Abstract
Spider silk is a biopolymer with a huge number of possible textile, technical, and biomedical applications. For several inventions prototype status could be achieved, while most ideas are still of speculative nature. This is especially true for applications which afford quantities of silk which are unrealistic to gain by traditional reeling methods but need industrial large-scale production. This could be realized by production of artificial silk fibers based on recombinant proteins. Recombinant silk production has also the advantage that genetic modifications allow for tailored proteins adapted to specific requirements. These approaches, however, are still thwarted by immense technical challenges due to the size and repetitive nature of silk proteins. Available data so far supports the notion that spider silk is highly cytocompatible and not immunogenic which renders it interesting for biomedical applications. Increasing knowledge about the molecular nature of the fibers and their particular characteristics inspires nanotechnology, e.g., microelectromechanical systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In their unique combination of tensile strength, elasticity, and light weight, spider silk fibers as well as fibers imitating spider silk are considered attractive for a number of technical applications. Low immunogenicity and a high biocompatibility support the notion of an ideal biomaterial which is further encouraged by the fact that spider silk is sterilizable at high temperatures (Gellynck et al. 2008).
Hence humans have been making use of spider silk for thousands of years in both technical and biomedical sense. Several old cultures like the Romans used cobwebs to stop wounds from bleeding (Newman and Newman 1995), and in native societies, like the Aborigines, spider silk was used as fishing lines for small fish, an application still practiced by the people of the Solomon Islands (Heim et al. 2009).
Since then, human use of spider silk has been disregarded, mostly due to the nonindustrial nature of spider silk reeling. Progressive technological developments, however, have prompted the demand of a more sustained production of industrial goods (US Congress 1993) among which the use of biopolymers, like spider silk or derivatives thereof, has a great potential. In contrast to synthetic fibers based on raw materials with limited availability and/or slow and environmental problematic degradation, spider silk in its natural, in processed, or in artificial forms is biodegradable and independent on fossil resources.
In a broader sense spider silk has inspired many adaptive solutions like silk-mimicking polymers or fibrillogenesis. Gosselin and coworkers became inspired to fabricate microfibers from a viscous polymer solution through liquid rope coiling instability which causes viscous solutions like honey to spontaneously form coils on surfaces. The researchers hypothesize that the process induces formation of bonds which equal the hydrogen bonding observed in spider silk fibers (Gosselin et al. 2012). A recently published study copied the beads-on-a-string morphology of flagelliform silk, which comprises the fibers of the capture spiral, by coating poly(dimethylsiloxane) on nylon fibers. The authors could demonstrate that the coating breaks spontaneously into regularly located droplets. The coated fibers demonstrated higher adhesion to glass plates attributed to the higher fiber–substrate contact area (Sahni et al. 2012).
Materials inspired by spider silk must not necessarily be of fibrillar nature as they might be applied as surface coatings or as thin and tough membranes. Silk protein-based foams and hydrogels with defined pore sizes have applications, e.g., as tissue scaffolds. Dispersed to microspheres, silk-derived peptides may be used for time-controlled drug delivery.
In our chapter we will summarize industrial as well as biomedical applications of spider silk and give a short discourse on the production of recombinant spider silk proteins and their use for spinning fibers. Future inventions and applications are discussed briefly.
