach 2003). This hypothesis is in agreement with the suggestion made by Tomlinson et al. (1992) that neutrophils take part in sperm cell selection in humans, removing superfluous, non-motile or damaged spermatozoa. Furthermore, in human (Vogelpoel and Verhoef 1985) and equine (Gorgens et al. 2005a) species, it has been reported that membrane-damaged sperm cells favour in vitro neutrophil migration.
It is not known exactly whether sperm cell phagocytosis is a selective or a random process, the former being more reliable than the latter. Indeed, under in vitro conditions boar spermatozoa are not phagocytosed by neutrophilic granulocytes when the antibodies and complements are absent (Schuberth et al. 2008), pointing to a selective process. Moreover, as stated above, neutrophils preferentially target sperm cells with intact mitochondrial membrane potential, which reflects a preference for certain sperm cell subpopulations.
8.4.2 Modulating the Decision of Immune Cells
Neutrophils play a key role in the recruitment, activation and programming of antigen-presenting cells (macrophages, dendritic cells) (Robertson 2007). These leucocytes secrete chemotactic signals that attract monocytes and dendritic cells, and influence macrophage differentiation to a pro- or anti-inflammatory state (Bennouna et al. 2003).
Although neutrophils release fewer molecules of cytokine than lymphocytes or macrophages, the number of neutrophilic cells during the inflammatory process is higher than that of mononuclear leucocytes and sources of cytokines.
Seminal plasma components, sperm cells and semen extender contribute to the regulation of neutrophil influx. Moreover, seminal plasma also takes the lead in the role that neutrophils play in the immunological response to insemination, so that it appears to shape the decision of neutrophils towards the activation or the suppression of other immune mechanisms (Schuberth et al. 2008).
8.4.3 The Biological Meaning of the Interactions Between Spermatozoa and Neutrophils
The biological meaning of the interactions between intact spermatozoa and neutrophils is still rather speculative. One of the most obvious reasons for spermatozoa–neutrophil interactions would be the initiation of sperm cell phagocytosis, even though this does not explain why neutrophils preferentially target viable spermatozoa. In this regard, it has been proposed that neutrophils play a negative-selection role removing those spermatozoa that are not able to bind epithelial cells and, therefore, are not considered fit for fertilisation (Schuberth et al. 2008). Another possible biological meaning is that the attachment of spermatozoa to epithelial cells and/or to neutrophils induces signals, triggering the subsequent inflammatory responses. This hypothesis is supported by previous reports showing that inseminating with extended spermatozoa induces an influx of neutrophils but inseminating with extender alone does not (Rozeboom et al. 1999).
Under natural conditions, when a high number of sperm cells reach the uterus a neutrophil-mediated selective process may not be so relevant. However, new insemination techniques (e.g. with sex-sorted spermatozoa) require considerably reduced sperm dosages and conventional AI does not yield a satisfactory reproductive performance under this condition. In contrast, fertility rates improve significantly when spermatozoa are deposited in the vicinity of the site of fertilisation (intrauterine and deep intrauterine insemination) (Vázquez et al. 2005). Intriguingly, the failure caused by using conventional insemination with low sperm dosages suggests the relevance of sperm interactions with the intrauterine environment and possible selection processes.
8.4.4 Maternal Immune Tolerance and Remodelling of the Uterine Tissue
In the swine tract, as in the female genital tract of other mammalian species (Moffett and Loke 2006; Robertson et al. 2009), the establishment of pregnancy needs a proper balance between the immune reaction against foreign pathogens and the tolerance to allo-antigens like those of the embryo (Taylor et al. 2009b). Thus, given that implantation and development of foetal trophoblastic cells and the pregnancy take place within the female reproductive tract, a control of potentially harmful maternal immune mechanisms against paternally derived antigenic epitopes is needed. In this regard, the term immunotolerance has been adopted to refer to different complex mechanisms, such as the induction of a variety of regulatory T-cells (Treg).
Within the uterine tissues, the concept of maternal immune tolerance is very important to explain the turn from a pro-inflammatory to an anti-inflammatory reaction. In this process, seminal plasma contains and/or induces the release of TGF-β and PGE2, which participate, along with an increase in progesterone levels, in the development of maternal immune tolerance (Schuberth et al. 2008; O’Leary et al. 2011). In fact, this mechanism is quite complex. Immediately after insemination, TGF-β favours an initial pro-inflammatory reaction (Schuberth et al. 2008) while it induces the generation of antigen-specific regulatory T-cells in regional draining lymph nodes at later stages (Rubtsov and Rudensky 2007). These antigen-specific regulatory T-cells seem to control the activation and proliferation of putative harmful effector-T-cells by producing interleukin-10 in the periphery (Zou 2006). Then, interleukin-10 inhibits the generation of pro-inflammatory T-helper 1 cells and enhances the generation of T-cells that produce anti-inflammatory cytokines (Schuberth et al. 2008). Generating anti-inflammatory cytokines inhibits the production of cytotoxic T-cells and complement-fixing antibodies. In this anti-inflammatory mechanism, PGE2, progesterone and progesterone-induced blocking factor are also involved (Zhang et al. 2007), stimulating the antigen-presenting cells to secrete interleukin-10 (Liu and Kelly 2008) and the activated T-cells to inhibit interleukin-12 release (Par et al. 2003).
Reorganisation of the endometrial tissue needs growth factors, necessary for angiogenesis (e.g. VEGF, vascular endothelial growth factor), and matrix metalloproteinases for the rearrangement and restructuring of the endometrial stroma (Aplin 2002; Curry and Osteen 2003; Das et al. 1997; Sunderkotter et al. 1994). Both seminal plasma and spermatozoa indirectly contribute to endometrial tissue remodelling, since this reorganisation depends on leucocytes (including semen-induced attracted neutrophils) and on local epithelial cells (Schuberth et al. 2008). Moreover, seminal plasma plays a dual role in controlling the sow’s immune response to mating/insemination, as it recruits macrophages and dendritic cells in the endometrial stromal tissue (McMaster et al. 1992; Robertson 2007; Robertson et al. 1996, 2009). First, macrophages and dendritic cells recruit foreign material, which then produce paternal antigen-specific T-cells that are inflammation-favouring T-cells. Later, macrophages and dendritic cells induce antigen-specific and regulatory T-cells (Robertson 2005, 2007). The seminal plasma-induced effects (i.e. cytokine expression, eicosanoid production) persist throughout early pregnancy (Robertson et al. 2006, 2009), are likely to be mediated by expansion of the Treg-cell pool and, thus, semen-induced attracted leucocytes modulate the course of inflammation and the mechanisms of tissue repair (Schuberth et al. 2008; O’Leary et al. 2011).
9 Conclusions
After mating or insemination, ejaculated spermatozoa are deposited in the female genital tract, being immediately and passively transported towards the oviduct throughout the uterus/uterine horns depending on the species. During this transit through the uterus, most of the male gametes are lost owing to immunological response, so that only a small number of them arrive at the distal portion of the isthmus, where they bind the oviductal epithelial cells. On the other hand, uterine epithelial cells are also able to bind viable spermatozoa, thereby suggesting that the uterus could protect viable spermatozoa from being removed with the backflow.
References
Aitken RJ, Kelly RW (1985) Analysis of the direct effects of prostaglandins on sperm function. J Reprod Fertil 73:139–146
Alghamdi AS, Foster DN, Troedsson MH (2004) Equine seminal plasma reduces sperm binding to polymorphonuclear neutrophils (PMNs) and improves the fertility of fresh semen inseminated into inflamed uteri. Reproduction 127:593–600
Aplin JD (2002) Endometrial extracellular matrix. In: Glasser JAS, Guidice LC, Tabibzadeh S (eds) The endometrium. Taylor and Francis, London, pp 294–307
Baker RD, Degen AA (1972) Transport of live and dead boar spermatozoa within the reproductive tract of gilts. J Reprod Fertil 28:369–377
Baldi E, Casano R, Falsetti C, Krausz C, Maggi M, Forti G (1991) Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl 12:323–330
Barratt CLR, Cooke ID (1991) Sperm transport in the human female reproductive tract-a dynamic interaction. Int J Androl 14:394–411
Belstra BA (2002) Review: intrauterine (transcervical) and fixed-times artificial insemination in swine. Ann Swine Report 25:2
Bendvold E, Svanborg K, Eneroth P, Gottlieb C, Bygdeman M (1984) The natural variations in prostaglandin concentration in human seminal fluid and its relation to sperm quality. Fertil Steril 41:743–747
Bennouna S, Bliss SK, Curiel TJ, Denkers EY (2003) Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol 171:6052–6058
Binks S, Pockley AG (1999) Modulation of leukocyte phagocytic and oxidative burst responses by human seminal plasma. Immunol Invest 28:353–364
Bischof RJ, Brandon MR, Lee CS (1994) Studies on the distribution of immune cells in the uteri of prepubertal and cycling gilts. J Reprod Immunol 26:111–129
Blackmore PF, Beebe SJ, Danforth DR (1990) Progesterone and 17-hydroxy-progesterone: novel stimulators of calcium influx in human sperm. J Biol Chem 265:1376–1380
Blanco AM, Palaoro L, Ahedo MI, Palamas M, Zanchetti F (1992) Phagocytosis of ejaculated spermatozoa. Acta Cytol 36:251–258
Blödow G, Bergfeld J, Kitzig M, Brüssow KP (1990) Steroid hormone levels in follicular fluid of pigs with spontaneous oestrus and synchronised ovulation. Arch Exp Vet Med 44:611–620
Brüssow KP, Kurth J, Blödow G (1988) Electrophysiological studies into uterus motoricity of gilts during estrus, following synchronization of ovulation. Arch Exp Vet Med 42:933–943
Casas I, Sancho S, Briz M, Pinart E, Bussalleu E, Yeste M, Bonet S (2010) Fertility after post-cervical artificial insemination with cryopreserved sperm from boar ejaculates of good and poor freezability. Anim Reprod Sci 118:69–76
Charbonnel B, Kremer M, Gerozissis K, Dray F (1982) Human cervical mucus contains large amounts of prostaglandins. Fertil Steril 38:109–111
Cheng H, Althouse GC, Hsu WH (2001) Prostaglandin F2α added to extended boar semen at processing elicits in vitro myometrial contractility after 72 hours of storage. Theriogenology 55:1901–1906
Claus R (1990) Physiological role of seminal components in the reproductive tract of the female pig. J Reprod Fertil Suppl 40:117–131
Claus R, Schams D (1990) Influence of mating and intra-uterine estradiol infusion on peripheral oxytocin concentrations in the sow. J Endocrinol 126:361–365
Claus R, Ellendorff F, Hoang-Vu C (1989) Spontaneous electromyographic activity throughout the cycle in the sow and its change by intrauterine estrogen infusion during estrus. J Reprod Fertil 87:543–551
Cox CI, Lesse HJ (1997) Retention of functional characteristics by bovine oviduct and uterine epithelia in vitro. Anim Reprod Sci 46:169–178
Curry TE Jr, Osteen KG (2003) The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24:428–465
Das SK, Yano S, Wang J, Edwards DR, Nagase H, Dey SK (1997) Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev Genet 21:44–54
DeMott RP, Lefebvre R, Suarez SS (1995) Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol Reprod 52(6):1395–1403
Edgerton LA, Kaminski MA, Silvia WJ (2000) Effects of progesterone and estradiol on uterine secretion of prostaglandin F2α in response to oxytocin in ovariectomised sows. Biol Reprod 62:365–369
Edström K (2009) The Porcine Cervix: Morphological changes and infiltration of immune cells during oestrus and dioestrus in the sow. Swedish University of Agricultural Sciences, Uppsala
Eiler H, Nalbandov AV (1977) Sex steroids in the follicular fluid and blood plasma during the estrous cycle of pigs. Endocrinology 100:331–338
Eisenbach M (2003) Why are sperm cells phagocytised by leukocytes in the female genital tract? Med Hypotheses 60:590–592
Ekhlasi-Hundrieser M, Gohr K, Wagner A, Tsolova M, Petrunkina A, Töpfer-Petersen E (2005) Spermadhesin AQN1 is a candidate receptor molecule involved in the formation of the oviductal sperm reservoir in the pig. Biol Reprod 73:536–545