2 Different Forms of Spider Silk
2.1 Natural Spider Silk
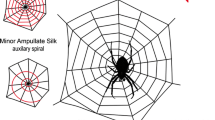
Spiders are unique among silk-producing arthropods as they depend on the production of silk lifelong. An outstanding example is the beauty and sophistication of a spider orb web (Fig. 36.1). Not only adapted to capture prey, these weavings practically serve as a sensual elongation of the spider’s body helping the animal to orient in their environment; to detect prey, enemies, and mates alike; and their webs have to fulfill these different tasks. As a result spider silk evolved as an outstanding fibrous biomaterial consisting almost entirely of large proteins solely encoded by the spider silk fibroin gene family (Gatesy et al. 2001). Spider silk proteins are of a modular nature consisting of nonrepetitive C- and N-terminal sections among which long repetitive parts consisting mainly of four characteristic amino acid motifs (An, GA, GGX, and GPGXn) are found (Gatesy et al. 2001; see also Blackledge 2013). Proteins show remarkable variability depending on allelic variants of which multiples can exist within species and even individual genomes although negative selection pressure keeps protein sizes in a range between 200 and 350 kDa (Chinali et al. 2010). They are expressed in specialized glands located in the opisthosoma of the spider. These glands are connected to the spinnerets by cone-shaped ducts in which the polymerization to fibers takes place. The conformational changes during the spinning process were studied by comparing the Raman spectra of the native silk dope and the dry silk dope probed in the region of the sac of the major ampullate glands of Nephila clavipes (Nephilidae) (Lefevre et al. 2011). This includes arrangement of the silk’s structural elements to pseudocrystalline regions of antiparallel β-sheets interspersed with elastic amorphous segments. This composition is made responsible for the remarkable mechanical properties of likewise strength, ascribed to the crystalline regions, and extensibility. The assembly of silk fibers has been especially well described for the dragline silk which is formed in the major ampullate glands of Araneidae spiders. Silk proteins secreted by specialized epithelial cells of the glands are stored at high concentrations in the lumen of the gland. On their way out, the proteins undergo a number of structural changes in a pH-regulated self-assembly process (reviewed by Heim et al. 2009). Higher flow rates are advantageous for fiber alignment which argues for a mechanical impact of flow and shear forces. Sponner and coworkers demonstrated that among the two proteins which mainly compose Nephila dragline silk, MaSp1 and MaSp2, MaSp2 is predominantly located in core regions of the fibers, whereas MaSp1 appears to be uniformly distributed along the radial axis which might also be important for the spinning process (Sponner et al. 2005).
Technical application of spider silk proteins and biopolymers basically depends on their specific properties resulting from their structure and composition. One of the most striking features of dragline spider silk is its ability to incorporate water into its amorphous parts. This causes entropy-driven recoiling of molecular chains leading to massive but reversible shrinking in contact with water or in a relative humidity greater than 60 % – a phenomenon called supercontraction (Liu et al. 2005). Agnarsson and coworkers exposed single dragline threads from N. clavipes to cyclic changes of humidity resulting in cycles of contraction able to produce an amazing amount of work, e.g., a single 40 mm-long fiber lifted at least 100 mg. The authors propose an energy-efficient and environmentally friendly biomimetic muscle with higher work density and higher stress resistance than most biological muscles (Agnarsson et al. 2009). Assuming a humidity-controlled atmosphere, cyclic spider silk contraction might be used in a variety of applications including sensors and microelectromechanical systems (MEMS) devices and is also interesting in biomedical applications where small steering elements are required (Bai et al. 2006). This is especially interesting as spider silk is the so far only known material which is apt to supercontract at physiological conditions.
Likewise, also a shape–memory effect has been assigned to spider silk. Spider silk fibers return to their original shape independent of external stimuli after a twisting deformation. By comparison, shape–memory nitinol, a nickel–titanium alloy, is needed to be heated to 90 °C under the same experimental conditions before recovery (Emile et al. 2006).
Natural spider silk can be harvested as dragline silk either with special devices, e.g., reeling machines (Fig. 36.2). Other possibilities include harvest of egg sac silk which can be used naturally or as regenerated silk. In the latter case natural silk fibers are solubilized to fibroin extracts which are subsequently processed into artificial fibers or foams. As an example Gellynck and coworkers used 9-M LiBr to dissolve egg sac silk, while hexafluoro-2-propanol and concentrated formic acid are also applicable. Production of scaffolds might include electrospinning procedures and salt-leaching processes (Gellynck et al. 2008; Zhou et al. 2008).
An example for a spider silk reeling machine as developed together by the Leibniz University Hannover and the Medical School Hannover. (a) The reeling machine is equipped with a clamping system (A) which can hold devices like spools or frames (B). The spider (C) is fixed by a piece of tissue and pins before the reeling machine. While the device fastened to the reeling machine rotates, dragline silk is pulled out of the spider. (b) A steel frame cross-weaved with spider dragline silk. (c) Scaffolds of cross-weaved spider silk can be used to study cellular growth and migration of spider silk fibers or for skin tissue engineering
2.2 Recombinant Spider Silk
Although cocoon silk from silk moths has been harvested for over 5,000 years, the domestication of spiders for large-scale silk collection is still impractical due to the animals’ territorial and cannibalistic nature. Additionally, the amount of silk which can be harvested from one single animal is six times lower in araneids than in the silkworms (Bombyx mori). Natural spider silk is subjected to considerable variability, e.g., to spinning forces and humidity which might be the reason for the remarkable differences in tensile strength stated in the published literature (Perez-Rigueiro et al. 2005).
A possible solution for this problem is the development of efficient strategies for synthetic spider silk production. The repetitive sequences encoding for the core structure of a fibroin protein were first identified for major ampullate silk of Nephila clavipes. A number of sequences for different types of silks and species followed as summarized, e.g., by Swanson et al. (2009). Transgenic systems used for production of spider silk are manifold and include bacteria, yeast, plants, silkworm, and mice (reviewed by Heim et al. (2009).
A number of studies concentrated on the production of spider silk in bacteria (see Heim et al. 2009 for an overview). The repetitive nature of spider silk caused a challenge to its biotechnological production, first in the construction of the appropriate expression systems as repetitive sequences are impossible to amplify by polymerase chain reaction and corresponding mRNAs are prone to obstructive secondary structures, second in the difficulty to produce sufficiently sized proteins as a consequence of tRNA depletion. In their pioneering work the work group around T. Scheibel developed a cloning strategy of seamlessly joining solid-phase synthesized oligonucleotides which allowed also for the adaption of codon usage (Heim et al. 2009). In their fundamental work, Lazaris and coworkers described the first successful attempt to produce spider silk protein in mammalian cell lines, e.g., baby hamster kidney cells (BHK). Lower molecular weight products predominated possibly due to inefficient transcription allegeable to complex secondary structures or to specific tRNA shortage in these highly repetitive genes. The protein was spun into fibers with a considerable tensile strength of 2.26 gpd (grams per denier: a unit which corresponds to the ultimate tensile strength) at its highest, but the values are still essentially lower than native spider silk which reportedly ranges between 7 and 11 gpd (Lazaris et al. 2002).
Spider silk production in the milk of transgenic goats has been proposed, although no results have been published so far. In spite of that, transgenic mice were generated expressing an artificial dragline analogue in their milk (cited after Heim et al. 2009).
Scheller et al. expressed synthetic genes in transgenic tobacco and potato plants. To this end, fragments adjusted to N. clavipes MaSp1 sequence were assembled to large units exploiting the modular nature of silk albeit lacking the nonrepetitive 3′ terminus of natural silk. The expressed proteins accumulated stably in the endoplasmic reticulum of leave and tuber cells and could be purified by chemical extraction followed by a heating step (Scheller et al. 2001). Xia et al. (2010) demonstrated that the strength of recombinantly produced spider silk fibers depends on the monomer proteins.
Recombinant spider silk production has been filed in a number of patents, and industrial production is currently arising. The chemical company DuPont has been interested in the production of artificial spider silk for 20 years. They recently filed a patent together with Toray Industries in which they claim a silk thread produced by transgenic silkworm with an intact fibroin H gene. The hybrid thread might contain spider silk dispersed in the silkworm fibroin or else fused to a polypeptide of the fibroin chain protein or might be inserted between the N-terminal and the C-terminal parts of the fibroin chain protein forming cysteine disulfide bridges fibroin L (Pat. No. US 2008287651 A1). EntoGenetics is also relying on transgenic silkworms raised for ballistic textile production. In their approach insertion of the appropriate spider DNA sequence into the silkworm gene replaces a significant portion of the native fibroin (Pat. No. EP 2244563 A1). Sigma-Aldrich has developed an approach to target specific sequences in the silkworm genome by CompoZr zinc finger nuclease technology (Pat. No. US 2011023154 A1). Together with Kraig Biocraft Laboratories, they announced a project to insert spider silk sequence, for instance, derived from the flagelliform gene to generate hybrid genes. Nexia Biotechnologies Ltd. developed an approach to generate transgenic mammals which they intended to use for production of recombinant spider silk in goats’ milk. Their projects were passed to the Advanced Foods and Materials Network since.
For scientific purposes, recombinant spider silk protein generally is intended for spider silk characterization, e.g., for structural investigations. These studies use variations of domain compositions to investigate the influence of protein size or multimer compositions on physicochemical and biomechanical properties or self-assembly into fibers. Other studies exploit the cytocompatible nature of silk for tissue engineering (reviewed by Hsia et al. 2011). The latter application does not necessarily depend on the production of strong fibers but makes use of different formats adapted to the requirements. Chemical and physical parameters, including temperature, pressure, solvents, and composition of the silk proteins, influence the morphology of the protein assemblies. Biomedical interesting forms include capsules and spheres usable as carriers and films and foams for tissue engineering purposes or as biomedical devices like dressings and implants (reviewed by Spiess et al. 2010). As an example, scaffolds of variable porosity were molded by a leaching process using recombinant spider silk proteins, in this case N. clavipes spidroin 1 produced in the yeast Pichia pastoris (Agapov et al. 2009).