Eldridge-White R, Easter RA, Heaton DM, O’Day MB, Petersen GC, Shanks RD, Tarbell MK, Sherwood OD (1989) Hormonal control of the cervix in pregnant gilts. I. Changes in the physical properties of the cervix correlate temporally with elevated serum levels of estrogen and relaxin. Endocrinology 125:2996–3003
Eslaminejad MB, Valojerdi MR, Ashtiani SK, Eftekhari-Yazdi P (2007) Light and electron microscopic study of epithelial cells from the human oviduct and uterus subcultured on extracellular matrix gel. J Reprod Med 52:503–512
Espey LL, Lipner H (1994) Ovulation. In: Knobil E, Neill JD (eds) The physiology of reproduction. Raven Press, New York, pp 725–780
Estienne MJ, Harper AF (2004) Semen characteristics and libido in boars treated repeatedly with PGF2α. J Anim Sci 82:1494–1498
Fazeli A, Duncan AE, Watson PF, Holt WV (1999) Sperm-Oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol Reprod 60:879–886
Finn CA, Porter DG (1975) The uterus. Elek Science, London, pp 149–165
First NL, Short RE, Peters JB, Stratman FW (1968) Transport of boar spermatozoa in estrual and luteal sows. J Anim Sci 27:1032–1036
Flower RJ (2006) Prostaglandins, bioassay and inflammation. Br J Pharmacol 147:182–192
Flowers B, Ziecik AJ, Caruolo EV (1991) Effects of human chorionic gonadotropin on contractile activity of steroid-primed pig myometrium. J Reprod Fertil 92:425–532
Friel AM, O’Reilly MW, Sexton DJ, Morrison JJ (2005) Specific PGF2α receptor (FP) antagonism and human uterine contractility in vitro. BJOG 112:1034–1042
Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C, Negishi H, Liu Z, Richards JS, Shimada M (2011) Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod 26:2799–2806
Galdiero F, Tufano MA, De Martino L, Capasso C, Porta R, Ravagnan G, Peluso G, Metafora S (1989) Inhibition of macrophage phagocytic activity by SV–IV, a major protein secreted from the rat seminal vesicle epithelium. J Reprod Immunol 16:269–284
Gamcik P, Mesaros P, Scvarc F (1980) Influence of prostaglandins on fertility of sheep with the use of deep-frozen sperm. Proceedings of the 9th international conference on animal reproduction and artificial insemination held in Madrid, Spain, vol 3, p 149
Gil J, Chico J, Gil O, López A (1998) Increasing swine prolificacy by adding Dinolytic to semen doses. Abstract. Proceedings of 15th IPVS congress held in Birmingham, UK, p 216
Gilbert RO, Fales MH (1996) The effect of bovine seminal plasma on the function and integrity of bovine neutrophils. Theriogenology 46:649–658
Gorgens A, Leibold W, Klug E, Schuberth HJ, Martinsson G, Zerbe H (2005a) Inseminate components are modulating the chemotactic activity of uterine polymorphonuclear granulocytes (PMN) of mares. Anim Reprod Sci 89:308–310
Gorgens A, Leibold W, Klug E, Schuberth HJ, Martinsson, Zerbe H (2005b) Influence of repeated artificial insemination (IS) on functional properties of uterine neutrophils of mares. Anim Reprod Sci 89:258–261
Gottlieb C, Svanborg K, Eneroth P, Bygdeman M (1988) Effects of prostaglandins on human sperm function and seminal adenosine triphosphate content. Fertil Steril 49:322–327
Green CE, Bredl J, Holt WV, Watson PF, Fazeli A (2001) Carbohydrate mediation of boar sperm binding to oviductal epithelial cells in vitro. Reproduction 122:305–315
Gustaffson B, Edqvist S, Einarsson S, Linge F (1975) Fertility of deep frozen ram semen supplemented with PGF2α. Acta Vet Scand 16:468–470
Gutsche S, von Wolff M, Strowitzki T, Thaler CJ (2003) Seminal plasma induces mRNA expression of IL-1beta, IL-6 and LIF in endometrial epithelial cells in vitro. Mol Hum Reprod 9:785–791
Hafez ESE (1993) Anatomy of female reproductive tract. In: Hafez ESE (ed) Reproduction in farm animals, 6th edn. Lea and Febiger, Philadelphia
Hawk HW (1983) Sperm survival and transport in the female reproductive tract. J Dairy Sci 66:2645–2660
Hazeleger W, Kemp B (1994) Farrowing rate and litter size after transcervical embryo transfer in sows. Reprod Domest Anim 29:481–487
Herrero MB, Viggiano JM, Boquet M, Gimeno MA (1997) Prostaglandin modulation of mouse and human sperm capacitation. Prostaglandins Leukot Essent Fatty Acids 57:279–284
Huleihel M, Lunenfeld E, Horowitz S, Levy A, Potashnik G, Mazor M, Glezerman M (1999) Expression of IL-12, IL-10, PGE2, sIL-2R and sIL-6R in seminal plasma of fertile and infertile men. Andrologia 31:283–288
Huleihel M, Lunenfeld E, Horowitz S, Levy A, Potashnik G, Mazor M, Glezerman M (2000) Involvement of serum and lipopolysaccharide in the production of interleukin-1- and interleukin-6-likemolecules byhumansperm cells. Am J Reprod Immunol 43:41–46
Jeremias JC, Mockel S, Witkin SS (1998) Human semen induces interleukin 10 and 70 kDa heat shock protein gene transcription and inhibits interferon-gamma messenger RNA production in peripheral blood mononuclear cells. Mol Hum Reprod 4:1084–1088
Jeremias JC, Bongiovanni AM, Witkin SS (1999) Induction of heat shock protein expression in cervical epithelial cells by human semen. Infect Dis Obstet Gynecol 7:17–22
Jiwakanon J, Berg M, Persson E, Fossum C, Dalin AM (2010) Cytokine expression in the gilt oviduct: effects of seminal plasma, spermatozoa and extender after insemination. Anim Reprod Sci 119:244–257
Jiwakanon J, Persson E, Berg M, Dalin AM (2011) Influence of seminal plasma, spermatozoa and semen extender on cytokine expression in the porcine endometrium after insemination. Anim Reprod Sci 123:210–220