2.3 Recombinant Proteins as Chimeric Proteins
An obvious advantage of recombinant spider silk proteins is the possibility to combine spider silk with foreign elements or design variations of the natural sequence to alter its properties. This kind of protein engineering facilitates certain steps of production or allows for adaption to specific applications. Such either the physicochemical properties may be changed or bioactive molecules may be integrated. As an example for the latter, Bini et al. (2006) inserted a CRGD motif into their 15mers based on Nephila clavipes MaSp1 to enhance cellular adhesion on their biomaterial. The RGD motif was originally identified on fibronectin and is recognized by cellular adhesion molecules, the integrins. The resulting recombinant protein tended to oligomerize due to the inserted Cys residues and showed enhanced solubility in water. Bone marrow-derived stem cells grown on silk films comprised of the recombinant proteins were able to differentiate into the osteogenic lineage although the measured calcium content stayed behind the respective controls of unmodified 15mers. 15mer repeats fused to recombinant dentin matrix protein (DMP)1 had calcium hydroxyapatite nucleating ability comparable like DMP1 expressed in hard tissues (Huang et al. 2007).
Beside combining spider silk segments with fibroin-derived stretches, Scheller and coworkers also generated a chimeric construct of spider silk and an elastin-based repetitive biopolymer with 100 repeats of the pentapeptide VPGXaaG with Xaa standing for either G, V, or A. Human chondrocytes kept on monolayer coating displayed higher cell number in cultures coated with the spider silk-elastin chimera than in any of the included controls. Additionally the cell morphology remained rounded instead of increasing flatness as a sign of dedifferentiation in two-dimensional culture (Scheller et al. 2004).
3 Cytocompatibility and Biocompatibility
Safety concerns and the wish to implicate silks in clinical use and as a biomaterial and scaffold for tissue engineering have inspired a number of studies describing cell and body reactions to silk exposure.
Widhe and coworkers used matrices from recombinant spider silk protein consisting of five glycine-rich alternating with four polyalanine stretches connected to a nonrepetitive globular C-terminal domain by a serine- and alanine-rich linker. The recombinant protein produced in Escherichia coli self-assembled into fibers which were also used to produce meshes and was cast into films and foams. The primary human fibroblasts used in this study were able to attach and grow on all different matrices almost alike, reaching highest cell counts on combinations of films and meshes (Widhe et al. 2010).
Recombinant protein based on spidroin 1 could be processed into three-dimensional porous scaffolds proposed for use in tissue engineering. 3T3 fibroblasts colonized the 700 μm-thick specimen from the surface, filling the deeper layers through channels between the pores after 14 days (Agapov et al. 2009).
In contrast to a number of studies describing production and cytocompatibility of artificially produced silks, studies reporting of the biocompatibility of native spider silk are limited. In their basic work concerning the local response to spider silk, Vollrath and coworkers implanted different forms of spider silk in pigs. At the same time spider silks and clinically established wound dressings were used for split-skin wounds over a 15-day period. Implantations were accompanied by lymphoplasmacellular infiltrations and a high number of giant cells; granulomatous reaction, however, was limited. Immune reaction to epicutaneously used spider silk was also low and comparable to controls. Nevertheless, any advantages by spider silk treatment could not be observed over the duration of the experiment (Vollrath et al. 2002).
In their study about the use of silk scaffolds for chondrocyte cultivation, Gellynck and coworkers processed silkworm cocoon silk, Araneus diadematus (Araneidae) dragline and egg sac silk. They produced nonwoven scaffolds from cocoon and egg sac silk by stitching the raw material on yarn grids before processing them to give pure silk fibers. Due to mild processing conditions, fiber structures were kept intact and chondrocytes could be observed on them for up to 6 weeks after seeding. Both types of silk were also completely dissolved and mended into salt-leached porous scaffolds in which the growth patterns of the cells could be influenced by pore sizes after silk particle size (Gellynck et al. 2008).
Human Schwann cells isolated from peripheral nerves adhered sufficiently on native spider silk fibers with high vitality rates after 48 h excluding any toxic or harmful effects on the cells. The silk could be collected directly from the major ampullate gland of adult female Nephila spiders and was used without any further treatment. A nerve conduit was developed based on these data consisting of spider silk fibers aligned within a decellularized vein in which the supplemented Schwann cells aligned along the fibers (Allmeling et al. 2006).
4 Medical Applications
4.1 Spider Silk as a Biomaterial for Surgical Sutures and in Regenerative Medicine
Biomaterials in use for biomedical applications have to meet a number of requirements like biocompatibility and a mild inflammatory response, biodegradability in a reasonable time, and specific structural and mechanical properties. Tensile strength and elasticity of spider silk fibers render them interesting for surgical sutures and tissue engineering purposes (Fig. 36.3), while its biocompatibility is a prerequisite for clinical application.
Despite the fact that silk-based sutures derived from B. mori cocoons have been used for over 100 years, questionable biocompatibility has led to displacement by synthetic materials including polydioxanone, polypropylene, and nylon. Nevertheless, silk sutures which provoke a low inflammatory response are still of clinical interest due to their beneficial biodegradability and their mechanical properties. Our group has used spider silk for braiding sutures of about 25 μm corresponding to microsurgical suture material 10–0 after USP definition. The sutures had a tensile strength of 0.7–0.8 MPa depending on the number of strands braided and a stress–strain ratio of 0.13 MPa, while 0.3 MPa tensile strength and 0.08 MPa stress–strain ratio were measured for commercial nylon suture (Kuhbier et al. 2011).
RGD modified 16mers and 32mers recombinantly produced in E. coli have been used as a wound dressing in a rat model of second-degree burn wounds. Healing of the burns was compared to equal wounds treated with collagen sponges or left untreated as a negative control. Spider silk proteins significantly promoted wound healing to a regenerated skin which appeared like normal skin on day 21. Differences to the collagen-treated groups, however, were only marginal, constricted mainly to an early reduction of immune cell infiltration (Baoyong et al. 2010).
Our group showed that natural dragline silk incorporated into isogenic or decellularized veins promoted peripheral nerve regeneration. Axon regrowth and remyelination were achieved, replacing 20 mm sciatic nerve in rats and 60 mm tibial nerve in adult sheep. The results obtained were comparable to autologous nerve transplants which is the current gold standard in clinical practice (Allmeling et al. 2008; Radtke et al. 2011).
Cross-woven spider silk fibers woven on steel frames provided a model framework for adherent cell growth which we used to study cellular reactions on spider silk (Fig. 36.1). Fibroblasts were seeded on the silk scaffolds and kept in short-term and long-term culture showing metabolic activity and high vitality rates. Observations on single cells were enabled by the single thread structure of the devices demonstrating the spindle-shaped and asymmetrical morphology of migrating cells (Fig. 36.4) (Kuhbier et al. 2010). The concept of cross-woven steel frames was later transferred to skin tissue engineering. The approach resulted in a bilayered structure of epidermis and dermis when corresponding cell lines were cultured at the air–liquid interface to induce skin cell differentiation (Fig. 36.1) (Wendt et al. 2011).
Fibroblasts adhere to spider silk. (a) Fibroblasts growing on spider silk have been stained with calcein AM/ethidium heterodimer. Green fluorescence indicates cell vitality. Dead cells, which would show red fluorescent nuclei due to the incorporation of ethidium heterodimer into the chromosomal DNA, are not visible. (b) Scanning electron microscopy of fibroblasts attached to spider silk fibers
The British company Oxford Biomaterials developed an approach to adapt silkworm protein to spider silk properties. Their material was called Spidrex® and is tested in number of biomedical products in form of spinout ventures: Neurotex Ltd. is transferring Spidrex® technology into nerve repair, Suturox Ltd. is focusing on Spidrex®-based sutures, and Orthox Ltd. takes advantage of the material’s biomechanical properties for bone and cartilage repair.
4.2 Spider Silk as Drug Delivery Vehicles
Drug delivery devices for controlled release of bioactive molecules are of potential interest for pharmaceutical, cosmetic, and food industry (Fig 36.5). Lammel and coworkers used engineered spider silk particles eADF4(C16) mimicking part of the ADF4 silk protein from Araneus diadematus. The generation of such particles can be controlled by potassium phosphate concentration which is an important advantage in biomedical application where often sensitive constituents are involved. At physiological pH these particles were negatively charged such as small molecules with positive net charge can diffuse into the interior. When loading is complete, transfer into aqueous or acidic release media might induce redistribution of the load to the surface accompanied by a constant release (Lammel et al. 2011).