Joyce CL, Nuzzo NA, Wilson L Jr, Zanevel LJ (1987) Evidence for a role of cyclooxygenase (prostaglandin synthetase) and prostaglandins in the sperm acrosome reaction and fertilization. J Androl 8:74–82
Kaeoket K, Persson E, Dalin AM (2001) The sow endometrium at different stages of the oestrous cycle: studies on morphological changes and infiltration by cells of the immune system. Anim Reprod Sci 65:95–114
Kaeoket K, Persson E, Dalin AM (2002) The influence of pre- and post-ovulatory insemination on sperm distribution in the oviduct, accessory sperm to the zona pellucida, fertilisation rate and embryo development in sows. Anim Reprod Sci 71:239–248
Kaeoket K, Persson E, Dalin AM (2003a) Influence of pre-ovulatory insemination and early pregnancy on the infiltration by cells of the immune system in the sow endometrium. Anim Reprod Sci 75:55–71
Kaeoket K, Persson E, Dalin AM (2003b) Influence of pre-ovulatory insemination and early pregnancy on the distribution of CD2, CD4, CD8 and MHC class II expressing cells in the sow endometrium. Anim Reprod Sci 76:231–244
Kalaydjiev SK, Dimitrova DK, Trifonova NL, Fichorova RN, Masharova NG, Raicheva YN, Simeonova MN, Todorova EI, Todorov VI, Nakov LS (2002) The age-related changes in the incidence of ‘natural’ anti-sperm antibodies suggest they are not auto-/isoantibodies. Am J Reprod Immunol 47:65–71
Katila T (1995) Onset and duration of uterine inflammatory response of mares after insemination with fresh semen. Biol Reprod Mono 1:515–517
Kingsley PJ, Rouzer CA, Saleh S, Marnett LJ (2005) Simultaneous analysis of prostaglandin glyceryl esters and prostaglandins by electrospray tandem mass spectrometry. Ann Biochem 343:203–211
Knobil E, Neill JD (1994) The physiology of reproduction, 2nd edn. Raven Press, New York
Kodithuwakku SP, Miyamoto A, Wijayagunawardane MP (2007) Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells. Reproduction 133:1087–1094
Kos M, Bilkei G (2004) Prostaglandin F2α supplemented semen improves reproductive performance in artificially inseminated sows. Anim Reprod Sci 80:113–120
Kotilainen T, Huhtinen M, Katila T (1994) Sperm-induced leukocytosis in the equine uterus. Theriogenology 41:629–636
Kunavongkrit A, Karlberg K, Einarsson S (1983) The relationship between plasma levels of estradiol-17B, progesterone and the consistency of the cervix in the sow. Theriogenology 20:61–66
Kaczmarek MM, Krawczynski K, Blitek A, Kiewisz J, Schams D, Ziecik AJ (2010) Seminal plasma affects prostaglandin synthesis in the porcine oviduct. Theriogenology 74:1207–1220
Langendijk P, Bouwman EG, Kidson A, Kirkwood RN, Soede NM, Kemp B (2002a) Functions of myometrial activity in sperm transport through the genital tract and fertilisation in sows. Reproduction 123:683–690
Langendijk P, Bouwman EG, Schams D, Soede NM, Kemp B (2002b) Effects of different sexual stimuli on oxytocin release, uterine activity and receptive behavior in estrous sows. Theriogenology 59:849–861
Langendijk P, Bouwman EG, Soede NM, Taverne MAM, Kemp B (2002c) Myometrial activity around estrus in sows: spontaneous activity and effects of estrogens, cloprostenol, seminal plasma and clenbuterol. Theriogenology 57:1563–1577
Langendijk P, Soede NM, Kemp B (2005) Uterine activity, sperm transport, and the role of boar stimuli around insemination in sows. Theriogenology 63:500–513
Levin RJ (2011) Can the controversy about the putative role of the human female orgasm in sperm transport be settled with our current physiological knowledge of coitus? J Sex Med 8:1566–1578
Li JC, Yamaguchi S, Funahashi H (2012) Boar seminal plasma or hen’s egg yolk decrease the in vitro chemotactic and phagocytotic activities of neutrophils when co-incubated with boar or bull sperm. Theriogenology 77:73–80
Lindblom B, Hamberger L, Wiqvist N (1978) Differentiated contractile effects of prostaglandins E and F on the isolated circular and longitudinal smooth muscle of the human oviduct. Fertil Steril 30:553–559
Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, Clapham DE (2012) The control of male fertility by spermatozoan ion channels. Annu Rev Physiol 74:453–475
Liu W, Kelly KA (2008) Prostaglandin E2 modulates dendritic cell function during chlamydial genital infection. Immunology 123:290–303
Lovell JW, Getty R (1968) Fate of semen in the uterus of the sow: histologic study of endometrium during the 27 h after natural service. Am J Vet Res 29:609–625
Madej A, Lang A, Brandt Y, Kindahl H, Madsen MT, Einarsson S (2005) Factors regulating ovarian function in pigs. Domest Anim Endocrinol 29:347–361
Maes DG, Mateusen B, Rijsselaere T, De Vliegher S, Van Soom A, de Kruif A (2003) Motility characteristics of boar spermatozoa after addition of prostaglandin F2α. Theriogenology 60:1435–1443
Maffeo G, Vigo D, Salvo R, Mattioli M, Seren E (1993) Myometrial activity following exposure of estrous gilts to boar pheromone (5 alpha adrost-16-en-3-one). Arch Vet Ital 44:83–91
Margalioth EJ, Bronson RA, Cooper GW (1988) Luteal phase sera and progesterone enhance sperm penetration in the hamster egg assay. Fertil Steril 50:117–122
Martínez EA, Vázquez JM, Roca J, Lucas X, Gil MA, Parrilla I, Vázquez JL, Day BN (2001a) Successful non-surgical deep intrauterine insemination with small numbers of spermatozoa in sows. Reproduction 122:289–296
Martínez EA, Vázquez JM, Roca J, Lucas X, Gil MA, Vázquez JL (2001b) Deep intrauterine insemination and embryo transfer in pigs. Reprod Suppl 58:301–311