The group of Kaplan made use of a spider silk repeat unit based on a consensus repeat derived from Nephila clavipes major ampullate spidroin They expressed the repeats as a 6mer in a fusion with a poly(l-lysine) stretch of 30 residues and different tumor-homing peptides in E. coli. The bioengineered proteins formed complexes with reporter gene carrying plasmids which could be used efficiently for in vitro and in vivo transfection (Numata et al. 2011). In their patent file Kaplan and Wang describe the production of silk fibroin microsphere under mild condition, making use of lipids as microsphere templates (US 2010/0028451 A1).
5 Spider Silk in Textiles and Art
Spider silk might be used as a tissue fiber at least as well as its more common analogue, the silkworm silk. Lightweight in combination with extreme material strength is promising for creating clothing which is conformable and durable. Its exclusiveness made spider silk tissue a symbol of human talent and inspiration for centuries. In the seventeenth and eighteenth centuries, different approaches tried to use spider silk for tissue production. Bon started to experiment with harvest of different silk types among them egg sacs of native spiders. His efforts resulted in knitted gloves with an astonishing weight of 21 g, while a production with common silk weighed 226 g (Peers 2012). Later, French naturalist Réaumur also tried to make stocking or gloves from spider egg sac silk but gave it up as unpractical (Lewis 1996).
Termeyer harvested egg sac silk but was able to reel spider silk also directly with a self-constructed winding machine. Although these trials received some appreciation, tissue production of spider silk could not be brought to industrial scale and was thus terminated (Peers 2012).
Peers and Godley worked with silk of Nephila madagascariensis, weaving a textile brocaded in a traditional Madagascarian way and an embroidered cape using the thread of millions of spiders for both. The resulting shining textiles are of the natural golden color of the undyed silk threads reflecting the light on the skilful patterns created by the artists (Peers 2012).
Spider silk has also inspired music. In their patent file US 2011/0174134 A1 E. und V., Mueller-Zierach describe instrument strings of synthetic spider silk which might especially benefit from the tensile strength of silk fibers. This idea has been realized with native spider silk by Osaki, who used his technical ability to harvest large quantities of spider silk to generate violin strings of distinctive timbre (Osaki 2012).
6 Spider Silk in Technical Applications
Less artificial and more profane applications include fabrics whose assignation depends on their elasticity and ability to absorb large quantities of kinetic energy. Examples are the use as protective clothing and body armor, like knife-resistant gloves and bulletproof vests. In their patent file DE 102009013861 A1, Takata (Petri AG) proposed the use of spider silk for gas bags and safety belt for passenger safety in cars. They want to use natural or recombinant spider silk either alone or in combination with silicone, polyurethane, or polyamides.
Ropes and nets from spider silk or spider silk-like fibers promise a great strength and durability as well as sustainability attractive for modern industrial production. Other conceptions depend less on fiber structures but use silk fibroins for production of films and panels or reinforce composite materials, for instance carriages able to absorb the kinetic energy of accidents. Culpepper suggested artificial spider silk stripes as an ankle support integrated into shoes to prevent involuntary lateral movements and ankle injuries (Pat. No. US7587841). Nevertheless, in domains needing high amounts of starting material, cost-benefit calculations render any use of native spider silk impossible which means development of artificial silk production processes which reliably reproduce spider silk properties is a prerequisite for such applications.
Meanwhile, applications making use of spider silk fibers in miniature have already been realized. As an example, spider silk was used as crosshairs in optical targeting devices such as guns and telescopes until the Second World War (Heim et al. 2009; Lewis 1996).
Silk fibroins are also interesting for production of films and coatings or for nanostructuring of surfaces usable, e.g., for nanoscaled steering devices or sensor systems (Fig. 36.5). In this sense, an invention filed recently describes photonic nanoimprinting of fibroin biopolymer films which might be used for different optical devices, like biophotonic sensors or optofluidic devices, or for biomedical applications including drug delivery and tissue engineering (Pat. No. US 2012/0034291 A1). Nanobioconjugates of gold nanoparticles bound on the surface of silk fibers of Pholcus phalangioides (Pholcidae) show ohmic electronic conduction. Exposition to the vapors of organic solvents, like methanol, leads to linearly enhanced electronic conduction probably due to fiber contraction. Spider silk prepared in such a manner could thus be used as a methanol sensor system (Singh et al. 2007).