Martínez EA, Vázquez JM, Parrilla I, Cuello C, Gil MA, Rodríguez-Martínez H, Roca J, Vázquez JL (2006) Incidence of unilateral fertilizations after low dose deep intrauterine insemination in spontaneously ovulating sows under field conditions. Reprod Domest Anim 41:41–47
Matthijs A, Harkema W, Engel B, Woelders H (2000) In vitro phagocytosis of boar spermatozoa by neutrophils from peripheral blood of sows. J Reprod Fertil 120:265–273
Matthijs A, Engel B, Woelders H (2003) Neutrophil recruitment and phagocytosis of boar spermatozoa after artificial insemination of sows, and the effects of inseminate volume, sperm dose and specific additives in the extender. Reproduction 125:357–367
Mattioli M, Galeati G, Conte F, Seren E (1986) Effect of 5α-androst-16-en-3-one on oxytocin release in oestrous sows. Theriogenology 25:399–403
Mburu JN, Einarsson S, Lundeheim N, Rodríguez-Martínez H (1996) Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim Reprod Sci 45:109–121
Mburu JN, Einarsson S, Kindahl H, Madej A, Rodríguez-Martínez H (1998) Effects of post-ovulatory food deprivation on oviductal sperm concentration, embryo development and hormonal profiles in the pig. Anim Reprod Sci 52:221–234
McMaster MT, Newton RC, Dey SK, Andrews GK (1992) Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol 148:1699–1705
Meredith MJ (1977) Clinical examination of the ovaries and cervix of the sow. Vet Rec 101:70–74
Michael CA, Schofield BM (1969) The influence of the ovarian hormones on the actomyosin content and the development of tension in uterine muscle. J Endocrinol 44:501–511
Mirando MA, Prince BC, Tysseling KA, Carnahan KG, Ludwig TE, Hoagland TA, Crain RC (1995) Proposed role for oxytocin in regulation of endometrial prostaglandin F2α secretion during luteolysis in swine. Adv Exp Med Biol 395:421–433
Moffett A, Loke C (2006) Immunology of placentation in eutherian mammals. Nat Rev Immunol 6:584–594
Moussad EE, Rageh MA, Wilson AK, Geisert RD, Brigstock DR (2002) Temporal and spatial expression of connective tissue growth factor (CCN2; CTGF) and transforming growth factor beta type 1 (TGF-beta1) at the utero-placental interface during early pregnancy in the pig. Mol Pathol 55:186–192
Mwanza AM, Englund P, Kindahl H, Lundeheim N, Einarsson S (2000) Effects of post-ovulatory food deprivation on the hormonal profiles, activity of the oviduct and ova transport in sows. Anim Reprod Sci 59:185–199
Mwanza AM, Razdan P, Hultén F, Einarsson S (2002a) Transport of fertilised and unfertilised ova in sows. Anim Reprod Sci 74:69–74
Mwanza AM, Einarsson S, Madej A, Lundeheim N, Rodríguez-Martínez H, Kindahl H (2002b) Postovulatory effect of repeated administration of prostaglandin F2α on the endocrine status, ova transport, binding of accessory spermatozoa to the zona pellucida and embryo development of recently ovulated sows. Theriogenology 58:1111–1124
Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6:173–182
Noguchi M, Yoshioka K, Itoh S, Suzuki C, Arai S, Wada Y, Hasegawa Y, Kaneko H (2010) Peripheral concentrations of inhibin A, ovarian steroids, and gonadotropins associated with follicular development throughout the estrous cycle of the sow. Reproduction 139:153–161
Norrby M (2010) Hormones and sex steroid receptors in the female pig. Effects of mating, artificial insemination, seminal plasma and genistein. Doctoral Thesis. Swedish University of Agricultural Sciences, Uppsala
Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR (2008) Innate Immunity in the female reproductive tract: role of sex hormones in regulating uterine epithelial cell protection against pathogens. Curr Womens Health Rev 4:102–117
O’Day MB, Winn RJ, Easter RA, Dziuk PJ, Sherwood OD (1989) Hormonal control of the cervix in pregnant gilts. II. Relaxin promotes changes in the physical properties of the cervix in ovariectomized hormone-treated pregnant gilts. Endocrinology 125:3004–3010
O’Day-Bowman MB, Winn RJ, Dziuk PJ, Lindley ER, Sherwood OD (1991) Hormonal control of the cervix in pregnant gilts. III. Relaxin’s influence on cervical biochemical properties in ovariectomized hormone-treated pregnant gilts. Endocrinology 129:1967–1975
O’Leary S, Robertson SA, Armstrong DT (2002) The influence of seminal plasma on ovarian function in pigs—a novel inflammatory mechanism? J Reprod Immunol 57:225–238
O’Leary S, Jasper MJ, Warnes GM, Armstrong DT, Robertson SA (2004) Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction 128:237–247
O’Leary S, Armstrong DT, Robertson SA (2011) Transforming growth factor-β (TGFβ) in porcine seminal plasma. Reprod Fertil Dev 23:748–758
Ofek I, Sharon N (1988) Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun 56:539–547
Olson DM, Zaragoza DB, Shalow MC, Cook JL, Mitchell BF, Grigsby P, Hirst J (2003) Myometrial activation and preterm labour: evidence supporting a role for the prostaglandin F receptor. A review. Placenta 24:S47–S54
Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P (2007) Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod 76:958–964
Palladino MA, Savarese MA, Chapman JL, Dughi MK, Plaska D (2008) Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol 60:541–555
Palter SF, Mulayim N, Senturk L, Arici A (2001) Interleukin-8 in the humans fallopian tube. J Clin Endocrinol Metab 86:2660–2667
Pandya IJ, Cohen J (1985) The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil Steril 43:417–421
Par G, Geli J, Kozma N, Varga P, Szekeres-Bartho J (2003) Progesterone regulates IL12 expression in pregnancy lymphocytes by inhibiting phospholipase A2. Am J Reprod Immunol 49:1–5
Peña FJ, Dominguez JC, Carbajo M, Anel L, Alegre B (1998) Treatment of swine summer infertility syndrome by means of oxytocin under field conditions. Theriogenology 49:829–836
Peña FJ, Dominguez JC, Pelaez J, Alegre B (2000) Intrauterine infusion of PGF2α at insemination enhances reproductive performance of sows during the low fertility season. Vet J 159:259–261
Pérez-Martínez S, Franchi AM, Viggiano JM, Herrero MB, Gimeno M (1998) Effect of prostaglandin F2α (PGF2α) on oviductal nitric oxide synthase (NOS) activity: possible role of endogenous NO on PGF2α-induced contractions in rat oviduct. Prost Other Lipid Mediat 56:155–166
Pursel VG, Schulman LL, Johnson LA (1978) Distribution and morphology of fresh and frozen-thawed sperm in the reproductive tract of gilts after artificial insemination. Biol Reprod 19:69–76
Razdan P, Mwanza A, Kindahl H, Hultén F, Einarsson S (2001) Effects of post-ovulatory food deprivation on ova transport, hormonal profiles and metabolic changes in sows. Acta Vet Scand 42:45–55
Rigby JP (1967) The cervix of the sow during estrous. Vet Rec 80:672–675
Robertson SA (2005) Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 322:43–52
Robertson SA (2007) Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci 85:E36–E44
Robertson SA, Mau VJ, Tremellen KP, Seamark RF (1996) Role of high molecular weight seminal vesicle proteins in eliciting the uterine inflammatory response to semen in mice. J Reprod Fertil 107:265–277
Robertson SA, O’Connell AC, Hudson SN, Seamark RF (2000) Granulocyte-macrophage colony-stimulating factor (GM-CSF) targets myeloid leukocytes in the uterus during the post-mating inflammatory response in mice. J Reprod Immunol 46:131–154
Robertson SA, O’Leary S, Armstrong DT (2006) Influence of semen on inflammatory modulators of embryo implantation. Soc Reprod Fertil Suppl 62:231–245
Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS (2009) Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 80:1036–1045
Rodríguez-Martínez H (2007) Role of the oviduct in sperm capacitation. Theriogenology 68(Suppl 1):S138–S146
Rodríguez-Martínez H, Nicander L, Viring S, Einarsson S, Larsson K (1990) Ultrastructure of the uterotubal junction in preovulatory pigs. Anat Histol Embryol 19:16–36
Rodríguez-Martínez H, Saravia F, Wallgren M, Tienthai P, Johannisson A, Vázquez JM, Martínez E, Roca J, Sanz L, Calvete JJ (2005) Boar spermatozoa in the oviduct. Theriogenology 63:514–535
Rodríguez-Martínez H, Saravia F, Wallgren M, Martínez EA, Sanz L, Roca J, Vázquez JM, Calvete JJ (2010) Spermadhesin PSP-I/PSP-II heterodimer induces migration of polymorphonuclear neutrophils into the uterine cavity of the sow. J Reprod Immunol 84:57–65
Romek M, Karasinski J (2011) Quantification of connexin43 gap junctions in porcine myometrium by confocal microscopy and stereology. Reprod Domest Anim 46:29–38
Rousseau JP, Ménézo Y (1993) Role of the female genital tract in the transport and survival of gametes and the fertilized egg. In: Thibault C, Levasseur MC, Hunter RHF (eds) Reproduction in mammals and man. Ellipses, Paris, pp 369–386
Roy AC, Ratnam SS (1992) Biosynthesis of prostaglandins by human spermatozoa in vitro and their role in acrosome reaction and fertilization. Mol Reprod Dev 33:303–306
Rozeboom KJ, Troedsson MH, Crabo BG (1998) Characterization of uterine leukocyte infiltration in gilts after artificial insemination. J Reprod Fertil 114:195–199
Rozeboom KJ, Troedsson MH, Molitor TW, Crabo BG (1999) The effect of spermatozoa and seminal plasma on leukocyte migration into the uterus of gilts. J Anim Sci 77:2201–2206
Rozeboom KJ, Troedsson MH, Rocha GR, Crabo BG (2001) The chemotactic properties of porcine seminal components toward neutrophils in vitro. J Anim Sci 79:996–1002
Rubtsov YP, Rudensky AY (2007) TGF-β signalling in control of T cell-mediated self-reactivity. Nat Rev Immunol 7:443–453
Schnurrbusch U, Erices J (1979) Results obtained from histochemical and biochemical examinations of uterus of gilts during estric cycle. Arch Exp Vet Med 33:909–932
Schuberth HJ, Taylor U, Zerbe H, Waberski D, Hunter R, Rath D (2008) Immunological responses to semen in the female genital tract. Theriogenology 70:1174–1181
Shalev Y, Shemesh M, Levinshal T, Marcus S, Breibart H (1994) Localization of cyclooxygenase and production of prostaglandins in bovine spermatozoa. J Reprod Fertil 101:405–413
Shille VM, Karlbom I, Einarsson S, Larsson K, Kindahl H, Edqvist LE (1979) Concentrations of progesterone and 15-keto-13,14-dihydroprostaglandin F2α in peripheral plasma during the estrous cycle and early pregnancy in gilts. Zentralbl Veterinarmed A 26:169–181
Shimizu Y, Yorimitsu A, Maruyama Y, Kubota T, Aso T, Bronson RA (1998) Prostaglandins induce calcium influx in human spermatozoa. Mol Hum Reprod 4:555–561
Smith DF, Toft DO (1993) Steroid receptors and their associated proteins. Mol Endocrinol 7:4–11
Smith JC, Nalbandov AV (1958) The role of hormones in the relaxation of the uterine portion of the cervix in swine. Am J Vet Res 19:15–18
Soede NM, Langendijk P, Kemp B (2011) Reproductive cycles in pigs. Anim Reprod Sci 124:251–258