7 Conclusion
In conclusion, spider silk is a biopolymer with a huge number of possible textile, technical, and biomedical applications. For several inventions prototype status could be achieved, while most ideas are still of speculative nature. This is especially true for applications which afford quantities of silk which are unrealistic to gain by traditional reeling methods but need industrial large-scale production. This could be realized by production of artificial silk fibers based on recombinant proteins. Recombinant silk production has also the advantage that genetic modifications allow for tailored proteins adapted to specific requirements. These approaches, however, are still thwarted by immense technical challenges due to the size and repetitive nature of silk proteins. Available data so far supports the notion that spider silk is highly cytocompatible and not immunogenic which renders it interesting for biomedical applications. Increasing knowledge about the molecular nature of the fibers and their particular characteristics inspires nanotechnology, e.g., MEMS.
References
Agapov II, Pustovalova OL, Moisenovich MM, Bogush VG, Sokolova OS, Sevastyanov VI, Debabov VG, Kirpichnikov MP (2009) Three-dimensional scaffold made from recombinant spider silk protein for tissue engineering. Dokl Biochem Biophys 426:127–130
Agnarsson I, Dhinojwala A, Sahni V, Blackledge TA (2009) Spider silk as a novel high performance biomimetic muscle driven by humidity. J Exp Biol 212:1990–1994
Allmeling C, Jokuszies A, Reimers K, Kall S, Choi CY, Brandes G, Kasper C, Scheper T, Guggenheim M, Vogt PM (2008) Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif 41:408–420
Allmeling C, Jokuszies A, Reimers K, Kall S, Vogt PM (2006) Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J Cell Mol Med 10:770–777
Bai J, Ma T, Chu W, Wang R, Silva L, Michal C, Chiao JC, Chiao M (2006) Regenerated spider silk as a new biomaterial for MEMS. Biomed Microdevices 8:317–323
Baoyong L, Jian Z, Denglong C, Min L (2010) Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns 36:891–896
Bini E, Foo CW, Huang J, Karageorgiou V, Kitchel B, Kaplan DL (2006) RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules 7:3139–3145
Blackledge TA (2013) Spider silk: molecular structure and function in webs. In: Nentwig W (ed) Spider ecophysiology. Springer, Heidelberg (this volume)
Chinali A, Vater W, Rudakoff B, Sponner A, Unger E, Grosse F, Guehrs KH, Weisshart K (2010) Containment of extended length polymorphisms in silk proteins. J Mol Evol 70:325–338
Emile O, Le Floch A, Vollrath F (2006) Biopolymers: shape memory in spider draglines. Nature 440:621
Gatesy J, Hayashi C, Motriuk D, Woods J, Lewis R (2001) Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science 291:2603–2605
Gellynck K, Verdonk PC, Van Nimmen E, Almqvist KF, Gheysens T, Schoukens G, Van Langenhove L, Kiekens P, Mertens J, Verbruggen G (2008) Silkworm and spider silk scaffolds for chondrocyte support. J Mater Sci Mater Med 19:3399–3409
Gosselin FP, Therriault D, Levesque M (2012) Microfabrication of a spider-silk analogue through the liquid rope coiling instability. Bull Am Physic Soc (APS March Meeting) 57(1):H52
Heim M, Keerl D, Scheibel T (2009) Spider silk: from soluble protein to extraordinary fiber. Angew Chem Int Ed Engl 48:3584–3596
Hsia Y, Gnesa E, Jeffery F, Tang S, Vierra C (2011) Spider silk composites and applications. In: Cuppoletti J (ed) Metal, ceramic and polymeric composites for various uses. InTech, Rijeka
Huang J, Wong C, George A, Kaplan DL (2007) The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials 28: 2358–2367
Kuhbier JW, Allmeling C, Reimers K, Hillmer A, Kasper C, Menger B, Brandes G, Guggenheim M, Vogt PM (2010) Interactions between spider silk and cells—NIH/3T3 fibroblasts seeded on miniature weaving frames. PLoS One 5:e12032
Kuhbier JW, Reimers K, Kasper C, Allmeling C, Hillmer A, Menger B, Vogt PM, Radtke C (2011) First investigation of spider silk as a braided microsurgical suture. J Biomed Mater Res B Appl Biomater 97:381–387
Lammel A, Schwab M, Hofer M, Winter G, Scheibel T (2011) Recombinant spider silk particles as drug delivery vehicles. Biomaterials 32:2233–2240
Lazaris A, Arcidiacono S, Huang Y, Zhou JF, Duguay F, Chretien N, Welsh EA, Soares JW, Karatzas CN (2002) Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science 295:472–476