Steinbach J, Smidt D (1970) Cyclical phenomena in the female genital tract of swine—Histological observations. J Anim Sci 30:573–577
Steverink DW, Soede NM, Bouwman EG, Kemp B (1998) Semen backflow after insemination and its effect on fertilisation results in sows. Anim Reprod Sci 54:109–119
Stratman FW, Self HL, Smith VR (1959) The effect of oxytocin on the fertility in gilts artificially inseminated with a low sperm concentration and semen volume. J Anim Sci 18:634–640
Stroband HWJ, Taverne N, Langenfeld K, Barends PMG (1986) The ultrastructure of the uterine epithelium of the pig during the estrous cycle and early pregnancy. Cell Tissue Res 246:81–89
Strzemienski PJ (1989) Effect of bovine seminal plasma on neutrophil phagocytosis of bull spermatozoa. J Reprod Fertil 87:519–528
Suarez SS, Pacey AA (2006) Sperm transport in the female reproductive tract. Hum Reprod Update 12:23–37
Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S (1997) Failure of parturition in mice lacking the prostaglandin F receptor. Science 277:681–683
Sumransap P, Tummaruk P, Kunavongkrit A (2007) Sperm distribution in the reproductive tract of sows after intrauterine insemination. Reprod Domest Anim 42:113–117
Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukoc Biol 55:410–422
Szurop L, Nagy A, Jochle W (1986) Stimulation of libido in pubertal and mature boars with prostaglandin F2α analogs: clinical observations. Reprod Domest Anim 21:83–86
Taneike T, Miyazaki H, Nakamura H, Ohga A (1990) Autonomic innervation of the circular and longitudonal layers in swine myometrium. Biol Reprod 45:831–840
Taylor U, Rath D, Zerbe H, Schuberth HJ (2008) Interaction of Intact Porcine Spermatozoa with epithelial cells and neutrophilic granulocytes during uterine passage. Reprod Domest Anim 43:166–175
Taylor U, Zerbe H, Seyfert HM, Rath D, Schuberth HJ (2009a) Binding of porcine spermatozoa to uterine epithelial cells modulates the female immune response and might indicate the formation of a pre-oviductal sperm reservoir. Soc Reprod Fertil Suppl 66:83–84
Taylor U, Zerbe H, Seyfert HM, Rath D, Baulain U, Langner KF, Schuberth HJ (2009b) Porcine spermatozoa inhibit post-breeding cytokine induction in uterine epithelial cells in vivo. Anim Reprod Sci 115:279–289
Taylor U, Schuberth HJ, Rath D, Michelmann HW, Sauter-Louis C, Zerbe H (2009c) Influence of inseminate components on porcine leucocyte migration in vitro and in vivo after pre- and post-ovulatory insemination. Reprod Domest Anim 44:180–188
Templeton AA, Cooper I, Kelly RC (1978) Prostaglandin concentration in the semen of fertile men. J Reprod Fertil 52:147–150
Thibault Ch, Levasseur MC, Hunter RHF (eds) (1993) Reproduction in mammals and man. Ellipses, Paris
Thilander G, Eriksson H, Edqvist LE, Rodríguez-Martínez H (1990) Progesterone and estradiol-17-β receptors in the porcine myometrium during the estrous cycle. Zentralbl Veterinarmed A 37:321–328
Thomas P, Meizel S (1988) An influx of extracellular calcium is required for initiation of human sperm acrosome reaction induced by human follicular fluid. Gamete Res 20:397–411
Tomlinson MJ, White A, Barratt CL, Bolton AE, Cooke ID (1992) The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocytes? Hum Reprod 7:517–522
Töpfer-Petersen E, Wagner A, Friedrich J, Petrunkina AM, Ekhlasi-Hundrieser M, Waberski D, Drommer W (2002) Function of the mammalian oviductal sperm reservoir. J Exp Zool 292:210–215
Töpfer-Petersen E, Ekhlasi-Hundrieser M, Tsolova M (2008) Glycobiology of fertilization in the pig. Int J Dev Biol 52:717–736
Taverne MAM (1982) Myometrial activity during pregnancy and parturition in the pig. In: Cole DJA, Foxcroft, GR, Eds. Control of Pig Production. Butterworths, London
Tremellen KP, Seamark RF, Robertson SA (1998) Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod 58:1217–1225
Troedsson MH, Desvousges A, Alghamdi AS, Dahms B, Dow CA, Hayna J, Valesco R, Collahan PT, Macpherson ML, Pozor M, Buhi WC (2005) Components in seminal plasma regulating sperm transport and elimination. Anim Reprod Sci 89:171–186
Tummaruk P, Tienthai P (2010) Number of spermatozoa in the crypts of the sperm reservoir at about 24 h after a low-dose intrauterine and deep intrauterine insemination in sows. Reprod Domest Anim 45:208–213
Tummaruk P, Sumransap P, Techakumphu M, Kunavongkrit A (2007) Distribution of spermatozoa and embryos in the female reproductive tract after unilateral deep intra uterine insemination in the pig. Reprod Domest Anim 42:603–609
Vázquez JM, Martínez EA, Roca J, Gil MA, Parrilla I, Cuello C, Parrilla I, Cuello C, Carvajal G, Lucas X, Vázquez JL (2005) Improving the efficiency of sperm technologies in pigs: the value of deep intrauterine insemination. Theriogenology 63:536–547
Verhoeff A, Garfield RE, Ramondt J, Wallenburg HC (1986) Electrical and mechanical uterine activity and gap junctions in estrogen-treated oophorectomized sheep. Am J Obstet Gynecol 155:1192–1196
Viring S, Einarsson S (1980) Influence of boar seminal plasma on the distribution of spermatozoa in the genital tract of gilts. Acta Vet Scand 21:598–606
Viring S, Einarsson S (1981) Sperm distribution within the genital tract of naturally inseminated gilts. Nord Vet Med 33:145–149
Vogelpoel FR, Verhoef J (1985) Activation of polymorphonuclear leukocytes by spermatozoa. Arch Androl 14:123–131
Von Döcke F, Worch H (1963) Investigations into uterine motility and mating reactions of sows. Reprod Domest Anim 7:169–178