Lefevre T, Boudreault S, Cloutier C, Pezolet M (2011) Diversity of molecular transformations involved in the formation of spider silks. J Mol Biol 405:238–253
Lewis RV (1996) Unraveling the weave of spider silk. Bioscience 46:636
Liu Y, Shao Z, Vollrath F (2005) Relationships between supercontraction and mechanical properties of spider silk. Nat Mater 4:901–905
Newman J, Newman C (1995) Oh what a tangled web: the medicinal uses of spider silk. Int J Dermatol 34:290–292
Numata K, Reagan MR, Goldstein RH, Rosenblatt M, Kaplan DL (2011) Spider silk-based gene carriers for tumor cell-specific delivery. Bioconjug Chem 22:1605–1610
Osaki S (2012) Spider silk violin strings with a unique packing structure generate a soft and profound timbre. Phys Rev Lett 108:154301
Peers S (2012) Golden spider silk. V&A Publishing, London
Perez-Rigueiro J, Elices M, Plaza G, Real JI, Guinea GV (2005) The effect of spinning forces on spider silk properties. J Exp Biol 208:2633–2639
Radtke C, Allmeling C, Waldmann KH, Reimers K, Thies K, Schenk HC, Hillmer A, Guggenheim M, Brandes G, Vogt PM (2011) Spider silk constructs enhance axonal regeneration and remyelination in long nerve defects in sheep. PLoS One 6:e16990
Sahni V, Labhasetwar DV, Dhinojwala A (2012) Spider silk inspired functional microthreads. Langmuir 28:2206–2210
Scheller J, Guhrs KH, Grosse F, Conrad U (2001) Production of spider silk proteins in tobacco and potato. Nat Biotechnol 19:573–577
Scheller J, Henggeler D, Viviani A, Conrad U (2004) Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res 13: 51–57
Singh A, Hede S, Sastry M (2007) Spider silk as an active scaffold in the assembly of gold nanoparticles and application of the gold-silk bioconjugate in vapor sensing. Small 3:466–473
Spiess K, Lammel A, Scheibel T (2010) Recombinant spider silk proteins for applications in biomaterials. Macromol Biosci 10:998–1007
Sponner A, Unger E, Grosse F, Weisshart K (2005) Differential polymerization of the two main protein components of dragline silk during fibre spinning. Nat Mater 4:772–775
Swanson BO, Anderson SP, Digiovine C, Ross RN, Dorsey JP (2009) The evolution of complex biomaterial performance: the case of spider silk. Integr Comp Biol 49:21–31
US Congress (1993) Biopolymers: making materials nature’s way. Background paper, Office of Technology Assessment, OTA-BP-E-102. US Government Printing Office, Washington DC
Vollrath F, Barth P, Basedow A, Engstrom W, List H (2002) Local tolerance to spider silks and protein polymers in vivo. In Vivo 16:229–234
Wendt H, Hillmer A, Reimers K, Kuhbier JW, Schafer-Nolte F, Allmeling C, Kasper C, Vogt PM (2011) Artificial skin—culturing of different skin cell lines for generating an artificial skin substitute on cross-weaved spider silk fibres. PLoS One 6:e21833
Widhe M, Bysell H, Nystedt S, Schenning I, Malmsten M, Johansson J, Rising A, Hedhammar M (2010) Recombinant spider silk as matrices for cell culture. Biomaterials 31:9575–9585
Xia XX, Qian ZG, Ki CS, Park YH, Kaplan DL, Lee SY (2010) Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc Natl Acad Sci USA 107:14059–14063
Zhou S, Peng H, Yu X, Zheng X, Cui W, Zhang Z, Li X, Wang J, Weng J, Jia W, Li F (2008) Preparation and characterization of a novel electrospun spider silk fibroin/poly(d,l-lactide) composite fiber. J Phys Chem B 112:11209–11216
Acknowledgments
The authors want to thank Kerstin Reimers and Jörn W. Kuhbier for the help with the manuscript and the images. We also thank Stefanie Michael for the beautiful image of a Nephila spider. We are also grateful to Cornelia Kasper and Nicholas Godley for fruitful collaboration and remarkable discussions. We apologize to all authors whose contributions to spider silk research could not be presented due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Allmeling, C., Radtke, C., Vogt, P.M. (2013). Technical and Biomedical Uses of Nature’s Strongest Fiber: Spider Silk. In: Nentwig, W. (eds) Spider Ecophysiology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-33989-9_36
Download citation
DOI: https://doi.org/10.1007/978-3-642-33989-9_36
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33988-2
Online ISBN: 978-3-642-33989-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)