Waberski D (1996) Effect of a transcervical infusion of seminal plasma prior to insemination on the fertilising competence of low numbers of boar spermatozoa at controlled AI-ovulation intervals. Anim Reprod Sci 44:165–173
Waberski D, Sudhoff H, Hahn T, Jungblut PW, Kallweit E, Calvete JJ, Ensslin M, Hoppen HO, Wintergalen N, Weitze KF, Töpfer-Petersen E (1995) Advanced ovulation in gilts by the intrauterine application of a low molecular mass pronase-sensitive fraction of boar seminal plasma. J Reprod Fertil 105:247–252
Waberski D, Soares JAG, Dearruda EB, Weitze KF (1996) Fertilizing-capacity of boar spermatozoa related to the application of seminal plasma, the interval AI-ovulation and sperm number. Reprod Domest Anim 31:299
Waberski D, Claassen R, Hahn T, Jungblut PW, Parvizi N, Kallweit E, Weitze KF (1997) LH profile and advancement of ovulation after transcervical infusion of seminal plasma at different stages of oestrus in gilts. J Reprod Fertil 109:29–34
Waberski D, Kremer H, Borchardt Neto G, Jungblut PW, Kallweit E, Weitze KF (1999) Studies on a local effect of boar seminal plasma on ovulation time in gilts. Zentralbl Veterinarmed A 46:431–438
Waberski D, Döhring A, Ardón F, Ritter N, Zerbe H, Schuberth HJ, Hewicker-Trautwein M, Weitze KF, Hunter RH (2006) Physiological routes from intra-uterine seminal contents to advancement of ovulation. Acta Vet Scand 48:13
Wagner A, Ekhlasi-Hundrieser M, Hettel C, Petrunkina A, Waberski D, Nimtz M, Töpfer-Petersen E (2002) Carbohydrate-based interactions of oviductal sperm reservoir formation-studies in the pig. Mol Reprod Dev 61:249–257
Walter I, Bavdek S (1997) Lectin binding pattern of porcine mucosa and endometrium during oestrous cycle. J Anat 190:299–307
Wånggren K, Lalitkumar PG, Stavreus-Evers A, Ståbi B, Gemzell-Danielsson K (2006) Prostaglandin E2 and F2α receptors in the human Fallopian tube before and after mifepristone treatment. Mol Hum Reprod 12:577–585
Wathes DC, Mann GE, Payne JH, Riley PR, Stevenson KR, Lamming GE (1996) Regulation of oxytocin, estradiol and progesterone receptor concentrations in different uterine regions by estradiol, progesterone and oxytocin in ovariectomized ewes. J Endocrinol 151:375–393
Weigel NL (1996) Steroid hormone receptors and their regulation by phosphorylation. Biochem J 319:657–667
Winn RJ, O’Day-Bowman MB, Sherwood OD (1993) Hormonal control of the cervix in pregnant gilts (IV). Relaxin promotes changes in the histological characteristics of the cervix that are associated with cervical softening during late pregnancy in gilts. Endocrinology 133:121–128
Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L (2005) Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 206:306–335
Woelders H, Matthijs A (2001) Phagocytosis of boar spermatozoa in vitro and in vivo. Reprod Suppl 58:113–127
Woelders H, Matthijs JJ, Bouwman EG, Soede NM (2000) Caffeine plus Ca2+ reduces uterine leukocyte recruitment and sperm phagocytosis and improves fertility in pig AI. In: Proceedings of the 14th international congress on animal reproduction, Stockholm 2000. Proc 2, 94; Abstract 15, p 20
Yáñiz JL, López-Gatius F, Hunter RH (2006) Scanning electron microscopy of the functional anatomy of the porcine oviductal mucosa. Anat Histol Embryol 35:28–34
Yeste M (2008) New insights into boar sperm function and survival from integrated laboratory and field studies. PhD Thesis. University of Girona. Girona
Yeste M, Briz M, Pinart E, Sancho S, Garcia-Gil N, Badia E, Bassols J, Pruneda A, Bussalleu E, Casas I, Bonet S (2008) Boar spermatozoa and prostaglandin F2α. Quality of boar sperm after the addition of prostaglandin F2α to the short-term extender over cooling time. Anim Reprod Sci 108:180–195
Yeste M, Lloyd RE, Briz M, Badia E, Bonet S, Holt WV (2009) Direct contact between boar spermatozoa and porcine oviductal epithelial cell (OEC) cultures is needed for optimal sperm survival in vitro. Anim Reprod Sci 113:263–278
Yeste M, Castillo-Martín M, Bonet S, Briz M (2012) Direct binding of boar ejaculate and epididymal spermatozoa to porcine epididymal epithelial cells is also needed to maintain sperm survival in in vitro co-culture. Anim Reprod Sci 131:181–193
Yoshida M, Yoshida K (2011) Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol Hum Reprod 17:457–465
Zhang Q, Collins V, Chakrabarty K, Rose JC, Wu WX (2007) Regulation of the prostaglandin enzymatic system by estradiol and progesterone in nonpregnant sheep cervix. Reproduction 133:1027–1034
Ziecik AJ, Derecka-Reszka KA, Rzucidlo SJ (1992) Extragonadal gonadotropin receptors, their distribution and function. J Physiol Pharmacol 43(Suppl 1):33–49
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Zuccarello D, Ferlin A, Garolla A, Menegazzo M, Perilli L, Ambrosini G, Foresta C (2011) How the human spermatozoa sense the oocyte: a new role of SDF1-CXCR4 signalling. Int J Androl 34:E554–E565
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Yeste, M., Castillo-Martín, M. (2013). Boar Spermatozoa Within the Uterine Environment. In: Bonet, S., Casas, I., Holt, W., Yeste, M. (eds) Boar Reproduction. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-35049-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-35049-8_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-35048-1
Online ISBN: 978-3-642-35049-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)